Osteoblasts in a Perfusion Flow Bioreactor—Tissue Engineered Constructs of TiO2 Scaffolds and Cells for Improved Clinical Performance
Abstract
:1. Introduction
- (1)
- Validate whether perfusion seeding of TiO2 scaffold versus static seeding is beneficial for bone scaffolds prior to clinical use based on in silico modeling.
- (2)
- Analyze the effect of shear fluid flow on the growth and differentiation of MC3T3-E1 cells and human osteoblasts cultured on TiO2 scaffolds.
- (3)
- Analyze the validity of the results obtained from the cell line osteoblasts compared to primary human osteoblasts.
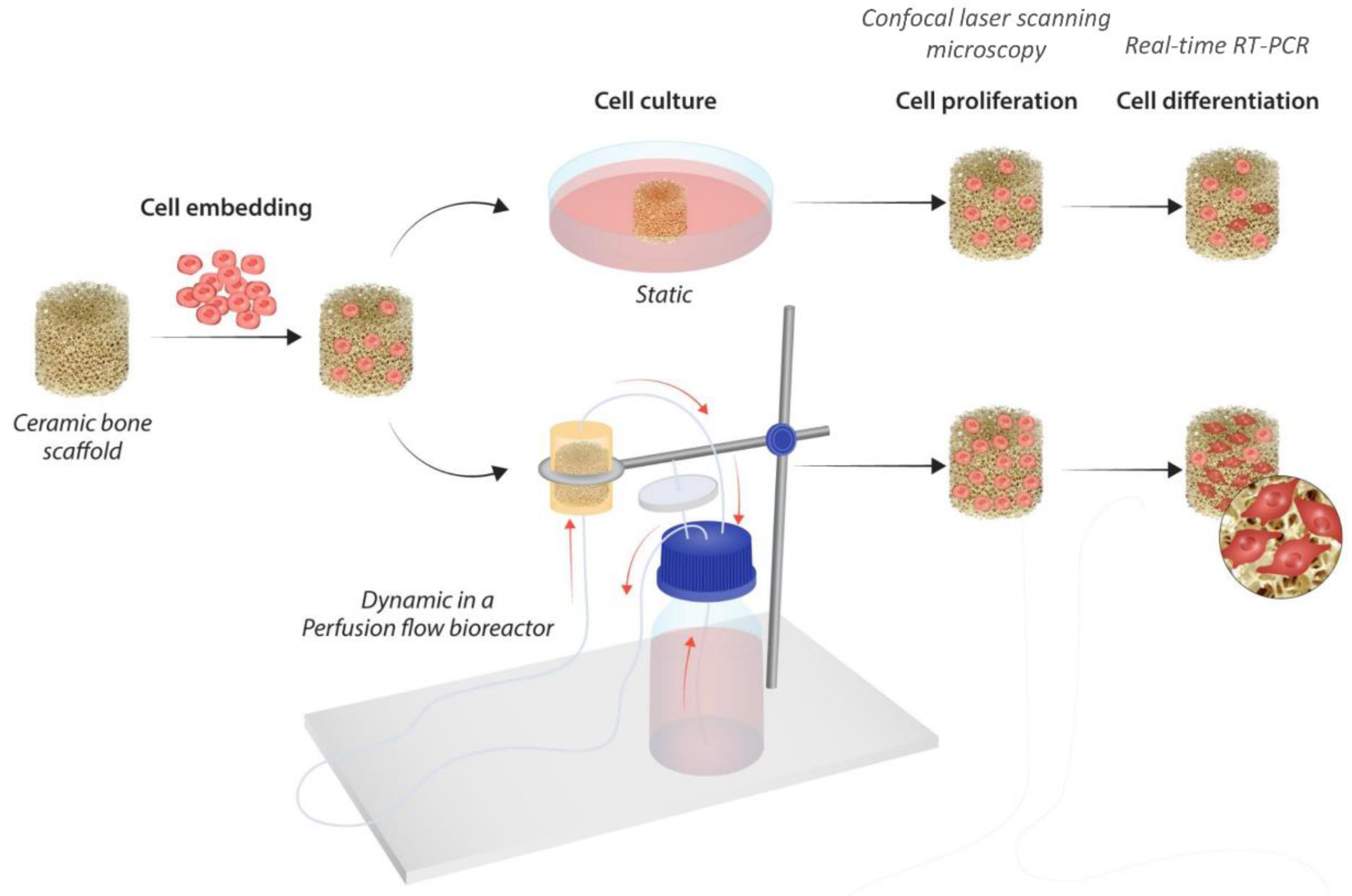
2. Materials and Methods
2.1. Fabrication of TiO2 Scaffolds
2.2. Cell Culture and Seeding
2.3. Perfusion Bioreactor System and Flow Culture
2.4. DNA Quantification
2.5. Confocal Laser Scanning Microscopy
2.6. RNA Isolation and Real-Time RT-PCR Analysis
2.7. Quantification of Proteins Secreted in the Cell Culture Medium
2.8. Statistical Analysis
3. Results
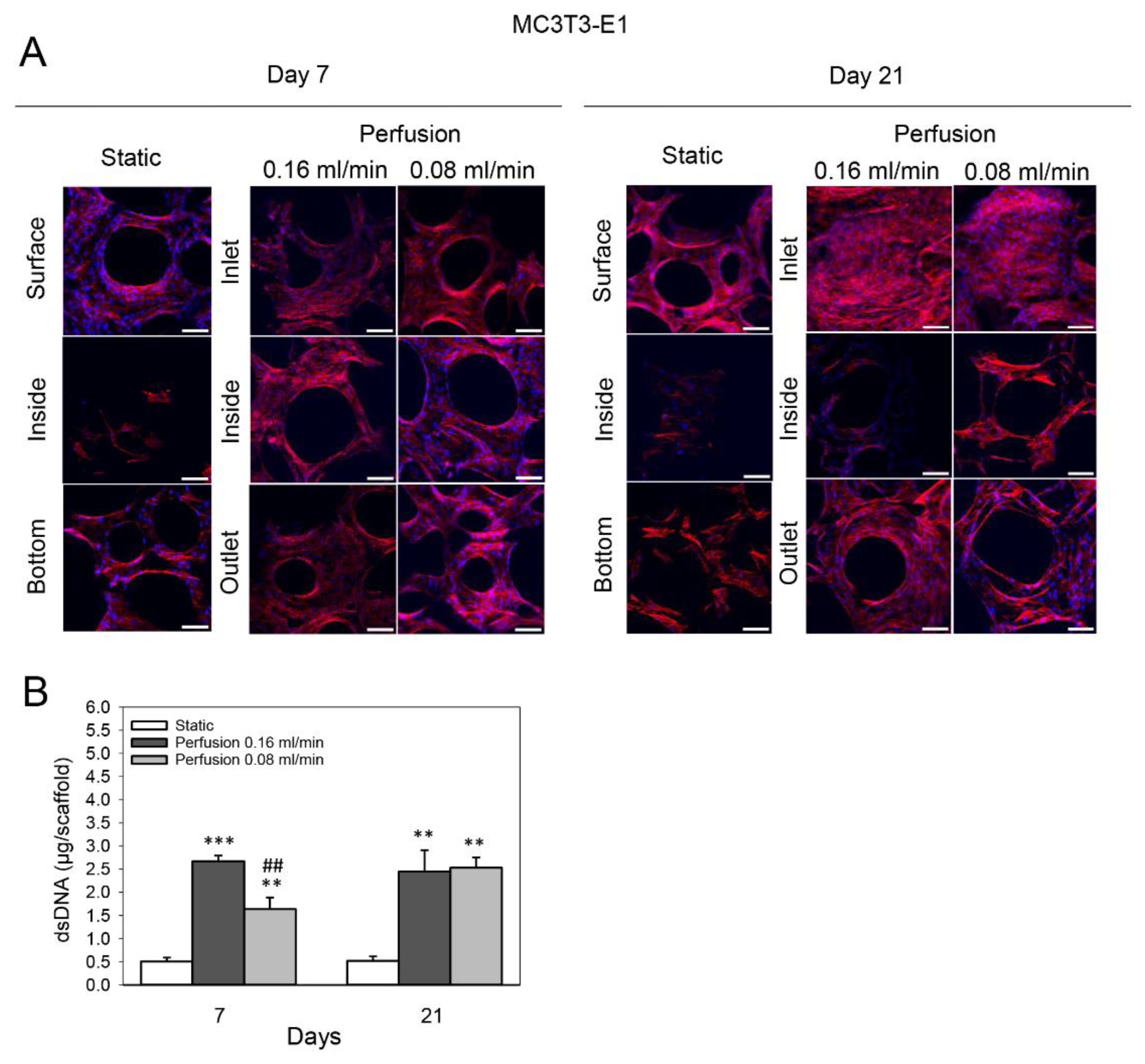
3.1. Effect of Medium Flow and Influence of Flow Rate on Growth and Distribution of MC3T3-E1 Cells Cultured on TiO2 Scaffolds
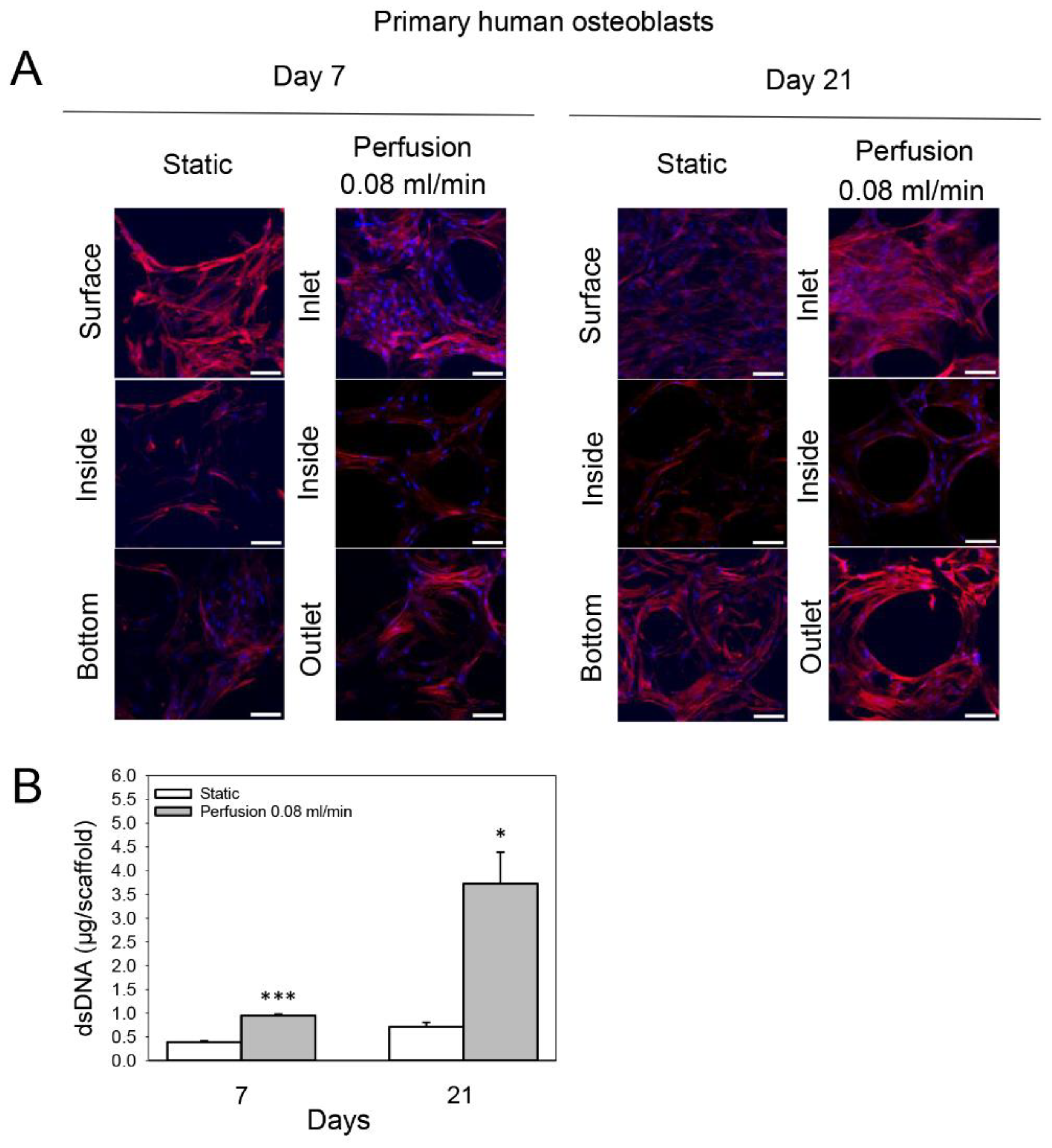
3.2. Effect of Medium Flow on Growth and Distribution of Primary Human Osteoblasts Cultured on TiO2 Scaffolds
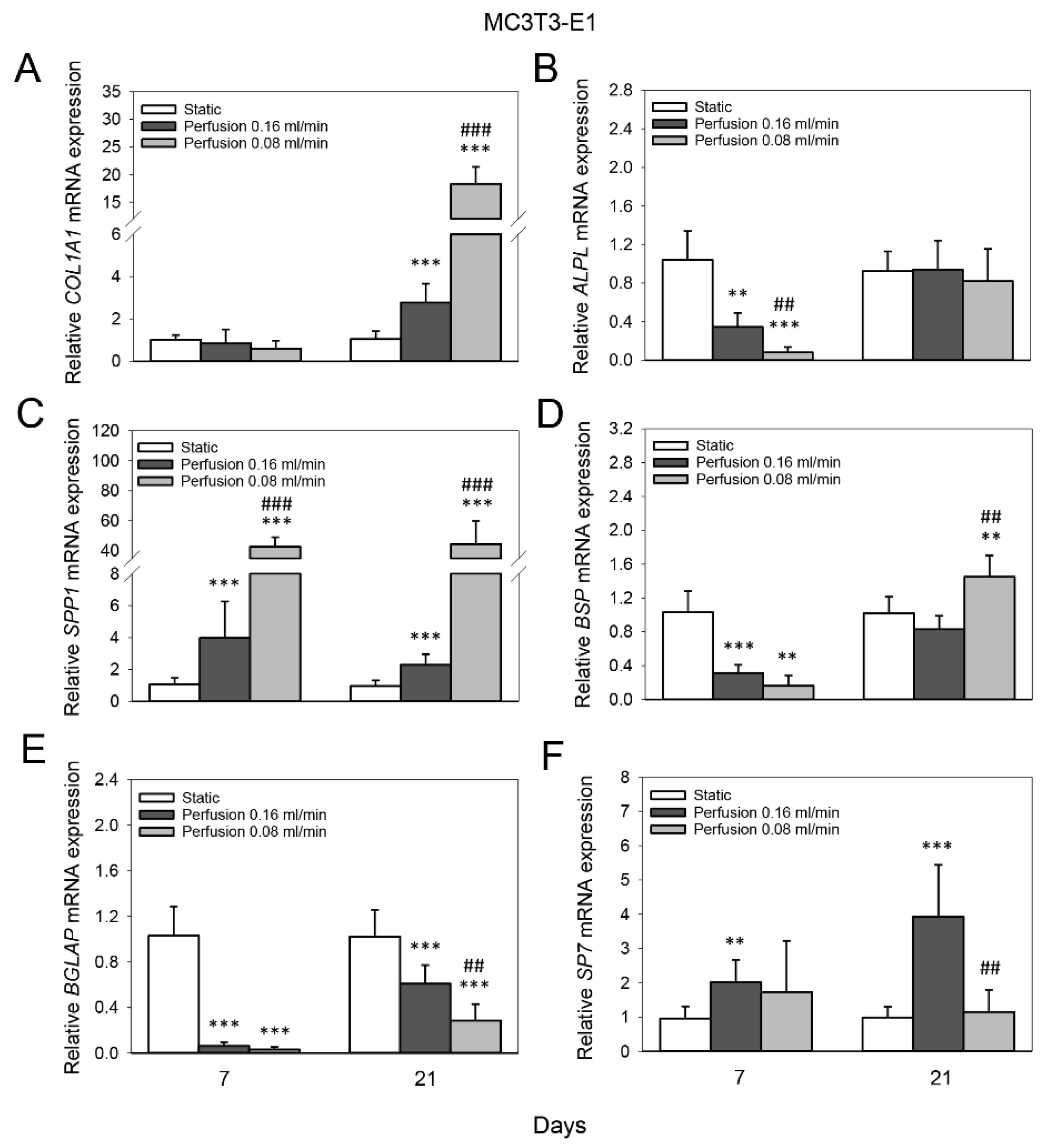
3.3. Effect of Medium Flow and Influence of Flow Rate on Osteogenic Gene Expression of MC3T3-E1 Cells Cultured on TiO2 Scaffolds
3.4. Effect of Medium Flow on Osteogenic Gene Expression of Primary Human Osteoblasts Cultured on TiO2 Scaffolds
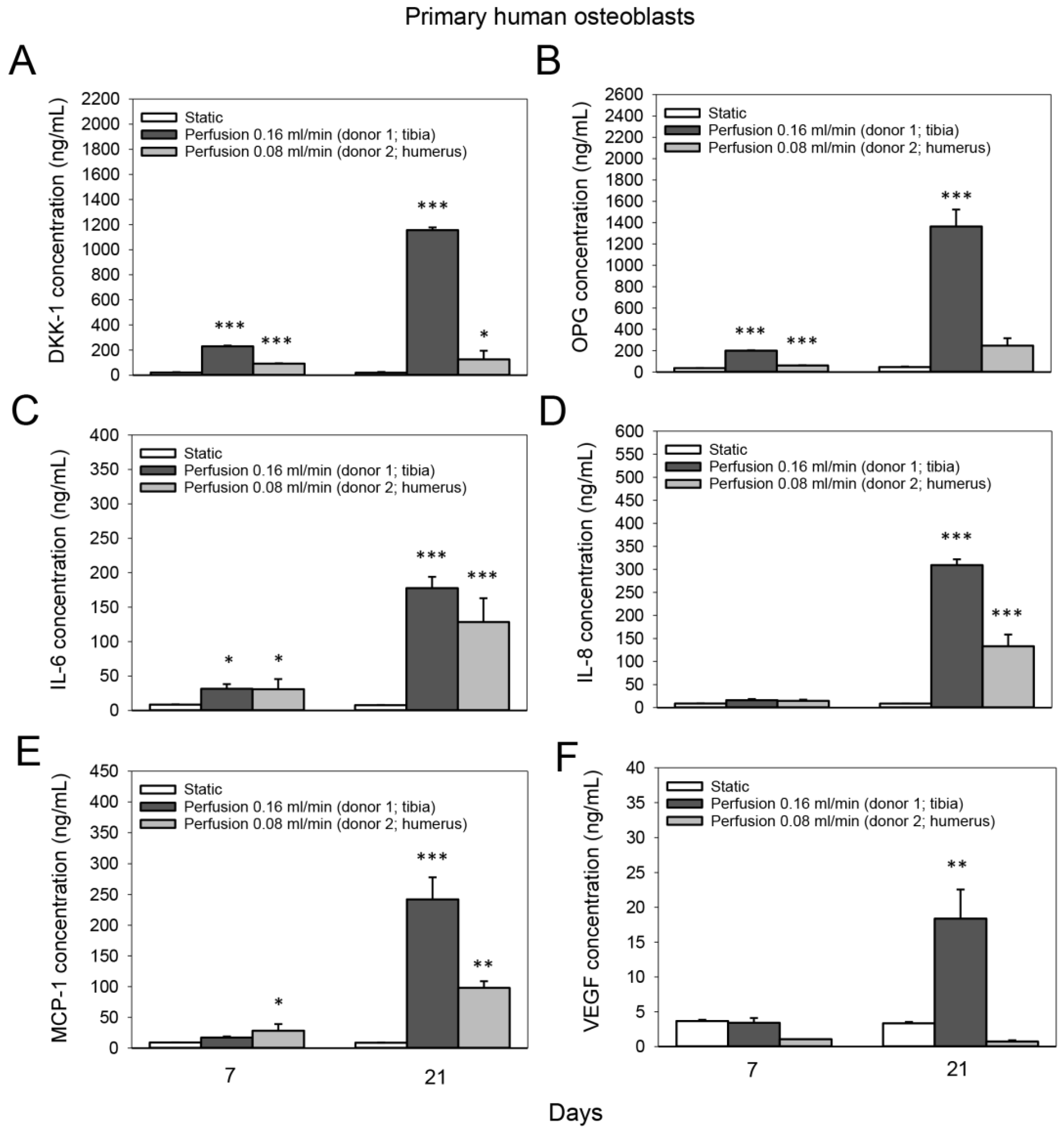
3.5. Effect of Medium Flow on the Secretion of Bone-Related Proteins from Primary Human Osteoblasts Cultured on TiO2 Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bostrom, M.P.G.; Seigerman, D.A. The Clinical Use of Allografts, Demineralized Bone Matrices, Synthetic Bone Graft Substitutes and Osteoinductive Growth Factors: A Survey Study. HSS J.®: Musculoskelet. J. Hosp. Spéc. Surg. 2005, 1, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 92–102. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Hannink, G. Injectable bone-graft substitutes: Current products, their characteristics and indications, and new developments. Injury 2011, 42, S30–S34. [Google Scholar] [CrossRef]
