The Shape of Human Red Blood Cells Suspended in Autologous Plasma and Serum
Abstract
1. Introduction
- A direct correlation of THR and spontaneous curvature of the membrane is likely.
- The variation of the mean THR between different donors is large.
- The aspect ratio of RBCs viewed face-on ranged on average from 1 to 1.48.
- In oval RBCs, the rim is thicker along the major axis than along the minor axis, an effect increasing with increasing aspect ratio.
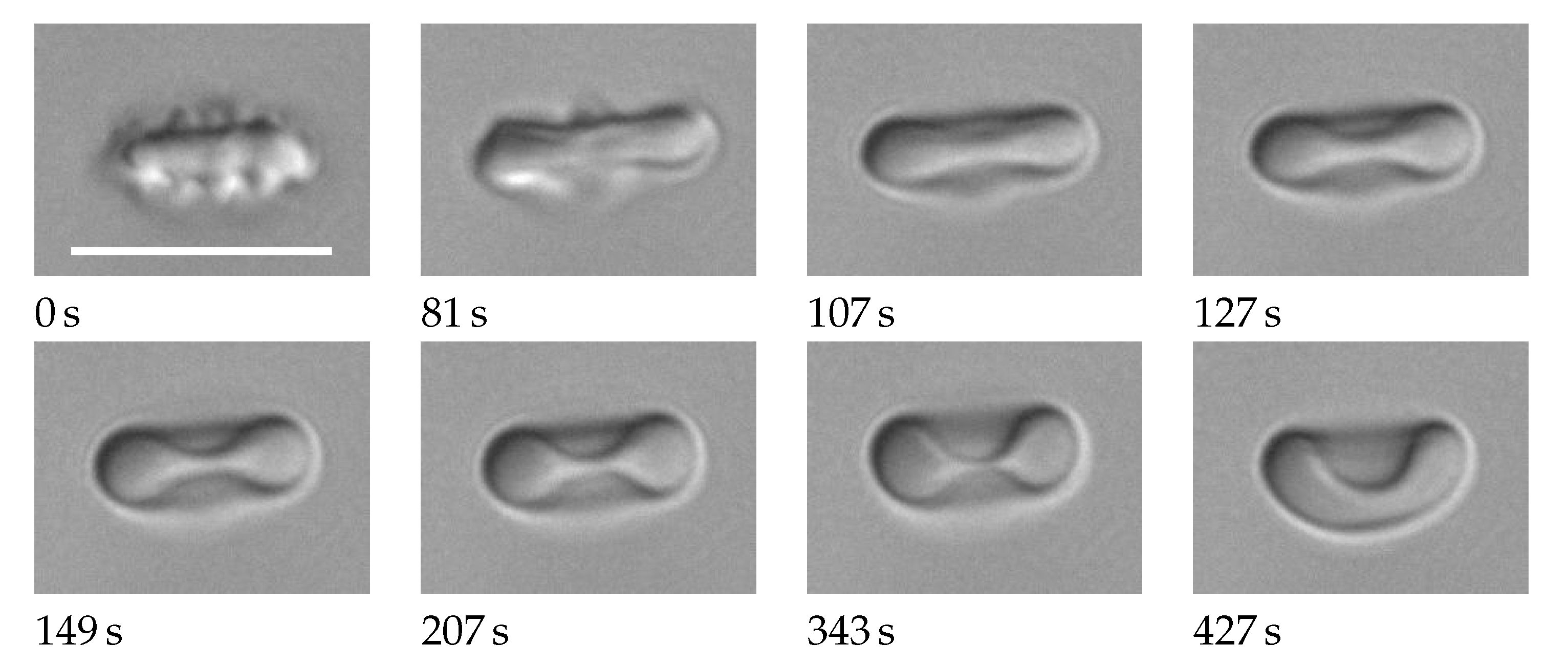
- Remodeling of the membrane skeleton occurs in vivo with a characteristic time on the order of 1 h.
2. Materials and Methods
2.1. Blood Sampling and Preparation
- code
- p Heparinized blood was pipetted into an Eppendorf vial and centrifuged at 5700 g for 8 min. The clear plasma supernatand was transferred into another Eppendorf vial. To obtain the final concentration of RBCs, heparinized blood was diluted with plasma in three steps 500 to 1000-fold.
