Regulation and Dysregulation of Endothelial Permeability during Systemic Inflammation
Abstract
:1. Introduction
2. Endothelial Barrier Characteristics in Vascular Quiescence and Inflammation
2.1. Composition of the Endothelial Barrier
2.2. Endothelial Permeability and Leukocyte Extravasation
3. Modulation of Endothelial Permeability during Systemic Inflammation—From Mechanisms to Targets
3.1. Characteristics of Inflammation-Induced Leakage
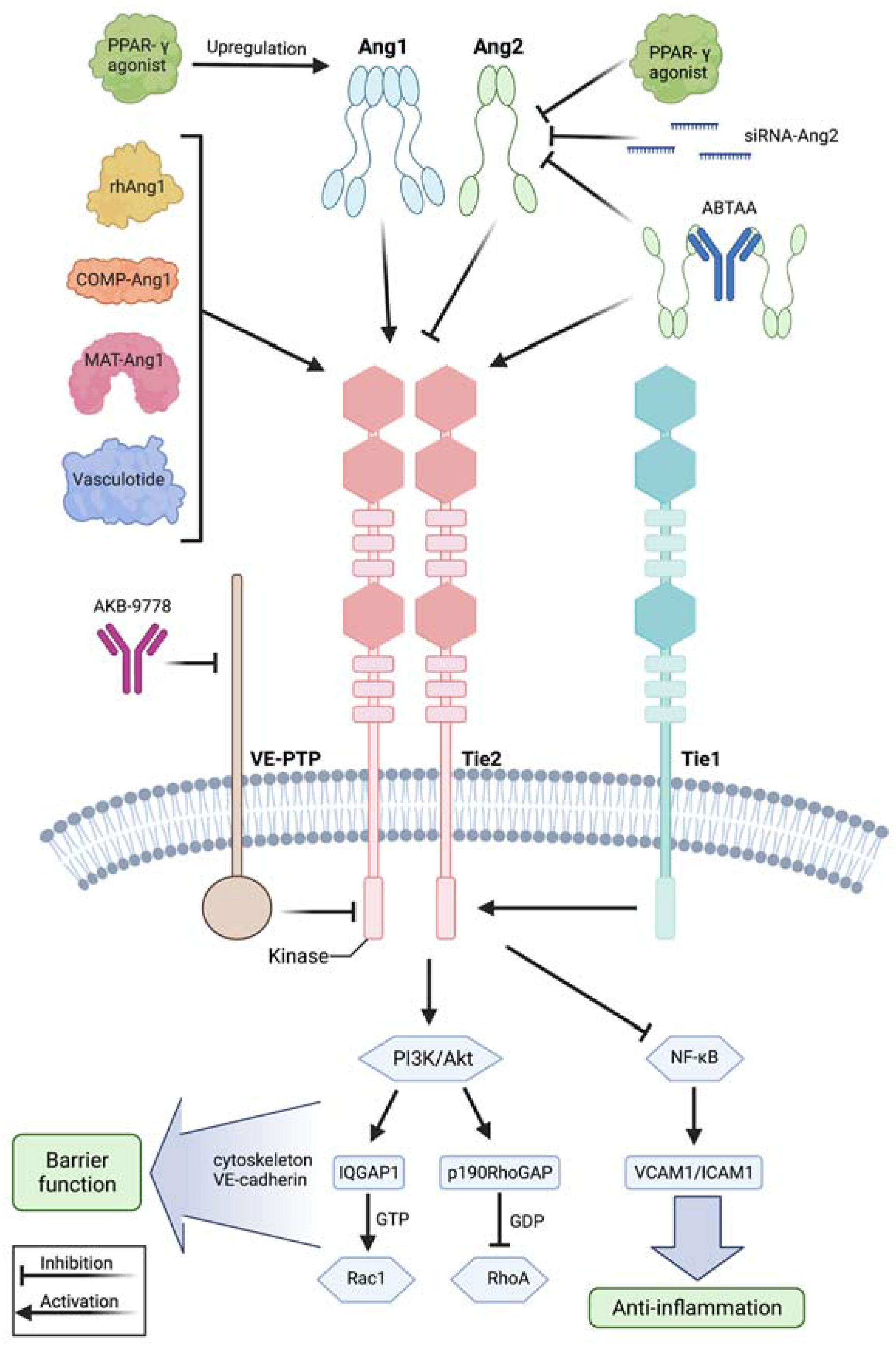
3.2. The Angiopoietin/Tie2 Axis and the Relevance of VE-Cadherin in Protecting Vascular Integrity
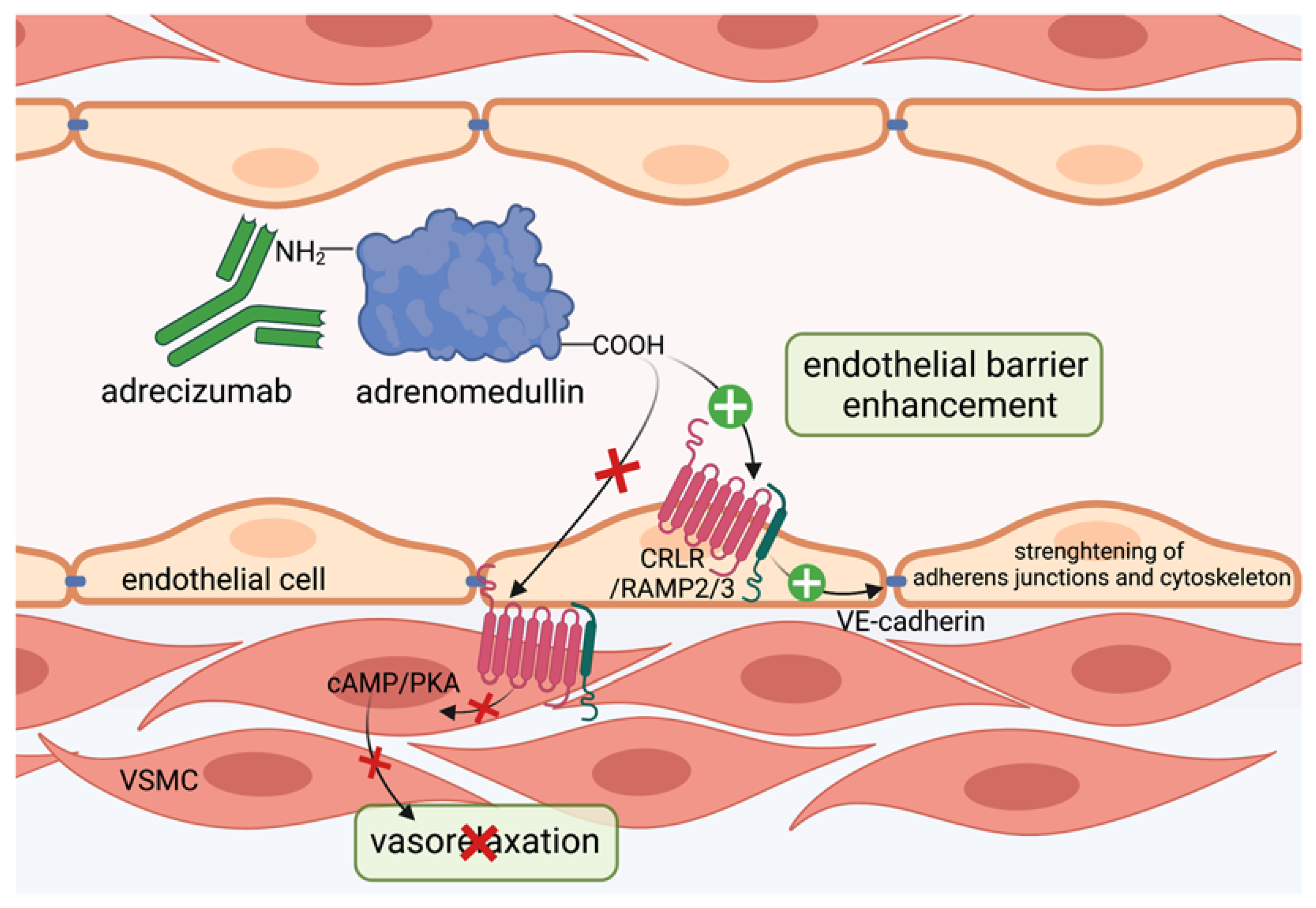
3.3. Targeting Adrenomedullin Protects the Vascular Barrier
3.4. VE-Cadherin as a Target to Seal the Endothelial Barrier
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aird, W.C. The Role of the Endothelium in Severe Sepsis and Multiple Organ Dysfunction Syndrome. Blood 2003, 101, 3765–3777. [Google Scholar] [CrossRef] [PubMed]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef]
- Henneke, P.; Golenbock, D.T. Innate Immune Recognition of Lipopolysaccharide by Endothelial Cells. Crit. Care Med. 2002, 30, S207–S213. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Malbrain, M.L.N.G.; Marik, P.E.; Witters, I.; Cordemans, C.; Kirkpatrick, A.W.; Roberts, D.J.; Van Regenmortel, N. Fluid Overload, de-Resuscitation, and Outcomes in Critically Ill or Injured Patients: A Systematic Review with Suggestions for Clinical Practice. Anaesthesiol. Intensive Ther. 2014, 46, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Teuwen, L.-A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The Vasculature Unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Crawford, T.C.; Magruder, J.T.; Grimm, J.C.; Suarez-Pierre, A.; Sciortino, C.M.; Mandal, K.; Zehr, K.J.; Conte, J.V.; Higgins, R.S.; Cameron, D.E.; et al. Complications After Cardiac Operations: All Are Not Created Equal. Ann. Thorac. Surg. 2017, 103, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Margraf, A.; Ludwig, N.; Zarbock, A.; Rossaint, J. Systemic Inflammatory Response Syndrome After Surgery: Mechanisms and Protection. Anesth. Analg. 2020, 131, 1693–1707. [Google Scholar] [CrossRef]
- Merino, J.G.; Latour, L.L.; Tso, A.; Lee, K.Y.; Kang, D.W.; Davis, L.A.; Lazar, R.M.; Horvath, K.A.; Corso, P.J.; Warach, S. Blood-Brain Barrier Disruption after Cardiac Surgery. Am. J. Neuroradiol. 2013, 34, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Abrahamov, D.; Levran, O.; Naparstek, S.; Refaeli, Y.; Kaptson, S.; Abu Salah, M.; Ishai, Y.; Sahar, G. Blood–Brain Barrier Disruption after Cardiopulmonary Bypass: Diagnosis and Correlation to Cognition. Ann. Thorac. Surg. 2017, 104, 161–169. [Google Scholar] [CrossRef] [Green Version]
- McDonald, D.M. Angiogenesis and Remodeling of Airway Vasculature in Chronic Inflammation. Am. J. Respir. Crit. Care Med. 2001, 164, S39–S45. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Benjamin, L.; Zeng, H.; Dvorak, A.M.; Dvorak, H.F. Vascular Permeability, Vascular Hyperpermeability and Angiogenesis. Angiogenesis 2008, 11, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funamoto, K.; Yoshino, D.; Matsubara, K.; Zervantonakis, I.K.; Funamoto, K.; Nakayama, M.; Masamune, J.; Kimura, Y.; Kamm, R.D. Endothelial Monolayer Permeability under Controlled Oxygen Tension. Integr. Biol. 2017, 9, 529–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, D.; Malik, A.B. Signaling Mechanisms Regulating Endothelial Permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; van der Poll, T. Endothelial Barrier Dysfunction in Septic Shock. J. Intern. Med. 2015, 277, 277–293. [Google Scholar] [CrossRef] [Green Version]
- Weinbaum, S.; Tarbell, J.M.; Damiano, E.R. The Structure and Function of the Endothelial Glycocalyx Layer. Annu. Rev. Biomed. Eng. 2007, 9, 121–167. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.M.J.; oude Egbrink, M.G.A. The Endothelial Glycocalyx: Composition, Functions, and Visualization. Pflüg. Arch.-Eur. J. Physiol. 2007, 454, 345–359. [Google Scholar] [CrossRef] [Green Version]
- Harding, I.C.; Mitra, R.; Mensah, S.A.; Nersesyan, A.; Bal, N.N.; Ebong, E.E. Endothelial Barrier Reinforcement Relies on Flow-Regulated Glycocalyx, a Potential Therapeutic Target. Biorheology 2019, 56, 131–149. [Google Scholar] [CrossRef]
- Pretini, V.; Koenen, M.H.; Kaestner, L.; Fens, M.H.A.M.; Schiffelers, R.M.; Bartels, M.; Van Wijk, R. Red Blood Cells: Chasing Interactions. Front. Physiol. 2019, 10, 945. [Google Scholar] [CrossRef] [Green Version]
- Kolářová, H.; Víteček, J.; Černá, A.; Černík, M.; Přibyl, J.; Skládal, P.; Potěšil, D.; Ihnatová, I.; Zdráhal, Z.; Hampl, A.; et al. Myeloperoxidase Mediated Alteration of Endothelial Function Is Dependent on Its Cationic Charge. Free Radic. Biol. Med. 2021, 162, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ishida, T.; Traub, O.; Coron, M.A.; Berk, B.C. Mechanotransduction in Endothelial Cells: Temporal Signaling Events in Response to Shear Stress. J. Vasc. Res. 1997, 34, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.Y. Protein Kinase Signaling in the Modulation of Microvascular Permeability. Vascul. Pharmacol. 2002, 39, 213–223. [Google Scholar] [CrossRef]
- Languino, L.R.; Gehlsen, K.R.; Wayner, E.; Carter, W.G.; Engvall, E.; Ruoslahti, E. Endothelial Cells Use Alpha 2 Beta 1 Integrin as a Laminin Receptor. J. Cell Biol. 1989, 109, 2455–2462. [Google Scholar] [CrossRef] [Green Version]
- Belkin, A.M.; Stepp, M.A. Integrins as Receptors for Laminins. Microsc. Res. Tech. 2000, 51, 280–301. [Google Scholar] [CrossRef]
- Kevil, C.G.; Okayama, N.; Trocha, S.D.; Kalogeris, T.J.; Coe, L.L.; Specian, R.D.; Davis, C.P.; Alexander, J.S. Expression of Zonula Occludens and Adherens Junctional Proteins in Human Venous and Arterial Endothelial Cells: Role of Occludin in Endothelial Solute Barriers. Microcirculation 1998, 5, 197–210. [Google Scholar] [CrossRef]
- Dejana, E.; Vestweber, D. The Role of VE-Cadherin in Vascular Morphogenesis and Permeability Control. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 116, pp. 119–144. ISBN 978-0-12-394311-8. [Google Scholar]