- Tiainen, H.; Wiedmer, D.; Haugen, H.J. Processing of highly porous TiO2 bone scaffolds with improved compressive strength. J. Eur. Ceram. Soc. 2013, 33, 15–24. [Google Scholar] [CrossRef]
- Tiainen, H.; Wohlfahrt, J.C.; Verket, A.; Lyngstadaas, S.P.; Haugen, H.J. Bone formation in TiO2 bone scaffolds in extraction sockets of minipigs. Acta Biomater. 2012, 8, 2384–2391. [Google Scholar] [CrossRef]
- Gómez-Florit, M.; Rubert, M.; Ramis, J.M.; Haugen, H.J.; Tiainen, H.; Lyngstadaas, S.P.; Monjo, M. TiO2 Scaffolds Sustain Differentiation of MC3T3-E1 Cells. J. Biomater. Tissue Eng. 2012, 2, 336–344. [Google Scholar] [CrossRef]
- Haugen, H.J.; Monjo, M.; Rubert, M.; Verket, A.; Lyngstadaas, S.P.; Ellingsen, J.E.; Rønold, H.J.; Wohlfahrt, J.C. Porous ceramic titanium dioxide scaffolds promote bone formation in rabbit peri-implant cortical defect model. Acta Biomater. 2013, 9, 5390–5399. [Google Scholar] [CrossRef]
- Jokinen, M.; Pätsi, M.; Rahiala, H.; Peltola, T.; Ritala, M.; Rosenholm, J.B. Influence of sol and surface properties onin vitro bioactivity of sol-gel-derived TiO2 and TiO2-SiO2 films deposited by dip-coating method. J. Biomed. Mater. Res. 1998, 42, 295–302. [Google Scholar] [CrossRef]
- Nygren, H.; Tengvall, P.; Lundström, I. The initial reactions of TiO2 with blood. J. Biomed. Mater. Res. 1997, 34, 487–492. [Google Scholar] [CrossRef]
- Le Thieu, M.K.; Homayouni, A.; Hæren, L.R.; Tiainen, H.; Verket, A.; Ellingsen, J.E.; Rønold, H.J.; Wohlfahrt, J.C.; Gonzalez Cantalapiedra, A.; Guzon Muñoz, F.M.; et al. Impact of simultaneous placement of implant and block bone graft substitute: An in vivo peri-implant defect model. Biomater. Res. 2021, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Le