Downregulation of miR-192 Alleviates Oxidative Stress-Induced Porcine Granulosa Cell Injury by Directly Targeting Acvr2a
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture and Reagents
2.3. GC Viability Assay
2.4. Detection of Intracellular ROS
2.5. Apoptosis Analysis
2.6. Caspase-3 Activity Assay
2.7. Small-RNA Library Construction and Sequencing
2.8. Data Analysis
2.9. Analysis of Differentially Expressed miRNAs
2.10. The Prediction of Target Genes of miRNAs
2.11. Quantitative Real-Time RT-PCR (qRT-PCR)
2.12. Western Blot Analysis
2.13. Statistical Analysis
3. Results
3.1. OS Reduces Viability and Induces Apoptosis in PGCs
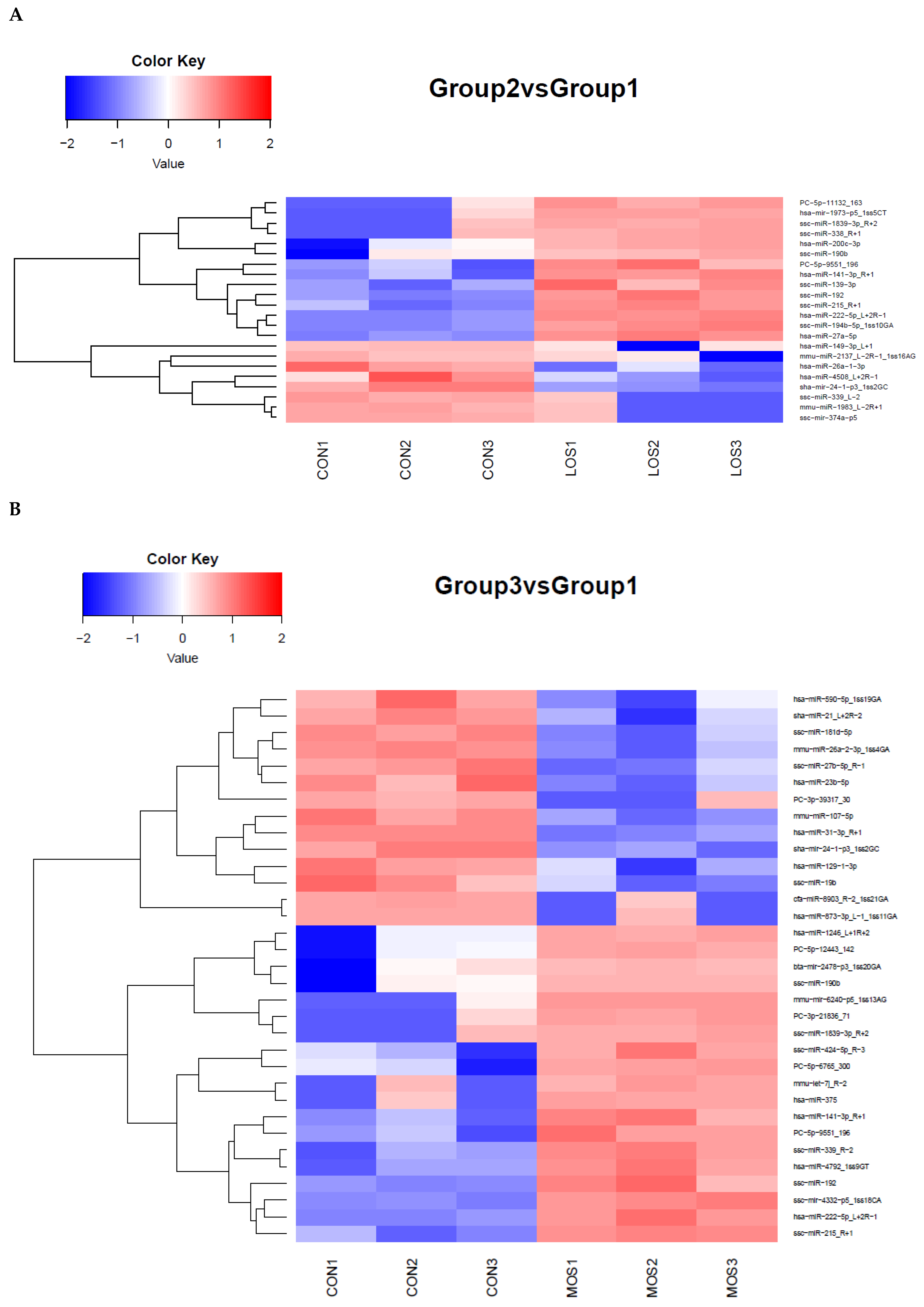
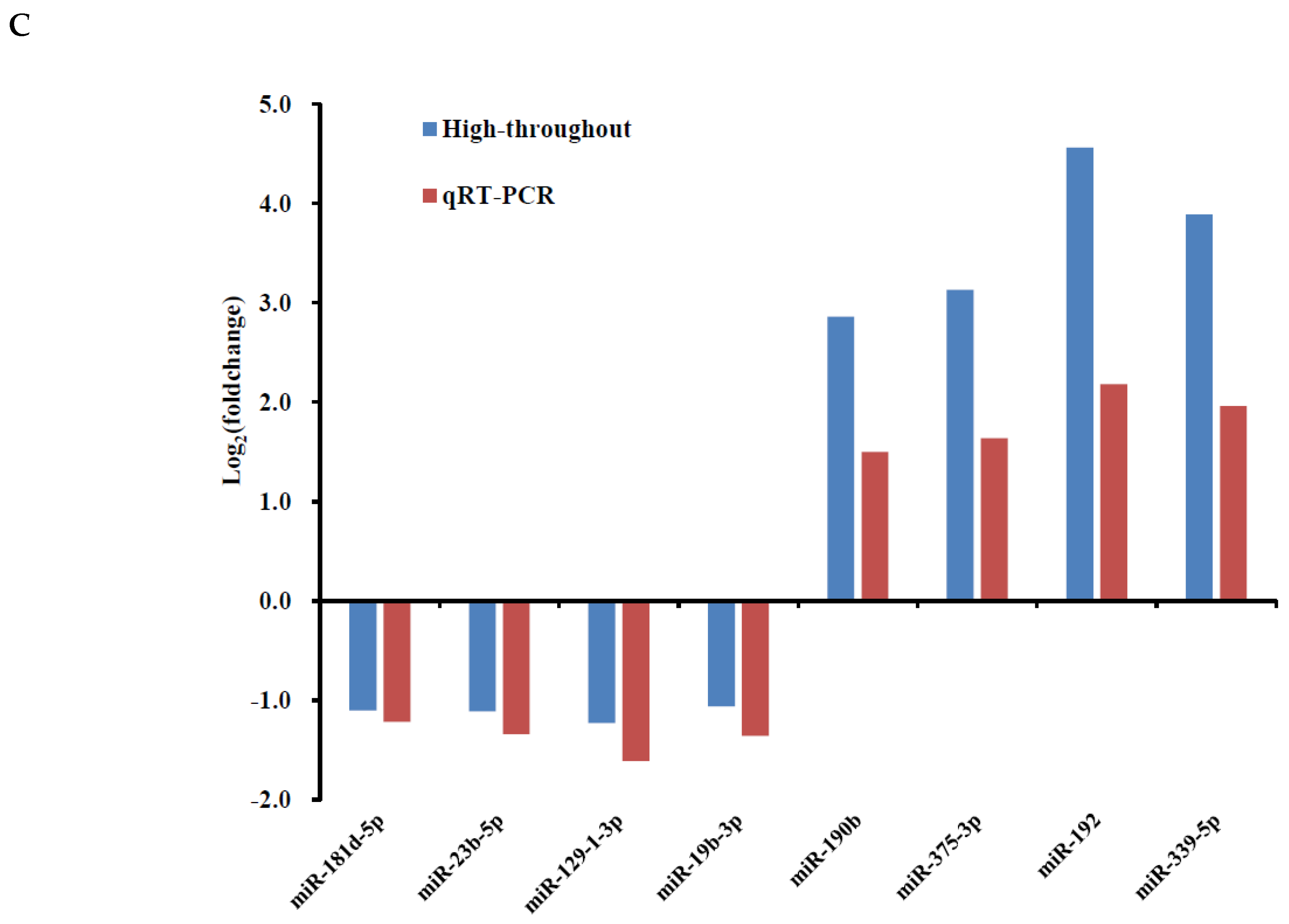
3.2. Identification of H2O2-Induced Changes in miRNA Expression in PGCs
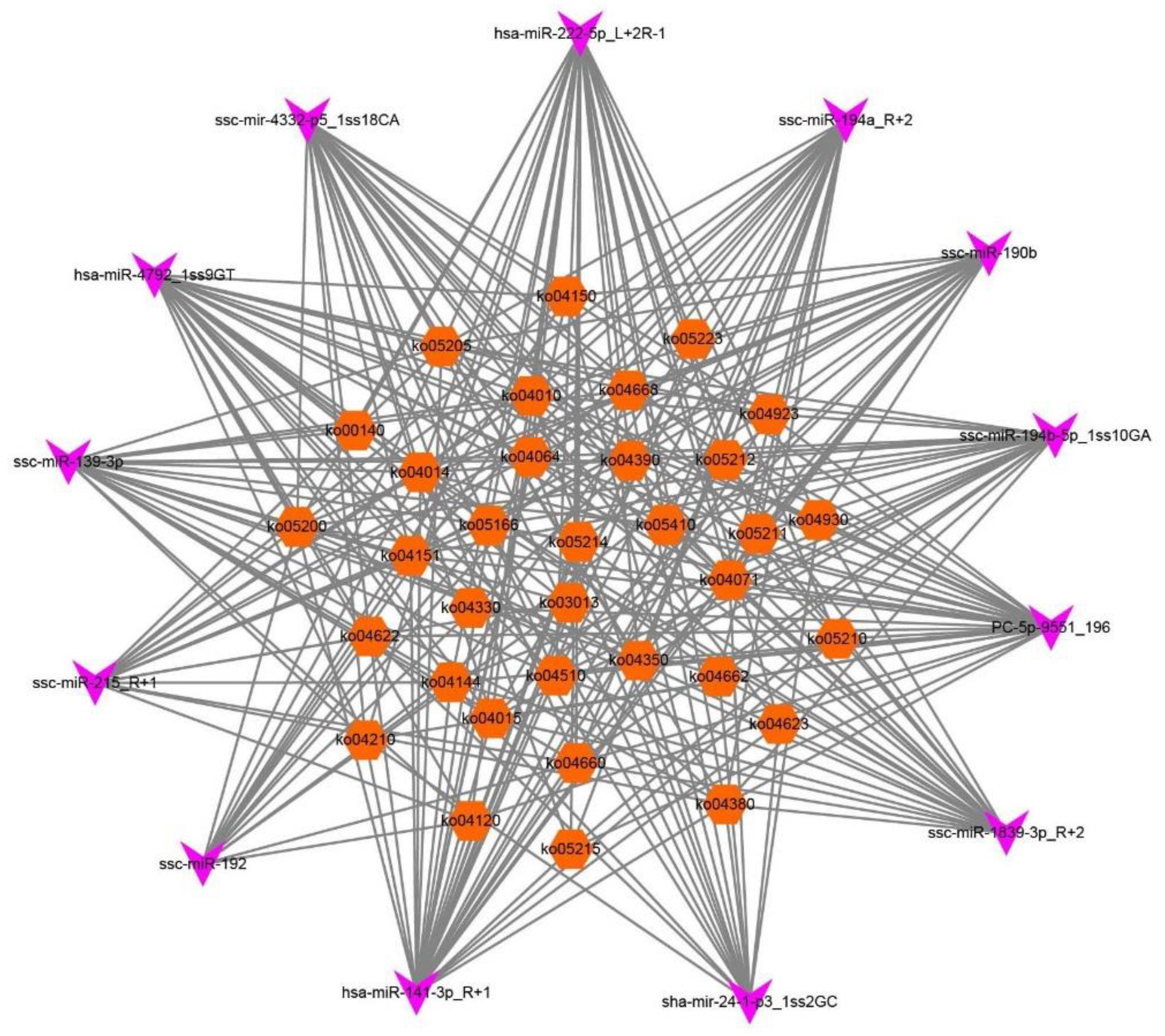
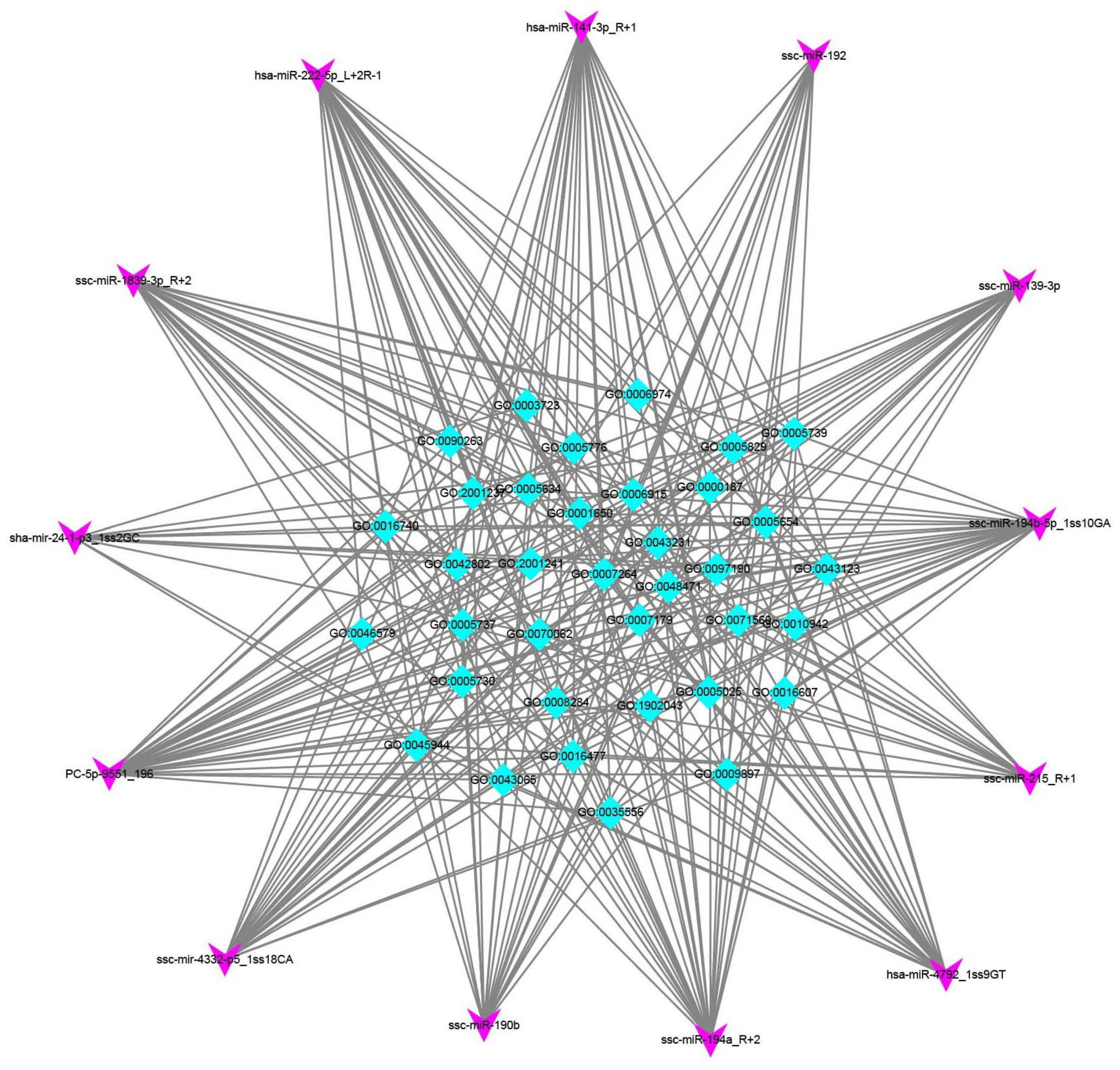
3.3. Functional Analysis of DEmRNAs
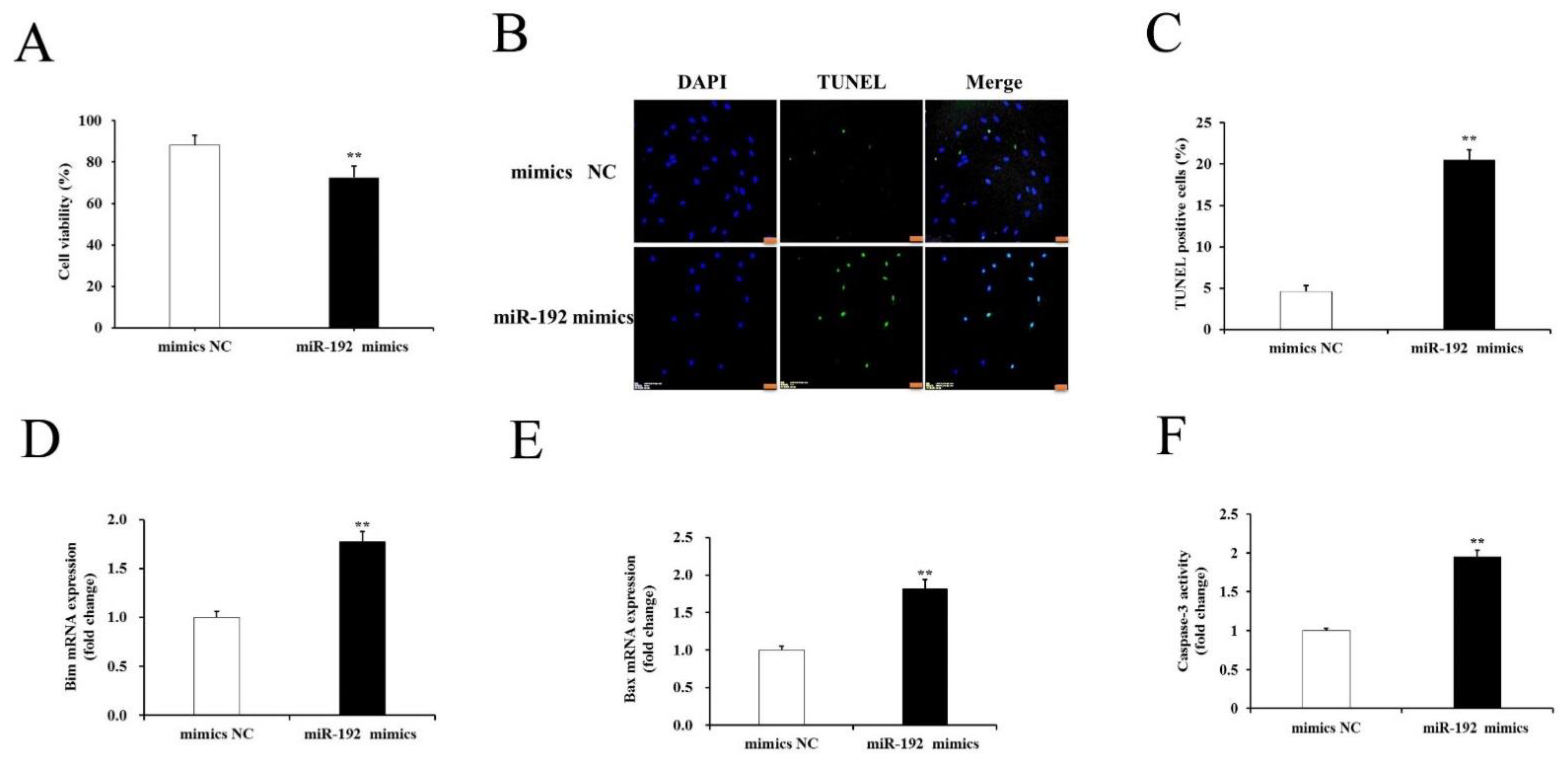
3.4. miR-192 Overexpression Promotes Porcine GCs Apoptosis
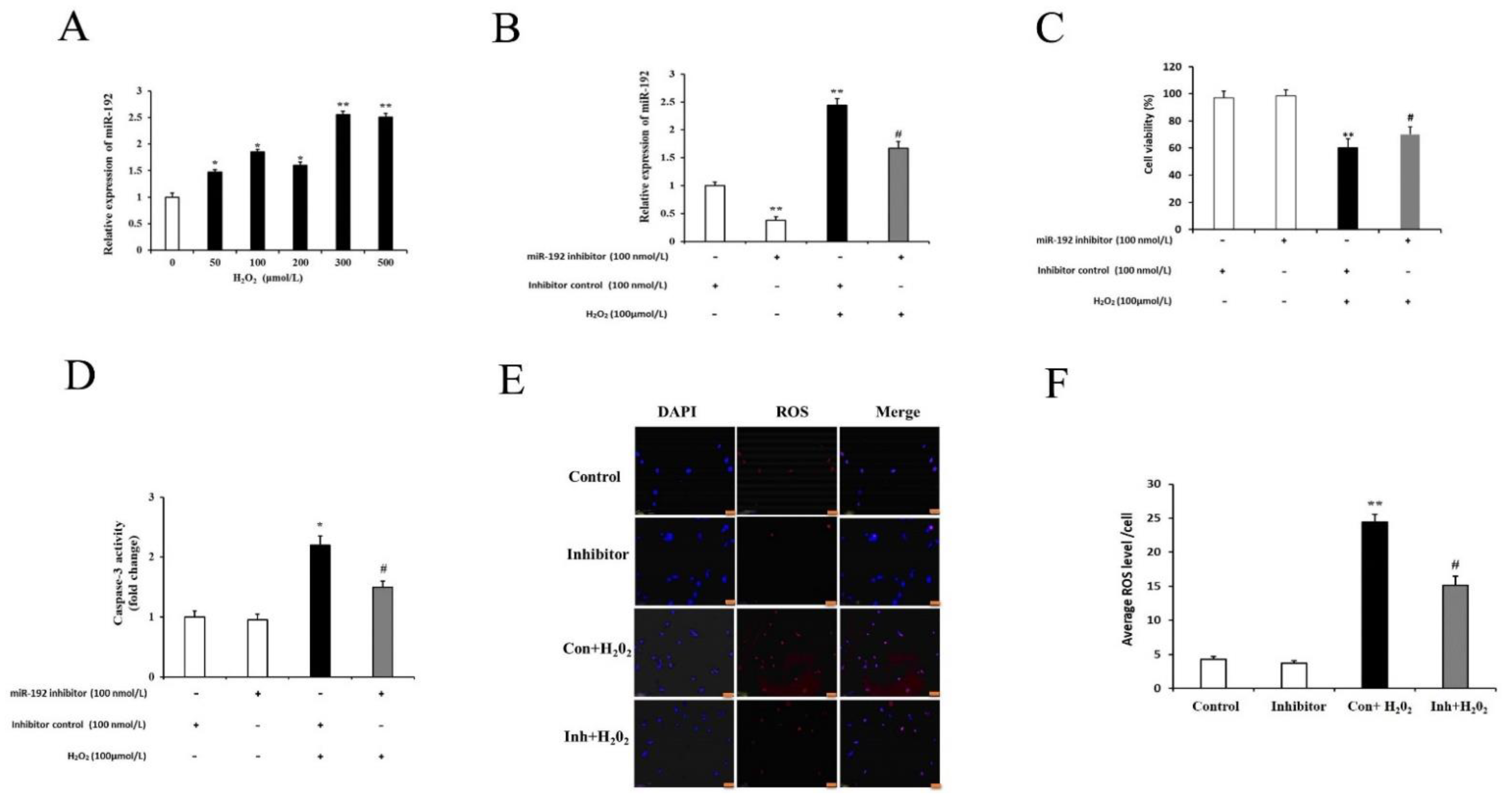
3.5. miR-192 Knockdown Counteracts H2O2-Induced PGC Injury
3.6. Acvr2a Is a miR-192 Target
3.7. miR 192a Knockdown Inhibits PGC Oxidative Damage through the Upregulation of Acvr2a and Suppression of Caspase-3 Activity
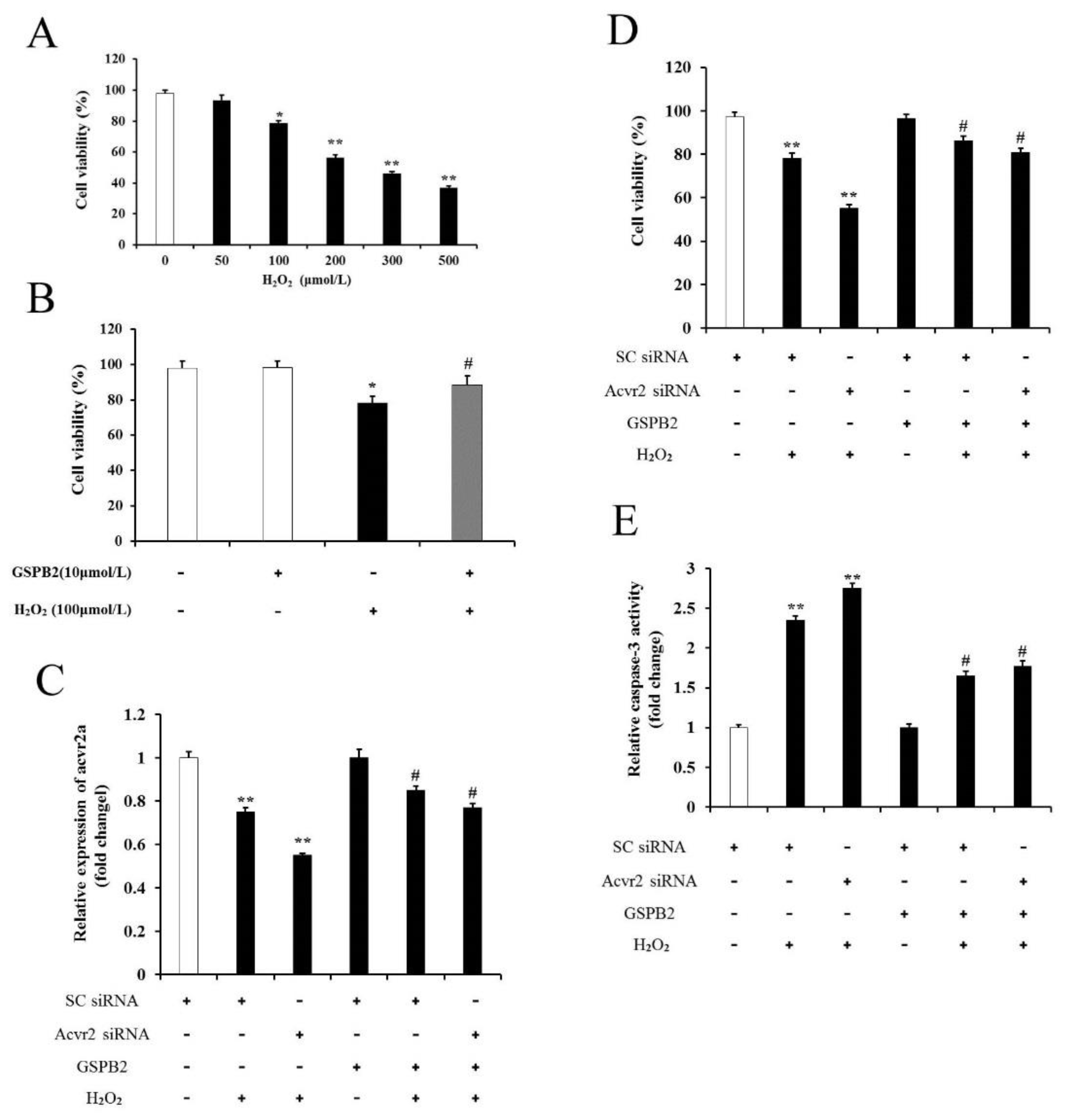
3.8. Acvr2a Is Required for GSPB2-Mediated PGC Protection against Oxidative Damage
3.9. GSPB2 Protects PGCs from H2O2-Induced Oxidative Damage through the Downregulation of miR-192
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.-Q.; Gao, B.-W.; Guo, H.-X.; Ren, Q.-L.; Wang, X.-W.; Chen, J.-F.; Wang, J.; Zhang, Z.-J.; Ma, Q.; Xing, B.-S. miR-181a promotes porcine granulosa cell apoptosis by targeting TGFBR1 via the activin signaling pathway. Mol. Cell. Endocrinol. 2019, 499, 110603. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Cheung, H.H.; Chan, C.L.-K.; Chan, W.Y. The Role of microRNAs in Ovarian Granulosa Cells in Health and Disease. Front. Endocrinol. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saller, S.; Kunz, L.; Berg, D.; Berg, U.; Lara, H.; Urra, J.; Hecht, S.; Pavlik, R.; Thaler, C.; Mayerhofer, A. Dopamine in human follicular fluid is associated with cellular uptake and metabolism-dependent generation of reactive oxygen species in granulosa cells: Implications for physiology and pathology. Hum. Reprod. 2014, 29, 555–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuseppina, B.; Bussolati, S.; Sujen Eleonora, S.; Francesca, G. Reactive oxygen species and anti-oxidant defences in swine follicular fluids. Reprod. Fertil. Dev. 2008, 20, 269. [Google Scholar]
- Zhang, J.-Q.; Gao, B.-W.; Wang, J.; Ren, Q.-L.; Chen, J.-F.; Ma, Q.; Zhang, Z.-J.; Xing, B.-S. Critical Role of FoxO1 in Granulosa Cell Apoptosis Caused by Oxidative Stress and Protective Effects of Grape Seed Procyanidin B2. Oxidative Med. Cell. Longev. 2016, 2016, 6147345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhang, Q.; Hu, Y.; Xu, L.; Jiang, Y.; Zhang, C.; Ding, L.; Jiang, R.; Sun, J.; Sun, H.; et al. miR-181a increases FoxO1 acetylation and promotes granulosa cell apoptosis via SIRT1 downregulation. Cell Death Dis. 2017, 8, e3088. [Google Scholar] [CrossRef]
- Luderer, U. Ovarian Toxicity from Reactive Oxygen Species. Vitam. Horm. 2014, 94, 99–127. [Google Scholar] [CrossRef] [Green Version]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of Reactive Oxygen Species and Antioxidants in Ovarian Toxicity1. Biol. Reprod. 2012, 86, 27. [Google Scholar] [CrossRef]
- Jia-Qing, Z.; Ming, S.; Cheng-Cheng, Z.; Feng-Xiang, Y.; Ze-Qun, L.; Nazim, A.; Shao-Chen, S.; Kui, L.; Hong-Lin, L. 3-nitropropionic acid induces ovarian oxidative stress and impairs follicle in mouse. PLoS ONE 2014, 9, e86589. [Google Scholar]
- Ming, S.; Fei, L.; Jiaqing, Z.; Yiting, T.; Wei-Kang, C.; Honglin, L. Involvement of the up-regulated foxo1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J. Biol. Chem. 2012, 287, 25727–25740. [Google Scholar]
