Distinct PKA Signaling in Cytosolic and Mitochondrial Compartments in Electrically Paced Atrial Myocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Use
2.2. Atrial Cell Isolation
2.3. Culture Procedure
2.4. Cell Infection with FRET Probes
2.5. Electrical Stimulation
2.6. FRET Measurements
2.7. Confocal Imaging
2.8. Ca2+ Measurements
2.9. Drugs
2.10. Statistics
3. Results
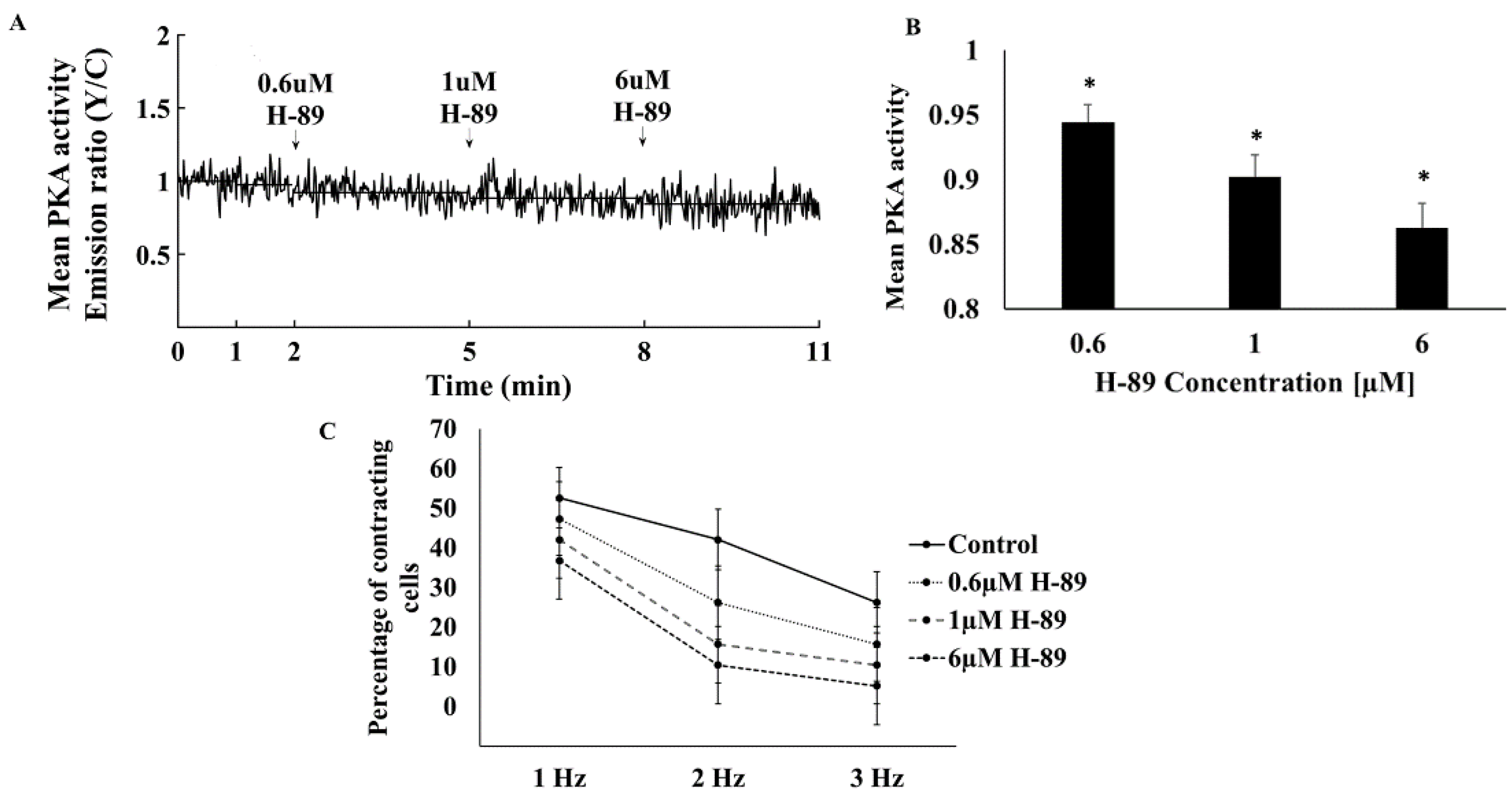
3.1. PKA Activity Mediates the Ability of Atrial Cells to Be Electrically Paced
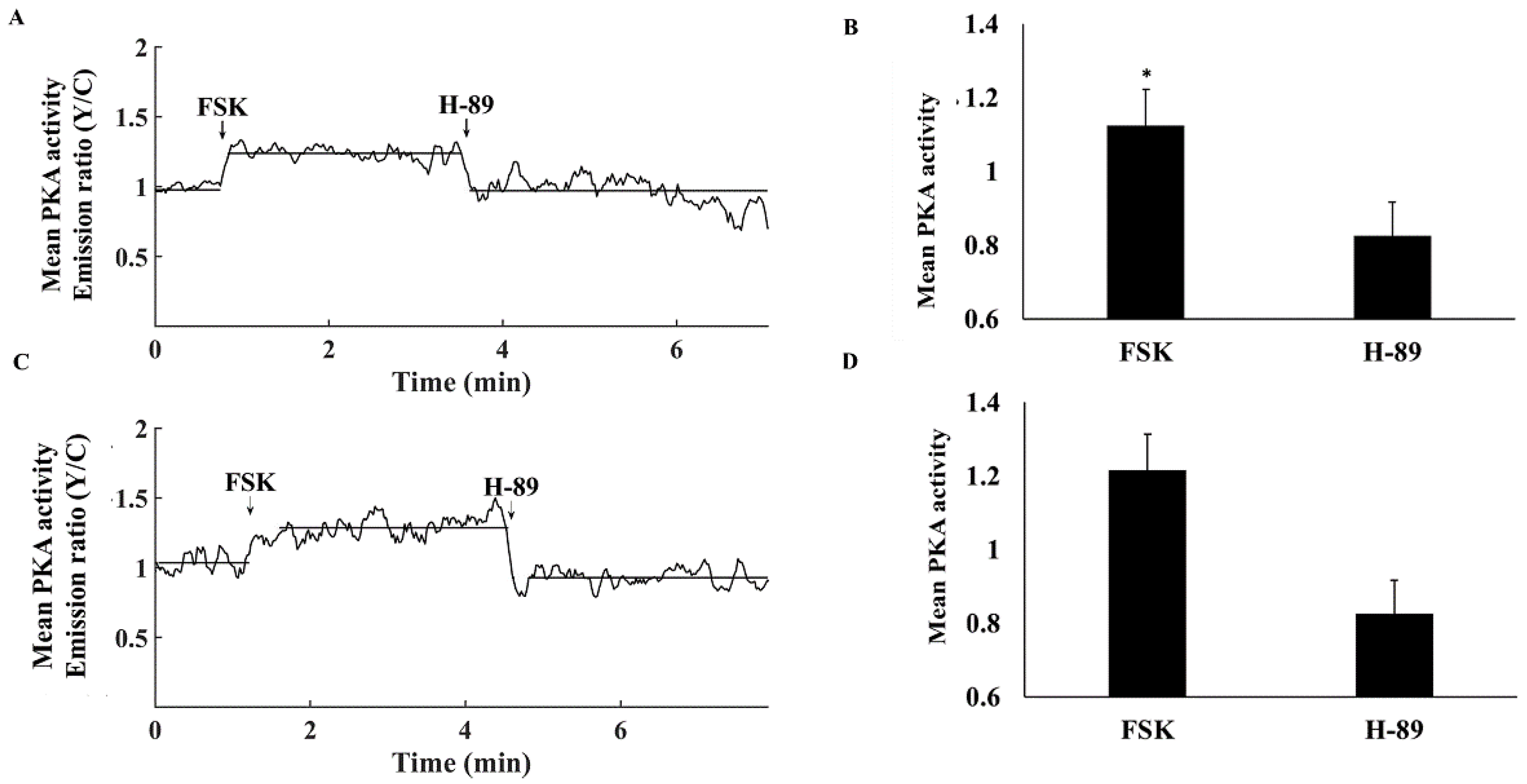
3.2. Ca2+-Activated AC Is an Important Regulator of PKA Activity in Atrial Cells
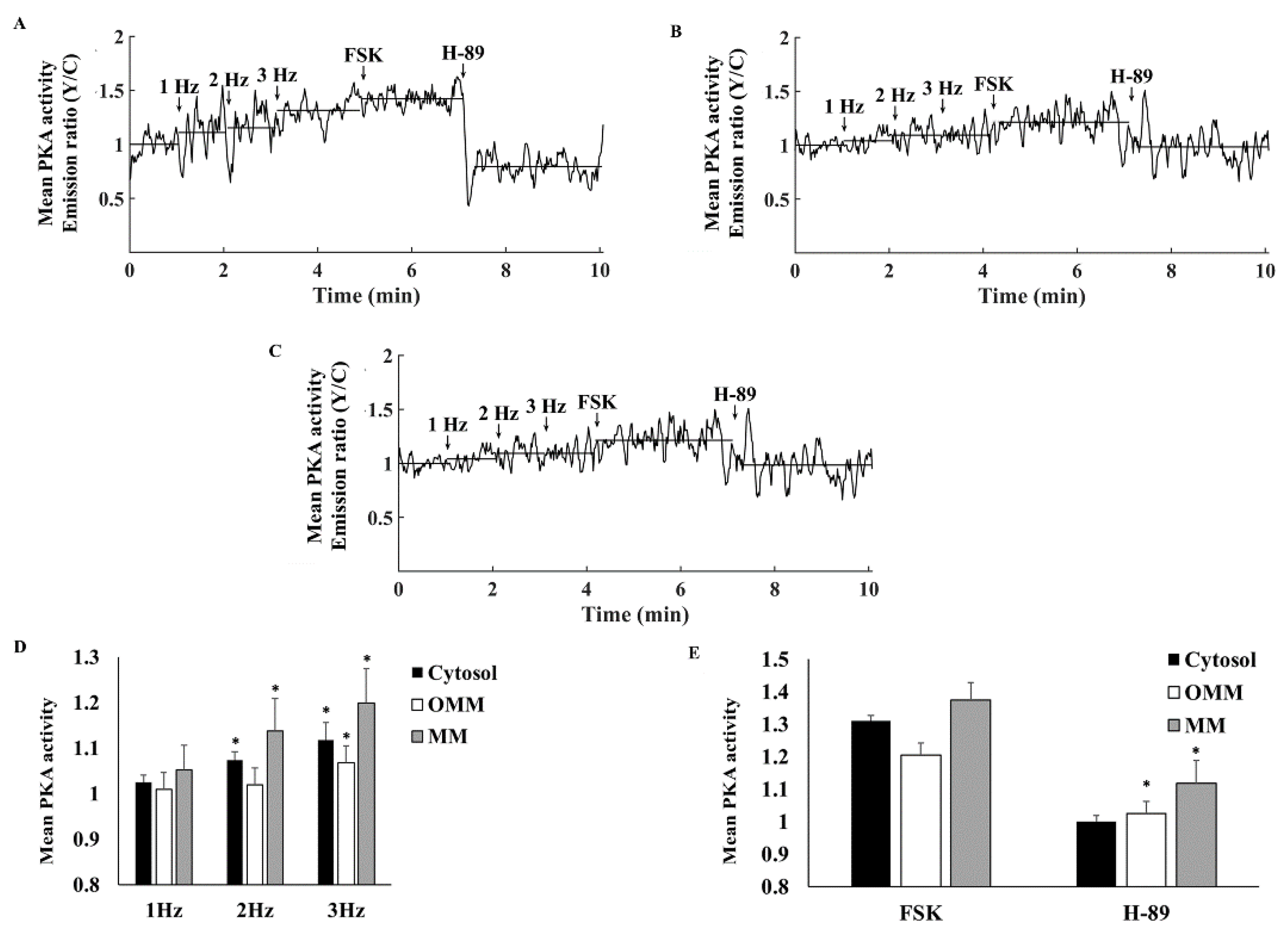
3.3. PKA Compartmentalization in the Cytosol, MM, and OMM
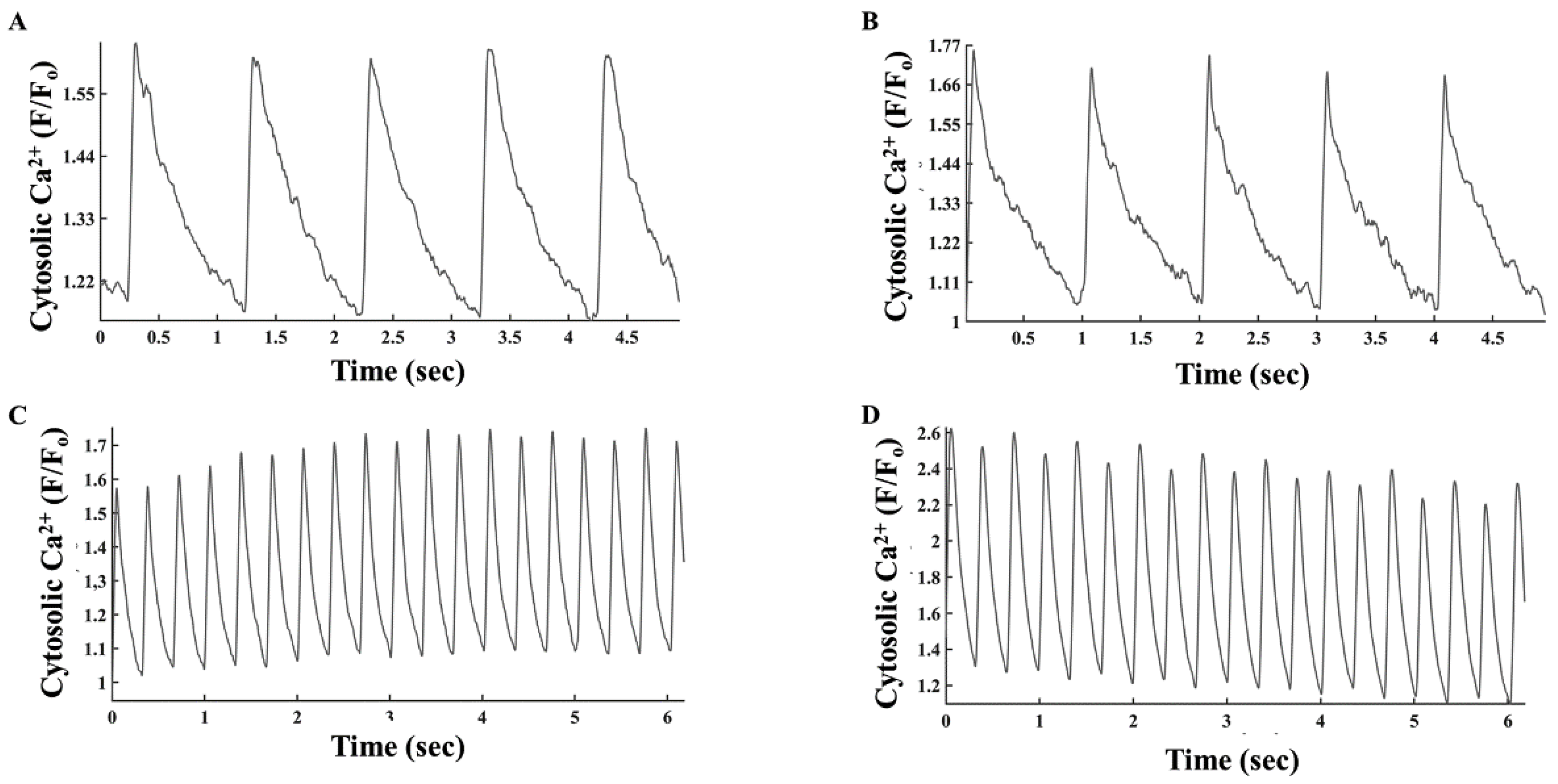
3.4. Electrical Stimulation Elicits Distinct PKA Activity in the Cytosol
4. Discussion
Study Limitation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ubersax, J.A.; Ferrell, J.E., Jr. Mechanisms of Specificity in Protein Phosphorylation. Nat. Rev. Mol. Cell Biol. 2007, 8, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, S.J.; Wasco, W.; MacLeod, J.; Satir, P.; Orr, G.A. Immunogold Localization of the Regulatory Subunit of a Type II CAMP-Dependent Protein Kinase Tightly Associated with Mammalian Sperm Flagella. J. Cell Biol. 1988, 107, 1809–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feliciello, A.; Gottesman, M.E.; Avvedimento, E.V. CAMP-PKA Signaling to the Mitochondria: Protein Scaffolds, MRNA and Phosphatases. Cell. Signal. 