Thieu, M.K.; Haugen, H.J.; Sanz-Esporrin, J.; Sanz, M.; Lyngstadaas, S.P.; Verket, A. Guided bone regeneration of chronic non-contained bone defects using a volume stable porous block TiO2 scaffold: An experimental in vivo study. Clin. Oral Im-plant. Res. 2021, 32, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Sikavitsas, V.I.; Bancroft, G.N.; Mikos, A.G. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J. Biomed. Mater. Res. 2002, 62, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Botchwey, E.A.; Pollack, S.R.; Levine, E.M.; Laurencin, C.T. Bone tissue engineering in a rotating bioreactor using a microcarrier matrix system. J. Biomed. Mater. Res. 2001, 55, 242–253. [Google Scholar] [CrossRef]
- Bancroft, G.N.; Sikavitsas, V.I.; Dolder, J.V.D.; Sheffield, T.L.; Ambrose, C.G.; Jansen, J.A.; Mikos, A.G. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc. Natl. Acad. Sci. USA 2002, 99, 12600–12605. [Google Scholar] [CrossRef] [Green Version]
- Grayson, W.L.; Bhumiratana, S.; Cannizzaro, C.; Chao, P.-H.G.; Lennon, D.P.; Caplan, A.; Vunjak-Novakovic, G. Effects of Initial Seeding Density and Fluid Perfusion Rate on Formation of Tissue-Engineered Bone. Tissue Eng. Part A 2008, 14, 1809–1820. [Google Scholar] [CrossRef]
- Janssen, F.W.; Oostra, J.; Van Oorschot, A.; van Blitterswijk, C. A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: In vivo bone formation showing proof of concept. Biomaterials 2006, 27, 315–323. [Google Scholar] [CrossRef]
- Goldstein, A. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials 2001, 22, 1279–1288. [Google Scholar] [CrossRef]
- Ishaug-Riley, S.L.; Crane, G.; Yaszemski, M.J.; Mikos, A.G. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials 1998, 19, 1405–1412. [Google Scholar] [CrossRef]
- Ishaug, S.L.; Crane, G.M.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J. Biomed. Mater. Res. 1997, 36, 17–28. [Google Scholar] [CrossRef]