- Vestweber, D. VE-Cadherin: The Major Endothelial Adhesion Molecule Controlling Cellular Junctions and Blood Vessel Formation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Wallez, Y.; Cand, F.; Cruzalegui, F.; Wernstedt, C.; Souchelnytskyi, S.; Vilgrain, I.; Huber, P. Src Kinase Phosphorylates Vascular Endothelial-Cadherin in Response to Vascular Endothelial Growth Factor: Identification of Tyrosine 685 as the Unique Target Site. Oncogene 2007, 26, 1067–1077. [Google Scholar] [CrossRef] [Green Version]
- Gavard, J. Endothelial Permeability and VE-Cadherin: A Wacky Comradeship. Cell Adhes. Migr. 2014, 8, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Potter, M.D.; Barbero, S.; Cheresh, D.A. Tyrosine Phosphorylation of VE-Cadherin Prevents Binding of P120- and β-Catenin and Maintains the Cellular Mesenchymal State. J. Biol. Chem. 2005, 280, 31906–31912. [Google Scholar] [CrossRef] [Green Version]
- Wessel, F.; Winderlich, M.; Holm, M.; Frye, M.; Rivera-Galdos, R.; Vockel, M.; Linnepe, R.; Ipe, U.; Stadtmann, A.; Zarbock, A.; et al. Leukocyte Extravasation and Vascular Permeability Are Each Controlled in Vivo by Different Tyrosine Residues of VE-Cadherin. Nat. Immunol. 2014, 15, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and Their Regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Mikelis, C.M.; Simaan, M.; Ando, K.; Fukuhara, S.; Sakurai, A.; Amornphimoltham, P.; Masedunskas, A.; Weigert, R.; Chavakis, T.; Adams, R.H.; et al. RhoA and ROCK Mediate Histamine-Induced Vascular Leakage and Anaphylactic Shock. Nat. Commun. 2015, 6, 6725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojciak-Stothard, B.; Ridley, A.J. Rho GTPases and the Regulation of Endothelial Permeability. Vascul. Pharmacol. 2002, 39, 187–199. [Google Scholar] [CrossRef]
- Wettschureck, N.; Strilic, B.; Offermanns, S. Passing the Vascular Barrier: Endothelial Signaling Processes Controlling Extravasation. Physiol. Rev. 2019, 99, 1467–1525. [Google Scholar] [CrossRef]
- Gavard, J.; Patel, V.; Gutkind, J.S. Angiopoietin-1 Prevents VEGF-Induced Endothelial Permeability by Sequestering Src through MDia. Dev. Cell 2008, 14, 25–36. [Google Scholar] [CrossRef] [Green Version]
- David, S.; Ghosh, C.C.; Mukherjee, A.; Parikh, S.M. Angiopoietin-1 Requires IQ Domain GTPase-Activating Protein 1 to Activate Rac1 and Promote Endothelial Barrier Defense. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2643–2652. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, I.; Heemskerk, N.; Kroon, J.; Schaefer, A.; van Rijssel, J.; Hoogenboezem, M.; van Unen, J.; Goedhart, J.; Gadella, T.W.J.; Yin, T.; et al. A Local VE-Cadherin/Trio-Based Signaling Complex Stabilizes Endothelial Junctions through Rac1. J. Cell Sci. 2015, 128, 3041–3054. [Google Scholar] [CrossRef] [Green Version]
- Knezevic, I.I.; Predescu, S.A.; Neamu, R.F.; Gorovoy, M.S.; Knezevic, N.M.; Easington, C.; Malik, A.B.; Predescu, D.N. Tiam1 and Rac1 Are Required for Platelet-Activating Factor-Induced Endothelial Junctional Disassembly and Increase in Vascular Permeability. J. Biol. Chem. 2009, 284, 5381–5394. [Google Scholar] [CrossRef] [Green Version]
- Koh, G.Y. Orchestral Actions of Angiopoietin-1 in Vascular Regeneration. Trends Mol. Med. 2013, 19, 31–39. [Google Scholar] [CrossRef]
- Vestweber, D. How Leukocytes Cross the Vascular Endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Aziz, M.; Wang, P. Role of Reverse Transendothelial Migration of Neutrophils in Inflammation. Biol. Chem. 2016, 397, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Burn, T.; Alvarez, J.I. Reverse Transendothelial Cell Migration in Inflammation: To Help or to Hinder? Cell. Mol. Life Sci. 2017, 74, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Hwa, C.; Aird, W.C. The History of the Capillary Wall: Doctors, Discoveries, and Debates. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H2667–H2679. [Google Scholar] [CrossRef] [Green Version]
- Filewod, N.C.; Lee, W.L. Inflammation without Vascular Leakage. Science Fiction No Longer? Am. J. Respir. Crit. Care Med. 2019, 200, 1472–1476. [Google Scholar] [CrossRef] [Green Version]
- Heemskerk, N.; Schimmel, L.; Oort, C.; van Rijssel, J.; Yin, T.; Ma, B.; van Unen, J.; Pitter, B.; Huveneers, S.; Goedhart, J.; et al. F-Actin-Rich Contractile Endothelial Pores Prevent Vascular Leakage during Leukocyte Diapedesis through Local RhoA Signalling. Nat. Commun. 2016, 7, 10493. [Google Scholar] [CrossRef] [Green Version]
- Phillipson, M.; Kaur, J.; Colarusso, P.; Ballantyne, C.M.; Kubes, P. Endothelial Domes Encapsulate Adherent Neutrophils and Minimize Increases in Vascular Permeability in Paracellular and Transcellular Emigration. PLoS ONE 2008, 3, e1649. [Google Scholar] [CrossRef] [Green Version]
- Petri, B.; Kaur, J.; Long, E.M.; Li, H.; Parsons, S.A.; Butz, S.; Phillipson, M.; Vestweber, D.; Patel, K.D.; Robbins, S.M.; et al. Endothelial LSP1 Is Involved in Endothelial Dome Formation, Minimizing Vascular Permeability Changes during Neutrophil Transmigration in Vivo. Blood 2011, 117, 942–952. [Google Scholar] [CrossRef]
- Sugiyama, M.G.; Armstrong, S.M.; Wang, C.; Hwang, D.; Leong-Poi, H.; Advani, A.; Advani, S.; Zhang, H.; Szaszi, K.; Tabuchi, A.; et al. The Tie2-Agonist Vasculotide Rescues Mice from Influenza Virus Infection. Sci. Rep. 2015, 5, 11030. [Google Scholar] [CrossRef] [Green Version]
- Gutbier, B.; Jiang, X.; Dietert, K.; Ehrler, C.; Lienau, J.; Van Slyke, P.; Kim, H.; Hoang, V.C.; Maynes, J.T.; Dumont, D.J.; et al. Vasculotide Reduces Pulmonary Hyperpermeability in Experimental Pneumococcal Pneumonia. Crit. Care 2017, 21, 274. [Google Scholar] [CrossRef] [Green Version]
- London, N.R.; Zhu, W.; Bozza, F.A.; Smith, M.C.P.; Greif, D.M.; Sorensen, L.K.; Chen, L.; Kaminoh, Y.; Chan, A.C.; Passi, S.F.; et al. Targeting Robo4-Dependent Slit Signaling to Survive the Cytokine Storm in Sepsis and Influenza. Sci. Transl. Med. 2010, 2, 23ra19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tracy, R.P. The Five Cardinal Signs of Inflammation: Calor, Dolor, Rubor, Tumor... and Penuria (Apologies to Aulus Cornelius Celsus, De Medicina, c. A.D. 25). J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1051–1052. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Evolving Functions of Endothelial Cells in Inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the Site of Inflammation: The Leukocyte Adhesion Cascade Updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Hinsbergh, V.W.M.; Collen, A.; Koolwijk, P. Role of Fibrin Matrix in Angiogenesis. Ann. N. Y. Acad. Sci. 2006, 936, 426–437. [Google Scholar] [CrossRef]
