Phosphodiesterase 10A (PDE10A): Regulator of Dopamine Agonist-Induced Gene Expression in the Striatum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Animals
2.3. 6-OHDA Lesions
2.4. Drug Treatment
2.5. Tissue Preparation and In Situ Hybridization Histochemistry
2.6. Analysis of Autoradiograms
2.7. Statistics
3. Results
3.1. Experiment 1: Relationship between PDE10A Expression and the Effects of Dopamine Depletion and Repeated L-DOPA and TP-10 Treatment in Adults
3.1.1. PDE10A Expression in the Striatum of Adults
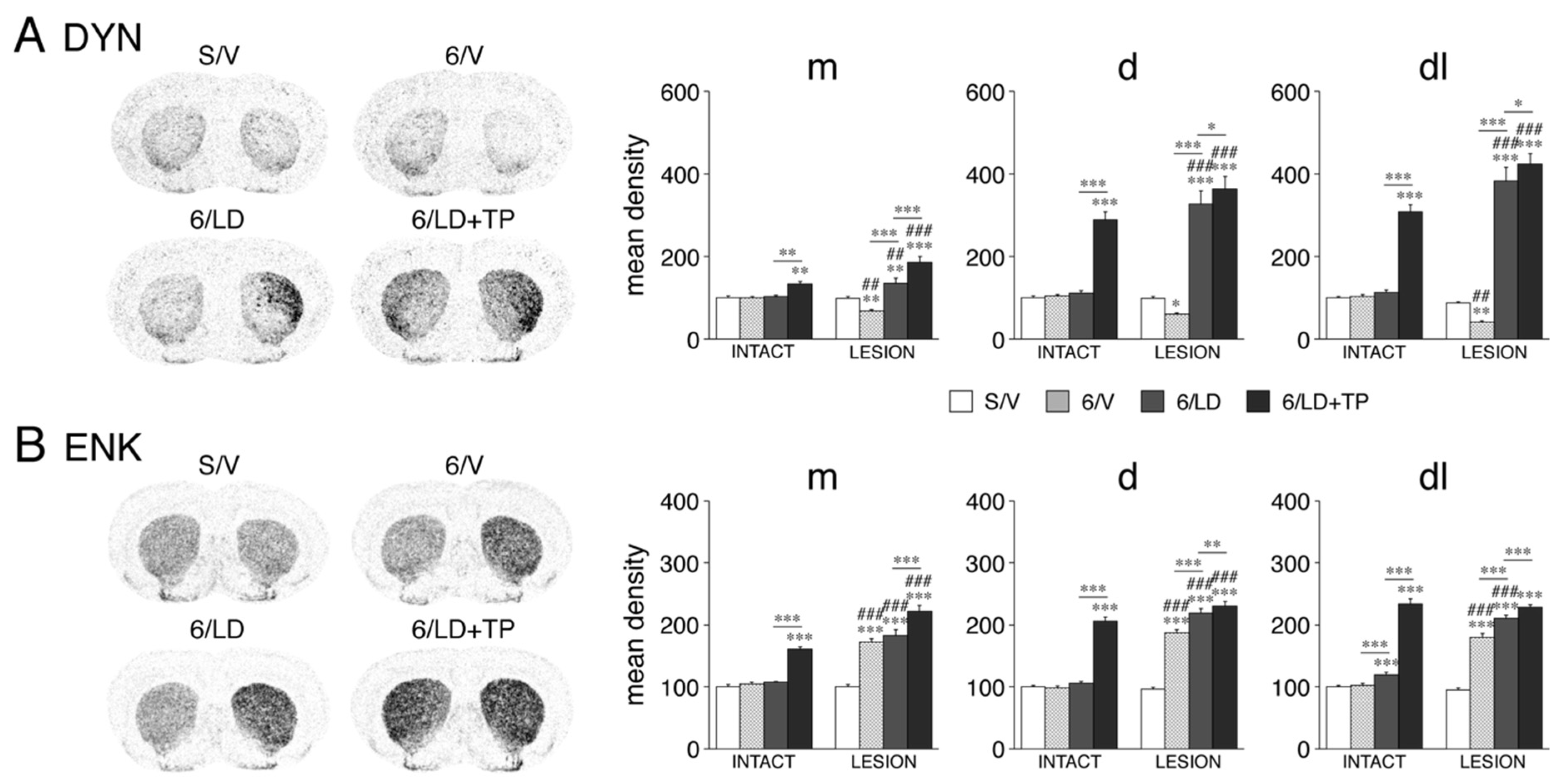
3.1.2. Dynorphin and Enkephalin Expression
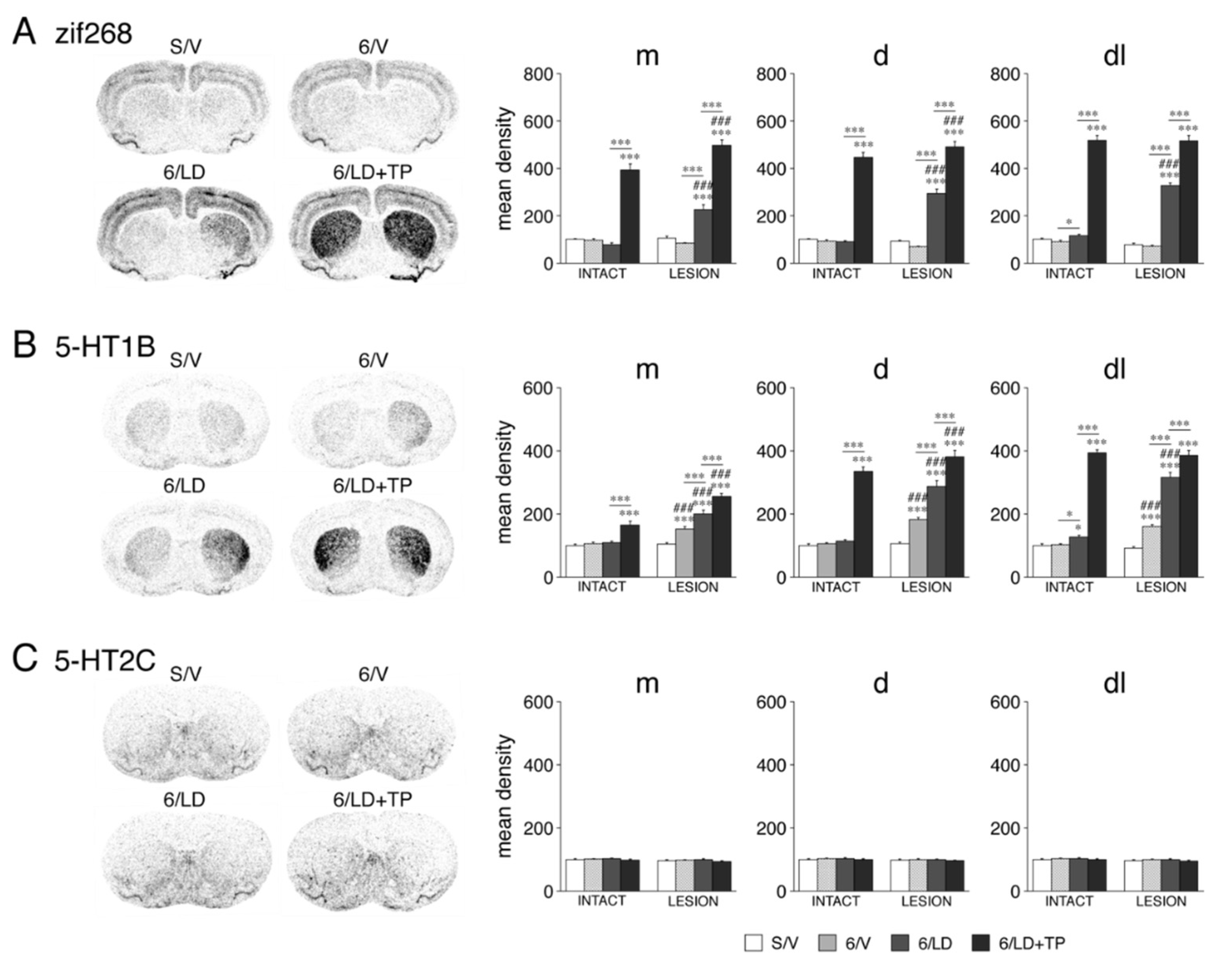
3.1.3. Zif268 Expression
3.1.4. 5-HT1B and 5-HT2C Serotonin Receptors: Selective Impact on 5-HT1B Expression
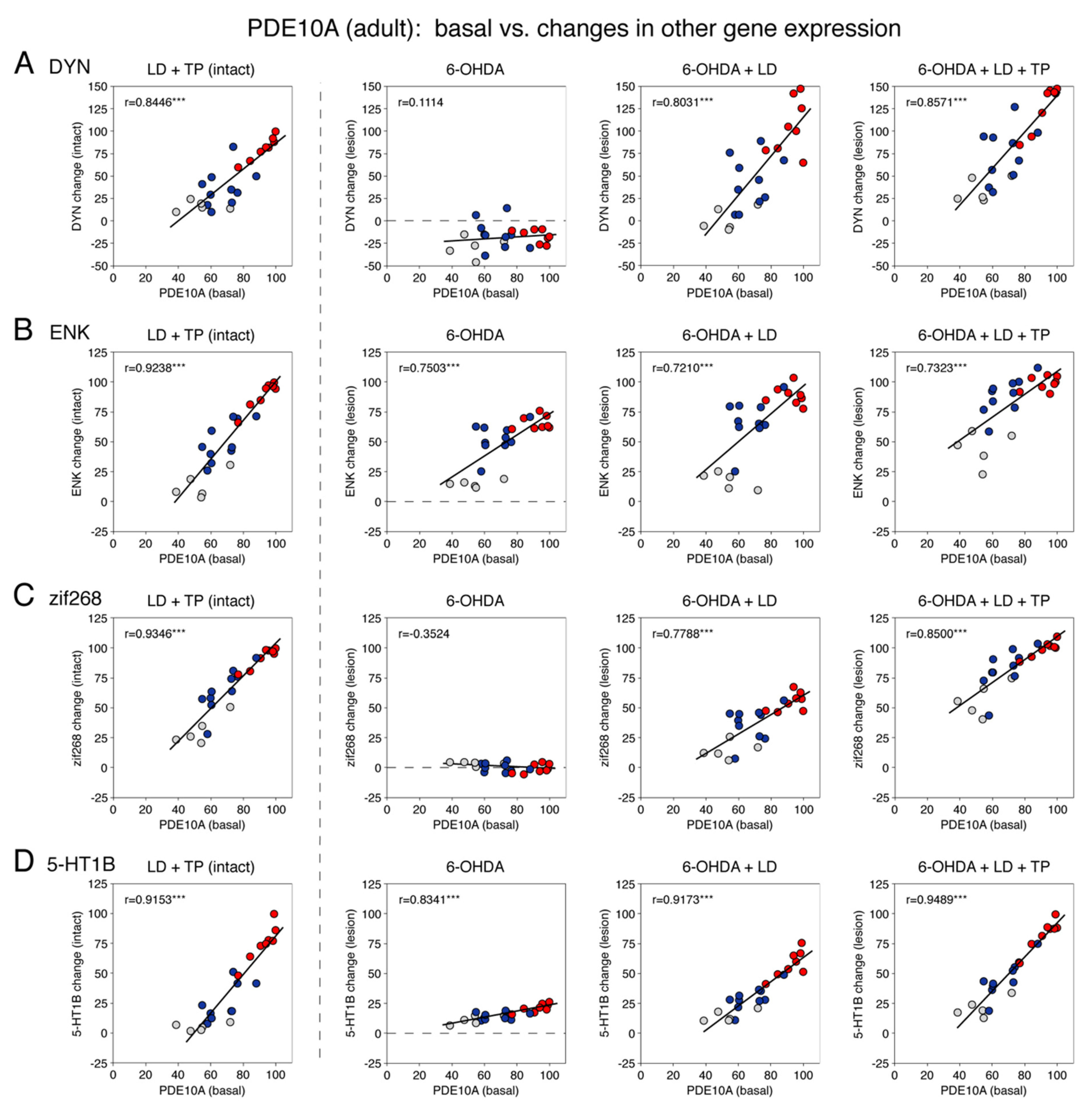
3.1.5. Relationship between Basal PDE10A Expression and the Effects of Dopamine Depletion and Drug Treatments on Gene Expression in Adults
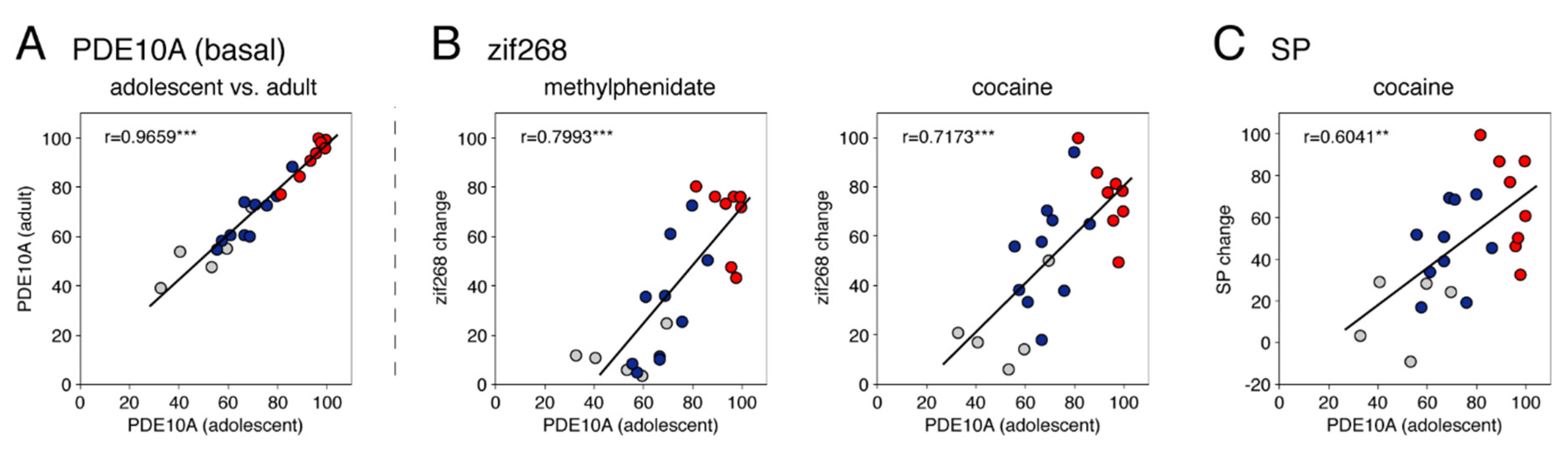
3.2. Experiment 2: Relationship between Basal Expression of PDE10A and the Effects of Acute Cocaine and Methylphenidate in Adolescents
3.2.1. PDE10A Expression in the Striatum of Adolescents
3.2.2. Zif268 Expression and Substance P Expression
4. Discussion
4.1. PDE10A Expression in the Striatum: Basal Expression and Effects of Dopamine Depletion and Drug Treatments
4.2. Changes in Gene Expression in Striatum after PDE10A Inhibition
4.3. Changes in Gene Expression after Dopamine Depletion: Relationship to Basal PDE10A Expression for D2-, but Not D1-, MSN Markers
4.4. Changes in Gene Expression Induced by L-DOPA Treatment after Dopamine Depletion: Relationship to Basal PDE10A Levels for D1- and D2-MSNs
4.5. Changes in Psychostimulant-Induced Gene Expression: Relationship to Basal PDE10A Levels
