A Wholistic View of How Bumetanide Attenuates Autism Spectrum Disorders
Abstract
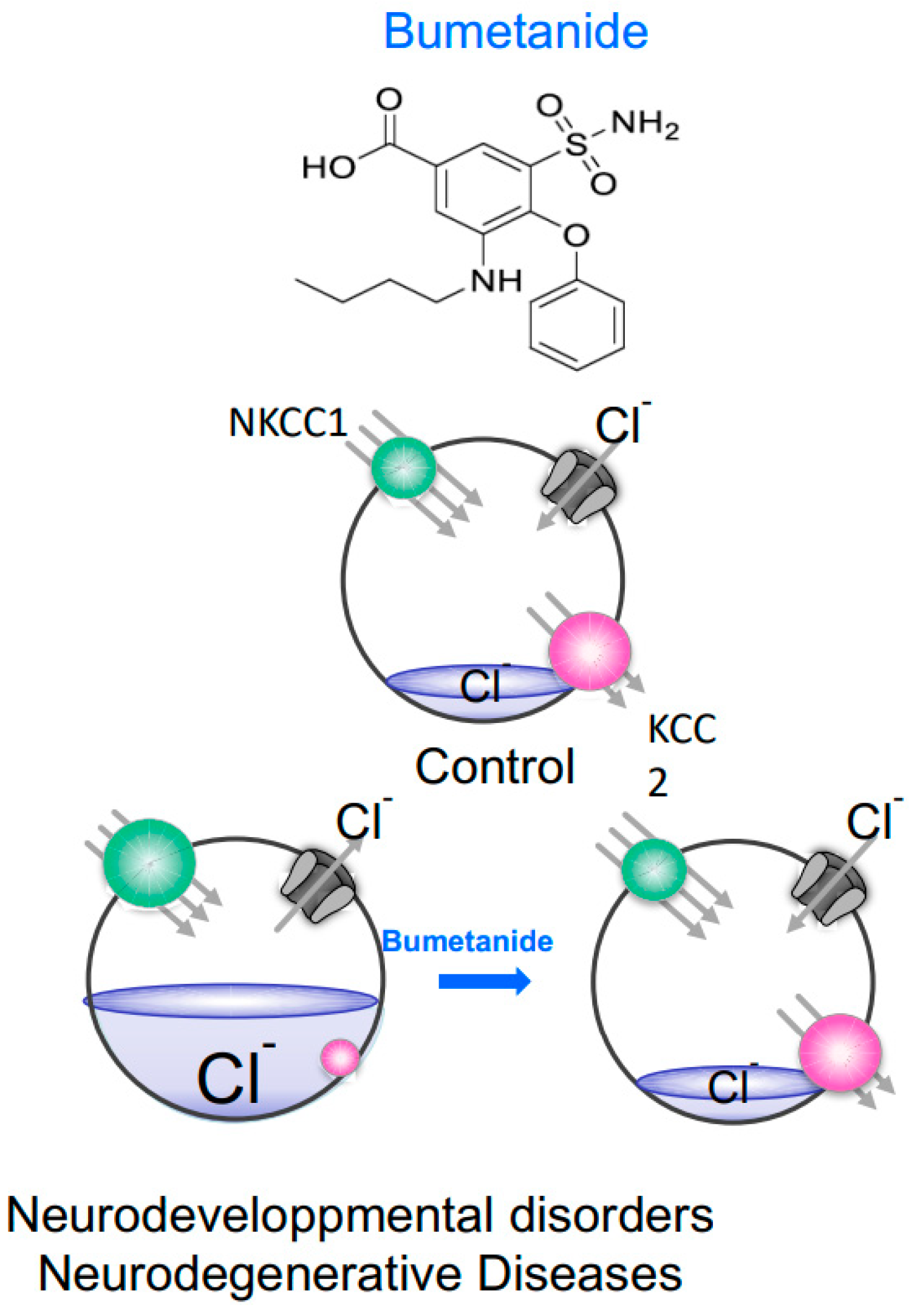
:1. Introduction
2. The NKCC1/KCC2 Activity Ratio and GABAergic Inhibition
3. The NKCC1/KCC2 Activity Ratio Is Perturbed in Brain Disorders
4. Brain Accessibility of Bumetanide
5. Peripheral Actions
5.1. Detrimental Effects on Inner Ear
5.2. CSF Production
5.3. Vasculature
5.4. Immune System
5.5. Enteric System
6. Conclusions—Brain and Peripheral Organs: A Continuum
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingram, T.T. Today’s drugs. Frusemide. Br. Med. J. 1964, 2, 1640–1641. [Google Scholar]
- Asbury, M.J.; Gatenby, P.B.; O’Sullivan, S.; Bourke, E. Bumetanide: Potent new “loop” diuretic. Br. Med. J. 1972, 1, 211–213. [Google Scholar] [CrossRef]
- Gamba, G.; Miyanoshita, A.; Lombardi, M.; Lytton, J.; Lee, W.-S.; Hediger, M.; Hebert, S.C. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J. Biol. Chem. 1994, 269, 17713–17722. [Google Scholar] [CrossRef]
- Oh, S.W.; Han, S.Y. Loop Diuretics in Clinical Practice. Electrolyte Blood Press. 2015, 13, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Walker, P.C.; Berry, N.S.; Edwards, D.J. Protein binding characteristics of bumetanide. Dev. Pharmacol. Ther. 1989, 12, 13–18. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, M.G. Pharmacokinetics and pharmacodynamics of intravenous bumetanide in mutant Nagase analbuminemic rats: Importance of globulin binding for the pharmacodynamic effects. Biopharm. Drug Dispos. 2001, 22, 147–156. [Google Scholar] [CrossRef]
- Delpire, E.; Lu, J.; England, R.; Dull, C.; Thorne, T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat. Genet. 1999, 22, 192–195. [Google Scholar] [CrossRef]
- Flagella, M.; Clarke, L.L.; Miller, M.L.; Erway, L.C.; Giannella, R.A.; Andringa, A.; Gawenis, L.R.; Kramer, J.; Duffy, J.J.; Doetschman, T.; et al. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J. Biol. Chem. 1999, 274, 26946–26955. [Google Scholar] [CrossRef] [Green Version]
- Grubb, B.R.; Pace, A.J.; Lee, E.; Koller, B.H.; Boucher, R.C. Alterations in airway ion transport in NKCC1-deficient mice. Am. J. Physiol. Cell Physiol. 2001, 281, C615–C623. [Google Scholar] [CrossRef]
- McDaniel, N.; Pace, A.J.; Spiegel, S.; Engelhardt, R.; Koller, B.H.; Seidler, U.; Lytle, C. Role of Na-K-2Cl cotransporter-1 in gastric secretion of nonacidic fluid and pepsinogen. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G550–G560. [Google Scholar] [CrossRef]
- Grubb, B.R.; Lee, E.; Pace, A.J.; Koller, B.H.; Boucher, R.C. Intestinal ion transport in NKCC1-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G707–G718. [Google Scholar] [CrossRef]