- Cueni, L.N.; Detmar, M. The Lymphatic System in Health and Disease. Lymphat. Res. Biol. 2008, 6, 109–122. [Google Scholar] [CrossRef]
- Lee, W.L.; Slutsky, A.S. Sepsis and Endothelial Permeability. N. Engl. J. Med. 2010, 363, 689–691. [Google Scholar] [CrossRef] [Green Version]
- Rossaint, J.; Zarbock, A. Pathogenesis of Multiple Organ Failure in Sepsis. Crit. Rev. Immunol. 2015, 35, 277–291. [Google Scholar] [CrossRef]
- An, R.; Pang, Q.-Y.; Liu, H. Association of Intra-operative Hypotension with Acute Kidney Injury, Myocardial Injury and Mortality in Non-cardiac Surgery: A Meta-analysis. Int. J. Clin. Pract. 2019, 73, e13394. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute Respiratory Distress Syndrome. Nat. Rev. Dis. Primer 2019, 5, 18. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Fakhouri, F.; Dou, L.; Bellien, J.; Burtey, S.; Frimat, M.; Jarrot, P.-A.; Kaplanski, G.; Le Quintrec, M.; Pernin, V.; et al. Endothelium Structure and Function in Kidney Health and Disease. Nat. Rev. Nephrol. 2019, 15, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Gomez, H.; Kellum, J.A. Sepsis-Induced Acute Kidney Injury Revisited: Pathophysiology, Prevention and Future Therapies. Curr. Opin. Crit. Care 2014, 20, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Grams, M.E.; Rabb, H. The Distant Organ Effects of Acute Kidney Injury. Kidney Int. 2012, 81, 942–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, A.A.; Postler, G.; Salhab, K.F.; Mendez, C.; Carey, L.C.; Rabb, H. Renal Ischemia/Reperfusion Leads to Macrophage-Mediated Increase in Pulmonary Vascular Permeability. Kidney Int. 1999, 55, 2362–2367. [Google Scholar] [CrossRef] [Green Version]
- Dumont, D.J.; Yamaguchi, T.P.; Conlon, R.A.; Rossant, J.; Breitman, M.L. Tek, a Novel Tyrosine Kinase Gene Located on Mouse Chromosome 4, Is Expressed in Endothelial Cells and Their Presumptive Precursors. Oncogene 1992, 7, 1471–1480. [Google Scholar]
- Dumont, D.J.; Gradwohl, G.; Fong, G.H.; Puri, M.C.; Gertsenstein, M.; Auerbach, A.; Breitman, M.L. Dominant-Negative and Targeted Null Mutations in the Endothelial Receptor Tyrosine Kinase, Tek, Reveal a Critical Role in Vasculogenesis of the Embryo. Genes Dev. 1994, 8, 1897–1909. [Google Scholar] [CrossRef] [Green Version]
- Parikh, S.M. Angiopoietins and Tie2 in Vascular Inflammation. Curr. Opin. Hematol. 2017, 24, 432–438. [Google Scholar] [CrossRef]
- Leligdowicz, A.; Richard-Greenblatt, M.; Wright, J.; Crowley, V.M.; Kain, K.C. Endothelial Activation: The Ang/Tie Axis in Sepsis. Front. Immunol. 2018, 9, 838. [Google Scholar] [CrossRef] [Green Version]
- Fiedler, U.; Scharpfenecker, M.; Koidl, S.; Hegen, A.; Grunow, V.; Schmidt, J.M.; Kriz, W.; Thurston, G.; Augustin, H.G. The Tie-2 Ligand Angiopoietin-2 Is Stored in and Rapidly Released upon Stimulation from Endothelial Cell Weibel-Palade Bodies. Blood 2004, 103, 4150–4156. [Google Scholar] [CrossRef]
- Mandriota, S.J.; Pepper, M.S. Regulation of Angiopoietin-2 MRNA Levels in Bovine Microvascular Endothelial Cells by Cytokines and Hypoxia. Circ. Res. 1998, 83, 852–859. [Google Scholar] [CrossRef]
- Korhonen, E.A.; Lampinen, A.; Giri, H.; Anisimov, A.; Kim, M.; Allen, B.; Fang, S.; D’Amico, G.; Sipilä, T.J.; Lohela, M.; et al. Tie1 Controls Angiopoietin Function in Vascular Remodeling and Inflammation. J. Clin. Investig. 2016, 126, 3495–3510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, S.; Mukherjee, A.; Ghosh, C.C.; Yano, M.; Khankin, E.V.; Wenger, J.B.; Karumanchi, S.A.; Shapiro, N.I.; Parikh, S.M. Angiopoietin-2 May Contribute to Multiple Organ Dysfunction and Death in Sepsis*. Crit. Care Med. 2012, 40, 3034–3041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, S.M. The Angiopoietin-Tie2 Signaling Axis in Systemic Inflammation. J. Am. Soc. Nephrol. 2017, 28, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, J.S.; Lahni, P.M.; Harmon, K.; Wong, H.R.; Doughty, L.A.; Carcillo, J.A.; Zingarelli, B.; Sukhatme, V.P.; Parikh, S.M.; Wheeler, D.S. Admission Angiopoietin Levels in Children with Septic Shock. Shock Augusta Ga 2007, 28, 650–654. [Google Scholar] [CrossRef]
- Ong, T.; McClintock, D.E.; Kallet, R.H.; Ware, L.B.; Matthay, M.A.; Liu, K.D. Ratio of Angiopoietin-2 to Angiopoietin-1 as a Predictor of Mortality in Acute Lung Injury Patients. Crit. Care Med. 2010, 38, 1845–1851. [Google Scholar] [CrossRef] [Green Version]
- Daly, C.; Wong, V.; Burova, E.; Wei, Y.; Zabski, S.; Griffiths, J.; Lai, K.-M.; Lin, H.C.; Ioffe, E.; Yancopoulos, G.D.; et al. Angiopoietin-1 Modulates Endothelial Cell Function and Gene Expression via the Transcription Factor FKHR (FOXO1). Genes Dev. 2004, 18, 1060–1071. [Google Scholar] [CrossRef] [Green Version]
- Fachinger, G.; Deutsch, U.; Risau, W. Functional Interaction of Vascular Endothelial-Protein-Tyrosine Phosphatase with the Angiopoietin Receptor Tie-2. Oncogene 1999, 18, 5948–5953. [Google Scholar] [CrossRef] [Green Version]
- Mammoto, T.; Parikh, S.M.; Mammoto, A.; Gallagher, D.; Chan, B.; Mostoslavsky, G.; Ingber, D.E.; Sukhatme, V.P. Angiopoietin-1 Requires P190 RhoGAP to Protect against Vascular Leakage in Vivo. J. Biol. Chem. 2007, 282, 23910–23918. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, T.; Horstkotte, J.; Schwab, C.; Pfetsch, V.; Weinmann, K.; Dietzel, S.; Rohwedder, I.; Hinkel, R.; Gross, L.; Lee, S.; et al. Angiopoietin 2 Mediates Microvascular and Hemodynamic Alterations in Sepsis. J. Clin. Investig. 2013, 123, 3436–3445. [Google Scholar] [CrossRef] [Green Version]
