Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives
Abstract
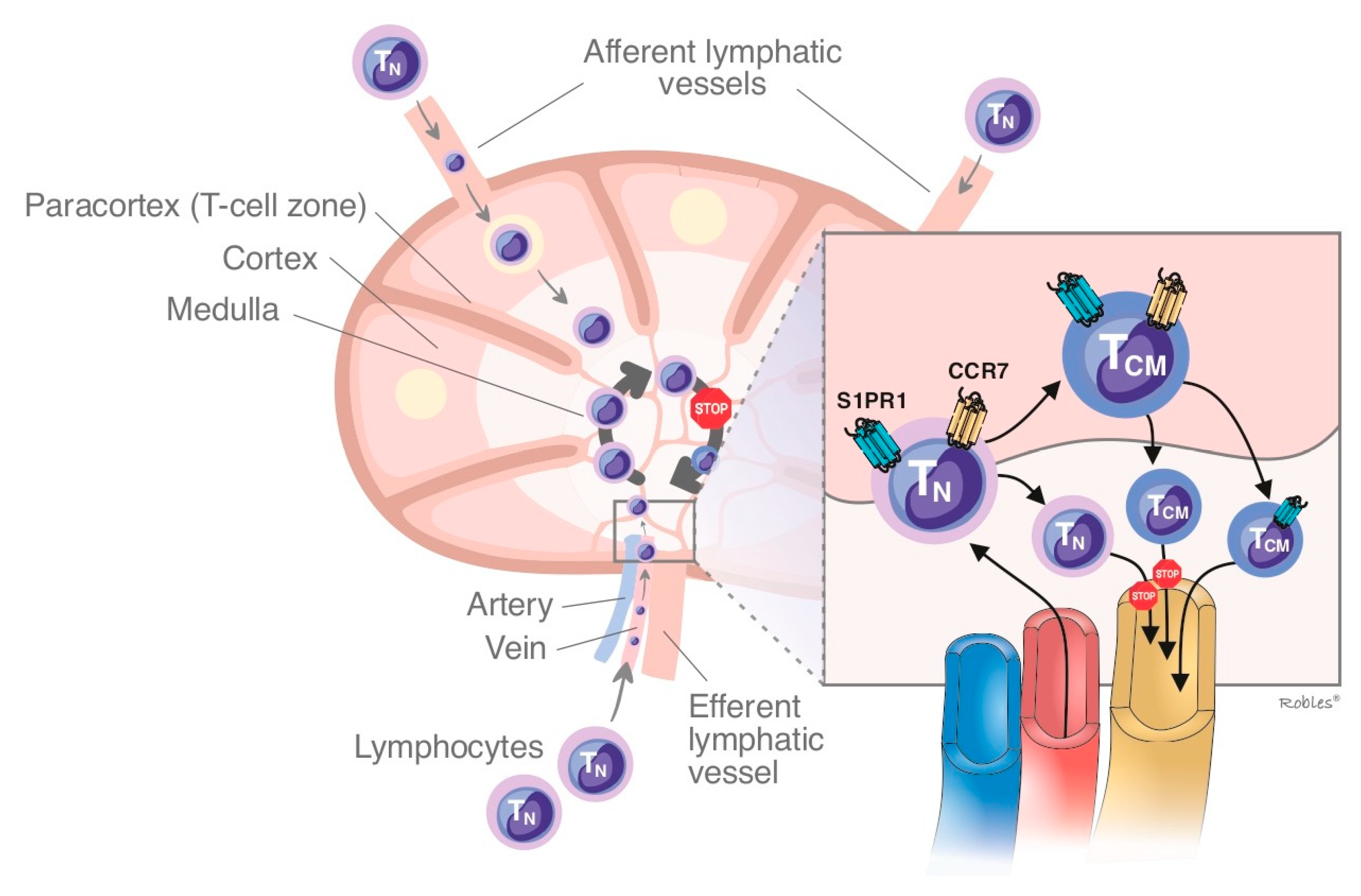
:1. Introduction
1.1. Mechanism of Action of S1P/S1PR
1.2. S1PR Isoforms
1.2.1. Sphingosine-1-Phosphate Receptor: Isoform 1 (S1PR1)
1.2.2. Sphingosine-1-Phosphate Receptor: Isoform 2 (S1PR2)
1.2.3. Sphingosine-1-Phosphate Receptor: Isoform 3 (S1PR3)
1.2.4. Sphingosine-1-Phosphate Receptor: Isoform 4 (S1PR4)
1.2.5. Sphingosine-1-Phosphate Receptor: Isoform 5 (S1PR5)
2. S1P Modulators
2.1. Fingolimod
2.2. Siponimod
2.3. Ozanimod
2.4. Ponesimod and Others S1P Modulators
2.4.1. Ponesimod
2.4.2. Others S1P Modulators
3. Similarities and Differences
3.1. Similarities
3.2. Differences
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Desai, N.N.; Olivera, A.; Seki, T.; Brooker, G.; Spiegel, S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J. Cell Biol. 1991, 114, 155–167. [Google Scholar] [CrossRef]
- Olivera, A.; Spiegel, S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 1993, 365, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kihara, Y.; Maceyka, M.; Spiegel, S.; Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 2014, 171, 3575–3594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1- phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Strub, G.M.; Maceyka, M.; Hait, N.C.; Milstien, S.; Spiegel, S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv. Exp. Med. Biol. 2010, 688, 141–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arish, M.; Alaidarous, M.; Ali, R.; Akhter, Y.; Rub, A. Implication of sphingosine-1-phosphate signaling in diseases: Molecular mechanism and therapeutic strategies. J. Recept. Signal Transduct. Res. 2017, 37, 437–446. [Google Scholar] [CrossRef]
- Leong, W.I.; Saba, J.D. S1P metabolism in cancer and other pathological conditions. Biochimie 2010, 92, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Saba, J.D.; Hla, T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ. Res. 2004, 94, 724–734. [Google Scholar] [CrossRef]
- Tani, M.; Ito, M.; Igarashi, Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell. Signal. 2007, 19, 229–237. [Google Scholar] [CrossRef]
- Snider, A.J.; Orr Gandy, K.A.; Obeid, L.M. Sphingosine kinase: Role in regulation of bioactive sphingolipid mediators in inflammation. Biochimie 2010, 92, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Stepanovska, B.; Huwiler, A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol. Res. 2019, 154, 104170. [Google Scholar] [CrossRef] [PubMed]
- Huwiler, A.; Pfeilschifter, J. New players on the center stage: Sphingosine 1-phosphate and its receptors as drug targets. Biochem. Pharm. 2008, 75, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleuser, B.; Maceyka, M.; Milstien, S.; Spiegel, S. Stimulation of nuclear sphingosine kinase activity by platelet-derived growth factor. FEBS Lett. 2001, 503, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Mandala, S.; Hajdu, R.; Bergstrom, J.; Quackenbush, E.; Xie, J.; Milligan, J.; Thornton, R.; Shei, G.J.; Card, D.; Keohane, C.; et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002, 296, 346–349. [Google Scholar] [CrossRef]