2005, 17, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Means, C.K.; Lygren, B.; Langeberg, L.K.; Jain, A.; Dixon, R.E.; Vega, A.L.; Gold, M.G.; Petrosyan, S.; Taylor, S.S.; Murphy, A.N.; et al. An Entirely Specific Type I A-Kinase Anchoring Protein That Can Sequester Two Molecules of Protein Kinase A at Mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, E1227–E1235. [Google Scholar] [CrossRef] [Green Version]
- Ghigo, A.; Mika, D. CAMP/PKA Signaling Compartmentalization in Cardiomyocytes: Lessons from FRET-Based Biosensors. J. Mol. Cell. Cardiol. 2019, 131, 112–121. [Google Scholar] [CrossRef]
- Lefkimmiatis, K.; Leronni, D.; Hofer, A.M. The Inner and Outer Compartments of Mitochondria Are Sites of Distinct CAMP/PKA Signaling Dynamics. J. Cell Biol. 2013, 202, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Vinogradova, T.M.; Kobrinsky, E.; Lakatta, E.G. Dual Activation of Phosphodiesterases 3 and 4 Regulates Basal Spontaneous Beating Rate of Cardiac Pacemaker Cells: Role of Compartmentalization? Front. Physiol. 2018, 9, 1301. [Google Scholar] [CrossRef]
- Derici, M.K.; Sadi, G.; Cenik, B.; Güray, T.; Demirel-Yilmaz, E. Differential Expressions and Functions of Phosphodiesterase Enzymes in Different Regions of the Rat Heart. Eur. J. Pharmacol. 2019, 844, 118–129. [Google Scholar] [CrossRef]