- code
- s After 30 min at room temperature, the serum vacutainer was centrifuged in a swing-out-rotor at 2000 g for 10 min. The clear serum supernatand was transferred into an Eppendorf vial. Heparinized blood was diluted with serum in the same way as described above for plasma.
- code
- sw Heparinized blood was washed three times with phosphate buffered saline (PBS, pH 7.4 1×, gibco, life technologies). A 50% suspension of the washed RBCs in PBS was diluted with serum in the same way as described above for plasma.
2.2. Microscopic Observation
2.3. Image Processing

2.4. Sorting Poikilocytes
- Stomatocytes
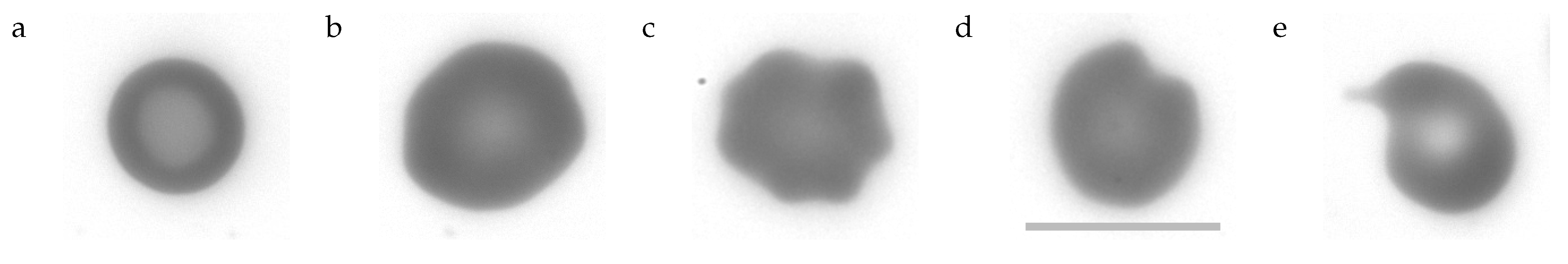
- can be described as cup-shaped RBCs (cf. Figure 1 last image). Their profile of GVs shows two minima and a maximum just as the profile of biconcave discocytes. However, what appears to be the rim or dimple in the profiles is actually the wall or bottom of the cup, respectively. The profile is less curved in the “dimple” region and the transition from the “rim” to the “dimple” is abrupt unlike in discocytes. Examples of the filtering procedure are shown in Supplementary Section S5. A typical stomatocyte is shown in Figure 4a.
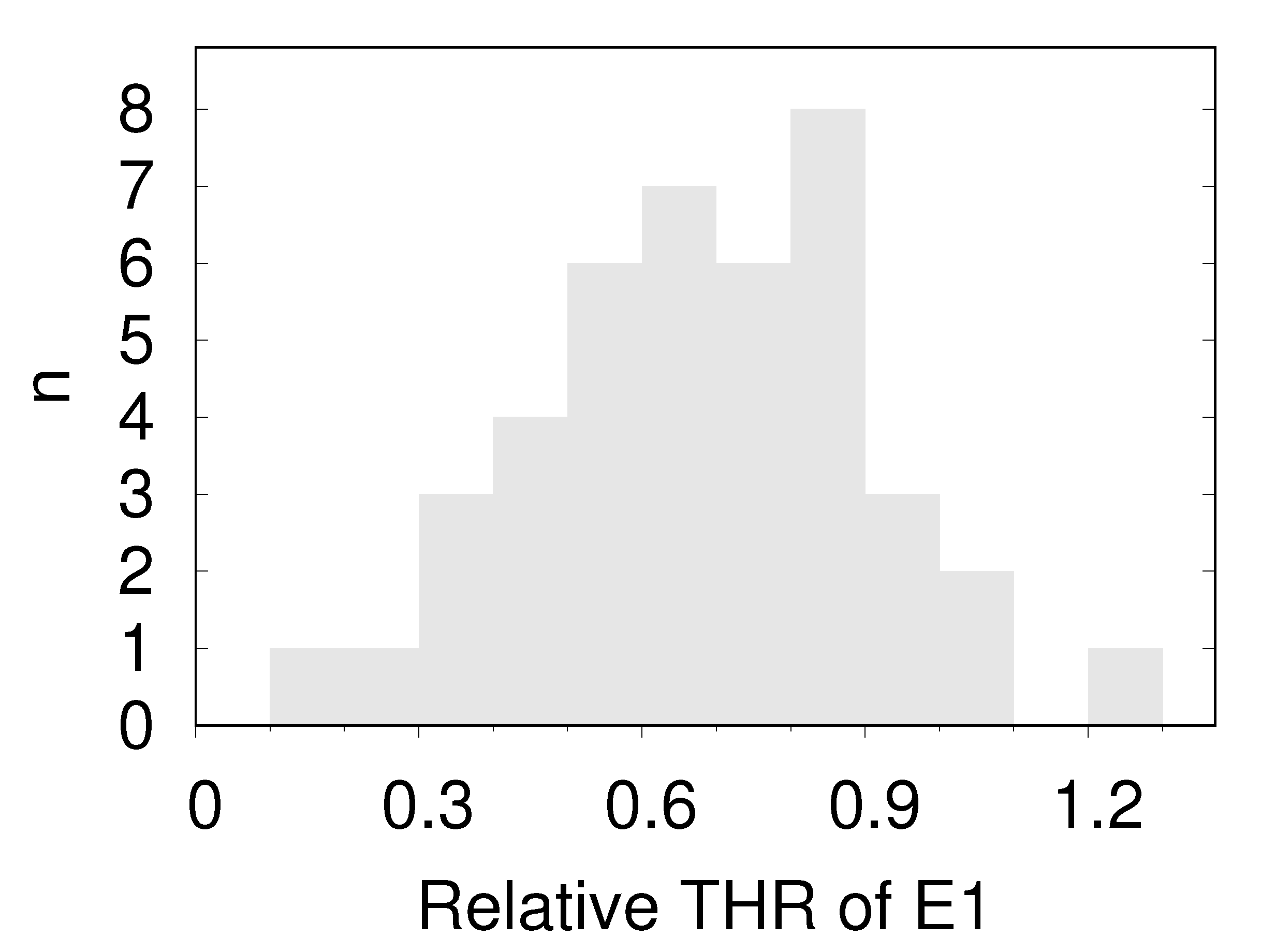
- Echinocytes type 1 (E1)
- oriented edge-on and sectioned microscopically through the center appear biconcave. In the face-on orientation, their outline is wavy. An example of the filtering procedure is shown in Supplementary Section S6. A typical E1 is shown in Figure 4b.
- Echinocytes type 2 (E2)
- are similar to E1 in that their overall shape is flat and their outline is wavy in the face-on orientation. In addition, E2s show spicules (cf. Figure 1 first image) or bumps (cf. Figure 1 second image) extending away from the mid-plane. In the present experiments these bumps are recognized as a local increase in GV. An example of the filtering procedure is shown in Supplementary Section S6. A typical E2 is shown in Figure 4c.
- Pitting type 1 (P1):
- The outline of some of these RBCs presented a single indentation in the face-on orientation. In others the curvature of the outline was zero or negative in places (the outline of a circle is defined as positive).The nomenclature (pitting) relates to the suggested origin of these shape (Section 4.7.2 and Section 4.7.3). A prominent example is shown in Figure 4d and all P1s found are shown in Supplementary Figures S10 and S11.
- Pitting type 2 (P2):
- Some of these RBCs showed a tongue protruding from the body of the RBC. Others were unusually small. A prominent example is shown in Figure 4e and all P2s found are shown in Supplementary Figure S12.