- Kaplan, M.R.; Plotkin, M.D.; Brown, D.; Hebert, S.C.; Delpire, E. Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal IMCD, the glomerular and extraglomerular mesangium and the glomerular afferent arteriole. J. Clin. Investig. 1996, 98, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Ecelbarger, C.A.; Terris, J.; Hoyer, J.R.; Nielsen, S.; Wade, J.B.; Knepper, M.A. Localization and regulation of the rat renal Na+-K+-2Cl− cotransporter, BSC-1. Am. J. Physiol. Ren. Physiol. 1996, 271, F619–F628. [Google Scholar] [CrossRef]
- Evans, R.L.; Park, K.; Turner, R.J.; Watson, G.E.; Nguyen, H.-V.; Dennett, M.R.; Hand, A.R.; Flagella, M.; Shull, G.E.; Melvin, J.E. Severe impairment of salivation in Na+/K+/2Cl− cotransporter (NKCC1)-deficient mice. J. Biol. Chem. 2000, 275, 26720–26726. [Google Scholar] [CrossRef]
- Kidokoro, M.; Nakamoto, T.; Mukaibo, T.; Kondo, Y.; Munemasa, T.; Imamura, A.; Masaki, C.; Hosokawa, R. Na+-K+-2Cl− cotransporter-mediated fluid secretion increases under hypotonic osmolarity in the mouse submandibular salivary gland. Am. J. Physiol. Ren. Physiol. 2014, 306, F1155–F1160. [Google Scholar] [CrossRef]
- Gosmanov, A.R.; Lindinger, M.I.; Thomason, D.B. Riding the tides: K+ concentration and volume regulation by muscle Na+-K+-2Cl− cotransport activity. News Physiol. Sci. 2003, 18, 196–200. [Google Scholar] [CrossRef]
- Garg, P.; Martin, C.F.; Elms, S.C.; Gordon, F.J.; Wall, S.M.; Garland, C.J.; Sutliff, R.L.; O’Neill, W.C. Effect of the Na-K-2Cl cotransporter NKCC1 on systemic blood pressure and smooth muscle tone. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2100–H2105. [Google Scholar] [CrossRef]
- Bulley, S.; Jaggar, J.H. Cl− channels in smooth muscle cells. Pflügers Arch. Eur. J. Physiol. 2014, 466, 5861–5872. [Google Scholar] [CrossRef] [Green Version]
- Tóth, K.; Lénárt, N.; Berki, P.; Fekete, R.; Szabadits, E.; Pósfai, B.; Cserép, C.; Alatshan, A.; Benkő, S.; Kiss, D.; et al. The NKCC1 ion transporter modulates microglial phenotype and inflammatory response to brain injury in a cell-autonomous manner. PLoS Biol. 2022, 20, e3001526. [Google Scholar] [CrossRef]
- Hung, C.M.; Peng, C.K.; Wu, C.P.; Huang, K.L. Bumetanide attenuates acute lung injury by suppressing macrophage activation. Biochem. Pharmacol. 2018, 156, 60–67. [Google Scholar] [CrossRef]
- Perry, J.S.A.; Morioka, S.; Medina, C.B.; Barron, B.; Raymond, M.H.; Lucas, C.D.; Onengut-Gumuscu, S.; Delpire, E.; Ravichandran, K.S. Interpreting an apoptotic corpse as anti-inflammatory involves a chloride sensing pathway. Nat. Cell Biol. 2019, 21, 1532–1543. [Google Scholar] [CrossRef]
- Pace, A.J.; Lee, E.; Athirakul, K.; Coffman, T.M.; O’Brien, D.A.; Koller, B.H. Failure of spermatogenesis in mouse lines deficient in the Na+-K+-2Cl− cotransporter. J. Clin. Investig. 2000, 105, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ari, Y.; Gaiarsa, J.L.; Tyzio, R.; Khazipov, R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 2007, 87, 1215–1284. [Google Scholar] [CrossRef]