- Gaspar, D.; Gomide, V.; Monteiro, F. The role of perfusion bioreactors in bone tissue engineering. Biomatter 2012, 2, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birru, B.; Mekala, N.K.; Parcha, S.R. Mechanistic role of perfusion culture on bone regeneration. J. Biosci. 2019, 44, 23. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.L.; Turner, C.H. Mechanotransduction and the functional response of bone to mechanical strain. Calcif. Tissue Res. 1995, 57, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Yassin, M.A.; Schwarz, T.; Hansmann, J.; Mustafa, K. Induction of osteogenic differentiation of bone marrow stromal cells on 3D polyester-based scaffolds solely by subphysiological fluidic stimulation in a laminar flow bioreactor. J. Tissue Eng. 2021, 12. [Google Scholar] [CrossRef]
- Moser, C.; Bardsley, K.; El Haj, A.J.; Alini, M.; Stoddart, M.J.; Bara, J.J. A Perfusion Culture System for Assessing Bone Marrow Stromal Cell Differentiation on PLGA Scaffolds for Bone Repair. Front. Bioeng. Biotechnol. 2018, 6. [Google Scholar] [CrossRef]
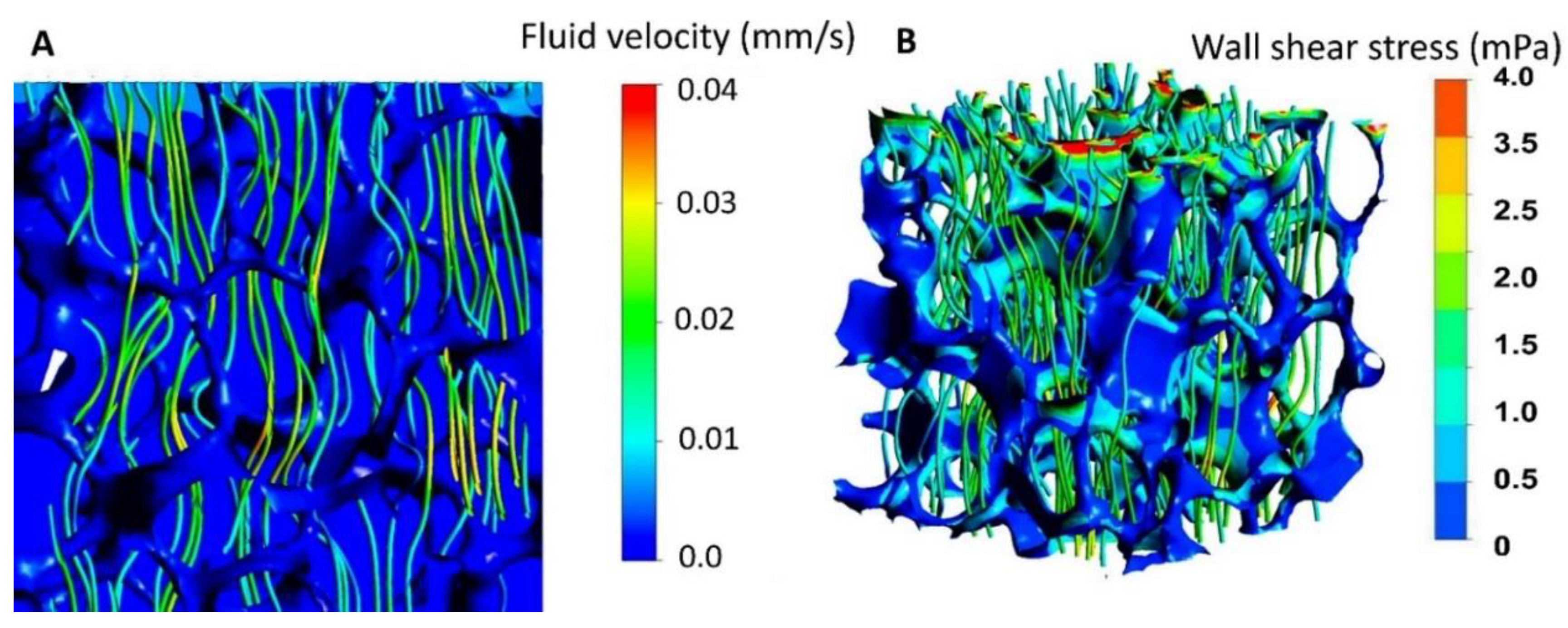
- Zhang, X.; Tiainen, H.; Haugen, H.J. Comparison of titanium dioxide scaffold with commercial bone graft materials through micro-finite element modelling in flow perfusion. Med. Biol. Eng. Comput. 2018, 57, 311–324. [Google Scholar] [CrossRef] [Green Version]
- Wittkowske, C.; Reilly, G.C.; Lacroix, D.; Perrault, C.M. In Vitro Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front. Bioeng. Biotechnol. 2016, 4, 87. [Google Scholar] [CrossRef] [Green Version]
- Tiainen, H.; Lyngstadaas, S.P.; Ellingsen, J.E.; Haugen, H.J. Ultra-porous titanium oxide scaffold with high compressive strength. J. Mater. Sci. Mater. Med. 2010, 21, 2783–2792. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Sampson, K.; Koo, S.; Gadola, C.; Vasiukhina, A.; Singh, A.; Spartano, A.; Gollapudi, R.; Duley, M.; Mueller, J.; James, P.F.; et al. Cultivation of hierarchical 3D scaffolds inside a perfusion bioreactor: Scaffold design and finite-element analysis of fluid flow. SN Appl. Sci. 2021, 3, 884. [Google Scholar] [CrossRef]
