TRPM7 Modulates Human Pancreatic Stellate Cell Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Treatments
2.2. Cell Transfection
2.3. RT-qPCR
2.4. Immunoblotting
2.5. Cell Viability Assays
2.6. Cell Cycle Analysis
2.7. Electrophysiological Recordings
2.8. Cell Imaging Experiments
2.9. Analysis of CAF Datasets
2.10. Statistical Analysis
3. Results
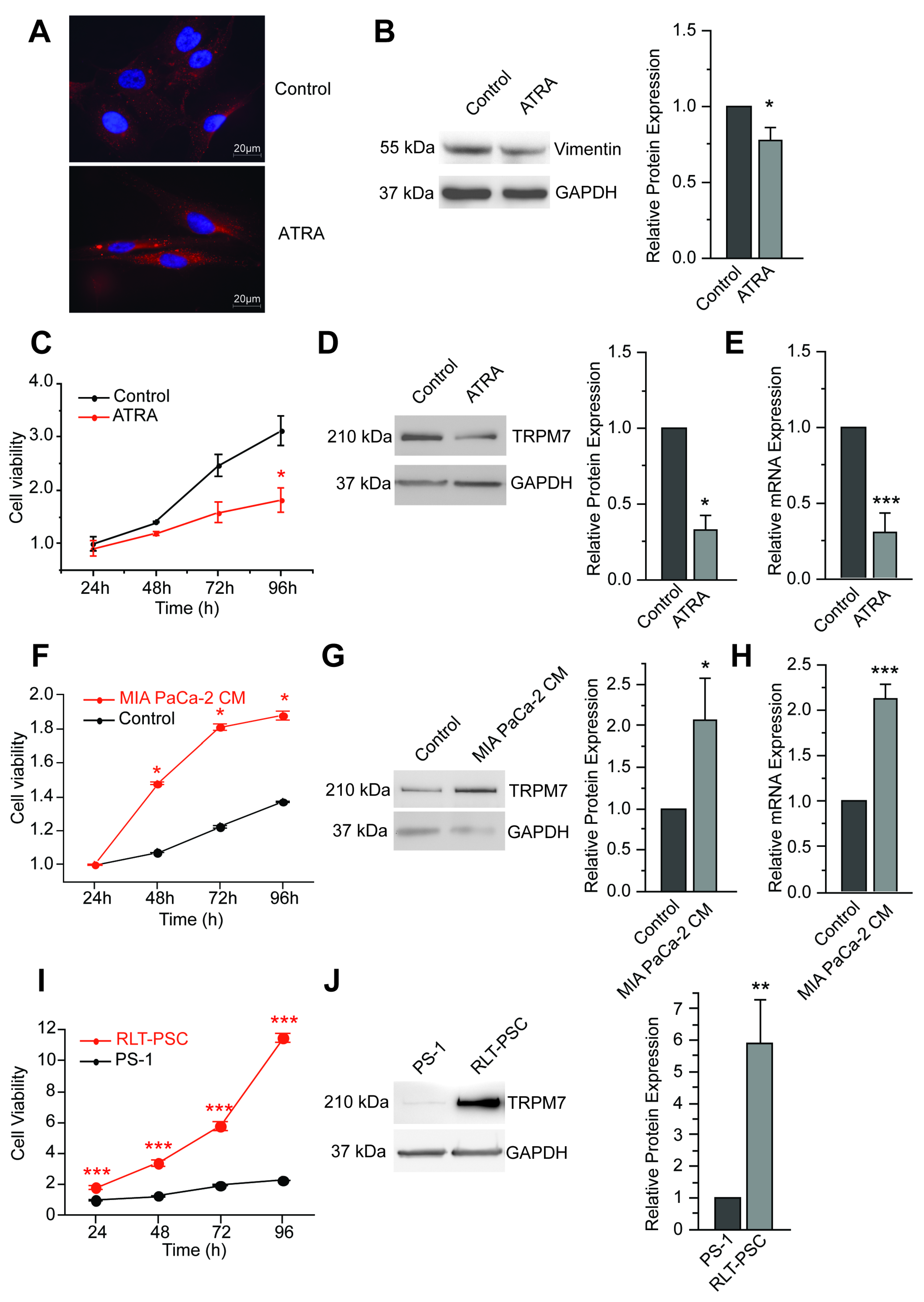
3.1. TRPM7 Is a Marker of Human Pancreatic Stellate Cell Activation
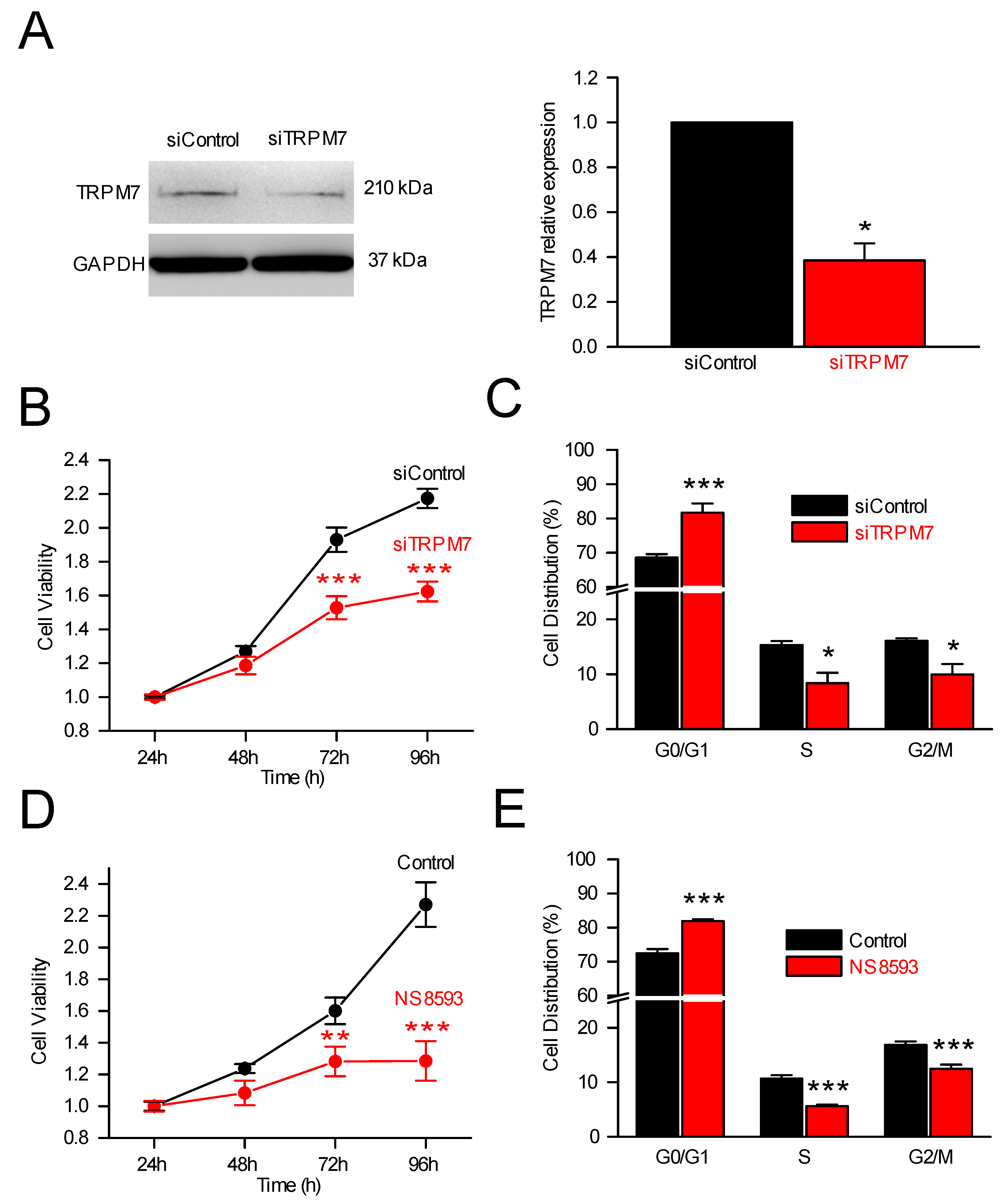
3.2. TRPM7 Regulates Cell Proliferation through Modulation of Cell Cycle Regulators
3.2.1. TRPM7, Cell Proliferation and Cell Cycle Analysis
3.2.2. TRPM7 Silencing and Expression of Cell Cycle Regulators
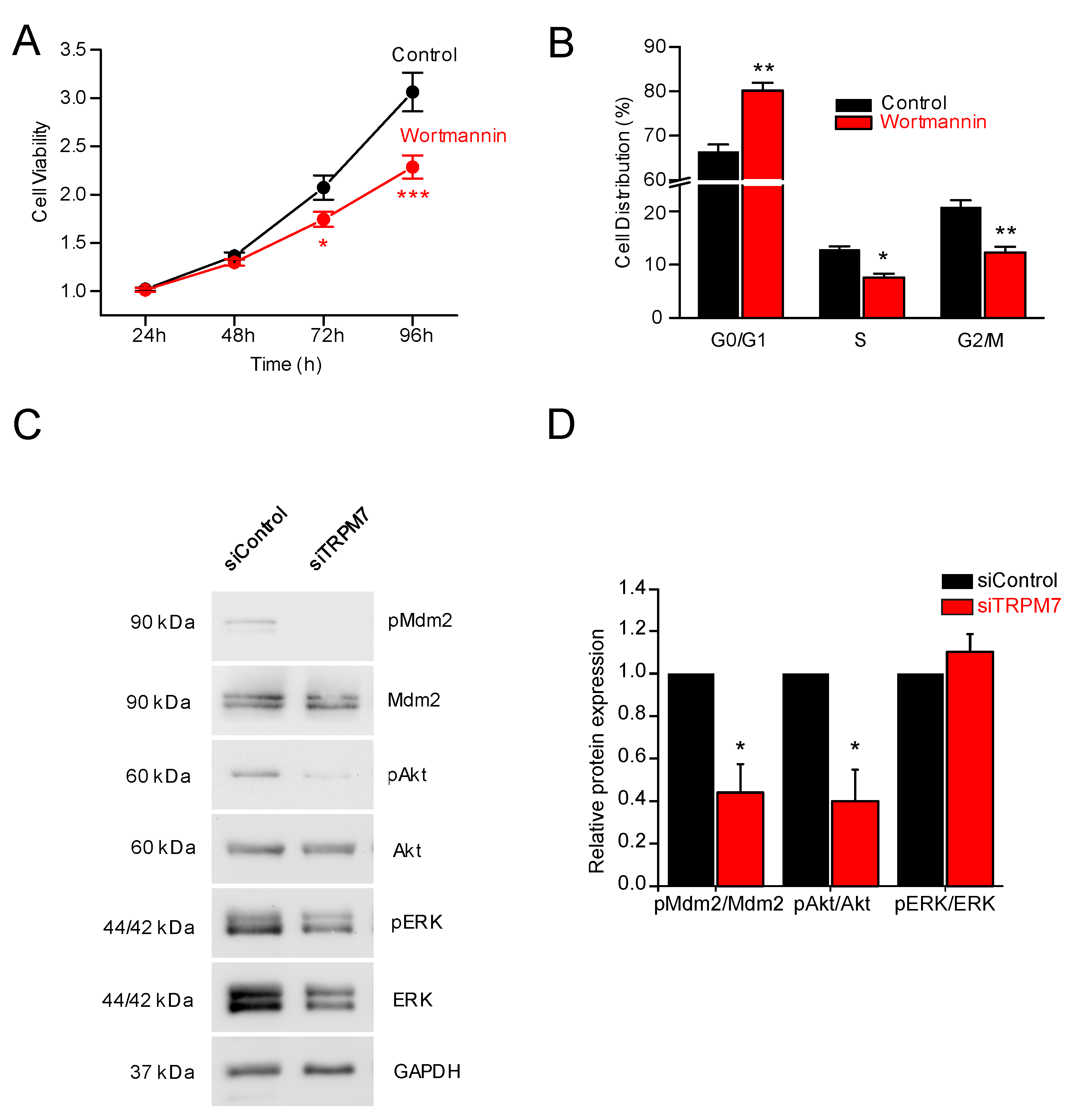
3.3. How Does TRPM7 Regulate Proliferation?
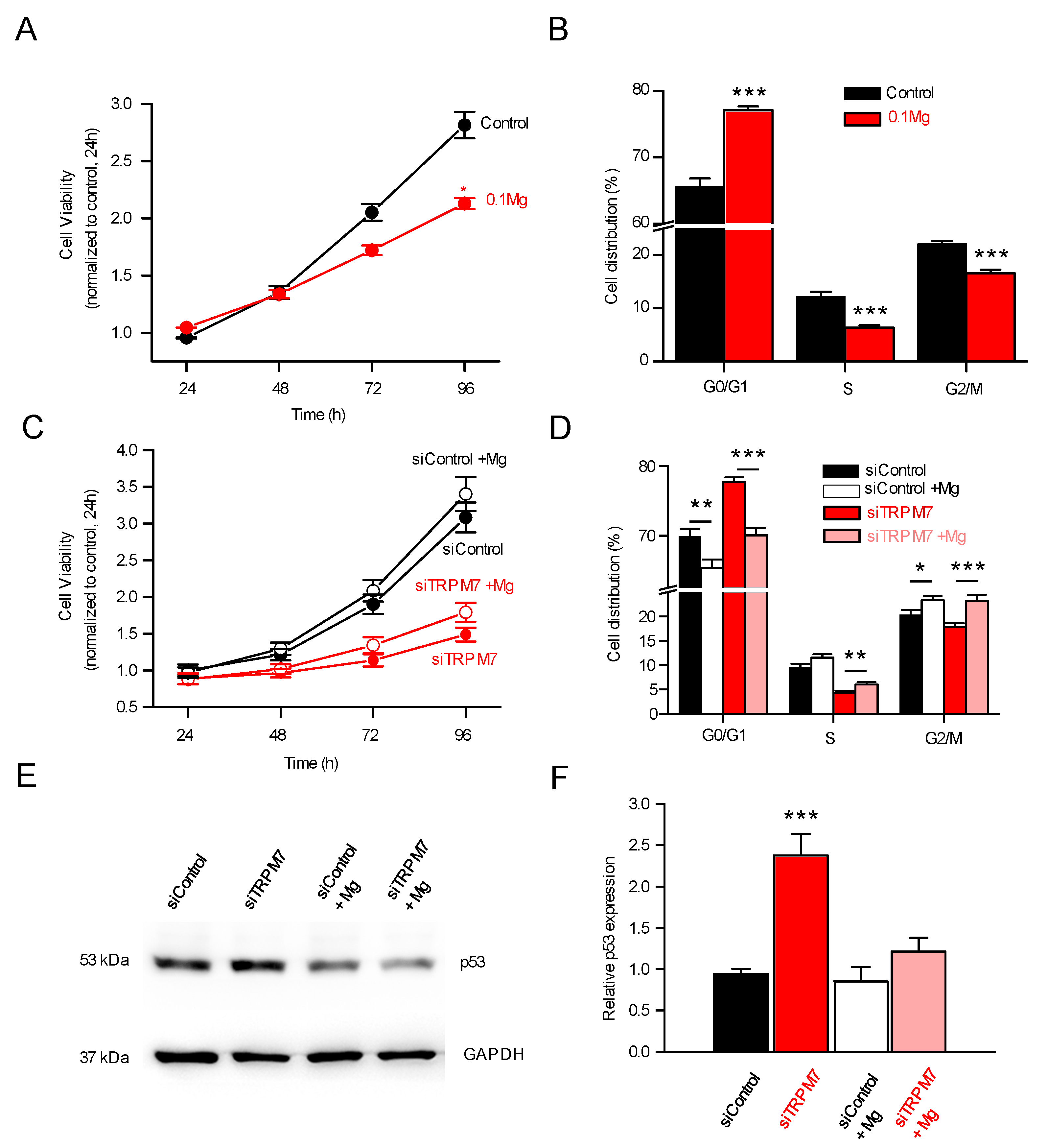
3.4. TRPM7 Regulates Proliferation through the Akt Pathway: Is the Process Calcium or Magnesium-Dependent?
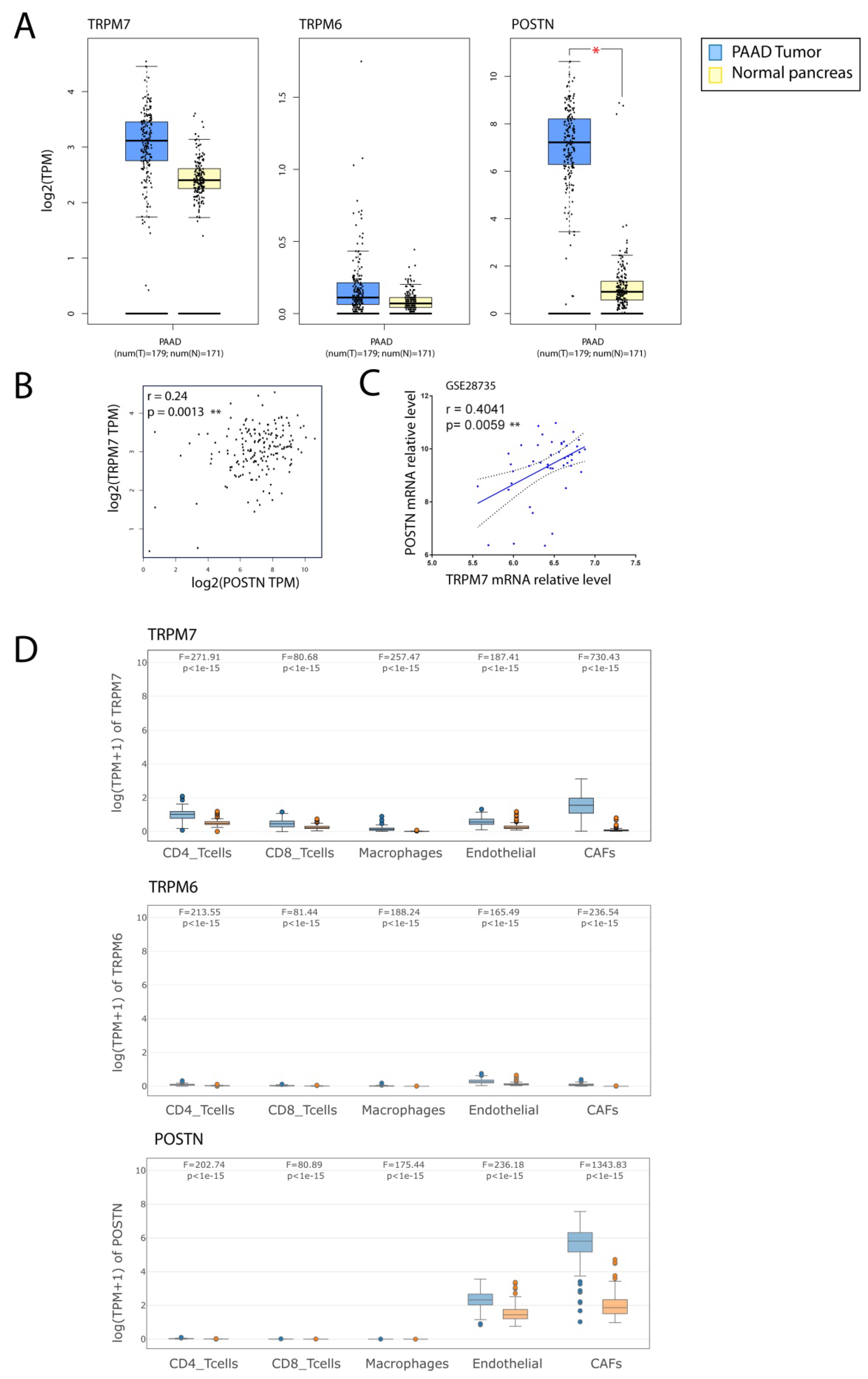
3.5. TRPM7 Expression Is Increased in Cancer-Associated Fibroblasts (CAFs)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phillips, P.A.; McCarroll, J.A.; Park, S.; Wu, M.J.; Pirola, R.; Korsten, M.; Wilson, J.S.; Apte, M.V. Rat pancreatic stellate cells secrete matrix metalloproteinases: Implications for extracellular matrix turnover. Gut 2003, 52, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Ferdek, P.E.; Jakubowska, M.A. Biology of pancreatic stellate cells—more than just pancreatic cancer. Pflügers Archiv-Eur. J. Physiol. 2017, 469, 1039–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.; Radhakrishnan, P. Pancreatic Stellate Cells: The Key Orchestrator of The Pancreatic Tumor Microenvironment. In Tumor Microenvironment: Non-Hematopoietic Cells; Birbrair, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 57–70. [Google Scholar]
- Wang, S.; Li, Y.; Xing, C.; Ding, C.; Zhang, H.; Chen, L.; You, L.; Dai, M.; Zhao, Y. Tumor microenvironment in chemoresistance, metastasis and immunotherapy of pancreatic cancer. Am. J. Cancer Res. 2020, 10, 1937–1953. [Google Scholar] [PubMed]
