DNA Methylation as an Early Indicator of Aging in Stored Seeds of “Exceptional” Species Populus nigra L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Assessment of Moisture Content (MC)
2.2. Germination
2.3. DNA Isolation and Assessment of Global DNA Methylation Levels
2.4. Statistical Analysis
3. Results
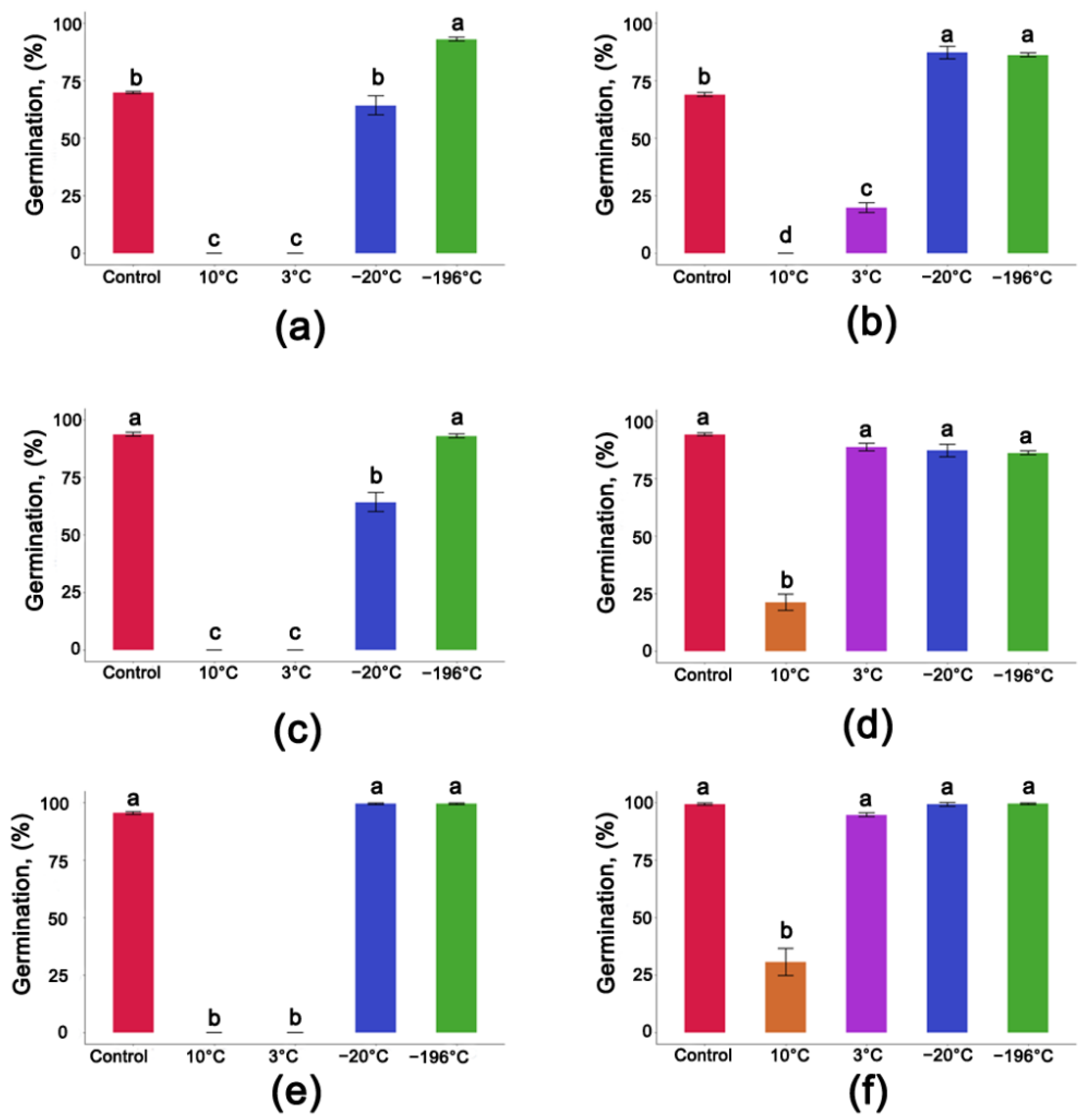
3.1. Seed Germination following Desiccation
3.2. DNA Methylation Level following Seed Desiccation
3.3. Seed Germination after One Year of Storage at Different Moisture Contents and Temperatures
3.4. DNA Methylation Level in Seeds after One Year of Storage at Different Moisture Contents and Temperatures
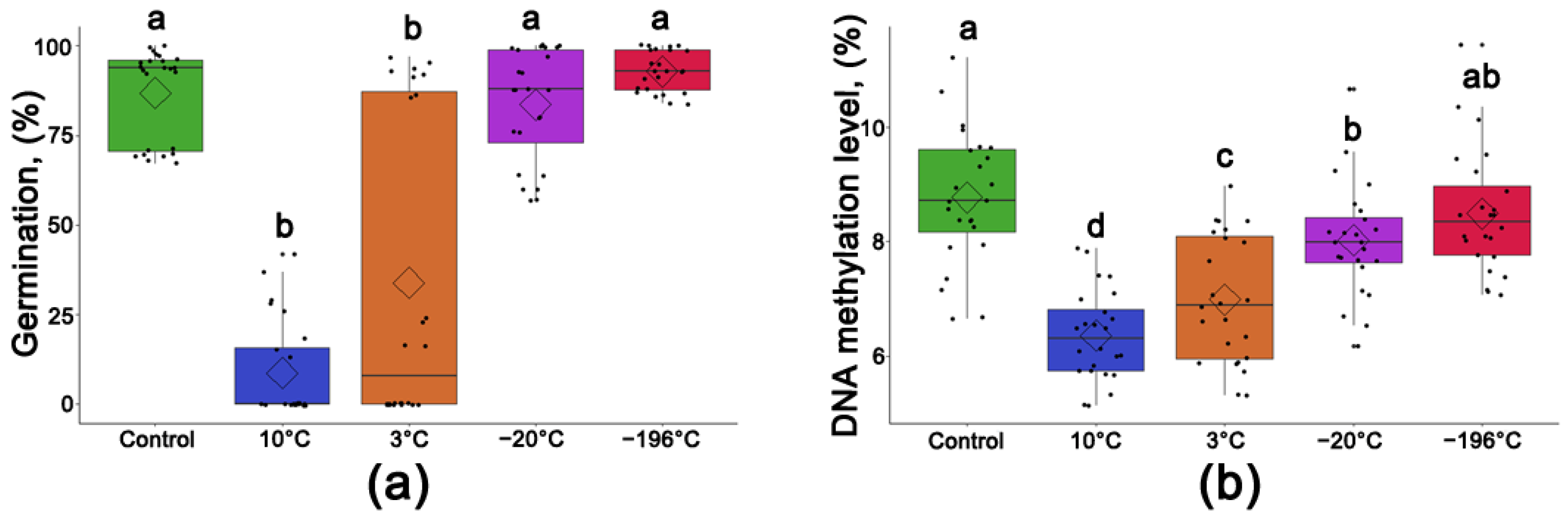
3.5. Average Germination Rate of Populus nigra L. Seeds after One Year of Storage at Different Temperatures
3.6. Average Level of DNA Methylation Level of Populus nigra L. Seeds after One Year of Storage at Different Temperatures
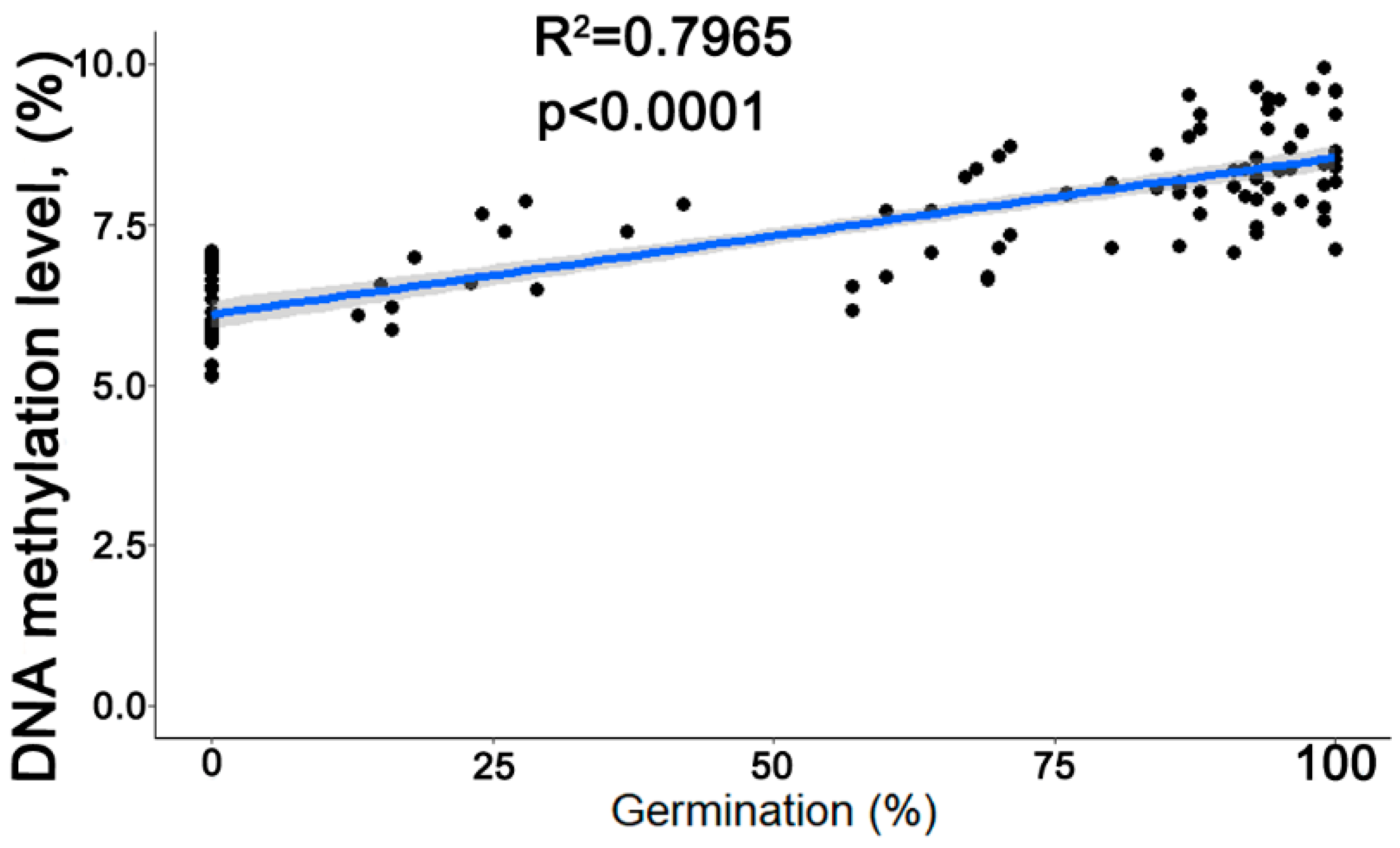
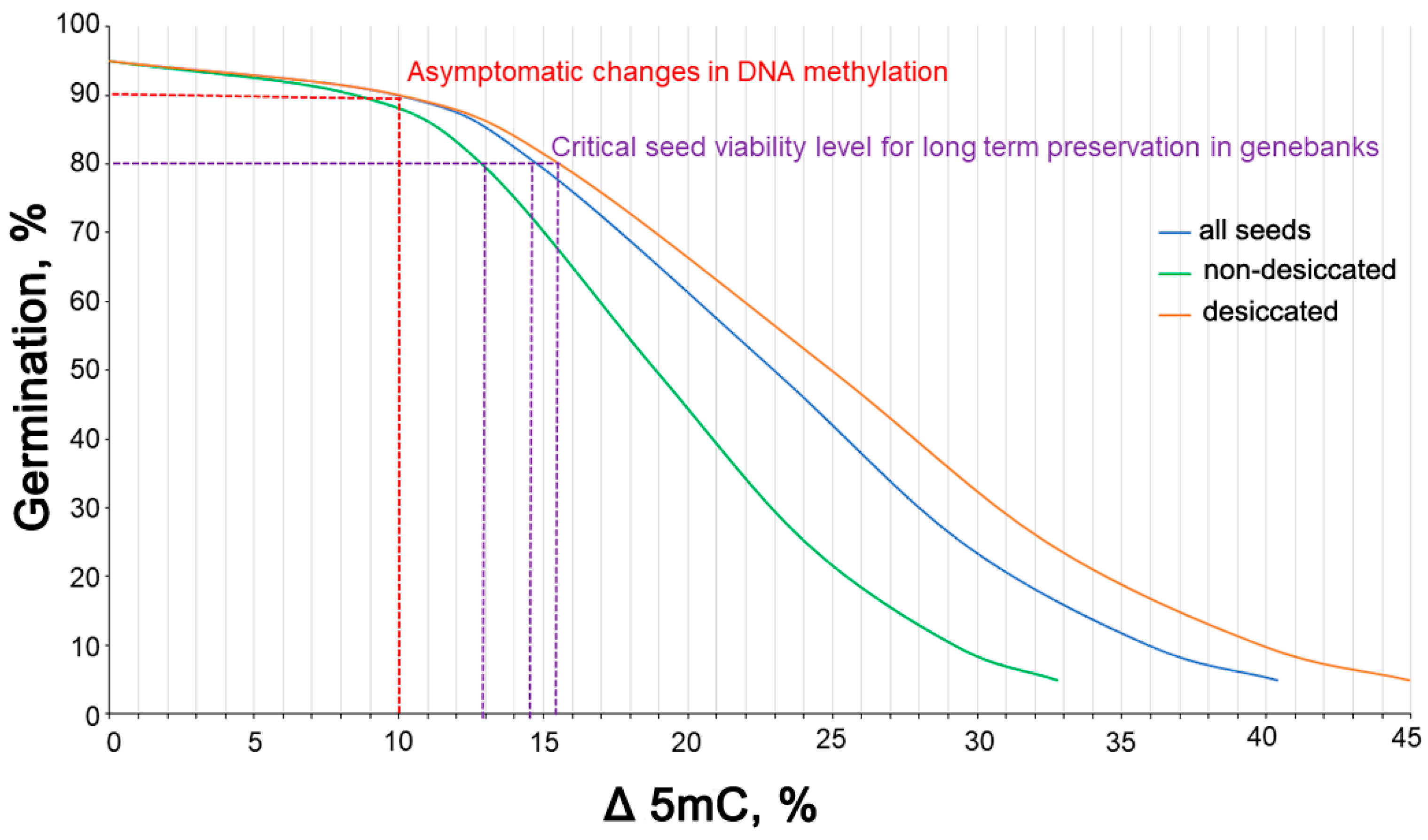
3.7. Correlation between the Rate of Germination and Global 5mC Levels and Determination of Threshold 5mC Values Resulting in a Change in the Viability of Stored Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hay, F.R.; Probert, R.J. Advances in Seed Conservation of Wild Plant Species: A Review of Recent Research. Conserv. Physiol. 2013, 1, cot030. [Google Scholar] [CrossRef]
- Merritt, D.J.; Dixon, K.W. Restoration Seed Banks—A Matter of Scale. Science 2011, 332, 424–425. [Google Scholar] [CrossRef]
- Convention on Biological Diversity, Global Strategy for Plant Conservation: 2011–2020; Botanic Gardens Conservation International: Richmond, UK, 2 April 2011.
- Sharrock, S. Plant Conservation Report 2020: A Review of Progress in Implementation of the Global Strategy for Plant Conservation 2011–2020; Secretariat of the Convention on Biological Diversity: Montréal, QC, Canada; Botanic Gardens Conservation International: Richmond, UK, 2020. [Google Scholar]