- Mikos, A.G.; Bao, Y.; Cima, L.G.; Ingber, N.E.; Vacanti, J.P.; Langer, R. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J. Biomed. Mater. Res. 1993, 27, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vázquez, F.; Cabañas, M.; Paris, J.; Lozano, D.; Vallet-Regí, M. Fabrication of novel Si-doped hydroxyapatite/gelatine scaffolds by rapid prototyping for drug delivery and bone regeneration. Acta Biomater. 2015, 15, 200–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Barradas, A.W.C.; van Blitterswijk, C.A.; de Boer, J.; Feijen, J.; Grijpma, D.W. Effects of the architecture of tissue engineering scaffolds on cell seeding and culturing. Acta Biomater. 2010, 6, 4208–4217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.C.; Arns, C.; Hutmacher, D.W.; Milthorpe, B.K.; Sheppard, A.; Knackstedt, M.A. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 2009, 30, 1440–1451. [Google Scholar] [CrossRef]
- Jones, J.R.; Atwood, R.C.; Poologasundarampillai, G.; Yue, S.; Lee, P. Quantifying the 3D macrostructure of tissue scaffolds. J. Mater. Sci. Mater. Electron. 2008, 20, 463–471. [Google Scholar] [CrossRef]
- Yeatts, A.; Fisher, J.P. Bone tissue engineering bioreactors: Dynamic culture and the influence of shear stress. Bone 2011, 48, 171–181. [Google Scholar] [CrossRef]
- Sandino, C.; Planell, J.; Lacroix, D. A finite element study of mechanical stimuli in scaffolds for bone tissue engineering. J. Biomech. 2008, 41, 1005–1014. [Google Scholar] [CrossRef]
- Mitsak, A.G.; Kemppainen, J.M.; Harris, M.T.; Hollister, S.J. Effect of Polycaprolactone Scaffold Permeability on Bone Regeneration in vivo. Tissue Eng. Part A 2011, 17, 1831–1839. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Xiong, Y.; Ito, K.; van Rietbergen, B.; Hofmann, S. Porous Geometry Guided Micro-mechanical Environment Within Scaffolds for Cell Mechanobiology Study in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 736489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gong, H. Simulation on tissue differentiations for different architecture designs in bone tissue engineering scaffold based on cellular structure model. J. Mech. Med. Biol. 2015, 15, 1550028. [Google Scholar] [CrossRef]