- Roy, C.; Lavoie, M.; Richard, G.; Archambault, A.; Lapointe, J. Evidence that oxidative stress is higher in replacement gilts than in multiparous sows. J. Anim. Physiol. Anim. Nutr. 2016, 100, 911–919. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Hu, C.J.; Zhao, X.C.; Xiao, K.L.; Deng, M.; Zhang, L.; Qiu, X.G.; Deng, J.P.; Yin, Y.L.; Tan, C.Q. Dietary energy sources during late gestation and lactation of sows: Effects on performance, glucolipid metabolism, oxidative status of sows, and their offspring1. J. Anim. Sci. 2019, 97, 4608–4618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Y.; Liu, H.; Pan, Z. Micrornas in ovarian follicular atresia and granulosa cell apoptosis. Reprod. Biol. Endocrinol. 2019, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Donadeu, F.X.; Schauer, S.N.; Sontakke, S.D. Involvement of miRNAs in ovarian follicular and luteal development. J. Endocrinol. 2012, 215, 323–334. [Google Scholar] [CrossRef]
- Xu, L.; Sun, H.; Zhang, M.; Jiang, Y.; Zhang, C.; Zhou, J.; Ding, L.; Hu, Y.; Yan, G. MicroRNA-145 protects follicular granulosa cells against oxidative stress-induced apoptosis by targeting Krüppel-like factor 4. Mol. Cell. Endocrinol. 2017, 452, 138–147. [Google Scholar] [CrossRef]
- Cao, R.; Wu, W.; Zhou, X.; Liu, K.; Li, B.; Huang, X.; Zhang, Y.; Liu, H. Let-7g induces granulosa cell apoptosis by targeting MAP3K1 in the porcine ovary. Int. J. Biochem. Cell Biol. 2015, 68, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.M.; Chun, J.L.; Lee, J.H.; Kim, E.Y.; Park, K.-S.; Lee, J.-H.; Daigneault, B.W.; Smith, G.W.; Kim, K.J.; Chang, K.-T.; et al. Follistatin Rescues Blastocyst Development of Poor Quality Porcine Cumulus-Oocyte Complexes by Delaying Meiotic Resumption with Decreased cGMP. Reprod. Sci. 2018, 25, 759–772. [Google Scholar] [CrossRef]