5. Functional Considerations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girault, J.A.; Greengard, P.; Nairn, A.C. Regulation of striatal signaling by protein phosphatases. In Handbook of Basal Ganglia Structure and Function; Steiner, H., Tseng, K.Y., Eds.; Academic Press/Elsevier: London, UK, 2017; pp. 583–607. [Google Scholar]
- Kelly, M.P.; Adamowicz, W.; Bove, S.; Hartman, A.J.; Mariga, A.; Pathak, G.; Reinhart, V.; Romegialli, A.; Kleiman, R.J. Select 3’,5’-cyclic nucleotide phosphodiesterases exhibit altered expression in the aged rodent brain. Cell Signal 2014, 26, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.J.; Chapin, D.S.; Cianfrogna, J.; Corman, M.L.; Hajos, M.; Harms, J.F.; Hoffman, W.E.; Lebel, L.A.; McCarthy, S.A.; Nelson, F.R.; et al. Preclinical characterization of selective phosphodiesterase 10A inhibitors: A new therapeutic approach to the treatment of schizophrenia. J. Pharmacol. Exp. Ther. 2008, 325, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grauer, S.M.; Pulito, V.L.; Navarra, R.L.; Kelly, M.P.; Kelley, C.; Graf, R.; Langen, B.; Logue, S.; Brennan, J.; Jiang, L.; et al. Phosphodiesterase 10A inhibitor activity in preclinical models of the positive, cognitive, and negative symptoms of schizophrenia. J. Pharmacol. Exp. Ther. 2009, 331, 574–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, G.; Maehara, S.; Chang, P.L.; Papa, S.M. A selective phosphodiesterase 10A inhibitor reduces L-Dopa-induced dyskinesias in Parkinsonian monkeys. Mov. Disord. 2018, 33, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Spigolon, G.; Fisone, G. Signal transduction in L-DOPA-induced dyskinesia: From receptor sensitization to abnormal gene expression. J. Neural Transm. 2018, 125, 1171–1186. [Google Scholar] [CrossRef] [Green Version]
- Threlfell, S.; Sammut, S.; Menniti, F.S.; Schmidt, C.J.; West, A.R. Inhibition of phosphodiesterase 10A increases the responsiveness of striatal projection neurons to cortical stimulation. J. Pharmacol. Exp. Ther. 2009, 328, 785–795. [Google Scholar] [CrossRef]