- Sung, K.-W.; Kirby, M.; McDonald, M.P.; Lovinger, D.M.; Delpire, E. Abnormal GABAA-receptor mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J. Neurosci. 2000, 20, 7531–7538. [Google Scholar] [CrossRef] [Green Version]
- Howard, H.C.; Mount, D.B.; Rochefort, D.; Byun, N.; Dupré, N.; Lu, J.; Fan, X.; Song, L.; Rivière, J.-B.; Prévost, C.; et al. Mutations in the K-Cl cotransporter KCC3 cause a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat. Genet. 2002, 32, 384–392. [Google Scholar] [CrossRef]
- Byun, N.; Delpire, E. Axonal and periaxonal swelling precede peripheral neurodegeneration in KCC3 knockout mice. Neurobiol. Dis. 2007, 28, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Mount, D.B.; Mercado, A.; Song, L.; Xu, J.; George, J.A.L.; Delpire, E.; Gamba, G. Cloning and Characterization of KCC3 and KCC4, new members of the cation-chloride cotransporter gene family. J. Biol. Chem. 1999, 274, 16355–16362. [Google Scholar] [CrossRef] [Green Version]
- Chabrol, F.P.; Eglen, S.J.; Sernagor, E. GABAergic control of retinal ganglion cell dendritic development. Neuroscience 2012, 227, 30–43. [Google Scholar] [CrossRef] [Green Version]
- Rohrbough, J.; Spitzer, N.C. Regulation of intracellulat Cl− levels by Na+-dependent Cl− cotransport distinguishes depolarizing from hyperpolarizing GABAA receptor-mediated responses in spinal neurons. J. Neurosci. 1996, 16, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ari, Y.; Cherubini, E.; Corradetti, R.; Gaiarsa, J.L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 1989, 416, 303–325. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Excitatory actions of gaba during development: The nature of the nurture. Nat. Rev. Neurosci. 2002, 3, 728–739. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Khazipov, R.; Leinekugel, X.; Caillard, O.; Gaiarsa, J.L. GABAA, NMDA and AMPA receptors: A developmentally regulated ‘ménage à trois’. Trends Neurosci. 1997, 20, 523–529. [Google Scholar] [CrossRef]
- Chen, J.; Kriegstein, A.R. A GABAergic projection from the zona incerta to cortex promotes cortical neuron development. Science 2015, 350, 554–558. [Google Scholar] [CrossRef] [Green Version]
- Clayton, G.H.; Owens, G.C.; Wolf, J.S.; Smith, R.L. Ontogeny of cation-Cl− cotransporter expression in rat neocortex. Brain Res. Dev. Brain Res. 1998, 109, 281–292. [Google Scholar] [CrossRef]
- Lu, J.; Karadsheh, M.; Delpire, E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J. Neurobiol. 1999, 39, 558–568. [Google Scholar] [CrossRef]
- Plotkin, M.D.; Snyder, E.Y.; Hebert, S.C.; Delpire, E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: A possible mechanism underlying GABA’s excitatory role in immature brain. J. Neurobiol. 1997, 33, 781–795. [Google Scholar] [CrossRef]