- Alsina, K.M.; Hulsurkar, M.; Brandenburg, S.; Kownatzki-Danger, D.; Lenz, C.; Urlaub, H.; Abu-Taha, I.; Kamler, M.; Chiang, D.Y.; Lahiri, S.K.; et al. Loss of Protein Phosphatase 1 Regulatory Subunit PPP1R3A Promotes Atrial Fibrillation. Circulation 2019, 140, 681–693. [Google Scholar] [CrossRef]
- Siddoway, B.A.; Altimimi, H.F.; Hou, H.; Petralia, R.S.; Xu, B.; Stellwagen, D.; Xia, H. An Essential Role for Inhibitor-2 Regulation of Protein Phosphatase-1 in Synaptic Scaling. J. Neurosci. 2013, 33, 11206. [Google Scholar] [CrossRef] [Green Version]
- Mika, D.; Leroy, J.; Vandecasteele, G.; Fischmeister, R. PDEs Create Local Domains of CAMP Signaling. J. Mol. Cell Cardiol. 2012, 52, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Westphal, R.S.; Anderson, K.A.; Means, A.R.; Wadzinski, B.E. A Signaling Complex of Ca2+-Calmodulin-Dependent Protein Kinase IV and Protein Phosphatase 2A. Science 1998, 280, 1258–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, T.P.; Terrar, D.A. Ca(2+)-Stimulated Adenylyl Cyclases Regulate the L-Type Ca(2+) Current in Guinea-Pig Atrial Myocytes. J. Physiol. 2012, 590, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, L.; Roderick, H.L.; Berridge, M.J.; Conway, S.J.; Bootman, M.D. The Spatial Pattern of Atrial Cardiomyocyte Calcium Signalling Modulates Contraction. J. Cell Sci. 2004, 117, 6327–6337. [Google Scholar] [CrossRef] [Green Version]