- Padovan-Neto, F.E.; Sammut, S.; Chakroborty, S.; Dec, A.M.; Threlfell, S.; Campbell, P.W.; Mudrakola, V.; Harms, J.F.; Schmidt, C.J.; West, A.R. Facilitation of corticostriatal transmission following pharmacological inhibition of striatal phosphodiesterase 10A: Role of nitric oxide-soluble guanylyl cyclase-cGMP signaling pathways. J. Neurosci. 2015, 35, 5781–5791. [Google Scholar] [CrossRef] [Green Version]
- Cenci, M.A.; Konradi, C. Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog. Brain Res. 2010, 183, 209–233. [Google Scholar]
- Kleiman, R.J.; Kimmel, L.H.; Bove, S.E.; Lanz, T.A.; Harms, J.F.; Romegialli, A.; Miller, K.S.; Willis, A.; des Etages, S.; Kuhn, M.; et al. Chronic suppression of phosphodiesterase 10A alters striatal expression of genes responsible for neurotransmitter synthesis, neurotransmission, and signaling pathways implicated in Huntington’s disease. J. Pharmacol. Exp. Ther. 2011, 336, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Gentzel, R.C.; Toolan, D.; Roberts, R.; Koser, A.J.; Kandebo, M.; Hershey, J.; Renger, J.J.; Uslaner, J.; Smith, S.M. The PDE10A inhibitor MP-10 and haloperidol produce distinct gene expression profiles in the striatum and influence cataleptic behavior in rodents. Neuropharmacology 2015, 99, 256–263. [Google Scholar] [CrossRef]
- Steiner, H.; Van Waes, V. Addiction-related gene regulation: Risks of exposure to cognitive enhancers vs. other psychostimulants. Prog. Neurobiol. 2013, 100, 60–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, H. Psychostimulant-induced gene regulation in striatal circuits. In Handbook of Basal Ganglia Structure and Function; Steiner, H., Tseng, K.Y., Eds.; Academic Press/Elsevier: London, UK, 2017; pp. 639–672. [Google Scholar]