- Ben-Ari, Y. NKCC1 Chloride Importer Antagonists Attenuate Many Neurological and Psychiatric Disorders. Trends Neurosci. 2017, 40, 536–554. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Sontheimer, H. Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration. Am. J. Physiol. Cell Physiol. 2011, 301, C541–C549. [Google Scholar] [CrossRef] [Green Version]
- Savardi, A.; Borgogno, M.; De Vivo, M.; Cancedda, L. Pharmacological tools to target NKCC1 in brain disorders. Trends Pharmacol. Sci. 2021, 42, 1009–1034. [Google Scholar] [CrossRef]
- Hyde, T.M.; Lipska, B.K.; Ali, T.; Mathew, S.V.; Law, A.J.; Metitiri, O.E.; Straub, R.E.; Ye, T.; Colantuoni, C.; Herman, M.M.; et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J. Neurosci. 2011, 31, 11088–11095. [Google Scholar] [CrossRef] [Green Version]
- Taubes, A.; Nova, P.; Zalocusky, K.A.; Kosti, I.; Bicak, M.; Zilberter, M.Y.; Hao, Y.; Yoon, S.Y.; Oskotsky, T.; Pineda, S.; et al. Experimental and real-world evidence supporting the computational repurposing of bumetanide for APOE4-related Alzheimer’s disease. Nat. Aging 2021, 1, 932–947. [Google Scholar] [CrossRef]
- Kahle, K.T.; Delpire, E. Kinase-KCC2 coupling: Cl− rheostasis, disease susceptibility, therapeutic target. J. Neurophysiol. 2016, 115, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Kim, J.; Zhou, L.; Wengert, E.; Zhang, L.; Wu, Z.; Carromeu, C.; Muotri, A.R.; Marchetto, M.C.; Gage, F.H.; et al. KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, 751–756. [Google Scholar] [CrossRef] [Green Version]
- Pisella, L.I.; Gaiarsa, J.L.; Diabira, D.; Zhang, J.; Khalilov, I.; Duan, J.; Kahle, K.T.; Medina, I. Impaired regulation of KCC2 phosphorylation leads to neuronal network dysfunction and neurodevelopmental pathology. Sci. Signal. 2019, 12. [Google Scholar] [CrossRef]
- Tyzio, R.; Nardou, R.; Ferrari, D.C.; Tsintsadze, T.; Shahrokhi, A.; Eftekhari, S.; Khalilov, I.; Tsintsadze, V.; Brouchoud, C.; Chazal, G.; et al. Oxytocin-Mediated GABA Inhibition During Delivery Attenuates Autism Pathogenesis in Rodent Offspring. Science 2014, 343, 675–679. [Google Scholar] [CrossRef]
- Zimmerman, A.W.; Connors, S.L. Neuroscience. Could autism be treated prenatally? Science 2014, 343, 620–621. [Google Scholar] [CrossRef]
- Deidda, G.; Parrini, M.; Naskar, S.; Bozarth, I.F.; Contestabile, A.; Cancedda, L. Reversing excitatory GABAAR signaling restores synaptic plasticity and memory in a mouse model of Down syndrome. Nat. Med. 2015, 21, 318–326. [Google Scholar] [CrossRef]