- Huwiler, A.; Zangemeister-Wittke, U. The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: Recent findings and new perspectives. Pharmacol. Ther. 2018, 185, 34–49. [Google Scholar] [CrossRef]
- Brinkmann, V.; Davis, M.D.; Heise, C.E.; Albert, R.; Cottens, S.; Hof, R.; Bruns, C.; Prieschl, E.; Baumruker, T.; Hiestand, P.; et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002, 277, 21453–21457. [Google Scholar] [CrossRef] [Green Version]
- Bigaud, M.; Guerini, D.; Billich, A.; Bassilana, F.; Brinkmann, V. Second generation S1P pathway modulators: Research strategies and clinical developments. Biochim. Biophys. Acta 2014, 1841, 745–758. [Google Scholar] [CrossRef]
- Schwab, S.R.; Pereira, J.P.; Matloubian, M.; Xu, Y.; Huang, Y.; Cyster, J.G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 2005, 309, 1735–1739. [Google Scholar] [CrossRef]
- Argraves, K.M.; Argraves, W.S. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J. Lipid. Res. 2007, 48, 2325–2333. [Google Scholar] [CrossRef] [Green Version]
- Christoffersen, C.; Obinata, H.; Kumaraswamy, S.B.; Galvani, S.; Ahnström, J.; Sevvana, M.; Egerer-Sieber, C.; Muller, Y.A.; Hla, T.; Nielsen, L.B.; et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 2011, 108, 9613–9618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaho, V.A.; Galvani, S.; Engelbrecht, E.; Liu, C.; Swendeman, S.L.; Kono, M.; Proia, R.L.; Steinman, L.; Han, M.H.; Hla, T. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 2015, 523, 342–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, K.; Hoshino, Y.; Suzuki, C.; Masubuchi, Y.; Yanagawa, Y.; Ohtsuki, M.; Sasaki, S.; Fujita, T. FTY720, a novel immunosuppressant possessing unique mechanisms. I. Prolongation of skin allograft survival and synergistic effect in combination with cyclosporine in rats. Transpl. Proc. 1996, 28, 1056–1059. [Google Scholar]
- Kihara, A.; Anada, Y.; Igarashi, Y. Mouse sphingosine kinase isoforms SPHK1a and SPHK1b differ in enzymatic traits including stability, localization, modification, and oligomerization. J. Biol. Chem. 2006, 281, 4532–4539. [Google Scholar] [CrossRef] [Green Version]
- Pitson, S.M.; Moretti, P.A.; Zebol, J.R.; Lynn, H.E.; Xia, P.; Vadas, M.A.; Wattenberg, B.W. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003, 22, 5491–5500. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, S.; Milstien, S. Functions of a new family of sphingosine-1-phosphate receptors. Biochim. Biophys. Acta 2000, 1484, 107–116. [Google Scholar] [CrossRef]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef]
- Oo, M.L.; Thangada, S.; Wu, M.T.; Liu, C.H.; Macdonald, T.L.; Lynch, K.R.; Lin, C.Y.; Hla, T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 2007, 282, 9082–9089. [Google Scholar] [CrossRef] [Green Version]
- Mullershausen, F.; Zecri, F.; Cetin, C.; Billich, A.; Guerini, D.; Seuwen, K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 2009, 5, 428–434. [Google Scholar] [CrossRef]
- Brinkmann, V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol. Ther. 2007, 115, 84–105. [Google Scholar] [CrossRef]