- Padovan-Neto, F.E.; Patterson, S.; Voelkner, N.M.; Altwal, F.; Beverley, J.A.; West, A.R.; Steiner, H. Selective regulation of 5-HT1B serotonin receptor expression in the striatum by dopamine depletion and repeated L-DOPA treatment: Relationship to L-DOPA-induced dyskinesias. Mol. Neurobiol. 2020, 57, 736–751. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Altwal, F.; Padovan-Neto, F.E.; Ritger, A.; Steiner, H.; West, A.R. Role of 5-HT1A receptor in vilazodone-mediated suppression of L-DOPA-induced dyskinesia and increased responsiveness to cortical input in striatal medium spiny neurons in an animal model of Parkinson’s disease. Molecules 2021, 26, 5790. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.; Kitai, S.T. Unilateral striatal dopamine depletion: Time-dependent effects on cortical function and behavioural correlates. Eur. J. Neurosci. 2001, 14, 1390–1404. [Google Scholar] [CrossRef] [PubMed]
- Van Waes, V.; Ehrlich, S.; Beverley, J.A.; Steiner, H. Fluoxetine potentiation of methylphenidate-induced gene regulation in striatal output pathways: Potential role for 5-HT1B receptor. Neuropharmacology 2015, 89, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Willuhn, I.; Sun, W.; Steiner, H. Topography of cocaine-induced gene regulation in the rat striatum: Relationship to cortical inputs and role of behavioural context. Eur. J. Neurosci. 2003, 17, 1053–1066. [Google Scholar] [CrossRef]
- Yano, M.; Steiner, H. Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience 2005, 132, 855–865. [Google Scholar] [CrossRef]
- Steiner, H.; Gerfen, C.R. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp. Brain Res. 1998, 123, 60–76. [Google Scholar] [CrossRef]
- Yano, M.; Steiner, H. Topography of methylphenidate (Ritalin)-induced gene regulation in the striatum: Differential effects on c-fos, substance P and opioid peptides. Neuropsychopharmacology 2005, 30, 901–915. [Google Scholar] [CrossRef] [Green Version]