- Liu, U.; Breman, E.; Cossu, T.; Kenney, S. The Conservation Value of Germplasm Stored at the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK. Biodivers. Conserv. 2018, 27, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of Seeds Stored in a Genebank: Species Characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Pence, V.C.; Bruns, E.B.; Meyer, A.; Pritchard, H.W.; Westwood, M.; Linsky, J.; Gratzfeld, J.; Helm-Wallace, S.; Liu, U.; Rivers, M.; et al. Gap Analysis of Exceptional Species—Using a Global List of Exceptional Plants to Expand Strategic Ex Situ Conservation Action beyond Conventional Seed Banking. Biol. Conserv. 2022, 266, 109439. [Google Scholar] [CrossRef]
- Pence, V.C.; Meyer, A.; Linsky, J.; Gratzfeld, J.; Pritchard, H.W.; Westwood, M.; Bruns, E.B. Defining Exceptional Species—A Conceptual Framework to Expand and Advance Ex Situ Conservation of Plant Diversity beyond Conventional Seed Banking. Biol. Conserv. 2022, 266, 109440. [Google Scholar] [CrossRef]
- Van Treuren, R.; Bas, N.; Kodde, J.; Groot, S.P.C.; Kik, C. Rapid Loss of Seed Viability in Ex Situ Conserved Wheat and Barley at 4 °C as Compared to −20 °C Storage. Conserv. Physiol. 2018, 6, 11333–11345. [Google Scholar] [CrossRef]
- Wiebach, J.; Nagel, M.; Börner, A.; Altmann, T.; Riewe, D. Age-Dependent Loss of Seed Viability Is Associated with Increased Lipid Oxidation and Hydrolysis. Plant Cell Environ. 2020, 43, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.-P.; Cueff, G.; Hegedus, D.; Rajjou, L.; Bentsink, L. A Role for Seed Storage Proteins in Arabidopsis Seed Longevity. J. Exp. Bot. 2015, 299, 272–279. [Google Scholar] [CrossRef] [Green Version]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying Alive: Molecular Aspects of Seed Longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, D.; Pritchard, H.W.; Walters, C. Dry Architecture: Towards the Understanding of the Variation of Longevity in Desiccation-Tolerant Germplasm. Seed Sci. Res. 2020, 30, 142–155. [Google Scholar] [CrossRef]
- Roqueiro, G.; Facorro, G.B.; Huarte, M.G.; Rubín de Celis, E.; García, F.; Maldonado, S.; Maroder, H. Effects of Photooxidation on Membrane Integrity in Salix Nigra Seeds. Ann. Bot-London 2010, 105, 1027–1034. [Google Scholar] [CrossRef] [Green Version]
- Zinsmeister, J.; Leprince, O.; Buitink, J. Molecular and Environmental Factors Regulating Seed Longevity. Biochem. J. 2020, 477, 305–323. [Google Scholar] [CrossRef]
- Gerna, D.; Ballesteros, D.; Arc, E.; Stöggl, W.; Seal, C.E.; Marami-Zonouz, N.; Na, C.S.; Kranner, I.; Roach, T. Does Oxygen Affect Ageing Mechanisms of Pinus Densiflora Seeds? A Matter of Cytoplasmic Physical State. J. Exp. Bot. 2022, 73, 2631–2649. [Google Scholar] [CrossRef]