- Lozovaya, N.; Nardou, R.; Tyzio, R.; Chiesa, M.; Pons-Bennaceur, A.; Eftekhari, S.; Bui, T.T.; Billon-Grand, M.; Rasero, J.; Bonifazi, P.; et al. Early alterations in a mouse model of Rett syndrome: The GABA developmental shift is abolished at birth. Sci. Rep. 2019, 9, 9276. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.; Dumon, C.; Guimond, D.; Tyzio, R.; Bonifazi, P.; Lozovaya, N.; Burnashev, N.; Ferrari, D.C.; Ben-Ari, Y. The GABA Developmental Shift Is Abolished by Maternal Immune Activation Already at Birth. Cereb. Cortex 2019, 29, 3982–3992. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef]
- Hu, D.; Yu, Z.L.; Zhang, Y.; Han, Y.; Zhang, W.; Lu, L.; Shi, J. Bumetanide treatment during early development rescues maternal separation-induced susceptibility to stress. Sci. Rep. 2017, 7, 11878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemonnier, E.; Degrez, C.; Phelep, M.; Tyzio, R.; Josse, F.; Grandgeorge, M.; Hadjikhani, N.; Ben-Ari, Y. A randomised controlled trial of bumetanide in the treatment of autism in children. Transl. Psychiatry 2012, 2, e202. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier, E.; Villeneuve, N.; Sonie, S.; Serret, S.; Rosier, A.; Roue, M.; Brosset, P.; Viellard, M.; Bernoux, D.; Rondeau, S.; et al. Effects of bumetanide on neurobehavioral function in children and adolescents with autism spectrum disorders. Transl. Psychiatry 2017, 7, e1056. [Google Scholar] [CrossRef]
- Wang, T.; Shan, L.; Miao, C.; Xu, Z.; Jia, F. Treatment Effect of Bumetanide in Children With Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 751575. [Google Scholar] [CrossRef]
- Hadjikhani, N.; Åsberg Johnels, J.; Zürcher, N.R.; Lassalle, A.; Guillon, Q.; Hippolyte, L.; Billstedt, E.; Ward, N.; Lemonnier, E.; Gillberg, C. Look me in the eyes: Constraining gaze in the eye-region provokes abnormally high subcortical activation in autism. Sci. Rep. 2017, 7, 3163. [Google Scholar] [CrossRef]
- Hadjikhani, N.; Åsberg Johnels, J.; Lassalle, A.; Zürcher, N.R.; Hippolyte, L.; Gillberg, C.; Lemonnier, E.; Ben-Ari, Y. Bumetanide for autism: More eye contact, less amygdala activation. Sci. Rep. 2018, 8, 3602. [Google Scholar] [CrossRef]
- Juarez-Martinez, E.L.; Sprengers, J.J.; Cristian, G.; Oranje, B.; van Andel, D.M.; Avramiea, A.E.; Simpraga, S.; Houtman, S.J.; Hardstone, R.; Gerver, C.; et al. Prediction of Behavioral Improvement Through Resting-State Electroencephalography and Clinical Severity in a Randomized Controlled Trial Testing Bumetanide in Autism Spectrum Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Shan, H.; Yu, J.; Dai, Y.; He, H.; Li, W.G.; Langley, C.; Sahakian, B.J.; Yao, Y.; et al. The immuno-behavioural covariation associated with the treatment response to bumetanide in young children with autism spectrum disorder. Transl. Psychiatry 2022, 12, 228. [Google Scholar] [CrossRef]
- Lemonnier, E.; Lazartigues, A.; Ben-Ari, Y. Treating Schizophrenia With the Diuretic Bumetanide: A Case Report. Clin. Neuropharmacol. 2016, 39, 115–117. [Google Scholar] [CrossRef] [Green Version]