- Seeger, T.F.; Bartlett, B.; Coskran, T.M.; Culp, J.S.; James, L.C.; Krull, D.L.; Lanfear, J.; Ryan, A.M.; Schmidt, C.J.; Strick, C.A.; et al. Immunohistochemical localization of PDE10A in the rat brain. Brain Res. 2003, 985, 113–126. [Google Scholar] [CrossRef]
- Heiman, M.; Heilbut, A.; Francardo, V.; Kulicke, R.; Fenster, R.J.; Kolaczyk, E.D.; Mesirov, J.P.; Surmeier, D.J.; Cenci, M.A.; Greengard, P. Molecular adaptations of striatal spiny projection neurons during levodopa-induced dyskinesia. Proc. Natl. Acad. Sci. USA 2014, 111, 4578–4583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgi, M.; Melchiorri, G.; Nuccetelli, V.; D’Angelo, V.; Martorana, A.; Sorge, R.; Castelli, V.; Bernardi, G.; Sancesario, G. PDE10A and PDE10A-dependent cAMP catabolism are dysregulated oppositely in striatum and nucleus accumbens after lesion of midbrain dopamine neurons in rat: A key step in parkinsonism physiopathology. Neurobiol. Dis. 2011, 43, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R.; Surmeier, D.J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strick, C.A.; James, L.C.; Fox, C.B.; Seeger, T.F.; Menniti, F.S.; Schmidt, C.J. Alterations in gene regulation following inhibition of the striatum-enriched phosphodiesterase, PDE10A. Neuropharmacology 2010, 58, 444–451. [Google Scholar] [CrossRef]
- Wilson, J.M.; Ogden, A.M.; Loomis, S.; Gilmour, G.; Baucum II, A.J.; Belecky-Adams, T.L.; Merchant, K.M. Phosphodiesterase 10A inhibitor, MP-10 (PF-2545920), produces greater induction of c-Fos in dopamine D2 neurons than in D1 neurons in the neostriatum. Neuropharmacology 2015, 99, 379–386. [Google Scholar] [CrossRef]