- Lefebvre, M.; Villar, M.; Boizot, N.; Delile, A.; Dimouro, B.; Lomenech, A.-M.; Teyssier, C. Variability in seeds’ physicochemical characteristics, germination and seedling growth within and between two French Populus nigra L. populations. Peer J. 2022, 2, 1502. [Google Scholar] [CrossRef]
- Żukowska, W.; Wójkiewicz, B.; Lewandowski, A. Trunks of Multi-Stem Black Poplars May Have Different Genotypes—Evidence from the Oder Valley in Poland. Dendrobiology 2021, 86, 1–7. [Google Scholar] [CrossRef]
- Vanden Broeck, A.; Cox, K.; Van Braeckel, A.; Neyrinck, S.; De Regge, N.; Van Looy, K. Reintroduced Native Populus Nigra in Restored Floodplain Reduces Spread of Exotic Poplar Species. Front Plant Sci. 2020, 11, 580653. [Google Scholar] [CrossRef]
- EU Biodiversity Strategy 2030 EU Floods Directive 2007/60/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32007L0060 (accessed on 7 February 2022).
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. An Intermediate Category of Seed Storage Behaviour? I. COFFEE. J. Exp. Bot. 1990, 41, 1167–1174. [Google Scholar] [CrossRef]
- Stanton, B.J. Biology of Populus and Its Implications for Management and Conservation; NRC Research Press: Ottawa, ON, Canada, 1996; ISBN 978-0-660-16506-6. [Google Scholar]
- Gosling, P. Raising Trees and Shrubs from Seed: Practice Guide. In Raising Trees and Shrubs from Seed: Practice Guide; CAB Direct: Edinburgh, UK, 2007. [Google Scholar]
- Suszka, J.; Plitta, B.P.; Michalak, M.; Bujarska-Borkowska, B.; Tylkowski, T.; Chmielarz, P. Optimal Seed Water Content and Storage Temperature for Preservation of Populus nigra L. Germplasm. Ann. For. Sci. 2014, 71, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Michalak, M.; Plitta, B.P.; Tylkowski, T.; Chmielarz, P.; Suszka, J. Desiccation Tolerance and Cryopreservation of Seeds of Black Poplar (Populus nigra L.), a Disappearing Tree Species in Europe. Eur. J. Forest Res. 2015, 134, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Mucha, J.; Szymańska, A.K.; Zadworny, M.; Tylkowski, T.; Michalak, M.; Suszka, J. Effect of Seed Storage Temperature on Fine Root Development and Mycorrhizal Colonization of Young Populus Nigra Seedlings. Ann. For. Sci. 2015, 72, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Kalemba, E.M.; Suszka, J.; Ratajczak, E. The Role of Oxidative Stress in Determining the Level of Viability of Black Poplar (Populus nigra) Seeds Stored at Different Temperatures. Funct. Plant Biol. 2015, 42, 630–642. [Google Scholar] [CrossRef] [Green Version]