- Mongillo, M.; McSorley, T.; Evellin, S.; Sood, A.; Lissandron, V.; Terrin, A.; Huston, E.; Hannawacker, A.; Lohse, M.J.; Pozzan, T.; et al. Fluorescence Resonance Energy Transfer-Based Analysis of CAMP Dynamics in Live Neonatal Rat Cardiac Myocytes Reveals Distinct Functions of Compartmentalized Phosphodiesterases. Circ. Res. 2004, 95, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Davoodi, M.; Segal, S.; Kirschner Peretz, N.; Kamoun, D.; Yaniv, Y. Semi-Automated Program for Analysis of Local Ca2+ Spark Release with Application for Classification of Heart Cell Type. Cell Calcium. 2017, 64, 83–90. [Google Scholar] [CrossRef]
- Kirschner Peretz, N.; Segal, S.; Arbel-Ganon, L.; Ben Jehuda, R.; Shemer, Y.; Eisen, B.; Davoodi, M.; Binah, O.; Yaniv, Y. A Method Sustaining the Bioelectric, Biophysical, and Bioenergetic Function of Cultured Rabbit Atrial Cells. Front. Physiol. 2017, 8, 584. [Google Scholar] [CrossRef] [Green Version]
- Yaniv, Y.; Ahmet, I.; Liu, J.; Lyashkov, A.E.; Guiriba, T.-R.; Okamoto, Y.; Ziman, B.D.; Lakatta, E.G. Synchronization of Sinoatrial Node Pacemaker Cell Clocks and Its Autonomic Modulation Impart Complexity to Heart Beating Intervals. Hear. Rhythm 2014, 11, 1210–1219. [Google Scholar] [CrossRef] [Green Version]
- Kaumann, A.J.; Birnbaumer, L. Desensitization of Kitten Atria to Chronotropic, Inotropic and Adenylyl Cyclase Stimulating Effects of (-)Isoprenaline. Naunyn. Schmiedebergs. Arch. Pharmacol. 1976, 293, 199–202. [Google Scholar] [CrossRef]