- Nishi, A.; Kuroiwa, M.; Miller, D.B.; O’Callaghan, J.P.; Bateup, H.S.; Shuto, T.; Sotogaku, N.; Fukuda, T.; Heintz, N.; Greengard, P.; et al. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J. Neurosci. 2008, 28, 10460–10471. [Google Scholar] [CrossRef] [Green Version]
- Cenci, M.A. Molecular mechanisms of L-DOPA-induced dyskinesia. In Handbook of Basal Ganglia Structure and Function; Steiner, H., Tseng, K.Y., Eds.; Elsevier: London, UK, 2017; pp. 857–871. [Google Scholar]
- Altwal, F.; Moon, C.; West, A.R.; Steiner, H. The multimodal serotonergic agent vilazodone inhibits L-DOPA-induced gene regulation in striatal projection neurons and associated dyskinesia in an animal model of Parkinson’s disease. Cells 2020, 9, 2265. [Google Scholar] [CrossRef]
- Uhl, G.R.; Navia, B.; Douglas, J. Differential expression of preproenkephalin and preprodynorphin mRNAs in striatal neurons: High levels of preproenkephalin expression depend on cerebral cortical afferents. J. Neurosci. 1988, 8, 4755–4764. [Google Scholar] [CrossRef] [Green Version]
- Pisani, A.; Bernardi, G.; Ding, J.; Surmeier, D.J. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007, 30, 545–553. [Google Scholar] [CrossRef]
- Reynolds, J.N.J.; Avvisati, R.; Dodson, P.D.; Fisher, S.D.; Oswald, M.J.; Wickens, J.R.; Zhang, Y.F. Coincidence of cholinergic pauses, dopaminergic activation and depolarisation of spiny projection neurons drives synaptic plasticity in the striatum. Nat. Commun. 2022, 13, 1296. [Google Scholar] [CrossRef]
- Hervé, D.; Lévi-Strauss, M.; Marey-Semper, I.; Verney, C.; Tassin, J.P.; Glowinski, J.; Girault, J.A. G(olf) and Gs in rat basal ganglia: Possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J. Neurosci. 1993, 13, 2237–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerfen, C.R. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum: Aberrant ERK1/2 signaling. In Handbook of Basal Ganglia Structure and Function; Steiner, H., Tseng, K.Y., Eds.; Academic Press/Elsevier: London, UK, 2010; pp. 491–500. [Google Scholar]