- Lukasz, A.; Hillgruber, C.; Oberleithner, H.; Kusche-Vihrog, K.; Pavenstädt, H.; Rovas, A.; Hesse, B.; Goerge, T.; Kümpers, P. Endothelial Glycocalyx Breakdown Is Mediated by Angiopoietin-2. Cardiovasc. Res. 2017, 113, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Gavrilovskaya, I.N.; Gorbunova, E.E.; Mackow, N.A.; Mackow, E.R. Hantaviruses Direct Endothelial Cell Permeability by Sensitizing Cells to the Vascular Permeability Factor VEGF, While Angiopoietin 1 and Sphingosine 1-Phosphate Inhibit Hantavirus-Directed Permeability. J. Virol. 2008, 82, 5797–5806. [Google Scholar] [CrossRef] [Green Version]
- Vuong, N.L.; Lam, P.K.; Ming, D.K.Y.; Duyen, H.T.L.; Nguyen, N.M.; Tam, D.T.H.; Duong Thi Hue, K.; Chau, N.V.; Chanpheaktra, N.; Lum, L.C.S.; et al. Combination of Inflammatory and Vascular Markers in the Febrile Phase of Dengue Is Associated with More Severe Outcomes. eLife 2021, 10, e67460. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, F.E.; Tangpukdee, N.; Opoka, R.O.; Lafferty, E.I.; Rajwans, N.; Hawkes, M.; Krudsood, S.; Looareesuwan, S.; John, C.C.; Liles, W.C.; et al. Serum Angiopoietin-1 and -2 Levels Discriminate Cerebral Malaria from Uncomplicated Malaria and Predict Clinical Outcome in African Children. PLoS ONE 2009, 4, e4912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fremont, R.D.; Koyama, T.; Calfee, C.S.; Wu, W.; Dossett, L.A.; Bossert, F.R.; Mitchell, D.; Wickersham, N.; Bernard, G.R.; Matthay, M.A.; et al. Acute Lung Injury in Patients With Traumatic Injuries: Utility of a Panel of Biomarkers for Diagnosis and Pathogenesis. J. Trauma Inj. Infect. Crit. Care 2010, 68, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, N.J.; Li, M.; Feng, R.; Bradfield, J.; Gallop, R.; Bellamy, S.; Fuchs, B.D.; Lanken, P.N.; Albelda, S.M.; Rushefski, M.; et al. ANGPT2 Genetic Variant Is Associated with Trauma-Associated Acute Lung Injury and Altered Plasma Angiopoietin-2 Isoform Ratio. Am. J. Respir. Crit. Care Med. 2011, 183, 1344–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Heijden, M.; van Nieuw Amerongen, G.P.; van Hinsbergh, V.W.M.; Groeneveld, A.J. The interaction of soluble TIE2 with angiopoietins and pulmonary vascular permeability in septic and nonseptic critically ill patients. Shock 2010, 33, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Witzenbichler, B.; Westermann, D.; Knueppel, S.; Schultheiss, H.-P.; Tschope, C. Protective Role of Angiopoietin-1 in Endotoxic Shock. Circulation 2005, 111, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Jung, Y.J.; Lee, A.S.; Lee, S.; Kang, K.P.; Lee, T.H.; Lee, S.Y.; Jang, K.Y.; Moon, W.S.; Choi, K.-S.; et al. COMP-Angiopoietin-1 Decreases Lipopolysaccharide-Induced Acute Kidney Injury. Kidney Int. 2009, 76, 1180–1191. [Google Scholar] [CrossRef] [Green Version]
- David, S.; Park, J.-K.; van Meurs, M.; Zijlstra, J.G.; Koenecke, C.; Schrimpf, C.; Shushakova, N.; Gueler, F.; Haller, H.; Kümpers, P. Acute Administration of Recombinant Angiopoietin-1 Ameliorates Multiple-Organ Dysfunction Syndrome and Improves Survival in Murine Sepsis. Cytokine 2011, 55, 251–259. [Google Scholar] [CrossRef]
- Alfieri, A.; Watson, J.J.; Kammerer, R.A.; Tasab, M.; Progias, P.; Reeves, K.; Brown, N.J.; Brookes, Z.L. Angiopoietin-1 Variant Reduces LPS-Induced Microvascular Dysfunction in a Murine Model of Sepsis. Crit. Care 2012, 16, R182. [Google Scholar] [CrossRef] [Green Version]
- Serghides, L.; McDonald, C.R.; Lu, Z.; Friedel, M.; Cui, C.; Ho, K.T.; Mount, H.T.J.; Sled, J.G.; Kain, K.C. PPARγ Agonists Improve Survival and Neurocognitive Outcomes in Experimental Cerebral Malaria and Induce Neuroprotective Pathways in Human Malaria. PLoS Pathog. 2014, 10, e1003980. [Google Scholar] [CrossRef] [PubMed]
- Boggild, A.K.; Krudsood, S.; Patel, S.N.; Serghides, L.; Tangpukdee, N.; Katz, K.; Wilairatana, P.; Liles, W.C.; Looareesuwan, S.; Kain, K.C. Use of Peroxisome Proliferator-Activated Receptor γ Agonists as Adjunctive Treatment for Plasmodium Falciparum Malaria: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2009, 49, 841–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiehl, T.; Thamm, K.; Kaufmann, J.; Schaeper, U.; Kirsch, T.; Haller, H.; Santel, A.; Ghosh, C.C.; Parikh, S.M.; David, S. Lung-Targeted RNA Interference Against Angiopoietin-2 Ameliorates Multiple Organ Dysfunction and Death in Sepsis. Crit. Care Med. 2014, 42, e654–e662. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.; Dierkes, M.; Küppers, V.; Vockel, M.; Tomm, J.; Zeuschner, D.; Rossaint, J.; Zarbock, A.; Koh, G.Y.; Peters, K.; et al. Interfering with VE-PTP Stabilizes Endothelial Junctions in Vivo via Tie-2 in the Absence of VE-Cadherin. J. Exp. Med. 2015, 212, 2267–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Frye, M.; Lee, B.L.; Reinardy, J.L.; McClung, J.M.; Ding, K.; Kojima, M.; Xia, H.; Seidel, C.; e Silva, R.L.; et al. Targeting VE-PTP Activates TIE2 and Stabilizes the Ocular Vasculature. J. Clin. Investig. 2014, 124, 4564–4576. [Google Scholar] [CrossRef] [Green Version]
- Campochiaro, P.A.; Sophie, R.; Tolentino, M.; Miller, D.M.; Browning, D.; Boyer, D.S.; Heier, J.S.; Gambino, L.; Withers, B.; Brigell, M.; et al. Treatment of Diabetic Macular Edema with an Inhibitor of Vascular Endothelial-Protein Tyrosine Phosphatase That Activates Tie2. Ophthalmology 2015, 122, 545–554. [Google Scholar] [CrossRef]
- Kümpers, P.; Gueler, F.; David, S.; Van Slyke, P.; Dumont, D.J.; Park, J.-K.; Bockmeyer, C.L.; Parikh, S.M.; Pavenstädt, H.; Haller, H.; et al. The Synthetic Tie2 Agonist Peptide Vasculotide Protects against Vascular Leakage and Reduces Mortality in Murine Abdominal Sepsis. Crit. Care 2011, 15, R261. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Lee, S.-J.; Kim, K.E.; Lee, H.S.; Oh, N.; Park, I.; Ko, E.; Oh, S.J.; Lee, Y.-S.; Kim, D.; et al. Amelioration of Sepsis by TIE2 Activation–Induced Vascular Protection. Sci. Transl. Med. 2016, 8, 335ra55. [Google Scholar] [CrossRef]
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A Novel Hypotensive Peptide Isolated from Human Pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560. [Google Scholar] [CrossRef]
- Ishiyama, Y.; Kitamura, K.; Ichiki, Y.; Nakamura, S.; Kida, O.; Kangawa, K.; Eto, T. Hemodynamic Effects of a Novel Hypotensive Peptide, Human Adrenomedullin, in Rats. Eur. J. Pharmacol. 1993, 241, 271–273. [Google Scholar] [CrossRef]