- Foss, F.W., Jr.; Mathews, T.P.; Kharel, Y.; Kennedy, P.C.; Snyder, A.H.; Davis, M.D.; Lynch, K.R.; Macdonald, T.L. Synthesis and biological evaluation of sphingosine kinase substrates as sphingosine-1-phosphate receptor prodrugs. Bioorg. Med. Chem. 2009, 17, 6123–6136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaire, B.P.; Lee, C.H.; Sapkota, A.; Lee, S.Y.; Chun, J.; Cho, H.J.; Nam, T.G.; Choi, J.W. Identification of Sphingosine 1-Phosphate Receptor Subtype 1 (S1P1) as a Pathogenic Factor in Transient Focal Cerebral Ischemia. Mol. Neurobiol. 2018, 55, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Mi, Y.; Pally, C.; Beerli, C.; Chen, A.; Guerini, D.; Hinterding, K.; Nuesslein-Hildesheim, B.; Tuntland, T.; Lefebvre, S.; et al. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem. Biol. 2006, 13, 1227–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quancard, J.; Bollbuck, B.; Janser, P.; Angst, D.; Berst, F.; Buehlmayer, P.; Streiff, M.; Beerli, C.; Brinkmann, V.; Guerini, D.; et al. A potent and selective S1P(1) antagonist with efficacy in experimental autoimmune encephalomyelitis. Chem. Biol. 2012, 19, 1142–1451. [Google Scholar] [CrossRef] [Green Version]
- Blankenbach, K.V.; Schwalm, S.; Pfeilschifter, J.; Meyer, Z.; Heringdorf, D. Sphingosine-1-Phosphate Receptor-2 Antagonists: Therapeutic Potential and Potential Risks. Front. Pharmacol. 2016, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Bigaud, M.; Dincer, Z.; Bollbuck, B.; Dawson, J.; Beckmann, N.; Beerli, C.; Fishli-Cavelti, G.; Nahler, M.; Angst, D.; Janser, P.; et al. Pathophysiological Consequences of a Break in S1P1-Dependent Homeostasis of Vascular Permeability Revealed by S1P1 Competitive Antagonism. PLoS ONE 2016, 11, e0168252. [Google Scholar] [CrossRef]
- Okazaki, H.; Ishizaka, N.; Sakurai, T.; Kurokawa, K.; Goto, K.; Kumada, M.; Takuwa, Y. Molecular Cloning of a Novel Putative G Protein-Coupled Receptor Expressed in the Cardiovascular System. Biochem. Biophys. Res. Commun. 1993, 190, 1104–1109. [Google Scholar] [CrossRef]
- Skoura, A.; Sanchez, T.; Claffey, K.; Mandala, S.M.; Proia, R.L.; Hla, T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J. Clin. Investig. 2007, 117, 2506–2516. [Google Scholar] [CrossRef] [Green Version]
- Kono, M.; Belyantseva, I.A.; Skoura, A.; Frolenkov, G.I.; Starost, M.F.; Dreier, J.L.; Lidington, D.; Bolz, S.S.; Friedman, T.B.; Hla, T.; et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. J. Biol. Chem. 2007, 282, 10690–10696. [Google Scholar] [CrossRef] [Green Version]
- Burczyk, M.; Burkhalter, M.D.; Blätte, T.; Matysik, S.; Caron, M.G.; Barak, L.S.; Philipp, M. Phenotypic regulation of the sphingosine 1-phosphate receptor miles apart by G protein-coupled receptor kinase 2. Biochemistry 2015, 54, 765–775. [Google Scholar] [CrossRef] [Green Version]