- Wei, H.-K.; Zhou, Y.; Jiang, S.; Tao, Y.-X.; Sun, H.; Peng, J.; Jiang, S. Feeding a DHA-enriched diet increases skeletal muscle protein synthesis in growing pigs: Association with increased skeletal muscle insulin action and local mRNA expression of insulin-like growth factor 1. Br. J. Nutr. 2013, 110, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fuschi, P.; Carrara, M.; Voellenkle, C.; Garcia-Manteiga, J.M.; Righini, P.; Maimone, B.; Sangalli, E.; Villa, F.; Specchia, C.; Picozza, M.; et al. Central role of the p53 pathway in the noncoding-RNA response to oxidative stress. Aging 2017, 9, 2559–2586. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.P.; Li, J.-W.; Tse, A.C.-K.; Chan, T.-F.; Wu, R.S.-S. Hypoxia alters steroidogenesis in female marine medaka through miRNAs regulation. Aquat. Toxicol. 2016, 172, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Vaux, D.L. Glucocorticoids can induce BIM to trigger apoptosis in the absence of BAX and BAK1. Cell Death Dis. 2020, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, H.; Jiang, Y.; Ding, L.; Wu, S.; Fang, T.; Yan, G.; Hu, Y. MicroRNA-181a Suppresses Mouse Granulosa Cell Proliferation by Targeting Activin Receptor IIA. PLoS ONE 2013, 8, e59667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbe, A.; Ramé, C.; Mellouk, N.; Estienne, A.; Bongrani, A.; Brossaud, A.; Riva, A.; Guérif, F.; Froment, P.; Dupont, J. Effects of Grape Seed Extract and Proanthocyanidin B2 on In Vitro Proliferation, Viability, Steroidogenesis, Oxidative Stress, and Cell Signaling in Human Granulosa Cells. Int. J. Mol. Sci. 2019, 20, 4215. [Google Scholar] [CrossRef] [Green Version]
- Miyun, T.T.; Ulrike, L. Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology 2006, 147, 1224–1236. [Google Scholar]
- Zhang, J.-Q.; Wang, X.-W.; Chen, J.-F.; Ren, Q.-L.; Wang, J.; Gao, B.-W.; Shi, Z.-H.; Zhang, Z.-J.; Bai, X.-X.; Xing, B.-S. Grape Seed Procyanidin B2 Protects Porcine Ovarian Granulosa Cells against Oxidative Stress-Induced Apoptosis by Upregulating let-7a Expression. Oxidative Med. Cell. Longev. 2019, 2019, 1076512. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Wen, X.; Wang, L.; Gao, J.; Wang, Z.; Zhang, C.; Zhang, P.; Lu, C.; Duan, L.; Tian, Y. Identification of MicroRNAs Involved in Growth Arrest and Apoptosis in Hydrogen Peroxide-Treated Human Hepatocellular Carcinoma Cell Line HepG2. Oxidative Med. Cell. Longev. 2016, 2016, 7530853. [Google Scholar] [CrossRef]
- Fatemi, N.; Sanati, M.H.; Shamsara, M.; Moayer, F.; Zavarehei, M.J.; Pouya, A.; Sayyahpour, F.; Ayat, H.; Gourabi, H. TBHP-induced oxidative stress alters microRNAs expression in mouse testis. J. Assist. Reprod. Genet. 2014, 31, 1287–1293. [Google Scholar] [CrossRef] [Green Version]
- Sohel, M.H.; Akyuz, B.; Konca, Y.; Arslan, K.; Sariozkan, S.; Cinar, M.U. Oxidative stress modulates the expression of apoptosis-associated microRNAs in bovine granulosa cells in vitro. Cell Tissue Res. 2019, 376, 295–308. [Google Scholar] [CrossRef]
- Ebrahimi, S.O.; Reiisi, S.; Shareef, S. miRNAs, oxidative stress, and cancer: A comprehensive and updated review. J. Cell. Physiol. 2020, 235, 8812–8825. [Google Scholar] [CrossRef]
- Chunxue, Z.; Jingtao, S.; Shuangbo, K.; Mei, Z.; Qun, Z.; Jidong, Z.; Xin, Z.; Nannan, K.; Yue, J.; Lijun, D. Microrna-181a promotes follicular granulosa cell apoptosis via sphingosine-1-phosphate receptor 1 expression downregulation. Biol. Reprod. 2019, 5, 975–985. [Google Scholar]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Capogrossi, M.C. Mir-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via zeb1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romay, M.C.; Che, N.; Becker, S.N.; Pouldar, D.; Hagopian, R.; Xiao, X.; Lusis, A.J.; Berliner, J.A.; Civelek, M. Regulation of nf-κb signaling by oxidized glycerophospholipid and il-1β induced mirs-21-3p and -27a-5p in human aortic endothelial cells. J. Lipid Res. 2015, 56, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tian, Y.; Liang, D.; Fu, Q.; Jia, L.; Wu, D.; Zhu, X. The Effects of Inhibition of MicroRNA-375 in a Mouse Model of Doxorubicin-Induced Cardiac Toxicity. Med. Sci. Monit. 