- Kato, J.; Kitamura, K. Bench-to-Bedside Pharmacology of Adrenomedullin. Eur. J. Pharmacol. 2015, 764, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Caron, K.M.; Smithies, O. Extreme Hydrops Fetalis and Cardiovascular Abnormalities in Mice Lacking a Functional Adrenomedullin Gene. Proc. Natl. Acad. Sci. USA 2001, 98, 615–619. [Google Scholar] [CrossRef]
- Dackor, R.T.; Fritz-Six, K.; Dunworth, W.P.; Gibbons, C.L.; Smithies, O.; Caron, K.M. Hydrops Fetalis, Cardiovascular Defects, and Embryonic Lethality in Mice Lacking the Calcitonin Receptor-Like Receptor Gene. Mol. Cell. Biol. 2006, 26, 2511–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czyzyk, T.A.; Ning, Y.; Hsu, M.-S.; Peng, B.; Mains, R.E.; Eipper, B.A.; Pintar, J.E. Deletion of Peptide Amidation Enzymatic Activity Leads to Edema and Embryonic Lethality in the Mouse. Dev. Biol. 2005, 287, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ-Crain, M.; Morgenthaler, N.G.; Struck, J.; Harbarth, S.; Bergmann, A.; Müller, B. Mid-Regional pro-Adrenomedullin as a Prognostic Marker in Sepsis: An Observational Study. Crit. Care 2005, 9, R816. [Google Scholar] [CrossRef] [Green Version]
- Marino, R.; Struck, J.; Maisel, A.S.; Magrini, L.; Bergmann, A.; Somma, S. Plasma Adrenomedullin Is Associated with Short-Term Mortality and Vasopressor Requirement in Patients Admitted with Sepsis. Crit. Care 2014, 18, R34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, O.H.M.; Bergenzaun, L.; Rydén, J.; Rosenqvist, M.; Melander, O.; Chew, M.S. Adrenomedullin and Endothelin-1 Are Associated with Myocardial Injury and Death in Septic Shock Patients. Crit. Care 2016, 20, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caironi, P.; Latini, R.; Struck, J.; Hartmann, O.; Bergmann, A.; Maggio, G.; Cavana, M.; Tognoni, G.; Pesenti, A.; Gattinoni, L.; et al. Circulating Biologically Active Adrenomedullin (Bio-ADM) Predicts Hemodynamic Support Requirement and Mortality During Sepsis. Chest 2017, 152, 312–320. [Google Scholar] [CrossRef]
- AdrenOSS-1 Study Investigators; Mebazaa, A.; Geven, C.; Hollinger, A.; Wittebole, X.; Chousterman, B.G.; Blet, A.; Gayat, E.; Hartmann, O.; Scigalla, P.; et al. Circulating Adrenomedullin Estimates Survival and Reversibility of Organ Failure in Sepsis: The Prospective Observational Multinational Adrenomedullin and Outcome in Sepsis and Septic Shock-1 (AdrenOSS-1) Study. Crit. Care 2018, 22, 354. [Google Scholar] [CrossRef] [Green Version]
- Hippenstiel, S.; Witzenrath, M.; Schmeck, B.; Hocke, A.; Krisp, M.; Krüll, M.; Seybold, J.; Seeger, W.; Rascher, W.; Schütte, H.; et al. Adrenomedullin Reduces Endothelial Hyperpermeability. Circ. Res. 2002, 91, 618–625. [Google Scholar] [CrossRef] [Green Version]
- Ertmer, C.; Morelli, A.; Rehberg, S.; Lange, M.; Hucklenbruch, C.; Van Aken, H.; Booke, M.; Westphal, M. Exogenous Adrenomedullin Prevents and Reverses Hypodynamic Circulation and Pulmonary Hypertension in Ovine Endotoxaemia. Br. J. Anaesth. 2007, 99, 830–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temmesfeld-Wollbrück, B.; Brell, B.; Dávid, I.; Dorenberg, M.; Adolphs, J.; Schmeck, B.; Suttorp, N.; Hippenstiel, S. Adrenomedullin Reduces Vascular Hyperpermeability and Improves Survival in Rat Septic Shock. Intensive Care Med. 2007, 33, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Sugo, S.; Minamino, N.; Kangawa, K.; Miyamoto, K.; Kitamura, K.; Sakata, J.; Eto, T.; Matsuo, H. Endothelial Cells Actively Synthesize and Secrete Adrenomedullin. Biochem. Biophys. Res. Commun. 1994, 201, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Minamino, N.; Isumi, Y.; Kangawa, K.; Dohi, K.; Matsuo, H. Adrenomedullin Production Is Correlated with Differentiation in Human Leukemia Cell Lines and Peripheral Blood Monocytes. FEBS Lett. 1998, 426, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Kubo, A.; Minamino, N.; Isumi, Y.; Katafuchi, T.; Kangawa, K.; Dohi, K.; Matsuo, H. Production of Adrenomedullin in Macrophage Cell Line and Peritoneal Macrophage. J. Biol. Chem. 1998, 273, 16730–16738. [Google Scholar] [CrossRef] [Green Version]
- Sugo, S.; Minamino, N.; Shoji, H.; Kangawa, K.; Kitamura, K.; Eto, T.; Matsuo, H. Interleukin-1, Tumor Necrosis Factor and Lipopolysaccharide Additively Stimulate Production of Adrenomedullin in Vascular Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 1995, 207, 25–32. [Google Scholar] [CrossRef]
- Smith, D.M.; Coppock, H.A.; Withers, D.J.; Owji, A.A.; Hay, D.L.; Choksi, T.P.; Chakravarty, P.; Legon, S.; Poyner, D.R. Adrenomedullin: Receptor and Signal Transduction. Biochem. Soc. Trans. 2002, 30, 432–437. [Google Scholar] [CrossRef] [Green Version]
- Kuwasako, K.; Kitamura, K.; Nagata, S.; Hikosaka, T.; Takei, Y.; Kato, J. Shared and Separate Functions of the RAMP-Based Adrenomedullin Receptors. Peptides 2011, 32, 1540–1550. [Google Scholar] [CrossRef]
- Meeran, K.; O’Shea, D.; Upton, P.D.; Small, C.J.; Ghatei, M.A.; Byfield, P.H.; Bloom, S.R. Circulating Adrenomedullin Does Not Regulate Systemic Blood Pressure but Increases Plasma Prolactin after Intravenous Infusion in Humans: A Pharmacokinetic Study 1. J. Clin. Endocrinol. Metab. 1997, 82, 95–100. [Google Scholar] [CrossRef]
- Schönauer, R.; Kaiser, A.; Holze, C.; Babilon, S.; Köbberling, J.; Riedl, B.; Beck-Sickinger, A.G. Fluorescently Labeled Adrenomedullin Allows Real-Time Monitoring of Adrenomedullin Receptor Trafficking in Living Cells: Fluorescent Adrenomedullin for Live Cell Receptor Trafficking. J. Pept. Sci. 2015, 21, 905–912. [Google Scholar] [CrossRef]
- Lewis, L.K.; Smith, M.W.; Brennan, S.O.; Yandle, T.G.; Richards, A.M.; Nicholls, M.G. Degradation of Human Adrenomedullin(1-52) by Plasma Membrane Enzymes and Identification of Metabolites. Peptides 1997, 18, 733–739. [Google Scholar] [CrossRef]
- Martínez, A.; Oh, H.-R.; Unsworth, E.J.; Bregonzio, C.; Saavedra, J.M.; Stetler-Stevenson, W.G.; Cuttitta, F. Matrix Metalloproteinase-2 Cleavage of Adrenomedullin Produces a Vasoconstrictor out of a Vasodilator. Biochem. J. 2004, 383, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Lisy, O.; Jougasaki, M.; Schirger, J.A.; Chen, H.H.; Barclay, P.T.; Burnett, J.C. Neutral Endopeptidase Inhibition Potentiates the Natriuretic Actions of Adrenomedullin. Am. J. Physiol.-Ren. Physiol. 1998, 275, F410–F414. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Callejero, L.; Pozo-Rodrigálvarez, A.; Martínez-Murillo, R.; Martínez, A. Lack of Adrenomedullin in Mouse Endothelial Cells Results in Defective Angiogenesis, Enhanced Vascular Permeability, Less Metastasis, and More Brain Damage. Sci. Rep. 2016, 6, 33495. [Google Scholar] [CrossRef]