2.5. Calculations
3. Results
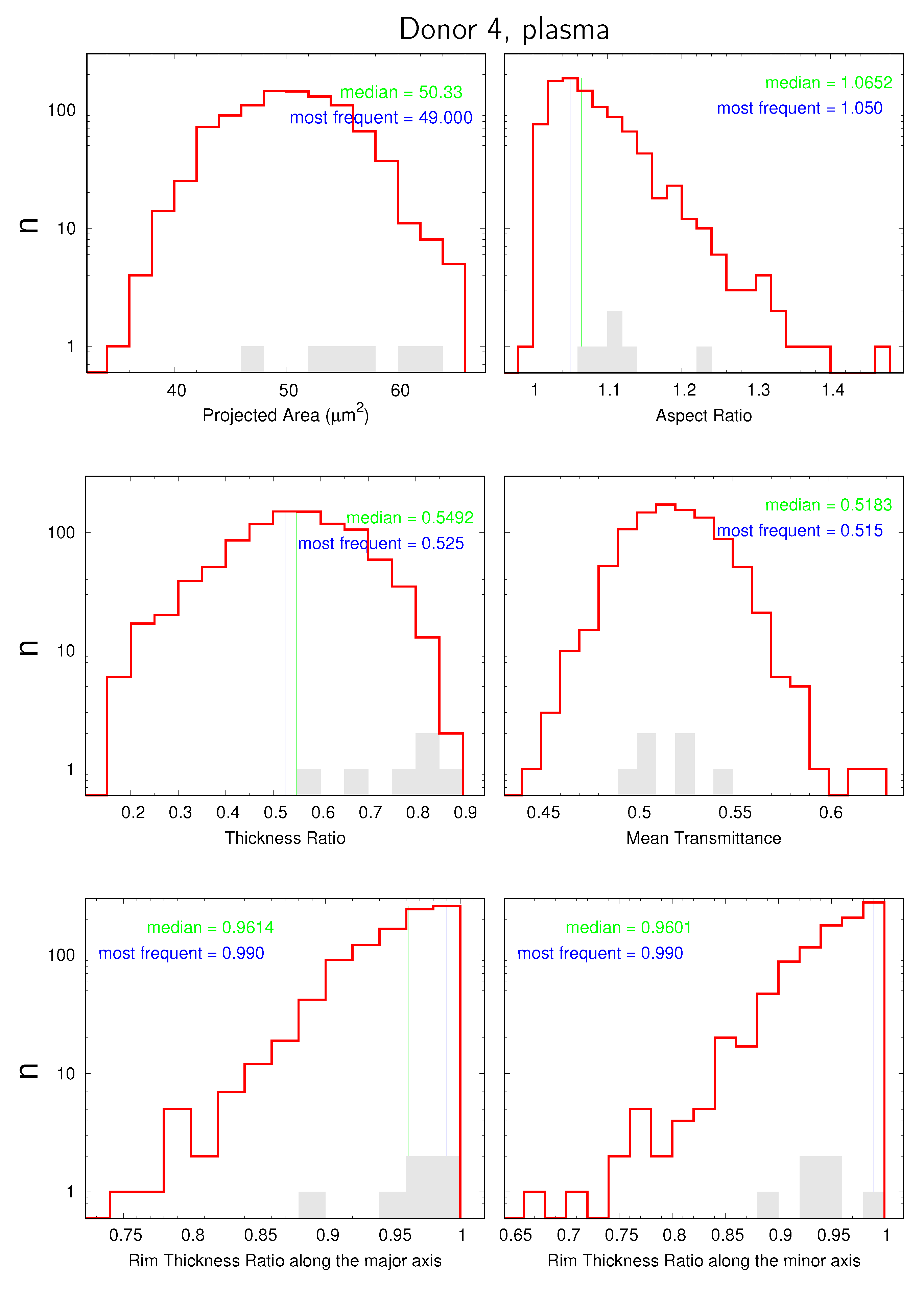
3.1. Distributions and Correlations
- the projected area;
- the aspect ratio;
- the THR;
- the mean transmittance;
- the ratio between rim thicknesses at the two extremities of the major axis;
- the ratio between rim thicknesses at the two extremities of the minor axis;
3.2. Lady’s Finger Asymmetry
3.3. Mean Values
4. Discussion
4.1. Method
4.2. RBC Diameter and Area
4.3. Influence of the External Environment on the Shape of the Red Cell
4.4. Thickness Ratio (THR)
4.5. Comparison between Plasma and Serum
4.6. Correlations
4.7. Deviations from Circular Symmetry
4.7.1. Determinants of RBC Shape
4.7.2. P1s
4.7.3. P2s
4.7.4. Aspect Ratio
4.8. Measurement
4.8.1. Fluctuations of GVs
4.8.2. Attachment to the Bottom of the Chamber
4.8.3. Effect of Gravity
5. Conclusions
- Based on the averages of eight donors and plasma suspension, the following ratios are suggested for a mean resting RBC: aspect ratio 1.07, mean THR 0.55, <LFA> 0.942. This data results in a THR along the major axis of 0.5664 and along the minor axis of 0.5336. Based on an elliptical outline and the average projected area of 51.5 µm², the average major and minor axes amount to 8.38 µm and 7.83 µm respectively.To provide an analytical description of the average shape is beyond the scope of this report. For models with a discrete description of the membrane, a sequence of steps to design the average shape is presented in Supplementary Section S12. The procedure follows the approach of Canham [40], Helfrich [41], Deuling and Helfrich [42].