- Phillips, P.A.; Yang, L.; Shulkes, A.; Vonlaufen, A.; Poljak, A.; Bustamante, S.; Warren, A.; Xu, Z.; Guilhaus, M.; Pirola, R.; et al. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc. Natl. Acad. Sci. USA 2010, 107, 17397–17402. [Google Scholar] [CrossRef] [Green Version]
- Blauer, M.; Laaninen, M.; Sand, J.; Laukkarinen, J. Reciprocal stimulation of pancreatic acinar and stellate cells in a novel long-term in vitro co-culture model. Pancreatology 2016, 16, 570–577. [Google Scholar] [CrossRef]
- Liu, J.S.; Cui, Z.J. Pancreatic Stellate Cells Serve as a Brake Mechanism on Pancreatic Acinar Cell Calcium Signaling Modulated by Methionine Sulfoxide Reductase Expression. Cells 2019, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, B.; Fellows, G.F.; Goodyer, C.G.; Wang, R. Activation of Pancreatic Stellate Cells Is Beneficial for Exocrine but Not Endocrine Cell Differentiation in the Developing Human Pancreas. Front. Cell Dev. Biol. 2021, 9, 694276. [Google Scholar] [CrossRef]
- Goulart, M.R.; Watt, J.; Siddiqui, I.; Lawlor, R.T.; Imrali, A.; Hughes, C.; Saad, A.; ChinAleong, J.; Hurt, C.; Cox, C.; et al. Pentraxin 3 is a stromally-derived biomarker for detection of pancreatic ductal adenocarcinoma. NPJ Precis. Oncol. 2021, 5, 61. [Google Scholar] [CrossRef]
- Thomas, D.; Radhakrishnan, P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol. Cancer 2019, 18, 14. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D Receptor-Mediated Stromal Reprogramming Suppresses Pancreatitis and Enhances Pancreatic Cancer Therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Kocher, H.M.; Basu, B.; Froeling, F.E.M.; Sarker, D.; Slater, S.; Carlin, D.; de Souza, N.M.; De Paepe, K.N.; Goulart, M.R.; Hughes, C.; et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 2020, 11, 4841. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Tijeras-Raballand, A.; Ragulan, C.; Cros, J.; Patil, Y.; Martinet, M.; Erkan, M.; Kleeff, J.; Wilson, J.; Apte, M.; et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J. Pathol. 2019, 248, 51–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybarczyk, P.; Gautier, M.; Hague, F.; Dhennin-Duthille, I.; Chatelain, D.; Kerr-Conte, J.; Pattou, F.; Regimbeau, J.M.; Sevestre, H.; Ouadid-Ahidouch, H. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J. Cancer 2012, 131, E851–E861. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.S.; Kazi, A.A.; Li, Q.; Yang, Z.; Berg, A.; Yee, R.K. Aberrant over-expression of TRPM7 ion channels in pancreatic cancer: Required for cancer cell invasion and implicated in tumor growth and metastasis. Biol. Open 2015, 4, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Auwercx, J.; Rybarczyk, P.; Kischel, P.; Dhennin-Duthille, I.; Chatelain, D.; Sevestre, H.; Van Seuningen, I.; Ouadid-Ahidouch, H.; Jonckheere, N.; Gautier, M. Mg2+ Transporters in Digestive Cancers. Nutrients 2021, 13, 210. [Google Scholar] [CrossRef]
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a Bifunctional Protein with Kinase and Ion Channel Activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Mittermeier, L.; Demirkhanyan, L.; Stadlbauer, B.; Breit, A.; Recordati, C.; Hilgendorff, A.; Matsushita, M.; Braun, A.; Simmons, D.G.; Zakharian, E.; et al. TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc. Natl. Acad. Sci. USA 2019, 116, 4706–4715. [Google Scholar] [CrossRef] [Green Version]
- Ryazanova, L.V.; Rondon, L.J.; Zierler, S.; Hu, Z.; Galli, J.; Yamaguchi, T.P.; Mazur, A.; Fleig, A.; Ryazanov, A.G. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat. Commun. 2010, 1, 109. [Google Scholar] [CrossRef] [Green Version]