- Tanaka, M.; Koyama, T.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Kawate, H.; Liu, T.; Xian, X.; Imai, A.; Zhai, L.; et al. The Endothelial Adrenomedullin-RAMP2 System Regulates Vascular Integrity and Suppresses Tumour Metastasis. Cardiovasc. Res. 2016, 111, 398–409. [Google Scholar] [CrossRef]
- Brell, B.; Temmesfeld-Wollbrück, B.; Altzschner, I.; Frisch, E.; Schmeck, B.; Hocke, A.C.; Suttorp, N.; Hippenstiel, S. Adrenomedullin Reduces Staphylococcus Aureus α-Toxin–Induced Rat Ileum Microcirculatory Damage. Crit. Care Med. 2005, 33, 819–826. [Google Scholar] [CrossRef]
- García Ponce, A.; Citalán Madrid, A.F.; Vargas Robles, H.; Chánez Paredes, S.; Nava, P.; Betanzos, A.; Zarbock, A.; Rottner, K.; Vestweber, D.; Schnoor, M. Loss of Cortactin Causes Endothelial Barrier Dysfunction via Disturbed Adrenomedullin Secretion and Actomyosin Contractility. Sci. Rep. 2016, 6, 29003. [Google Scholar] [CrossRef] [Green Version]
- Schnoor, M.; Lai, F.P.L.; Zarbock, A.; Kläver, R.; Polaschegg, C.; Schulte, D.; Weich, H.A.; Oelkers, J.M.; Rottner, K.; Vestweber, D. Cortactin Deficiency Is Associated with Reduced Neutrophil Recruitment but Increased Vascular Permeability in Vivo. J. Exp. Med. 2011, 208, 1721–1735. [Google Scholar] [CrossRef] [Green Version]
- Farmer, P.J.; Bernier, S.G.; Lepage, A.; Guillemette, G.; Regoli, D.; Sirois, P. Permeability of Endothelial Monolayers to Albumin Is Increased by Bradykinin and Inhibited by Prostaglandins. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2001, 280, L732–L738. [Google Scholar] [CrossRef]
- Langeler, E.G.; van Hinsbergh, V.W. Norepinephrine and Iloprost Improve Barrier Function of Human Endothelial Cell Monolayers: Role of CAMP. Am. J. Physiol.-Cell Physiol. 1991, 260, C1052–C1059. [Google Scholar] [CrossRef]
- Stelzner, T.J.; Weil, J.V.; O’Brien, R.F. Role of Cyclic Adenosine Monophosphate in the Induction of Endothelial Barrier Properties. J. Cell. Physiol. 1989, 139, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, P. The Cyclic AMP Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011148. [Google Scholar] [CrossRef] [PubMed]
- Cullere, X.; Shaw, S.K.; Andersson, L.; Hirahashi, J.; Luscinskas, F.W.; Mayadas, T.N. Regulation of Vascular Endothelial Barrier Function by Epac, a CAMP-Activated Exchange Factor for Rap GTPase. Blood 2005, 105, 1950–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park-Windhol, C.; D’Amore, P.A. Disorders of Vascular Permeability. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 251–281. [Google Scholar] [CrossRef]
- Kopperud, R.K.; Rygh, C.B.; Karlsen, T.V.; Krakstad, C.; Kleppe, R.; Hoivik, E.A.; Bakke, M.; Tenstad, O.; Selheim, F.; Lidén, Å.; et al. Increased Microvascular Permeability in Mice Lacking Epac1 (Rapgef3). Acta Physiol. 2017, 219, 441–452. [Google Scholar] [CrossRef]
- Ando, K.; Fukuhara, S.; Moriya, T.; Obara, Y.; Nakahata, N.; Mochizuki, N. Rap1 Potentiates Endothelial Cell Junctions by Spatially Controlling Myosin II Activity and Actin Organization. J. Cell Biol. 2013, 202, 901–916. [Google Scholar] [CrossRef] [Green Version]
- de Kreuk, B.-J.; Gingras, A.R.; Knight, J.D.; Liu, J.J.; Gingras, A.-C.; Ginsberg, M.H. Heart of Glass Anchors Rasip1 at Endothelial Cell-Cell Junctions to Support Vascular Integrity. eLife 2016, 5, e11394. [Google Scholar] [CrossRef]
- Birukova, A.A.; Zagranichnaya, T.; Alekseeva, E.; Bokoch, G.M.; Birukov, K.G. Epac/Rap and PKA Are Novel Mechanisms of ANP-Induced Rac-Mediated Pulmonary Endothelial Barrier Protection. J. Cell. Physiol. 2008, 215, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Birukova, A.A.; Zagranichnaya, T.; Fu, P.; Alekseeva, E.; Chen, W.; Jacobson, J.R.; Birukov, K.G. Prostaglandins PGE2 and PGI2 Promote Endothelial Barrier Enhancement via PKA- and Epac1/Rap1-Dependent Rac Activation. Exp. Cell Res. 2007, 313, 2504–2520. [Google Scholar] [CrossRef] [Green Version]
- Nishimatsu, H.; Suzuki, E.; Nagata, D.; Moriyama, N.; Satonaka, H.; Walsh, K.; Sata, M.; Kangawa, K.; Matsuo, H.; Goto, A.; et al. Adrenomedullin Induces Endothelium-Dependent Vasorelaxation via the Phosphatidylinositol 3-Kinase/Akt–Dependent Pathway in Rat Aorta. Circ. Res. 2001, 89, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, R.; Mitsui-Saito, M.; Ozaki, H.; Karaki, H. Effects of Adrenomedullin and Calcitonin Gene-Related Peptide on Contractions of the Rat Aorta and Porcine Coronary Artery: Adrenomedullin and CGRP in Isolated Vessels. Br. J. Pharmacol. 1998, 123, 1645–1654. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, S.; Hirata, Y.; Iwasaki, H.; Sato, K.; Watanabe, T.X.; Inui, T.; Nakajima, K.; Sakakibara, S.; Marumo, F. Structure-Activity Relationship of Adrenomedullin, a Novel Vasodilatory Peptide, in Cultured Rat Vascular Smooth Muscle Cells. Endocrinology 1994, 135, 2454–2458. [Google Scholar] [CrossRef]
- Geven, C.; Peters, E.; Schroedter, M.; Struck, J.; Bergmann, A.; McCook, O.; Radermacher, P.; Kox, M.; Pickkers, P. Effects of the Humanized Anti-Adrenomedullin Antibody Adrecizumab (HAM8101) on Vascular Barrier Function and Survival in Rodent Models of Systemic Inflammation and Sepsis. Shock 2018, 50, 648–654. [Google Scholar] [CrossRef]
- Pugin, J. Adrenomedullin: A Vasodilator to Treat Sepsis? Crit. Care 2014, 18, 152. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, J.; Caron, A.; Ruël, N. Biodistribution, Plasma Kinetics and Quantification of Single-Pass Pulmonary Clearance of Adrenomedullin. Clin. Sci. 2005, 109, 97–102. [Google Scholar] [CrossRef]