- Hervé, D. Identification of a specific assembly of the g protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front. Neuroanat. 2011, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakagami, H.; Sawamura, Y.; Kondo, H. Synchronous patchy pattern of gene expression for adenylyl cyclase and phosphodiesterase but discrete expression for G-protein in developing rat striatum. Mol. Brain Res. 1995, 33, 185–191. [Google Scholar] [CrossRef]
- Hervé, D.; Le Moine, C.; Corvol, J.C.; Belluscio, L.; Ledent, C.; Fienberg, A.A.; Jaber, M.; Studler, J.M.; Girault, J.A. Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J. Neurosci. 2001, 21, 4390–4399. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonate, R.; Kurek, G.; Hrabak, M.; Patterson, S.; Padovan-Neto, F.; West, A.R.; Steiner, H. Phosphodiesterase 10A (PDE10A): Regulator of Dopamine Agonist-Induced Gene Expression in the Striatum. Cells 2022, 11, 2214. https://doi.org/10.3390/cells11142214
Bonate R, Kurek G, Hrabak M, Patterson S, Padovan-Neto F, West AR, Steiner H. Phosphodiesterase 10A (PDE10A): Regulator of Dopamine Agonist-Induced Gene Expression in the Striatum. Cells. 2022; 11(14):2214. https://doi.org/10.3390/cells11142214
Chicago/Turabian StyleBonate, Ryan, Gabriela Kurek, Michael Hrabak, Santanna Patterson, Fernando Padovan-Neto, Anthony R. West, and Heinz Steiner. 2022. "Phosphodiesterase 10A (PDE10A): Regulator of Dopamine Agonist-Induced Gene Expression in the Striatum" Cells 11, no. 14: 2214. https://doi.org/10.3390/cells11142214
APA StyleBonate, R., Kurek, G., Hrabak, M., Patterson, S., Padovan-Neto, F., West, A. R., & Steiner, H. (2022). Phosphodiesterase 10A (PDE10A): Regulator of Dopamine Agonist-Induced Gene Expression in the Striatum. Cells, 11(14), 2214. https://doi.org/10.3390/cells11142214