- Besides the distribution of the shear modulus of the membrane skeleton [43], the variation of the shape within the RBC population could be considered.
- Considering the short characteristic time of remodeling and the various shapes the RBCs assume during circulation, a more general concept for the reference configuration of the membrane skeleton could be adopted than that of a stress free shape.
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, E.; Fung, Y.C. Improved Measurements of the Erythrocyte Geometry. Microvasc. Res. 1972, 4, 335–347. [Google Scholar] [CrossRef]
- Fung, Y.C.; Tsang, W.C.O.; Patitucci, P. High-Resolution Data on the Geometry of Red Blood Cells. Biorheology 1981, 18, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.E.G. On the shape of human red blood cells interacting with flat artificial surfaces—The ‘glass effect’. Biochim. Biophys. Acta 1990, 1036, 193–201. [Google Scholar] [CrossRef]
- Bessis, M. Red Cell Shapes. An Illustrated Classification and its Rationale. Nouv. Rev. Française d’Hématologie 1972, 12, 721–746. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ponder, E.; Saslow, G. The Measurement of the Diameter of Erythrocytes. IV. The Effect of Anticoagulants and of Variations in Drying and Fixing. Exp. Physiol. 1929, 19, 319–328. [Google Scholar] [CrossRef]
- Ponder, E. The Measurement of the Diameter of Erythrocytes. V.-The Relation of the Diameter to the Thickness. Exp. Physiol. 1930, 20, 29–39. [Google Scholar] [CrossRef]
- Rand, R.P.; Burton, A.C. Area and volume changes in hemolysis of single erythrocytes. J. Cell Comp. Physiol. 1963, 61, 245–253. [Google Scholar] [CrossRef]
- Ponder, E.; Millar, W.C. The Measurement of the Diameter of Erythrocytes. I. The Mean Diameter of the Red Cells in Man. Exp. Physiol. 1924, 14, 67–82. [Google Scholar] [CrossRef]
- Roma, P.M.S.; Siman, L.; Amaral, F.T.; Agero, U.; Mesquita, O.N. Total three-dimensional imaging of phase objects using defocusing microscopy: Application to red blood cells. Appl. Phys. Lett. 2014, 104, 251107. [Google Scholar] [CrossRef]
- Park, Y.; Best, C.A.; Kuriabova, T.; Henle, M.L.; Feld, M.S.; Levine, A.J.; Popescu, G. Measurement of the nonlinear elasticity of red blood cell membranes. Phys. Rev. E 2011, 83, 051925. [Google Scholar] [CrossRef] [PubMed]
- Gilev, K.; Iastrebova, E.; Strokotov, D.; Yurkin, M.; Karmadonova, N.; Chernyshev, A.; Lomivorotov, V.; Maltsev, V. Advanced consumable-free morphological analysis of intact red blood cells by a compact scanning flow cytometer. Cytom. Part A 2017, 91, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Canham, P.B.; Burton, A.C. Distribution of Size and Shape in Populations of Normal Human Red Cells. Circ. Res. 1968, 22, 405–422. [Google Scholar] [CrossRef]
- Gifford, S.C.; Derganc, J.; Shevkoplyas, S.S.; Yoshida, T.; Bitensky, M.W. A detailed study of time-dependent changes in human red blood cells: From reticulocyte maturation to erythrocyte senescence. Br. J. Haematol. 2006, 135, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Deuticke, B. Transformation and Restoration of Biconcave Shape of Human Erythrocytes Induced by Amphiphilic Agents and Changes of Ionic Environment. Biochim. Biophys. Acta 1968, 163, 494–500. [Google Scholar] [CrossRef]