- Watkins, H.; Au, M.; Bobby, R.; Archbold, J.; Abdul-Manan, N.; Moore, J.; Middleditch, M.; Williams, G.; Brimble, M.; Dingley, A.; et al. Identification of Key Residues Involved in Adrenomedullin Binding to the AM 1 Receptor: Key Residues for Adrenomedullin Binding. Br. J. Pharmacol. 2013, 169, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.-L.; Belousoff, M.J.; Fletcher, M.M.; Zhang, X.; Khoshouei, M.; Deganutti, G.; Koole, C.; Furness, S.G.B.; Miller, L.J.; Hay, D.L.; et al. Structure and Dynamics of Adrenomedullin Receptors AM 1 and AM 2 Reveal Key Mechanisms in the Control of Receptor Phenotype by Receptor Activity-Modifying Proteins. ACS Pharmacol. Transl. Sci. 2020, 3, 263–284. [Google Scholar] [CrossRef]
- Struck, J.; Hein, F.; Karasch, S.; Bergmann, A. Epitope Specificity of Anti-Adrenomedullin Antibodies Determines Efficacy of Mortality Reduction in a Cecal Ligation and Puncture Mouse Model. Intensive Care Med. Exp. 2013, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Geven, C.; Bergmann, A.; Kox, M.; Pickkers, P. Vascular Effects of Adrenomedullin and the Anti-Adrenomedullin Antibody Adrecizumab in Sepsis. Shock 2018, 50, 132–140. [Google Scholar] [CrossRef]
- Wagner, K.; Wachter, U.; Vogt, J.A.; Scheuerle, A.; McCook, O.; Weber, S.; Gröger, M.; Stahl, B.; Georgieff, M.; Möller, P.; et al. Adrenomedullin Binding Improves Catecholamine Responsiveness and Kidney Function in Resuscitated Murine Septic Shock. Intensive Care Med. Exp. 2013, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Laterre, P.-F.; Pickkers, P.; Marx, G.; Wittebole, X.; Meziani, F.; Dugernier, T.; Huberlant, V.; Schuerholz, T.; François, B.; Lascarrou, J.-B.; et al. Safety and Tolerability of Non-Neutralizing Adrenomedullin Antibody Adrecizumab (HAM8101) in Septic Shock Patients: The AdrenOSS-2 Phase 2a Biomarker-Guided Trial. Intensive Care Med. 2021, 47, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Besnier, E.; Brakenhielm, E.; Richard, V.; Tamion, F. Does Anti-VEGF Bevacizumab Improve Survival in Experimental Sepsis? Crit. Care 2017, 21, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeger, M.; Tear, G.; Ferres-Marco, D.; Goodman, C.S. Mutations Affecting Growth Cone Guidance in Drosophila: Genes Necessary for Guidance toward or Away from the Midline. Neuron 1993, 10, 409–426. [Google Scholar] [CrossRef]
- Xian, J.; Clark, K.J.; Fordham, R.; Pannell, R.; Rabbitts, T.H.; Rabbitts, P.H. Inadequate Lung Development and Bronchial Hyperplasia in Mice with a Targeted Deletion in the Dutt1/Robo1 Gene. Proc. Natl. Acad. Sci. USA 2001, 98, 15062–15066. [Google Scholar] [CrossRef] [Green Version]
- Grieshammer, U.; Ma, L.; Plump, A.S.; Wang, F.; Tessier-Lavigne, M.; Martin, G.R. SLIT2-Mediated ROBO2 Signaling Restricts Kidney Induction to a Single Site. Dev. Cell 2004, 6, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Sabatier, C.; Plump, A.S.; Ma, L.; Brose, K.; Tamada, A.; Murakami, F.; Lee, E.Y.-H.P.; Tessier-Lavigne, M. The Divergent Robo Family Protein Rig-1/Robo3 Is a Negative Regulator of Slit Responsiveness Required for Midline Crossing by Commissural Axons. Cell 2004, 117, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Medioni, C.; Bertrand, N.; Mesbah, K.; Hudry, B.; Dupays, L.; Wolstein, O.; Washkowitz, A.J.; Papaioannou, V.E.; Mohun, T.J.; Harvey, R.P.; et al. Expression of Slit and Robo Genes in the Developing Mouse Heart. Dev. Dyn. 2010, 239, 3303–3311. [Google Scholar] [CrossRef] [Green Version]
- Meisner, M. Pathobiochemistry and Clinical Use of Procalcitonin. Clin. Chim. Acta 2002, 323, 17–29. [Google Scholar] [CrossRef]
- Assicot, M.; Bohuon, C.; Gendrel, D.; Raymond, J.; Carsin, H.; Guilbaud, J. High Serum Procalcitonin Concentrations in Patients with Sepsis and Infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef]
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin in Sepsis and Systemic Inflammation: A Harmful Biomarker and a Therapeutic Target: Procalcitonin as Marker and Therapeutic Target in Sepsis. Br. J. Pharmacol. 2010, 159, 253–264. [Google Scholar] [CrossRef]
- Arora, S.; Singh, P.; Singh, P.M.; Trikha, A. Procalcitonin Levels in Survivors and Nonsurvivors of Sepsis: Systematic Review and Meta-Analysis. Shock 2015, 43, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Liaskou-Antoniou, L.; Adamis, G.; Panagaki, A.; Melachroinopoulos, N.; Drakou, E.; Marousis, K.; Chrysos, G.; Spyrou, A.; Alexiou, N.; et al. Procalcitonin to Reduce Long-Term Infection-Associated Adverse Events in Sepsis. A Randomized Trial. Am. J. Respir. Crit. Care Med. 2021, 203, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Schuetz, P.; Albrich, W.; Mueller, B. Procalcitonin for Diagnosis of Infection and Guide to Antibiotic Decisions: Past, Present and Future. BMC Med. 2011, 9, 107. [Google Scholar] [CrossRef] [Green Version]
- Müller, B.; White, J.C.; Nylén, E.S.; Snider, R.H.; Becker, K.L.; Habener, J.F. Ubiquitous Expression of the Calcitonin-I Gene in Multiple Tissues in Response to Sepsis 1. J. Clin. Endocrinol. Metab. 2001, 86, 396–404. [Google Scholar] [CrossRef]
- Linscheid, P.; Seboek, D.; Schaer, D.J.; Zulewski, H.; Keller, U.; Müller, B. Expression and Secretion of Procalcitonin and Calcitonin Gene-Related Peptide by Adherent Monocytes and by Macrophage-Activated Adipocytes*. Crit. Care Med. 2004, 32, 1715–1721. [Google Scholar] [CrossRef]
- Nylen, E.S.; Whang, K.T.; Snider, R.H.; Steinwald, P.M.; White, J.C.; Becker, K.L. Mortality Is Increased by Procalcitonin and Decreased by an Antiserum Reactive to Procalcitonin in Experimental Sepsis. Crit. Care Med. 1998, 26, 1001–1006. [Google Scholar] [CrossRef]
- Martinez, J.M.; Wagner, K.E.; Snider, R.H.; Nylen, E.S.; Muller, B.; Sarani, B.; Becker, K.L.; White, J.C. Late Immunoneutralization of Procalcitonin Arrests the Progression of Lethal Porcine Sepsis. Surg. Infect. 2001, 2, 193–203. [Google Scholar] [CrossRef]
- Wagner, K.E.; Martinez, J.M.; Vath, S.D.; Snider, R.H.; Nylén, E.S.; Becker, K.L.; Müller, B.; White, J.C. Early Immunoneutralization of Calcitonin Precursors Attenuates the Adverse Physiologic Response to Sepsis in Pigs. Crit. Care Med. 2002, 30, 2313–2321. [Google Scholar] [CrossRef]
- Tavares, E.; Maldonado, R.; Miñano, F.J. Immunoneutralization of Endogenous Aminoprocalcitonin Attenuates Sepsis-Induced Acute Lung Injury and Mortality in Rats. Am. J. Pathol. 2014, 184, 3069–3083. [Google Scholar] [CrossRef] [PubMed]