- Shi, Y. Serine/Threonine Phosphatases: Mechanism through Structure. Cell 2009, 139, 468–484. [Google Scholar] [CrossRef] [Green Version]
- Molina, C.E.; Leroy, J.; Richter, W.; Xie, M.; Scheitrum, C.; Lee, I.O.; Maack, C.; Rucker-Martin, C.; Donzeau-Gouge, P.; Verde, I.; et al. Cyclic Adenosine Monophosphate Phosphodiesterase Type 4 Protects against Atrial Arrhythmias. J. Am. Coll Cardiol 2012, 59, 2182–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, Y.H.; Ehrlich, J.R.; Qi, X.; Hébert, T.E.; Chartier, D.; Nattel, S. Adrenergic Control of a Constitutively Active Acetylcholine-Regulated Potassium Current in Canine Atrial Cardiomyocytes. Cardiovasc. Res. 2007, 74, 406–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.G.; Hüser, J.; Blatter, L.A.; Lipsius, S.L. Withdrawal of Acetylcholine Elicits Ca2+-Induced Delayed Afterdepolarizations in Cat Atrial Myocytes. Circulation 1997, 96, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Covian, R.; Balaban, R.S. Cardiac Mitochondrial Matrix and Respiratory Complex Protein Phosphorylation. Am. J. Physiol. Hear. Circ. Physiol. 2012, 303, H940–H966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bera, A.K.; Ghosh, S.; Das, S. Mitochondrial VDAC Can Be Phosphorylated by Cyclic AMP-Dependent Protein Kinase. Biochem. Biophys. Res. Commun. 