- Ponder, E. On the Spherical Form of the Mammalian Erythrocyte. J. Exp. Biol. 1929, 6, 387–398. [Google Scholar] [CrossRef]
- Korpman, R.A.; Dorrough, D.C.; Brailsford, J.D.; Bull, B.S. The Red Cell Shape as an Indicator of Membrane Structure. Blood Cells 1977, 3, 315–334. [Google Scholar]
- Fischer, T.M. Bending stiffness of lipid bilayers. I. Bilayer couple or single-layer bending? Biophys. J. 1992, 63, 1328–1335. [Google Scholar] [CrossRef][Green Version]
- Lim, H.W.G.; Wortis, M.; Mukhopadhyay, R. Red Blood Cell Shapes and Shape Transformations: Newtonian Mechanics of a Composite Membrane: Sections 2.1–2.4. In Soft Matter; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2009; pp. 83–139. [Google Scholar] [CrossRef]
- Peng, Z.; Mashayekh, A.; Zhu, Q. Erythrocyte responses in low-shear-rate flows: Effects of non-biconcave stress-free state in the cytoskeleton. J. Fluid Mech. 2014, 742, 96–118. [Google Scholar] [CrossRef]
- Lim, G.H.W.; Wortis, M.; Mukhopadhyay, R. Stomatocyte–discocyte–echinocyte sequence of the human red blood cell: Evidence for the bilayer–couple hypothesis from membrane mechanics. Proc. Nat. Acad. Sci. USA 2002, 99, 16766–16769. [Google Scholar] [CrossRef]
- Khairy, K.; Foo, J.; Howard, J. Shapes of Red Blood Cells: Comparison of 3D Confocal Images with the Bilayer-Couple Model. Cell. Mol. Bioeng. 2008, 1, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.; Nowak, R.B.; Zhou, S.; Giannetto, M.; Gokhin, D.S.; Papoin, J.; Ghiran, I.C.; Blanc, L.; Wan, J.; Fowler, V.M. Myosin IIA interacts with the spectrin-actin membrane skeleton to control red blood cell membrane curvature and deformability. Proc. Natl. Acad. Sci. USA 2018, 115, E4377–E4385. [Google Scholar] [CrossRef] [PubMed]
- Lew, V.L.; Tiffert, T. On the Mechanism of Human Red Blood Cell Longevity: Roles of Calcium, the Sodium Pump, PIEZO1, and Gardos Channels. Front. Physiol. 2017, 8, 977. [Google Scholar] [CrossRef] [PubMed]
- Danielczok, J.G.; Terriac, E.; Hertz, L.; Petkova-Kirova, P.; Lautenschläger, F.; Laschke, M.W.; Kaestner, L. Red Blood Cell Passage of Small Capillaries Is Associated with Transient Ca2+-mediated Adaptations. Front. Physiol. 2017, 8, 979. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.S. Heinz Body Hemolytic Anemia: “Bite Cells”—A Clue to Diagnosis. Arch. Intern. Med. 1976, 136, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.C.; Schwartz, B.; White, J.G. Heinz-Body Anemia: “Bite Cell” Variant—A Light and Electron Microscopic Study. Am. J. Hematol. 1983, 15, 135–146. [Google Scholar] [CrossRef]
- Yoo, D.; Lessin, L. Drug-Associated “Bite Cell” Hemolytic Anemia. Am. J. Med. 1992, 92, 243–248. [Google Scholar] [CrossRef]
- Dumaswala, U.J.; Greenwalt, T.J. Human erythrocytes shed exocytic vesicles in vivo. Transfusion 1984, 24, 490–492. [Google Scholar] [CrossRef]
- Ciana, A.; Achilli, C.; Gaur, A.; Minetti, G. Membrane Remodelling and Vesicle Formation During Ageing of Human Red Blood Cells. Cell Physiol. Biochem. 2017, 42, 1127–1138. [Google Scholar] [CrossRef]