- Pawłowski, T.; Klupczyńska, E.; Staszak, A.; Suszka, J. Proteomic Analysis of Black Poplar (Populus nigra L.) Seed Storability. Ann. For. Sci. 2019, 76, 104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lang, Z.; Zhu, J.-K. Dynamics and Function of DNA Methylation in Plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Pirredda, M.; González-Benito, M.E.; Martín, C.; Mira, S. Genetic and Epigenetic Stability in Rye Seeds under Different Storage Conditions: Ageing and Oxygen Effect. Plants 2020, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Mira, S.; Pirredda, M.; Martín-Sánchez, M.; Marchessi, J.E.; Martín, C. DNA Methylation and Integrity in Aged Seeds and Regenerated Plants. Seed Sci. Res. 2020, 30, 92–100. [Google Scholar] [CrossRef]
- Walters, C. Understanding the Mechanisms and Kinetics of Seed Aging. Seed Sci. Res. 1998, 8, 223–244. [Google Scholar] [CrossRef]
- Gianella, M.; Balestrazzi, A.; Ravasio, A.; Mondoni, A.; Börner, A.; Guzzon, F. Comparative Seed Longevity under Genebank Storage and Artificial Ageing: A Case Study in Heteromorphic Wheat Wild Relatives. Plant Biol. (Stuttg) 2022, 1342. [Google Scholar] [CrossRef]
- Michalak, M.; Plitta-Michalak, B.; Naskręt-Barciszewska, M.; Barciszewski, J.; Bujarska-Borkowska, B.; Chmielarz, P. Global 5-Methylcytosine Alterations in DNA during Ageing of Quercus Robur Seeds. Ann. Bot-London 2015, 116, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Barciszewska, M.Z.; Barciszewska, A.M.; Rattan, S.I.S. TLC-Based Detection of Methylated Cytosine: Application to Aging Epigenetics. Biogerontology 2007, 8, 673–678. [Google Scholar] [CrossRef]
- Plitta, B.P.; Michalak, M.; Bujarska-Borkowska, B.; Barciszewska, M.Z.; Barciszewski, J.; Chmielarz, P. Effect of Desiccation on the Dynamics of Genome-Wide DNA Methylation in Orthodox Seeds of Acer platanoides L. Plant Physiol. Biochem. 2014, 85, 71–77. [Google Scholar] [CrossRef]