- Ryazanov, A.G.; Ward, M.D.; Mendola, C.E.; Pavur, K.S.; Dorovkov, M.V.; Wiedmann, M.; Erdjument-Bromage, H.; Tempst, P.; Parmer, T.G.; Prostko, C.R.; et al. Identification of a new class of protein kinases represented by eukaryotic elongation factor-2 kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 4884–4889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, S.; Alanazi, R.; Ji, D.; Bandura, J.; Luo, Z.W.; Fleig, A.; Feng, Z.P.; Sun, H.S. Role of TRPM7 kinase in cancer. Cell Calcium 2021, 96, 102400. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef] [Green Version]
- Montell, C. Mg2+ homeostasis: The Mg2+nificent TRPM chanzymes. Curr. Biol. 2003, 13, R799–R801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castiglioni, S.; Maier, J.A.M. Magnesium and cancer: A dangerous liason. Magnes. Res. 2011, 24, S92–S100. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Huang, C.; Meng, X.; Wu, B.; Ma, T.; Liu, X.; Zhu, Q.; Zhan, S.; Li, J. TGF-β1-elevated TRPM7 channel regulates collagen expression in hepatic stellate cells via TGF-β1/Smad pathway. Toxicol. Appl. Pharmacol. 2014, 280, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Men, R.; Wen, M.; Hu, X.; Liu, X.; Yang, L. Blockage of TRPM7 channel induces hepatic stellate cell death through endoplasmic reticulum stress-mediated apoptosis. Life Sciences 2014, 94, 37–44. [Google Scholar] [CrossRef]
- Froeling, F.E.; Mirza, T.A.; Feakins, R.M.; Seedhar, A.; Elia, G.; Hart, I.R.; Kocher, H.M. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. Am. J. Pathol. 2009, 175, 636–648. [Google Scholar] [CrossRef] [Green Version]
- Jesnowski, R.; Fürst, D.; Ringel, J.; Chen, Y.; Schrödel, A.; Kleeff, J.; Kolb, A.; Schareck, W.D.; Löhr, M. Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: Deactivation is induced by matrigel and N-acetylcysteine. Lab. Investig. 2005, 85, 1276–1291. [Google Scholar] [CrossRef]