- Ciana, A.; Achilli, C.; Minetti, G. Spectrin and Other Membrane-Skeletal Components in Human Red Blood Cells of Different Age. Cell Physiol. Biochem. 2017, 42, 1139–1152. [Google Scholar] [CrossRef]
- Fischer, T.M. Creep and stress relaxation of human red cell membrane. Biomech. Model Mechanobiol. 2017, 16, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Waugh, R.; Evans, E.A. Thermoelasticity of red blood cell membrane. Biophys. J. 1979, 26, 115–131. [Google Scholar] [CrossRef]
- Seifert, U. Configurations of fluid membranes and vesicles. Adv. Phys. 1997, 46, 13–137. [Google Scholar] [CrossRef]
- Park, Y.; Best, C.A.; Auth, T.; Gov, N.S.; Safran, S.A.; Popescu, G.; Suresh, S.; Feld, M.S. Metabolic remodeling of the human red blood cell membrane. Proc. Nat. Acad. Sci. USA 2010, 107, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.A.; Bhaduri, B.; Popescu, G.; Levine, A.J. Geometric localization of thermal fluctuations in red blood cells. Proc. Nat. Acad. Sci. USA 2017, 114, 2865–2870. [Google Scholar] [CrossRef]
- Rappaz, B.; Barbul, A.; Hoffmann, A.; Boss, D.; Korenstein, R.; Depeursinge, C.D.; Magistretti, P.J.; Marquet, P. Spatial analysis of erythrocyte membrane fluctuations by digital holographic microscopy. Blood Cells Mol. Dis. 2009, 42, 228–232. [Google Scholar] [CrossRef]
- Mohandas, N.; Johnson, A.; Wyatt, J.; Croisille, L.; Reeves, J.; Tycko, D.; Groner, W. Automated quantitation of cell density distribution and hyperdense cell fraction in RBC disorders. Blood 1989, 74, 442–447. [Google Scholar] [CrossRef]
- Kraus, M.; Seifert, U.; Lipowsky, R. Gravity-Induced Shape Transformations of Vesicles. Europhys. Lett. (EPL) 1995, 32, 431–436. [Google Scholar] [CrossRef]
- Canham, P.B. The Minimum Energy of Bending as a Possible Explanation of the Biconcave Shape of the Human Red Blood Cell. J. Theoret. Biol. 1970, 26, 61–81. [Google Scholar] [CrossRef]
- Helfrich, W. Elastic properties of lipid bilayers: Theory and possible experiments. Z. Naturforsch. C Biosci. 1973, 28, 693–703. [Google Scholar] [CrossRef]
- Deuling, H.J.; Helfrich, W. Red Blood Cell Shapes as Explained on the Basis of Curvature Elasticity. Biophys. J. 1976, 16, 861–868. [Google Scholar] [CrossRef]
- Reichel, F.; Mauer, J.; Nawaz, A.A.; Gompper, G.; Guck, J.; Fedosov, D.A. High-Throughput Microfluidic Characterization of Erythrocyte Shapes and Mechanical Variability. Biophys. J. 2019, 117, 14–24. [Google Scholar] [CrossRef] [PubMed]






| Plasma, n = 8 | Serum, n = 7 | |||||
|---|---|---|---|---|---|---|
| MV | SD | CV | MV | SD | CV | |
| projected area | 50.97 | 1.80599 | 0.03543 | 50.27 | 2.63877 | 0.05249 |
| aspect ratio | 1.063 | 0.00841 | 0.00791 | 1.066 | 0.00882 | 0.00827 |
| thickness ratio (THR) | 0.554 | 0.06405 | 0.11561 | 0.609 | 0.05487 | 0.09010 |
| mean transmittance | 0.529 | 0.01832 | 0.03463 | 0.527 | 0.01733 | 0.03288 |
| rim thickness ratio along the major axis | 0.959 | 0.00644 | 0.00672 | 0.960 | 0.00554 | 0.00577 |
| rim thickness ratio along the minor axis | 0.959 | 0.00443 | 0.00462 | 0.959 | 0.00521 | 0.00543 |
| Plasma, n = 8 | Serum, n = 7 | |||||
|---|---|---|---|---|---|---|
| MV | SD | CV | MV | SD | CV | |