- Plitta-Michalak, B.P.; Naskręt-Barciszewska, M.Z.; Kotlarski, S.; Tomaszewski, D.; Tylkowski, T.; Barciszewski, J.; Chmielarz, P.; Michalak, M. Changes in Genomic 5-Methylcytosine Level Mirror the Response of Orthodox (Acer platanoides L.) and Recalcitrant (Acer pseudoplatanus L.) Seeds to Severe Desiccation. Tree Physiol. 2018, 38, 617–629. [Google Scholar] [CrossRef] [Green Version]
- Michalak, M.; Barciszewska, M.Z.; Barciszewski, J.; Plitta, B.P.; Chmielarz, P. Global Changes in DNA Methylation in Seeds and Seedlings of Pyrus Communis after Seed Desiccation and Storage. PLoS ONE 2013, 8, e70693. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.J.; de Santana, D.G.; Araújo, L.B. de Why Analyze Germination Experiments Using Generalized Linear Models? J. Seed Sci. 2018, 40, 281–287. [Google Scholar] [CrossRef]
- Jardim Amorim, D.; Pereira dos Santos, A.R.; Nunes da Piedade, G.; Quelvia de Faria, R.; Amaral da Silva, E.A.; Pereira Sartori, M.M. The Use of the Generalized Linear Model to Assess the Speed and Uniformity of Germination of Corn and Soybean Seeds. Agronomy 2021, 11, 588. [Google Scholar] [CrossRef]
- Warton, D.; Hui, F. The Arcsine Is Asinine: The Analysis of Proportions in Ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Waterworth, W.M.; Bray, C.M.; West, C.E. Seeds and the Art of Genome Maintenance. Front. Plant Sci. 2019, 10, 706. [Google Scholar] [CrossRef] [Green Version]
- Plitta-Michalak, B.P.; Ramos, A.A.; Pupel, P.; Michalak, M. Oxidative Damage and DNA Repair in Desiccated Recalcitrant Embryonic Axes of Acer Pseudoplatanus L. BMC Plant Biol. 2022, 22, 40. [Google Scholar] [CrossRef]
- Russo, G.; Landi, R.; Pezone, A.; Morano, A.; Zuchegna, C.; Romano, A.; Muller, M.T.; Gottesman, M.E.; Porcellini, A.; Avvedimento, E.V. DNA Damage and Repair Modify DNA Methylation and Chromatin Domain of the Targeted Locus: Mechanism of Allele Methylation Polymorphism. Sci. Rep. 2016, 6, 33222. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.-Q.; Zhang, Y.-J.; Zhou, S.-R.; Hu, W.-Y.; Wu, X.-T.; Ye, Y.-J.; Wu, X.-X.; Xiao, Y.-P.; Li, X.; Xue, H.-W. Global Analysis Reveals the Crucial Roles of DNA Methylation during Rice Seed Development. Plant Physiol. 2015, 168, 1417–1432. [Google Scholar] [CrossRef] [Green Version]