- Lemonnier, E.; Robin, G.; Degrez, C.; Tyzio, R.; Grandgeorge, M.; Ben-Ari, Y. Treating Fragile X syndrome with the diuretic bumetanide: A case report. Acta Paediatr. 2013, 102, e288–e290. [Google Scholar] [CrossRef]
- van Andel, D.M.; Sprengers, J.J.; Oranje, B.; Scheepers, F.E.; Jansen, F.E.; Bruining, H. Effects of bumetanide on neurodevelopmental impairments in patients with tuberous sclerosis complex: An open-label pilot study. Mol. Autism 2020, 11, 30. [Google Scholar] [CrossRef]
- Bruining, H.; Passtoors, L.; Goriounova, N.; Jansen, F.; Hakvoort, B.; de Jonge, M.; Poil, S.S. Paradoxical Benzodiazepine Response: A Rationale for Bumetanide in Neurodevelopmental Disorders? Pediatrics 2015, 136, e539–e543. [Google Scholar] [CrossRef] [Green Version]
- Nardou, R.; Yamamoto, S.; Chazal, G.; Bhar, A.; Ferrand, N.; Dulac, O.; Ben-Ari, Y.; Khalilov, I. Neuronal chloride accumulation and excitatory GABA underlie aggravation of neonatal epileptiform activities by phenobarbital. Brain 2011, 134, 987–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozovaya, N.; Eftekhari, S.; Cloarec, R.; Gouty-Colomer, L.A.; Dufour, A.; Riffault, B.; Billon-Grand, M.; Pons-Bennaceur, A.; Oumar, N.; Burnashev, N.; et al. GABAergic inhibition in dual-transmission cholinergic and GABAergic striatal interneurons is abolished in Parkinson disease. Nat. Commun. 2018, 9, 1422. [Google Scholar] [CrossRef] [PubMed]
- Damier, P.; Hammond, C.; Ben-Ari, Y. Bumetanide to Treat Parkinson Disease: A Report of 4 Cases. Clin. Neuropharmacol. 2016, 39, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Cleary, R.T.; Sun, H.; Huynh, T.; Manning, S.M.; Li, Y.; Rotenberg, A.; Talos, D.M.; Kahle, K.T.; Jackson, M.; Rakhade, S.N.; et al. Bumetanide enhances phenobarbital efficacy in a rat model of hypoxic neonatal seizures. PLoS ONE 2013, 8, e57148. [Google Scholar] [CrossRef]
- Römermann, K.; Fedrowitz, M.; Hampel, P.; Kaczmarek, E.; Töllner, K.; Erker, T.; Sweet, D.H.; Löscher, W. Multiple blood-brain barrier transport mechanisms limit bumetanide accumulation, and therapeutic potential, in the mammalian brain. Neuropharmacology 2017, 117, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Löscher, W.; Kaila, K. Reply to the commentary by Ben-Ari and Delpire: Bumetanide and neonatal seizures: Fiction versus reality. Epilepsia 2021, 62, 941–946. [Google Scholar] [CrossRef]
- Kaila, K.; Löscher, W. Bumetanide for neonatal seizures: No light in the pharmacokinetic/dynamic tunnel. Epilepsia 2022, 63, 1868–1873. [Google Scholar] [CrossRef]