| projected area | 51.15 | 5.3243 | 0.1043 | 50.45 | 5.2811 | 0.1049 |
| aspect ratio | 1.077 | 0.0576 | 0.0534 | 1.081 | 0.0628 | 0.0580 |
| thickness ratio (THR) | 0.545 | 0.1341 | 0.2501 | 0.593 | 0.1346 | 0.2294 |
| mean transmittance | 0.530 | 0.0234 | 0.0440 | 0.528 | 0.0257 | 0.0487 |
| rim thickness ratio along the major axis | 0.950 | 0.0396 | 0.0418 | 0.951 | 0.0393 | 0.0413 |
| rim thickness ratio along the minor axis | 0.950 | 0.0413 | 0.0435 | 0.950 | 0.0406 | 0.0427 |
| Plasma, n = 8 | Serum, n = 7 | |||||
|---|---|---|---|---|---|---|
| MV | SD | CV | MV | SD | CV | |
| <LFA> = LFA at the median of THR | 0.942 | 0.00845 | 0.00897 | 0.944 | 0.00684 | 0.00725 |
| Suspending Medium | Total Number of RBCs | Number of Donors or Groups | RBC Orientation | Optical Method | Mean Value (µm) | SD (µm) | Reference |
|---|---|---|---|---|---|---|---|
| plasma | 1917 | 6 donors | face-on | bright field image | 8.55 | 0.54 | [9] |
| serum | ≈2000 | 5 donors | edge-on | bright field image | 8.55 | 0.41 | [7] |
| saline | 1016 | 7 donors | edge-on | bright field image | 8.069 | 0.547 | [13] |
| saline | 1267 | 7 donors | face-on | bright field image | 8.063 | 0.429 | [13] |
| saline | 50 | 1 donor | face-on | interference holography | 7.82 | 0.62 | [1] |
| saline | 2853 | 4 groups | face-on | interference holography | 7.66 | 0.67 | [2] |
| saline | >22,000 | 22 donors | any | light scattering | 6.42 | 0.763 | [12] |
| plasma | 4882 | 8 donors | face-on | distribution of transmittance | 8.123 | 0.425 | this work |
| serum | 5029 | 7 donors | face-on | distribution of transmittance | 8.071 | 0.425 | this work |
| Suspending Medium | Total Number of RBCs | Number of Donors or Groups | RBC Orientation | Optical Method | Mean Value | Reference |
|---|---|---|---|---|---|---|
| serum | ≈2000 | 5 donors | edge-on | bright field image | 0.424 | [7] |
| saline | 50 | 1 donor | face-on | interference holography | 0.314 | [1] |
| saline | 2853 | 4 groups | face-on | interference holography | 0.509 | [2] |
| saline | 25 | not specified | face-on | defocusing microscopy | 0.50 1 | [10] |
| saline | 20 | not specified | face-on | diffraction phase microscopy | 0.27 1 | [11] |
| saline | >22,000 | 22 donors | any | light scattering | 0.627 | [12] |
| plasma | 4882 | 8 donors | face-on | distribution of transmittance | 0.550 | this work |
| serum | 5029 | 7 donors | face-on | distribution of transmittance | 0.601 | this work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, T.M. The Shape of Human Red Blood Cells Suspended in Autologous Plasma and Serum. Cells 2022, 11, 1941. https://doi.org/10.3390/cells11121941
Fischer TM. The Shape of Human Red Blood Cells Suspended in Autologous Plasma and Serum. Cells. 2022; 11(12):1941. https://doi.org/10.3390/cells11121941
Chicago/Turabian StyleFischer, Thomas M. 2022. "The Shape of Human Red Blood Cells Suspended in Autologous Plasma and Serum" Cells 11, no. 12: 1941. https://doi.org/10.3390/cells11121941
APA StyleFischer, T. M. (2022). The Shape of Human Red Blood Cells Suspended in Autologous Plasma and Serum. Cells, 11(12), 1941. https://doi.org/10.3390/cells11121941