- Vanlaeys, A.; Fouquet, G.; Kischel, P.; Hague, F.; Pasco-Brassart, S.; Lefebvre, T.; Rybarczyk, P.; Dhennin-Duthille, I.; Brassart, B.; Ouadid-Ahidouch, H.; et al. Cadmium exposure enhances cell migration and invasion through modulated TRPM7 channel expression. Lab. Investig. 2020, 94, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Rybarczyk, P.; Vanlaeys, A.; Brassart, B.; Dhennin-Duthille, I.; Chatelain, D.; Sevestre, H.; Ouadid-Ahidouch, H.; Gautier, M. The Transient Receptor Potential Melastatin 7 Channel Regulates Pancreatic Cancer Cell Invasion through the Hsp90alpha/uPA/MMP2 pathway. Neoplasia 2017, 19, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J. Gen. Physiol. 2002, 119, 487–507. [Google Scholar] [CrossRef] [Green Version]
- Racle, J.; de Jonge, K.; Baumgaertner, P.; Speiser, D.E.; Gfeller, D. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, Z.; Zhang, W.; Ye, Z.; Liu, F. GEPIA2021: Integrating multiple deconvolution-based analysis into GEPIA. Nucleic Acids Res. 2021, 49, W242–W246. [Google Scholar] [CrossRef]
- McCarroll, J.A.; Phillips, P.A.; Santucci, N.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Vitamin A inhibits pancreatic stellate cell activation: Implications for treatment of pancreatic fibrosis. Gut 2006, 55, 79–89. [Google Scholar] [CrossRef]
- Froeling, F.E.M.; Feig, C.; Chelala, C.; Dobson, R.; Mein, C.E.; Tuveson, D.A.; Clevers, H.; Hart, I.R.; Kocher, H.M. Retinoic Acid-Induced Pancreatic Stellate Cell Quiescence Reduces Paracrine Wnt-β-Catenin Signaling to Slow Tumor Progression. Gastroenterology 2011, 141, 1486–1497.e14. [Google Scholar] [CrossRef]
- Sbisa, E.; Catalano, D.; Grillo, G.; Licciulli, F.; Turi, A.; Liuni, S.; Pesole, G.; De Grassi, A.; Caratozzolo, M.F.; D’Erchia, A.M.; et al. p53FamTaG: A database resource of human p53, p63 and p73 direct target genes combining in silico prediction and microarray data. BMC Bioinf. 2007, 8 (Suppl. 1), S20. [Google Scholar] [CrossRef] [Green Version]