- Kravets, O.; Sokolova, D.O. Epigenetic Mechanisms Regulating Seed Germination Rate. Cytol. Genet. 2017, 51, 346–351. [Google Scholar] [CrossRef]
- Iwasaki, M.; Hyvärinen, L.; Piskurewicz, U.; Lopez-Molina, L. Non-Canonical RNA-Directed DNA Methylation Participates in Maternal and Environmental Control of Seed Dormancy. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Kawakatsu, T.; Huang, S.-S.C.; Jupe, F.; Sasaki, E.; Schmitz, R.J.; Urich, M.A.; Castanon, R.; Nery, J.R.; Barragan, C.; He, Y.; et al. Epigenomic Diversity in a Global Collection of Arabidopsis Thaliana Accessions. Cell 2016, 166, 492–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plitta-Michalak, B.P.; Naskręt-Barciszewska, M.Z.; Barciszewski, J.; Chmielarz, P.; Michalak, M. Epigenetic Integrity of Orthodox Seeds Stored under Conventional and Cryogenic Conditions. Forests 2021, 12, 288. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Nery, J.R.; Castanon, R.; Ecker, J.R. Dynamic DNA Methylation Reconfiguration during Seed Development and Germination. Genome Biol. 2017, 18, 171. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, O.; Jasencakova, Z.; Vaillant, I.; Gendrel, A.-V.; Colot, V.; Schubert, I.; Tourmente, S. Changes in 5S RDNA Chromatin Organization and Transcription during Heterochromatin Establishment in Arabidopsis. The Plant Cell 2003, 15, 2929–2939. [Google Scholar] [CrossRef] [Green Version]
- Douet, J.; Blanchard, B.; Cuvillier, C.; Tourmente, S. Interplay of RNA Pol IV and ROS1 during Post-Embryonic 5S RDNA Chromatin Remodeling. Plant Cell Physiol. 2008, 49, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Plitta-Michalak, B.P.; Litkowiec, M.; Michalak, M. Epigenetic Marks, DNA Damage Markers, or Both? The Impact of Desiccation and Accelerated Aging on Nucleobase Modifications in Plant Genomic DNA. Cells 2022, 11, 1748. [Google Scholar] [CrossRef]
- Walters, C.; Ballesteros, D.; Vertucci, V.A. Structural Mechanics of Seed Deterioration: Standing the Test of Time. Plant Sci. 2010, 179, 565–573. [Google Scholar] [CrossRef]
- Xia, F.; Wang, X.; Li, M.; Mao, P. Mitochondrial Structural and Antioxidant System Responses to Aging in Oat (Avena Sativa L.) Seeds with Different Moisture Contents. Plant Physiol. Biochem. 2015, 94, 122–129. [Google Scholar] [CrossRef]