- Skoura, A.; Michaud, J.; Im, D.S.; Thangada, S.; Xiong, Y.; Smith, J.D.; Hla, T. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Thromb. Vasc. Biol. 2011, 31, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, A.; Takasugi, H.; Ohnuma, S.; Koide, Y.; Sakurai, A.; Takeda, S.; Hasegawa, T.; Sasamori, J.; Konno, T.; Hayashi, K.; et al. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: Investigation based on a new S1P3 receptor antagonist. Mol. Pharmacol. 2010, 77, 704–713. [Google Scholar] [CrossRef]
- Awojoodu, A.O.; Ogle, M.E.; Sefcik, L.S.; Bowers, D.T.; Martin, K.; Brayman, K.L.; Lynch, K.R.; Peirce-Cottler, S.M.; Botchwey, E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 13785–13790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, K.; Kohno, M.; Kadoya, M.; Nagahara, H.; Fujii, W.; Seno, T.; Yamamoto, A.; Oda, R.; Fujiwara, H.; Kubo, T.; et al. Knock out of S1P3 receptor signaling attenuates inflammation and fibrosis in bleomycin-induced lung injury mice model. PLoS ONE 2014, 9, e106792. [Google Scholar] [CrossRef]
- Niessen, F.; Schaffner, F.; Furlan-Freguia, C.; Pawlinski, R.; Bhattacharjee, G.; Chun, J.; Derian, C.K.; Andrade-Gordon, P.; Rosen, H.; Ruf, W. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature 2008, 452, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.G.; Vincent, K.P.; Repetto, E.; Nguyen, N.; Brown, S.J.; Abgaryan, L.; Riley, S.W.; Leaf, N.B.; Cahalan, S.M.; Kiosses, W.B.; et al. Bitopic Sphingosine 1-Phosphate Receptor 3 (S1P3) Antagonist Rescue from Complete Heart Block: Pharmacological and Genetic Evidence for Direct S1P3 Regulation of Mouse Cardiac Conduction. Mol. Pharmacol. 2016, 89, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Golfier, S.; Kondo, S.; Schulze, T.; Takeuchi, T.; Vassileva, G.; Achtman, A.H.; Gräler, M.H.; Abbondanzo, S.J.; Wiekowski, M.; Kremmer, E.; et al. Shaping of terminal megakaryocyte differentiation and proplatelet development by sphingosine-1-phosphate receptor S1P4. FASEB J. 2010, 24, 4701–4710. [Google Scholar] [CrossRef]
- Gräler, M.H.; Grosse, R.; Kusch, A.; Kremmer, E.; Gudermann, T.; Lipp, M. The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J. Cell. Biochem. 2003, 89, 507–519. [Google Scholar] [CrossRef]
- Guerrero, M.; Urbano, M.; Velaparthi, S.; Schaeffer, M.T.; Brown, S.J.; Crisp, M.; Ferguson, J.; Hodder, P.; Rosen, H.; Oldstone, M.; et al. Identification of a novel agonist of the sphingosine 1-phos-phate receptor 4 (S1P4). In Probe Reports from the NIH Molecular Libraries Program; Bethesda: Rockville, MD, USA, 2010. [Google Scholar]
- Niedernberg, A.; Scherer, C.R.; Busch, A.E.; Kostenis, E. Comparative analysis of human and rat S1P(5) (edg8): Differential expression profiles and sensitivities to antagonists. Biochem. Pharmacol. 2002, 64, 1243–1250. [Google Scholar] [CrossRef]
- Im, D.S.; Clemens, J.; Macdonald, T.L.; Lynch, K.R. Characterization of the human and mouse sphingosine 1-phosphate receptor, S1P5 (Edg-8): Structure-activity relationship of sphingosine1-phosphate receptors. Biochemistry 2001, 40, 14053–14060. [Google Scholar] [CrossRef]