- Saunders, N.R.; Liddelow, S.A.; Dziegielewska, K.M. Barrier mechanisms in the developing brain. Front. Pharmacol. 2012, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Ek, C.J.; Habgood, M.D.; Dziegielewska, K.M.; Saunders, N.R. Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica). J. Comp. Neurol. 2006, 496, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Caley, D.W.; Maxwell, D.S. Development of the blood vessels and extracellular spaces during postnatal maturation of rat cerebral cortex. J. Comp. Neurol. 1970, 138, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Horani, M.H.; Mooradian, A.D. Effect of diabetes on the blood brain barrier. Curr. Pharm. Des. 2003, 9, 833–840. [Google Scholar] [CrossRef]
- Prasad, S.; Sajja, R.K.; Naik, P.; Cucullo, L. Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J. Pharmacovigil. 2014, 2, 125. [Google Scholar] [CrossRef]
- Süß, P.; Rothe, T.; Hoffmann, A.; Schlachetzki, J.C.M.; Winkler, J. The Joint-Brain Axis: Insights From Rheumatoid Arthritis on the Crosstalk Between Chronic Peripheral Inflammation and the Brain. Front. Immunol. 2020, 11, 612104. [Google Scholar] [CrossRef]
- Lai, P.H.; Wang, T.H.; Zhang, N.Y.; Wu, K.C.; Yao, C.J.; Lin, C.J. Changes of blood-brain-barrier function and transfer of amyloid beta in rats with collagen-induced arthritis. J. Neuroinflamm. 2021, 18, 35. [Google Scholar] [CrossRef]
- Kim, J.; Chuang, H.C.; Wolf, N.K.; Nicolai, C.J.; Raulet, D.H.; Saijo, K.; Bilder, D. Tumor-induced disruption of the blood-brain barrier promotes host death. Dev. Cell 2021, 56, 2712–2721. [Google Scholar] [CrossRef]
- de Senna, P.N.; Xavier, L.L.; Bagatini, P.B.; Saur, L.; Galland, F.; Zanotto, C.; Bernardi, C.; Nardin, P.; Gonçalves, C.A.; Achaval, M. Physical training improves non-spatial memory, locomotor skills and the blood brain barrier in diabetic rats. Brain Res. 2015, 1618, 75–82. [Google Scholar] [CrossRef]
- Chupel, M.U.; Minuzzi, L.G.; Furtado, G.E.; Santos, M.L.; Hogervorst, E.; Filaire, E.; Teixeira, A.M. Exercise and taurine in inflammation, cognition, and peripheral markers of blood-brain barrier integrity in older women. Appl. Physiol. Nutr. Metab. 2018, 43, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Li, X.; Chauhan, A.; Sheikh, A.M.; Patil, S.; Chauhan, V.; Li, X.M.; Ji, L.; Brown, T.; Malik, M. Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Alberts, I.; Li, X. Brain IL-6 and autism. Neuroscience 2013, 252, 320–325. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Patel, A.B.; Doyle, R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry 2016, 6, e844. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, H.V. Theoretical aspects of autism: Causes—A review. J. Immunotoxicol. 2011, 8, 68–79. [Google Scholar] [CrossRef]
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism 2016, 7, 49. [Google Scholar] [CrossRef] [Green Version]