- Olivares, A.L.; Marsal, È.; Planell, J.A.; Lacroix, D. Finite element study of scaffold architecture design and culture conditions for tissue engineering. Biomaterials 2009, 30, 6142–6149. [Google Scholar] [CrossRef] [PubMed]
- Cartmell, S.H.; Porter, B.D.; García, A.J.; Guldberg, R.E. Effects of Medium Perfusion Rate on Cell-Seeded Three-Dimensional Bone Constructs in Vitro. Tissue Eng. 2003, 9, 1197–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, R.J.; O’Brien, F.J. Influence of Shear Stress in Perfusion Bioreactor Cultures for the Development of Three-Dimensional Bone Tissue Constructs: A Review. Tissue Eng. Part B Rev. 2010, 16, 587–601. [Google Scholar] [CrossRef]
- Mueller, S.M.; Mizuno, S.; Gerstenfeld, L.C.; Glowacki, J. Medium Perfusion Enhances Osteogenesis by Murine Osteosarcoma Cells in Three-Dimensional Collagen Sponges. J. Bone Miner. Res. 1999, 14, 2118–2126. [Google Scholar] [CrossRef]
- Bjerre, L.; Bünger, C.E.; Kassem, M.; Mygind, T. Flow perfusion culture of human mesenchymal stem cells on silicate-substituted tricalcium phosphate scaffolds. Biomaterials 2008, 29, 2616–2627. [Google Scholar] [CrossRef]
- Su, W.-T.; Wang, Y.-T.; Chou, C.-M. Optimal fluid flow enhanced mineralization of MG-63 cells in porous chitosan scaffold. J. Taiwan Inst. Chem. Eng. 2014, 45, 1111–1118. [Google Scholar] [CrossRef]
- Butler, W.T. The Nature and Significance of Osteopontin. Connect. Tissue Res. 1989, 23, 123–136. [Google Scholar] [CrossRef]
- Golub, E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Feng, J.Q.; Dusevich, V.M.; Sinha, K.; Zhang, H.; Darnay, B.G.; de Crombrugghe, B. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc. Natl. Acad. Sci. USA 2010, 107, 12919–12924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinlapabodin, S.; Amornsudthiwat, P.; Damrongsakkul, S.; Kanokpanont, S. An axial distribution of seeding, proliferation, and osteogenic differentiation of MC3T3-E1 cells and rat bone marrow-derived mesenchymal stem cells across a 3D Thai silk fibroin/gelatin/hydroxyapatite scaffold in a perfusion bioreactor. Mater. Sci. Eng. C 2016, 58, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Kreke, M.; Huckle, W.; Goldstein, A. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone 2005, 36, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B. Molecular Mechanisms Mediating Proliferation/Differentiation Interrelationships During Progressive Development of the Osteoblast Phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Pinzone, J.J.; Hall, B.M.; Thudi, N.K.; Vonau, M.; Qiang, Y.-W.; Rosol, T.J.; Shaughnessy, J.D., Jr. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 2009, 113, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, F.; Duplomb, L.; Baud’Huin, M.; Brounais, B. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 2009, 20, 19–28. [Google Scholar] [CrossRef]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef]
- Hong, K.H.; Ryu, J.; Han, K.H. Monocyte chemoattractant protein-1–induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood 2005, 105, 1405–1407. [Google Scholar] [CrossRef] [Green Version]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Jaasma, M.J.; O’Brien, F.J. Mechanical Stimulation of Osteoblasts Using Steady and Dynamic Fluid Flow. Tissue Eng. Part A 2008, 14, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.M.; Sun, H.B.; Roeder, R.K.; Burr, D.B.; Turner, C.H.; Yokota, H. Osteoblast Responses One Hour After Load-Induced Fluid Flow in a Three-Dimensional Porous Matrix. Calcif. Tissue Res. 2005, 76, 261–271. [Google Scholar] [CrossRef] [PubMed]

| Protein | Gene | Primer Sequence (5′–3′) |
|---|---|---|
| Mouse glyceraldehyde-3-phosphate dehydrogenase | m-GAPDH m-GAPDH | f ACCCAGAAGACTGTGGATGG r CACATTGGG-GGTAGGAACAC |
| Mouse collagen type I alpha 1 | m-COL1A1 m-COL1A1 | f AGAGC-ATGACCGATGGATTC r CCTTCTTGAGGTTGCCAGTC |
| Mouse alkaline phosphatase | m-ALPL m-ALPL | f AACCCAGACACAAGCATTCC r GAGAGCGAAGGGTC-AGTCAG |