- Jaillard, C.; Harrison, S.; Stankoff, B.; Aigrot, M.S.; Calver, A.R.; Duddy, G.; Walsh, F.S.; Pangalos, M.N.; Arimura, N.; Kaibuchi, K.; et al. Edg8/S1P5: An oligodendroglial receptor with dual function on process retraction and cell survival. J. Neurosci. 2005, 25, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Arlt, O.; Schmidt, H.; Huwiler, A.; Angioni, C.; Pfeilschifter, J.M.; Schwiebs, A.; Radeke, H.H. Subcellular distribution of FTY720 and FTY720-phosphate in immune cells - another aspect of Fingolimod action relevant for therapeutic application. Biol. Chem. 2015, 396, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhry, B.Z.; Cohen, J.A.; Conway, D.S. Sphingosine 1-phosphate receptor modulators for the treatment of multiple sclerosis. Neurotherapeutics 2017, 14, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Pyne, S. Sphingosine 1-phosphate receptor 1 signaling in mammalian cells. Molecules 2017, 22, 344. [Google Scholar] [CrossRef] [Green Version]
- Kappos, L.; Radue, E.W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef] [Green Version]
- Kappos, L.; Antel, J.; Comi, G.; Montalban, X.; O’Connor, P.; Polman, C.H.; Haas, T.; Korn, A.A.; Karlsson, G.; Radue, E.W.; et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 355, 1124. [Google Scholar] [CrossRef] [Green Version]
- Hla, T.; Brinkmann, V. Sphingosine 1-phosphate (S1P). Physiology and the effects of S1P receptor modulation. Neurology 2011, 76, S3–S8. [Google Scholar] [CrossRef]
- Hunter, S.F.; Bowen, J.D.; Reder, A.T. The direct effects of fingolimod in the central nervous system: Implications for relapsing multiple sclerosis. CNS Drugs 2016, 30, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Calabresi, P.A.; Radue, E.W.; Goodin, D.; Jeffery, D.; Rammohan, K.W.; Reder, A.T.; Vollmer, T.; Agius, M.A.; Kappos, L.; Stites, T.; et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014, 13, 545–556. [Google Scholar] [CrossRef]
- Cohen, J.A.; Khatri, B.; Barkhof, F.; Comi, G.; Hartung, H.P.; Montalban, X.; Pelletier, J.; Stites, T.; Ritter, S.; von Rosenstiel, P.; et al. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: Results from the extension of the randomised TRANSFORMS study. J. Neurol. Neurosurg. Psychiatry 2016, 87, 468–475. [Google Scholar] [CrossRef] [Green Version]
- Khatri, B.; Barkhof, F.; Comi, G.; Hartung, H.P.; Kappos, L.; Montalban, X.; Pelletier, J.; Stites, T.; Wu, S.; Holdbrook, F.; et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: A randomised extension of the TRANSFORMS study. Lancet Neurol. 2011, 10, 520–529. [Google Scholar] [CrossRef]
- Lublin, F.; Miller, D.H.; Freedman, M.S.; Cree, B.A.C.; Wolinsky, J.S.; Weiner, H.; Lubetzki, C.; Hartung, H.P.; Montalban, X.; Uitdehaag, B.M.J.; et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2016, 387, 1075–1084. [Google Scholar] [CrossRef]
- Ziemssen, T.; Lang, M.; Tackenberg, B.; Schmidt, S.; Albrecht, H.; Klotz, L.; Haas, J.; Lassek, C.; Couto, C.A.; Findlay, J.A.; et al. Real-world persistence and benefit-risk profile of fingolimod over 36 months in Germany. Value Health 2015, 18, A749. [Google Scholar] [CrossRef] [Green Version]