- Chhabra, R.; Shabnam; Singh, T. Seed Aging, Storage and Deterioration: An Irresistible Physiological Phenomenon. Agric. Rev. 2019, 40, 234–238. [Google Scholar] [CrossRef] [Green Version]
- Gehring, M.; Reik, W.; Henikoff, S. DNA Demethylation by DNA Repair. Trends Genet. 2009, 25, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of Orthodox Seeds during Ageing: Influencing Factors, Physiological Alterations and the Role of Reactive Oxygen Species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef] [PubMed]
- González-Benito, M.E.; Ibáñez, M.Á.; Pirredda, M.; Mira, S.; Martín, C. Application of the MSAP Technique to Evaluate Epigenetic Changes in Plant Conservation. Internat. J. Mol. Sci. 2020, 21, 7459. [Google Scholar] [CrossRef]
- Ogneva, Z.V.; Dubrovina, A.S.; Kiselev, K.V. Age-Associated Alterations in DNA Methylation and Expression of Methyltransferase and Demethylase Genes in Arabidopsis Thaliana. Biol. Plant 2016, 60, 628–634. [Google Scholar] [CrossRef]
- Colville, L.; Pritchard, H.W. Seed Life Span and Food Security. New Phytol. 2019, 224, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Jeremias, G.; Barbosa, J.; Marques, S.M.; Asselman, J.; Gonçalves, F.J.M.; Pereira, J.L. Synthesizing the Role of Epigenetics in the Response and Adaptation of Species to Climate Change in Freshwater Ecosystems. Mol. Ecol. 2018, 27, 2790–2806. [Google Scholar] [CrossRef] [Green Version]
- Quadrana, L.; Colot, V. Plant Transgenerational Epigenetics. Annu. Rev. Genet. 2016, 50, 467–491. [Google Scholar] [CrossRef]
- Sow, M.D.; Le Gac, A.-L.; Fichot, R.; Lanciano, S.; Delaunay, A.; Le Jan, I.; Lesage-Descauses, M.-C.; Citerne, S.; Caius, J.; Brunaud, V.; et al. RNAi Suppression of DNA Methylation Affects the Drought Stress Response and Genome Integrity in Transgenic Poplar. New Phytol. 2021, 232, 80–97. [Google Scholar] [CrossRef]
- Feng, S.; Jacobsen, S.E.; Reik, W. Epigenetic Reprogramming in Plant and Animal Development. Science 2010, 330, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, Y.; Diao, S.; Zhong, S.; Wu, S.; Wang, L.; Su, X.; Zhang, B. Assessment of Epigenetic and Phenotypic Variation in Populus Nigra Regenerated via Sequential Regeneration. Front. Plant Sci. 2021, 12, 1373. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, Z. Small DNA Methylation, Big Player in Plant Abiotic Stress Responses and Memory. Front. Plant Sci. 2020, 11, 595603. [Google Scholar] [CrossRef] [PubMed]