- Chubanov, V.; Mederos y Schnitzler, M.; Meissner, M.; Schafer, S.; Abstiens, K.; Hofmann, T.; Gudermann, T. Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br. J. Pharmacol. 2012, 166, 1357–1376. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Zhan, S.; Huang, C.; Cheng, X.; Lv, X.; Si, H.; Li, J. TRPM7 channel regulates PDGF-BB-induced proliferation of hepatic stellate cells via PI3K and ERK pathways. Toxicol. Appl. Pharmacol. 2013, 272, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Kozak, J.A.; Kerschbaum, H.H.; Cahalan, M.D. Distinct Properties of CRAC and MIC Channels in RBL Cells. J. Gen. Physiol. 2002, 120, 221–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkan, M.; Hausmann, S.; Michalski, C.W.; Fingerle, A.A.; Dobritz, M.; Kleeff, J.; Friess, H. The role of stroma in pancreatic cancer: Diagnostic and therapeutic implications. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 454–467. [Google Scholar] [CrossRef]
- Neesse, A.; Algül, H.; Tuveson, D.A.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: A changing paradigm. Gut 2015, 64, 1476–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apte, M.; Pirola, R.; Wilson, J. Pancreatic stellate cells: A starring role in normal and diseased pancreas. Front. Physiol. 2012, 3, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaster, R.; Hilgendorf, I.; Fitzner, B.; Brock, P.; Sparmann, G.; Emmrich, J.; Liebe, S. Regulation of pancreatic stellate cell function in vitro: Biological and molecular effects of all-trans retinoic acid. Biochem. Pharmacol. 2003, 66, 633–641. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Huang, Y.; Huang, C. Inhibition of transient receptor potential melastain 7 channel increases HSCs apoptosis induced by TRAIL. Life Sci. 2012, 90, 612–618. [Google Scholar] [CrossRef]
- Tani, D.; Monteilh-Zoller, M.K.; Fleig, A.; Penner, R. Cell cycle-dependent regulation of store-operated ICRAC and Mg2+-nucleotide-regulated MagNuM (TRPM7) currents. Cell Calcium 2007, 41, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Hanano, T.; Hara, Y.; Shi, J.; Morita, H.; Umebayashi, C.; Mori, E.; Sumimoto, H.; Ito, Y.; Mori, Y.; Inoue, R. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J. Pharmacol. Sci. 2004, 95, 403–419. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Meng, Z.; Liu, T.; Wang, G.; Qian, G.; Cao, T.; Guan, X.; Dan, H.; Xiao, Y.; Wang, X. Decreased TRPM7 inhibits activities and induces apoptosis of bladder cancer cells via ERK1/2 pathway. Oncotarget 2016, 7, 72941. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci 2019, 20, 1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deason-Towne, F.; Perraud, A.-L.; Schmitz, C. The Mg2+ transporter MagT1 partially rescues cell growth and Mg2+ uptake in cells lacking the channel-kinase TRPM7. FEBS Lett. 2011, 585, 2275–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofer, A.M.; Machen, T.E. Technique for in situ measurement of calcium in intracellular inositol 1,4,5-trisphosphate-sensitive stores using the fluorescent indicator mag-fura-2. Proc. Natl. Acad. Sci. USA 1993, 90, 2598–2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faouzi, M.; Kilch, T.; Horgen, F.D.; Fleig, A.; Penner, R. The TRPM7 channel kinase regulates store-operated calcium entry. J. Physiol. 2017, 595, 3165–3180. [Google Scholar] [CrossRef] [Green Version]
- Ferdek, P.E.; Jakubowska, M.A.; Gerasimenko, J.V.; Gerasimenko, O.V.; Petersen, O.H. Bile acids induce necrosis in pancreatic stellate cells dependent on calcium entry and sodium-driven bile uptake. J. Physiol. 2016, 594, 6147–6164. [Google Scholar] [CrossRef] [Green Version]
- Petersen, O.H.; Gerasimenko, J.V.; Gerasimenko, O.V.; Gryshchenko, O.; Peng, S. The roles of calcium and ATP in the physiology and pathology of the exocrine pancreas. Physiol. Rev. 2021, 101, 1691–1744. [Google Scholar] [CrossRef]
- Callera, G.E.; He, Y.; Yogi, A.; Montezano, A.C.; Paravicini, T.; Yao, G.; Touyz, R.M. Regulation of the novel Mg2+ transporter transient receptor potential melastatin 7 (TRPM7) cation channel by bradykinin in vascular smooth muscle cells. J. Hypertens. 2009, 27, 155–166. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Sun, S.; Wang, Z.; Liu, P.; Liu, S.; Jiang, J. Bradykinin promotes migration and invasion of hepatocellular carcinoma cells through TRPM7 and MMP2. Exp. Cell Res. 2016, 349, 68–76. [Google Scholar] [CrossRef]