- Gold, R.; Comi, G.; Palace, J.; Siever, A.; Gottschalk, R.; Bijarnia, M.; von Rosenstiel, P.; Tomic, D.; Kappos, L.; FIRST Study Investigators. Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: A phase 3b, open-label study. J. Neurol. 2014, 261, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, N.Y.; Weigel, C.; Reimann, C.M.; Gräler, M.H. Modulating sphingosine 1-phosphate signaling with DOP or FTY720 alleviates vascular and immune defects in mouse sepsis. Eur. J. Immunol. 2016, 46, 2767–2777. [Google Scholar] [CrossRef] [PubMed]
- Patmanathan, S.N.; Yap, L.F.; Murray, P.G.; Paterson, I.C. The antineoplastic properties of FTY720: Evidence for the repurposing of fingolimod. J. Cell. Mol. Med. 2015, 19, 2329–2340. [Google Scholar] [CrossRef] [Green Version]
- Gergely, P.; Nuesslein-Hildesheim, B.; Guerini, D.; Brinkmann, V.; Traebert, M.; Bruns, C.; Pan, S.; Gray, N.S.; Hinterding, K.; Cooke, N.G.; et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br. J. Pharmacol. 2012, 167, 1035–1047. [Google Scholar] [CrossRef]
- Gentile, A. Siponimod (BAF312) prevents synaptic neurodegeneration in experimental multiple sclerosis. J. Neuroinflammation 2016, 13, 207. [Google Scholar] [CrossRef] [Green Version]
- Tiwari-Woodruff, S. Hana Yamate-Morgan, Maria Sekyi, Kelli Lauderdale, Jonathan Hasselmann, Anna Schubart. The Sphingosine 1-phosphate (S1P) Receptor Modulator, Siponimod Decreases Oligodendrocyte Cell Death and Axon Demyelination in a Mouse Model of Multiple Sclerosis (I10.011). Neurology 2016, 86, P5.325. [Google Scholar]
- Selmaj, K.; Li, D.K.; Hartung, H.P.; Hemmer, B.; Kappos, L.; Freedman, M.S.; Stüve, O.; Rieckmann, P.; Montalban, X.; Ziemssen, T.; et al. Siponimod for patients with relapsing-remitting multiple sclerosis (BOLD): An adaptive, dose-ranging, randomised, phase 2 study. Lancet Neurol. 2013, 12, 756–767. [Google Scholar] [CrossRef]
- Hammond, E.R. Perspectives on safety and efficacy—The BOLD phase 2 extension study of siponimod in relapsing-remitting multiple sclerosis. JAMA Neurol. 2016, 73, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet 2018, 391, 263–1273. [Google Scholar] [CrossRef]
- Sanna, M.G.; Liao, J.; Jo, E.; Alfonso, C.; Ahn, M.Y.; Peterson, M.S.; Webb, B.; Lefebvre, S.; Chun, J.; Gray, N.; et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J. Biol. Chem. 2004, 279, 13839–13848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, F.L.; Clemons, B.; Brooks, J.; Brahmachary, E.; Powell, R.; Dedman, H.; Desale, H.G.; Timony, G.A.; Martinborough, E.; Rosen, H.; et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1 ) and receptor-5 (S1P5 ) agonist with autoimmune disease-modifying activity. Br. J. Pharmacol. 2016, 173, 1778–1792. [Google Scholar] [CrossRef] [Green Version]
- Tran, J.Q.; Hartung, J.P.; Peach, R.J.; Boehm, M.F.; Rosen, H.; Smith, H.; Brooks, J.L.; Timony, G.A.; Olson, A.D.; Gujrathi, S.; et al. Results From the First-in-Human Study With Ozanimod, a Novel, Selective Sphingosine-1-Phosphate Receptor Modulator. J. Clin. Pharmacol. 2017, 57, 988–996. [Google Scholar] [CrossRef]
- Celgene Corporation. ZEPOSIA® (Ozanimod) Capsules: US Prescribing Information. Available online: http://www.accessdata.fda.gov/ (accessed on 20 April 2020).