| Mouse bone sialoprotein | m-BSP m-BSP | f GAAA-ATGGAGACGGCGATAG r ACCCGAGAGTGTGGAAAGTG |
| Mouse osteopontin | m-SPP1 m-SPP1 | f TCTGCGGCAGGCATTCTCGG r GTCA-CTTTCACCGGGAGGGAGGA |
| Mouse osteocalcin | m-BGLAP m-BGLAP | f CCGGGAGCAG-TGTGAGCTTA r TAGATGC-GTTTGTAGGCGGTC |
| Mouse osterix | m-SP7 m-SP7 | f AC-TGGCTAGGTGGTGGTCAG r GGTAGGGAGC-TGGGTTAAGG |
| Human-glyceraldehyde-3-phos-phate dehydrogenase | h-GAPDH h-GAPDH | f CTCTGCTCCTCCTGTTCGAC r ACGACCAAATCCGTTGACTC |
| Human collagen type I alpha 1 | h-COL1A1 h-COL1A1 | f CCAAATCCG-ATGTTTCTGCT r CATCTCCCCTTCGTTTTTGA |
| Human alkaline phosphatase | h-ALPL h-ALPL | f AGACGCGCCTGGTAGTTGT r GACAAGAAGCCCTTCACTGC |
| Human osteopontin | h-SPP1 h-SPP1 | f TGAGGTGATGTCCTCGTCTG r GCC-GAGGTGATAGTGTGGTT |
| Human osteocalcin | h-BGLAP h-BGLAP | f GCTTCACCCTCGAAATGGTA r GCAAGTAGCGCCAATCTAGG |
| Human osterix | h-SP7 h-SP7 | f TACCCC-ATCTCCCTTGACTG r GCTGCAAGCTCTCCATAACC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröder, M.; Reseland, J.E.; Haugen, H.J. Osteoblasts in a Perfusion Flow Bioreactor—Tissue Engineered Constructs of TiO2 Scaffolds and Cells for Improved Clinical Performance. Cells 2022, 11, 1995. https://doi.org/10.3390/cells11131995
Schröder M, Reseland JE, Haugen HJ. Osteoblasts in a Perfusion Flow Bioreactor—Tissue Engineered Constructs of TiO2 Scaffolds and Cells for Improved Clinical Performance. Cells. 2022; 11(13):1995. https://doi.org/10.3390/cells11131995
Chicago/Turabian StyleSchröder, Maria, Janne Elin Reseland, and Håvard Jostein Haugen. 2022. "Osteoblasts in a Perfusion Flow Bioreactor—Tissue Engineered Constructs of TiO2 Scaffolds and Cells for Improved Clinical Performance" Cells 11, no. 13: 1995. https://doi.org/10.3390/cells11131995
APA StyleSchröder, M., Reseland, J. E., & Haugen, H. J. (2022). Osteoblasts in a Perfusion Flow Bioreactor—Tissue Engineered Constructs of TiO2 Scaffolds and Cells for Improved Clinical Performance. Cells, 11(13), 1995. https://doi.org/10.3390/cells11131995