| Primary Antibody | Supplier | Dilution | Species |
|---|---|---|---|
| TRPM7 | Abcam (ab109438) | 1:1000 | Rabbit |
| Vimentin | ThermoFisher (MA5-16409) | 1:1000 | Rabbit |
| pERK1/2 | Cell Signaling (9101S) | 1:1000 | Rabbit |
| ERK1/2 | Cell Signaling (9102S) | 1:1000 | Rabbit |
| pAkt | Cell Signaling (9271S) | 1:250 | Rabbit |
| Akt | Cell Signaling (9272S) | 1:500 | Rabbit |
| pMdm2 | Abcam (ab170880) | 1:1000 | Rabbit |
| Mdm2 | Abcam (ab38618) | 1:1000 | Rabbit |
| PCNA | Abcam (ab29) | 1:1000 | Mouse |
| Cyclin-E1 | Abcam (ab133266) | 1:500 | Rabbit |
| CDK2 | Cell Signaling (2546S) | 1:500 | Rabbit |
| p53 | Santa Cruz (sc126) | 1:500 | Mouse |
| GAPDH | Abcam (ab8245) | 1:4000 | Mouse |
| HRP-linked Anti-rabbit IgG | Cell Signaling (7074S) | NA | Goat |
| HRP-linked Anti-mouse IgG | Cell Signaling (7076S) | NA | Horse |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auwercx, J.; Kischel, P.; Lefebvre, T.; Jonckheere, N.; Vanlaeys, A.; Guénin, S.; Radoslavova, S.; Van Seuningen, I.; Ouadid-Ahidouch, H.; Kocher, H.M.; et al. TRPM7 Modulates Human Pancreatic Stellate Cell Activation. Cells 2022, 11, 2255. https://doi.org/10.3390/cells11142255
Auwercx J, Kischel P, Lefebvre T, Jonckheere N, Vanlaeys A, Guénin S, Radoslavova S, Van Seuningen I, Ouadid-Ahidouch H, Kocher HM, et al. TRPM7 Modulates Human Pancreatic Stellate Cell Activation. Cells. 2022; 11(14):2255. https://doi.org/10.3390/cells11142255
Chicago/Turabian StyleAuwercx, Julie, Philippe Kischel, Thibaut Lefebvre, Nicolas Jonckheere, Alison Vanlaeys, Stéphanie Guénin, Silviya Radoslavova, Isabelle Van Seuningen, Halima Ouadid-Ahidouch, Hemant M. Kocher, and et al. 2022. "TRPM7 Modulates Human Pancreatic Stellate Cell Activation" Cells 11, no. 14: 2255. https://doi.org/10.3390/cells11142255