- Taylor Meadows, K.R.; Steinberg, M.W.; Clemons, B.; Stokes, M.E.; Opiteck, G.J.; Peach, R.; Scott, F.L. Ozanimod (RPC1063), a selective S1PR1 and S1PR5 modulator, reduces chronic inflammation and alleviates kidney pathology in murine systemic lupus erythematosus. PLoS One 2018, 13, e0193236. [Google Scholar] [CrossRef]
- Cohen, J.A.; Arnold, D.L.; Comi, G.; Bar-Or, A.; Gujrathi, S.; Hartung, J.P.; Cravets, M.; Olson, A.; Frohna, P.A.; Selmaj, K.W.; et al. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 373–381. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A phase 2/3, Multi-Center, Randomized, Double-Blind, Placebo-Controlled [Part A] and Double-Blind, Double-Dummy, Active-Controlled [part b], Parallel Group Study to Evaluate the Efficacy and Safety of RPC1063 Administered Orally to Relapsing Multiple Sclerosis Patients 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT02047734?term=NCT02047734&rank=1 (accessed on 6 August 2017).
- ClinicalTrials.gov. A Multicenter, Longitudinal, Open-Label, Single-Arm Study Describing Cognitive Processing Speed Changes in Relapsing Multiple Sclerosis Subjects Treated With Ozanimod (RPC-1063). Available online: https://clinicaltrials.gov/ct2/show/NCT04140305 (accessed on 6 August 2017).
- Sandborn, W.J.; Feagan, B.G.; D’Haens, G.; Wolf, D.C.; Jovanovic, I.; Hanauer, S.B.; Ghosh, S.; Petersen, A.; Hua, S.Y.; Lee, J.H.; et al. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2021, 385, 1280–1291. [Google Scholar] [CrossRef]
- Piali, L.; Froidevaux, S.; Hess, P.; Nayler, O.; Bolli, M.H.; Schlosser, E.; Kohl, C.; Steiner, B.; Clozel, M. The selective sphingosine 1-phosphate receptor 1 agonist ponesimod protects against lymphocyte-mediated tissue inflammation. J. Pharmacol. Exp. Ther. 2011, 337, 547. [Google Scholar] [CrossRef] [Green Version]
- Dash, R.P.; Rais, R.; Srinivas, N.R. Ponesimod, a selective sphingosine 1-phosphate (S1P1) receptor modulator for autoimmune diseases: Review of clinical pharmacokinetics and drug disposition. Xenobiotica 2018, 48, 442. [Google Scholar] [CrossRef]
- D’Ambrosio, D.; Freedman, M.S.; Prinz, J. Ponesimod, a selective S1P1 receptor modulator: A potential treatment for multiple sclerosis and other immune-mediated diseases. Ther. Adv. Chronic. Dis. 2016, 7, 18–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ambrosio, D.; Steinmann, J.; Brossard, P.; Dingemanse, J. Differential effects of ponesimod, a selective S1P1 receptor modulator, on blood-circulating human T cell subpopulations. Immunopharmacol. Immunotoxicol. 2015, 37, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Brossard, P.; Derendorf, H.; Xu, J.; Maatouk, H.; Halabi, A.; Dingemanse, J. Pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P1 receptor modulator, in the first-in-human study. Br. J. Clin. Pharmacol. 2013, 76, 888–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappos, L.; Fox, R.J.; Burcklen, M.; Freedman, M.S.; Havrdová, E.K.; Hennessy, B.; Hohlfeld, R.; Lublin, F.; Montalban, X.; Pozzilli, C.; et al. Ponesimod Compared With Teriflunomide in Patients With Relapsing Multiple Sclerosis in the Active-Comparator Phase 3 OPTIMUM Study: A Randomized Clinical Trial. JAMA Neurol. 2021, 78, 558–567. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Safety and Efficacy Extension Study of ONO-4641 [MSC2430913A] in Patients with Relapsing-Remitting Multiple Sclerosis 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT01226745?term=ONO-4641&rank=1 (accessed on 6 August 2017).
- Xu, J.; Gray, F.; Henderson, A.; Hicks, K.; Yang, J.; Thompson, P.; Oliver, J. Safety, pharmacokinetics, pharmacodynamics, and bioavailability of GSK2018682, a sphingosine-1-phosphate receptor modulator, in healthy volunteers. Clin. Pharmacol. Drug Dev. 2014, 3, 170–178. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Phase II, Multicentre, Randomised, Double-Blind, Parallel Group, Placebo-Controlled, Dose-Finding Study to Evaluate the Safety and Efficacy of Three Different Oral Doses of MT-1303 Administered for a Period of 24 Weeks in Subjects with Relapsing-Remitting Multiple Sclerosis 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT01742052term=MT-1303&rank=5 (accessed on 6 August 2017).