2020, 26, e920557-1–e920557-13. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xi, J.; Liu, S. MiR-139-3p induces cell apoptosis and inhibits metastasis of cervical cancer by targeting NOB1. Biomed. Pharmacother. 2016, 83, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Diez-Fraile, A.; Lammens, T.; Tilleman, K.; Witkowski, W.; Verhasselt, B.; Sutter, P.D.; Benoit, Y.; Espeel, M.; D’Herde, K. Age-associated differential microrna levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum. Fertil. 2014, 17, 90–98. [Google Scholar] [CrossRef]
- Wei, L.; Geng, L.; Yong, C. Mir-19b alleviates mpp+-induced neuronal cytotoxicity via targeting the hapln4/mapk pathway in sh-sy5y cells. RSC Adv. 2018, 8, 10706–10714. [Google Scholar]
- Shen, M.; Liu, Z.; Li, B.; Teng, Y.; Zhang, J.; Tang, Y.; Sun, S.-C.; Liu, H. Involvement of FoxO1 in the effects of follicle-stimulating hormone on inhibition of apoptosis in mouse granulosa cells. Cell Death Dis. 2014, 5, e1475. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Li, L.; Liu, H.; Sun, S.-C. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy 2017, 13, 1364–1385. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Lin, Y.; Zou, X.; Li, Z.; Liao, Y.; Zhang, H. Endothelial cell injury and dysfunction induced by silver nanoparticles through oxidative stress via ikk/nf-κb pathways. Biomaterials 2014, 35, 6657–6666. [Google Scholar] [CrossRef]
- Liu, S.; Shen, M.; Li, C.; Wei, Y.; Meng, X.; Li, R.; Cao, Y.; Wu, W.; Liu, H. PKCδ contributes to oxidative stress-induced apoptosis in porcine ovarian granulosa cells via activating JNK. Theriogenology 2019, 131, 89–95. [Google Scholar] [CrossRef]