1995, 209, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, K.L.; Maldonado, E.N.; Lemasters, J.J.; Rostovtseva, T.K.; Bezrukov, S.M. Phosphorylation of Voltage-Dependent Anion Channel by Serine/Threonine Kinases Governs Its Interaction with Tubulin. PLoS ONE 2011, 6, e25539. [Google Scholar] [CrossRef] [Green Version]
- Limbutara, K.; Kelleher, A.; Yang, C.R.; Raghuram, V.; Knepper, M.A. Phosphorylation Changes in Response to Kinase Inhibitor H89 in PKA-Null Cells. Sci. Rep. 2019, 9, 2814. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirschner Peretz, N.; Segal, S.; Weiser-Bitoun, I.; Yaniv, Y. Distinct PKA Signaling in Cytosolic and Mitochondrial Compartments in Electrically Paced Atrial Myocytes. Cells 2022, 11, 2261. https://doi.org/10.3390/cells11142261
Kirschner Peretz N, Segal S, Weiser-Bitoun I, Yaniv Y. Distinct PKA Signaling in Cytosolic and Mitochondrial Compartments in Electrically Paced Atrial Myocytes. Cells. 2022; 11(14):2261. https://doi.org/10.3390/cells11142261
Chicago/Turabian StyleKirschner Peretz, Noa, Sofia Segal, Ido Weiser-Bitoun, and Yael Yaniv. 2022. "Distinct PKA Signaling in Cytosolic and Mitochondrial Compartments in Electrically Paced Atrial Myocytes" Cells 11, no. 14: 2261. https://doi.org/10.3390/cells11142261
APA StyleKirschner Peretz, N., Segal, S., Weiser-Bitoun, I., & Yaniv, Y. (2022). Distinct PKA Signaling in Cytosolic and Mitochondrial Compartments in Electrically Paced Atrial Myocytes. Cells, 11(14), 2261. https://doi.org/10.3390/cells11142261