- Alping, P.; Askling, J.; Burman, J.; Fink, K.; Fogdell-Hahn, A.; Gunnarsson, M.; Hillert, J.; Langer-Gould, A.; Lycke, J.; Nilsson, P.; et al. Cancer Risk for Fingolimod, Natalizumab, and Rituximab in Multiple Sclerosis Patients. Ann. Neurol. 2020, 87, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Riddy, D.M.; Stamp, C.; Sykes, D.A.; Charlton, S.J.; Dowling, M.R. Reassessment of the pharmacology of Sphingosine-1-phosphate S1P3 receptor ligands using the DiscoveRx PathHunter and Ca2+ release functional assays. Br. J. Pharmacol. 2012, 167, 868–880. [Google Scholar] [CrossRef] [Green Version]
- ANZCTR. Available online: http://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000545763 (accessed on 7 April 2020).
- Baldin, E.; Lugaresi, A. Ponesimod for the treatment of relapsing multiple sclerosis. Expert Opin. Pharmacother. 2020, 21, 1955–1964. [Google Scholar] [CrossRef]
- Olsson, T.; Boster, A.; Fernández, Ó.; Freedman, M.S.; Pozzilli, C.; Bach, D.; Berkani, O.; Mueller, M.S.; Sidorenko, T.; Radue, E.W.; et al. Oral ponesimod in relapsing-remitting multiple sclerosis: A randomised phase II trial. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1198–1208. [Google Scholar] [CrossRef] [Green Version]
- Hatcher, S.E.; Waubant, E.; Nourbakhsh, B.; Crabtree-Hartman, E.; Graves, J.S. Rebound Syndrome in Patients with Multiple Sclerosis After Cessation of Fingolimod Treatment. JAMA Neurol. 2016, 73, 790–794. [Google Scholar] [CrossRef] [Green Version]
- Kamel, H.; Iadecola, C. Brain-immune interactions and ischemic stroke: Clinical implications. Arch. Neurol. 2012, 69, 576–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Zhang, N.; Ren, L.; Yan, Y.; Sun, N.; Li, Y.J.; Han, W.; Xue, R.; Liu, Q.; Hao, J.; et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc. Natl. Acad. Sci. USA 2014, 111, 18315–18320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Hao, J.; Zhang, N.; Ren, L.; Sun, N.; Li, Y.J.; Yan, Y.; Huang, D.; Yu, C.; Shi, F.D. Fingolimod for the treatment of intracerebral hemorrhage: A 2-arm proof-of-concept study. JAMA Neurol. 2014, 71, 1092–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Drug | Receptor Selectivity | Genotyping Needed | Dosing | Elimination T1/2 | Lymphocyte Restoration after Discontinuation |
|---|---|---|---|---|---|
| Fingolimod (Gilenya®) (FTY720) | S1PR1 S1PR3 S1PR4 S1PR5 | No | 0.5 mg/d | 7 days | 6 weeks |
| Siponimod (Mayzent®) (BAF312) | S1PR1 S1PR5 | Yes | Depending on genotype: CYP2C9*1*1 and CYP2C9*1*2: 2mg/d CYP2C9*2*2 and CYP2C9*1*3: 1mg/d CYP2C9*2*3 and CYP2C9*3*3: contraindicated | 30 h | 1–10 days |
| Ozanimod (Zeposia®) (RPC1063) | S1PR1 S1PR5 | No | 0.92 mg/d | 19–20 h | 4–12 weeks |
| Ponesimod (Ponvory®) (ACT128800) | S1PR1 | No | 20 mg/d | 33 h | 7 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo, G.Á.; Cedeño, R.R.; Casadevall, M.P.; Ramió-Torrentà, L. Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives. Cells 2022, 11, 2058. https://doi.org/10.3390/cells11132058
Bravo GÁ, Cedeño RR, Casadevall MP, Ramió-Torrentà L. Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives. Cells. 2022; 11(13):2058. https://doi.org/10.3390/cells11132058
Chicago/Turabian StyleBravo, Gary Álvarez, René Robles Cedeño, Marc Puig Casadevall, and Lluís Ramió-Torrentà. 2022. "Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives" Cells 11, no. 13: 2058. https://doi.org/10.3390/cells11132058
APA StyleBravo, G. Á., Cedeño, R. R., Casadevall, M. P., & Ramió-Torrentà, L. (2022). Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway Modulators, from Current Insights to Future Perspectives. Cells, 11(13), 2058. https://doi.org/10.3390/cells11132058