- Wang, X.-L.; Wu, Y.; Tan, L.-B.; Tian, Z.; Liu, J.-H.; Zhu, D.-S.; Zeng, S.-M. Follicle-stimulating Hormone Regulates Pro-apoptotic Protein Bcl-2-interacting Mediator of Cell Death-Extra Long (BimEL)-induced Porcine Granulosa Cell Apoptosis. J. Biol. Chem. 2012, 287, 10166–10177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Lin, L.; Haq, I.U.; Zeng, S.M. Inhibition of nf-κb promotes autophagy via jnk signaling pathway in porcine granulosa cells. Biochem. Biophys. Res. Commun. 2016, 473, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shen, M.; Jiang, Y.; Sun, S.-C.; Liu, H. Melatonin reduces oxidative damage in mouse granulosa cells via restraining JNK-dependent autophagy. Reproduction 2018, 155, 307–319. [Google Scholar] [CrossRef]
- He, T.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Oxidative stress impairs tgf-beta responsiveness by coordinate down-regulation of type ii tgf-beta receptor and smad3 in primary human skin fibroblasts. J. Investig. Dermatol. 2009, 129, S41. [Google Scholar]
- Lane, K.L.; Talati, M.; Austin, E.; Hemnes, A.R.; Johnson, J.A.; Fessel, J.P.; Blackwell, T.; Mernaugh, R.L.; Robinson, L.; Fike, C.; et al. Oxidative Injury is a Common Consequence of BMPR2 Mutations. Pulm. Circ. 2011, 1, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Kairisalo, M.; Bonomo, A.; Hyrskyluoto, A.; Mudò, G.; Belluardo, N.; Korhonen, L.; Lindholm, D. Resveratrol reduces oxidative stress and cell death and increases mitochondrial antioxidants and XIAP in PC6.3-cells. Neurosci. Lett. 2011, 488, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Mobley, J.A.; Brueggemeier, R.W. Estrogen receptor-mediated regulation of oxidative stress and DNA damage in breast cancer. Carcinogenesis 2004, 25, 3–9. [Google Scholar] [CrossRef]
- Ebert, M.S.; Sharp, P.A. Roles for MicroRNAs in Conferring Robustness to Biological Processes. Cell 2012, 149, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Geula, H.; Hermona, S. Cholinesterase-targeting micrornas identified in silico affect specific biological processes. Front. Mol. Neurosci. 2011, 4, 28. [Google Scholar]
- Feng, S.; Cong, S.; Zhang, X.; Bao, X.; Wang, W.; Li, H.; Wang, Z.; Wang, G.; Xu, J.; Du, B.; et al. MicroRNA-192 targeting retinoblastoma 1 inhibits cell proliferation and induces cell apoptosis in lung cancer cells. Nucleic Acids Res. 2011, 39, 6669–6678. [Google Scholar] [CrossRef] [PubMed]