| Seed Lot | Moisture Content (%) | Correlation Coefficients | Correlation Significance |
|---|---|---|---|
| Seed lot No. 1 # | 14.5 | 0.87 | p < 0.0001 |
| Seed lot No. 1 # | 7.6 | 0.822 | p < 0.0001 |
| Seed lot No. 2 # | 13.6 | 0.84 | p < 0.0001 |
| Seed lot No. 2 # | 7.8 | 0.723 | p = 0.0003 |
| Seed lot No. 3 # | 12.5 | 0.802 | p < 0.0001 |
| Seed lot No. 3 # | 7.6 | 0.694 | p = 0.0007 |
| Non-desiccated * | 12.5–14.5 | 0.822 | p < 0.0001 |
| Desiccated * | 7.6–7.8 | 0.775 | p < 0.0001 |
| Assumed Germination Level | Predicted DNA Methylation Level (%) 1 | ||
|---|---|---|---|
| All Seeds | Non-Desiccated Seeds | Desiccated Seeds | |
| 95% | 9.375 ± 0.382 | 8.767 ± 0.435 | 9.859 ± 0.48 |
| 90% | 8.347 ± 0.237 | 8.018 ± 0.28 | 8.742 ± 0.369 |
| 80% * | 7.989 ± 0.181 * | 7.638 ± 0.216 * | 8.208 ± 0.274 * |
| 50% | 7.221 ± 0.147 | 7.111 ± 0.161 | 7.294 ± 0.265 |
| 25% | 6.613 ± 0.198 | 6.657 ± 0.197 | 6.57 ± 0.394 |
| 10% | 6.005 ± 0.283 | 6.204 ± 0.278 | 5.845 ± 0.565 |
| 5% | 5.591 ± 0.347 | 5.895 ± 0.344 | 5.353 ± 0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, M.; Plitta-Michalak, B.P.; Naskręt-Barciszewska, M.Z.; Barciszewski, J.; Chmielarz, P. DNA Methylation as an Early Indicator of Aging in Stored Seeds of “Exceptional” Species Populus nigra L. Cells 2022, 11, 2080. https://doi.org/10.3390/cells11132080
Michalak M, Plitta-Michalak BP, Naskręt-Barciszewska MZ, Barciszewski J, Chmielarz P. DNA Methylation as an Early Indicator of Aging in Stored Seeds of “Exceptional” Species Populus nigra L. Cells. 2022; 11(13):2080. https://doi.org/10.3390/cells11132080
Chicago/Turabian StyleMichalak, Marcin, Beata Patrycja Plitta-Michalak, Mirosława Zofia Naskręt-Barciszewska, Jan Barciszewski, and Paweł Chmielarz. 2022. "DNA Methylation as an Early Indicator of Aging in Stored Seeds of “Exceptional” Species Populus nigra L." Cells 11, no. 13: 2080. https://doi.org/10.3390/cells11132080
APA StyleMichalak, M., Plitta-Michalak, B. P., Naskręt-Barciszewska, M. Z., Barciszewski, J., & Chmielarz, P. (2022). DNA Methylation as an Early Indicator of Aging in Stored Seeds of “Exceptional” Species Populus nigra L. Cells, 11(13), 2080. https://doi.org/10.3390/cells11132080