- Baranowsky, A.; Appelt, J.; Kleber, C.; Lange, T.; Ludewig, P.; Jahn, D.; Pandey, P.; Keller, D.; Rose, T.; Schetler, D.; et al. Procalcitonin Exerts a Mediator Role in Septic Shock Through the Calcitonin Gene-Related Peptide Receptor. Crit. Care Med. 2021, 49, e41–e52. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.-M.; Van Aken, C.; Butschkau, A.; Bierhansl, L.; Kellner, P.; Schleusener, V.; Seggewiss, J.; Vollmar, B.; Nöldge-Schomburg, G.; Roesner, J.P. Procalcitonin Impairs Endothelial Cell Function and Viability. Anesth. Analg. 2017, 124, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Doß, S.; Ehler, J.; Mencke, T.; Wagner, N.-M. Procalcitonin Impairs Liver Cell Viability and Function In Vitro: A Potential New Mechanism of Liver Dysfunction and Failure during Sepsis? BioMed Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Struck, J.; Strebelow, M.; Tietz, S.; Alonso, C.; Morgenthaler, N.G.; van der Hoeven, J.G.; Pickkers, P.; Bergmann, A. Method for the Selective Measurement of Amino-Terminal Variants of Procalcitonin. Clin. Chem. 2009, 55, 1672–1679. [Google Scholar] [CrossRef] [Green Version]
- Weglöhner, W.; Struck, J.; Fischer-Schulz, C.; Morgenthaler, N.G.; Otto, A.; Bohuon, C.; Bergmann, A. Isolation and Characterization of Serum Procalcitonin from Patients with Sepsis. Peptides 2001, 22, 2099–2103. [Google Scholar] [CrossRef]
- Wrenger, S.; Kähne, T.; Bohuon, C.; Weglöhner, W.; Ansorge, S.; Reinhold, D. Amino-Terminal Truncation of Procalcitonin, a Marker for Systemic Bacterial Infections, by Dipeptidyl Peptidase IV (DP IV). FEBS Lett. 2000, 466, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Sexton, P.M.; Christopoulos, G.; Christopoulos, A.; Nylen, E.S.; Snider, R.H.; Becker, K.L. Procalcitonin Has Bioactivity at Calcitonin Receptor Family Complexes: Potential Mediator Implications in Sepsis*. Crit. Care Med. 2008, 36, 1637–1640. [Google Scholar] [CrossRef]
- Brabenec, L.; Müller, M.; Hellenthal, K.E.; Karsten, O.S.; Pryvalov, H.; Otto, M.; Holthenrich, A.; Matos, A.L.L.; Weiss, R.; Kintrup, S.; et al. Targeting Procalcitonin Protects Vascular Barrier Integrity. [published online ahead of print, 2022 Jun 14]. Am. J. Respir Crit. Care Med. [CrossRef]
- Minami, E.; Ito, S.; Sugiura, T.; Fujita, Y.; Sasano, H.; Sobue, K. Markedly Elevated Procalcitonin in Early Postoperative Period in Pediatric Open Heart Surgery: A Prospective Cohort Study. J. Intensive Care 2014, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Klingele, M.; Bomberg, H.; Schuster, S.; Schäfers, H.-J.; Groesdonk, H.V. Prognostic Value of Procalcitonin in Patients after Elective Cardiac Surgery: A Prospective Cohort Study. Ann. Intensive Care 2016, 6, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisner, M. Update on Procalcitonin Measurements. Ann. Lab. Med. 2014, 34, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.L.; Salvatore, C.A. Targeting a Family B GPCR/RAMP Receptor Complex: CGRP Receptor Antagonists and Migraine: RAMPs and CGRP Receptor Antagonists. Br. J. Pharmacol. 2012, 166, 66–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messerer, D.A.C.; Datzmann, T.; Baranowsky, A.; Peschel, L.; Hoffmann, A.; Gröger, M.; Amling, M.; Wepler, M.; Nussbaum, B.L.; Jiang, S.; et al. Systemic Calcitonin Gene-Related Peptide Receptor Antagonism Decreases Survival in a Large Animal Model of Polymicrobial Sepsis: Blinded Randomised Controlled Laboratory Trial. Br. J. Anaesth. 2022, 128, S0007091221008606. [Google Scholar] [CrossRef]
- Kröller-Schön, S.; Knorr, M.; Hausding, M.; Oelze, M.; Schuff, A.; Schell, R.; Sudowe, S.; Scholz, A.; Daub, S.; Karbach, S.; et al. Glucose-Independent Improvement of Vascular Dysfunction in Experimental Sepsis by Dipeptidyl-Peptidase 4 Inhibition. Cardiovasc. Res. 2012, 96, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Steven, S.; Hausding, M.; Kröller-Schön, S.; Mader, M.; Mikhed, Y.; Stamm, P.; Zinßius, E.; Pfeffer, A.; Welschof, P.; Agdauletova, S.; et al. Gliptin and GLP-1 Analog Treatment Improves Survival and Vascular Inflammation/Dysfunction in Animals with Lipopolysaccharide-induced Endotoxemia. Basic Res. Cardiol. 2015, 110, 6. [Google Scholar] [CrossRef]
- Bergman, A.; Ebel, D.; Liu, F.; Stone, J.; Wang, A.; Zeng, W.; Chen, L.; Dilzer, S.; Lasseter, K.; Herman, G.; et al. Absolute Bioavailability of Sitagliptin, an Oral Dipeptidyl Peptidase-4 Inhibitor, in Healthy Volunteers. Biopharm. Drug Dispos. 2007, 28, 315–322. [Google Scholar] [CrossRef]
- Williams-Herman, D.; Engel, S.S.; Round, E.; Johnson, J.; Golm, G.T.; Guo, H.; Musser, B.J.; Davies, M.J.; Kaufman, K.D.; Goldstein, B.J. Safety and Tolerability of Sitagliptin in Clinical Studies: A Pooled Analysis of Data from 10,246 Patients with Type 2 Diabetes. BMC Endocr. Disord. 2010, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Deacon, C.F. A Review of Dipeptidyl Peptidase-4 Inhibitors. Hot Topics from Randomized Controlled Trials. Diabetes Obes. Metab. 2018, 20 (Suppl. S1), 34–46. [Google Scholar] [CrossRef]
- Buchtele, N.; Schwameis, M.; Schoergenhofer, C.; Derhaschnig, U.; Firbas, C.; Karch, R.; Nix, D.; Schenk, R.; Jilma, B. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Parenterally Administered Dutogliptin: A Prospective Dose-escalating Trial. Br. J. Clin. Pharmacol. 2020, 86, 979–990. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellenthal, K.E.M.; Brabenec, L.; Wagner, N.-M. Regulation and Dysregulation of Endothelial Permeability during Systemic Inflammation. Cells 2022, 11, 1935. https://doi.org/10.3390/cells11121935
Hellenthal KEM, Brabenec L, Wagner N-M. Regulation and Dysregulation of Endothelial Permeability during Systemic Inflammation. Cells. 2022; 11(12):1935. https://doi.org/10.3390/cells11121935
Chicago/Turabian StyleHellenthal, Katharina E. M., Laura Brabenec, and Nana-Maria Wagner. 2022. "Regulation and Dysregulation of Endothelial Permeability during Systemic Inflammation" Cells 11, no. 12: 1935. https://doi.org/10.3390/cells11121935
APA StyleHellenthal, K. E. M., Brabenec, L., & Wagner, N.-M. (2022). Regulation and Dysregulation of Endothelial Permeability during Systemic Inflammation. Cells, 11(12), 1935. https://doi.org/10.3390/cells11121935