- Schang, G.; Ongaro, L.; Schultz, H.; Wang, Y.; Zhou, X.; Brûlé, E.; Boehm, U.; Lee, S.-J.; Bernard, D.J. Murine FSH Production Depends on the Activin Type II Receptors ACVR2A and ACVR2B. Endocrinology 2020, 161, bqaa056. [Google Scholar] [CrossRef] [PubMed]
- Woo, I.; Christenson, L.K.; Gunewardena, S.; Ingles, S.A.; Thomas, S.; Ahmady, A.; Chung, K.; Bendikson, K.; Paulson, R.; McGinnis, L.K. Micro-RNAs involved in cellular proliferation have altered expression profiles in granulosa of young women with diminished ovarian reserve. J. Assist. Reprod. Genet. 2018, 35, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, L.; Zhou, X.; Cui, Y.; Boehm, U.; Bernard, D.J. Gonadotrope-specific deletion of the BMP type 2 receptor does not affect reproductive physiology in mice. Biol. Reprod. 2019, 102, 639–646. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Kumar, T.R.; Bradley, A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature 1995, 374, 356–360. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Ren, Q.; Chen, J.; Lv, L.; Wang, J.; Shen, M.; Xing, B.; Wang, X. Downregulation of miR-192 Alleviates Oxidative Stress-Induced Porcine Granulosa Cell Injury by Directly Targeting Acvr2a. Cells 2022, 11, 2362. https://doi.org/10.3390/cells11152362
Zhang J, Ren Q, Chen J, Lv L, Wang J, Shen M, Xing B, Wang X. Downregulation of miR-192 Alleviates Oxidative Stress-Induced Porcine Granulosa Cell Injury by Directly Targeting Acvr2a. Cells. 2022; 11(15):2362. https://doi.org/10.3390/cells11152362
Chicago/Turabian StyleZhang, Jiaqing, Qiaoling Ren, Junfeng Chen, Lingyan Lv, Jing Wang, Ming Shen, Baosong Xing, and Xianwei Wang. 2022. "Downregulation of miR-192 Alleviates Oxidative Stress-Induced Porcine Granulosa Cell Injury by Directly Targeting Acvr2a" Cells 11, no. 15: 2362. https://doi.org/10.3390/cells11152362