- Pressler, R.M.; Boylan, G.B.; Marlow, N.; Blennow, M.; Chiron, C.; Cross, J.H.; de Vries, L.S.; Hallberg, B.; Hellström-Westas, L.; Jullien, V.; et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): An open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol. 2015, 14, 469–477. [Google Scholar] [CrossRef]
- Smit, E.; Liu, X.; Gill, H.; Sabir, H.; Jary, S.; Thoresen, M. Factors associated with permanent hearing impairment in infants treated with therapeutic hypothermia. J. Pediatr. 2013, 163, 995–1000. [Google Scholar] [CrossRef]
- Schneider, W.J.; Becher, E.C. Acute transient hearing loss after ethacrynic acid therapy. Arch. Int. Med. 1966, 117, 715–717. [Google Scholar] [CrossRef]
- Morrison, J.C.; Fort, A.T.; Fish, S.A. Case report. Diuretic induced ototoxicity in preeclampsia. J. Tenn. Med. Assoc. 1971, 64, 36–37. [Google Scholar]
- Stone, W.J.; Bennett, W.M.; Cutler, R.E. Long-term bumetanide treatment of patients with edema due to renal disease. Cooperative studies. J. Clin. Pharmacol. 1981, 21, 587–980. [Google Scholar] [CrossRef] [PubMed]
- Wangemann, P.; Liu, J.; Marcus, D.C. Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vascularis in vitro. Hear. Res. 1995, 84, 19–29. [Google Scholar] [CrossRef]
- Macnamara, E.F.; Koehler, A.E.; D’Souza, P.; Estwick, T.; Lee, P.; Vezina, G.; Members of the Undiagnosed Diseases Network; Fauni, H.; Braddock, S.R.; Torti, E.; et al. Kilquist Syndrome: A Novel Syndromic Hearing Loss Disorder Caused by Homozygous Deletion of SLC12A2. Hum. Mut. 2019, 40, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Stodberg, T.; Magnusson, M.; Lesko, N.; Wredenberg, A.; Martin Munoz, D.; Stranneheim, H.; Wedell, A. SLC12A2 mutations cause NKCC1 deficiency with encephalopathy and impaired secretory epithelia. Neurol. Genet. 2020, 6, e478. [Google Scholar] [CrossRef]
- McNeill, A.; Lovino, E.; Bedoukian, E.; Cox, H.; Dean, J.; Goudie, D.; Kumar, A.; Newbury-Ecob, R.; Fallerini, C.; Lopergolo, D.; et al. SLC12A2 variants cause a neurodevelopmental disorder or cochleovestibular defect. Brain 2020, 143, 2380–2387. [Google Scholar] [CrossRef]
- Mutai, H.; Wasano, K.; Momozawa, Y.; Kamatani, Y.; Miya, F.; Masuda, S.; Morimoto, N.; Nara, K.; Takahashi, S.; Tsunoda, T.; et al. Variants encoding a restricted carboxy-terminal domain of SLC12A2 cause hereditary hearing loss in humans. PLoS Genet. 2020, 16, e1008643. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chu, H.; Chen, J.; Zhou, L.; Chen, Q.; Yu, Y.; Wu, Z.; Wang, S.; Lai, Y.; Pan, C.; et al. Age-related change in the expression of NKCC1 in the cochlear lateral wall of C57BL/6J mice. Acta Otolaryngol. 2014, 134, 1047–1051. [Google Scholar] [CrossRef]
- Brown, R.D. Cochlear N1 depression produced by the new “loop” diuretic, bumetanide, in cats. Neuropharmacology 1975, 14, 547–553. [Google Scholar] [CrossRef]
- Brown, R.D.; Manno, J.E.; Daigneault, E.A.; Manno, B.R. Comparative acute ototoxicity of intravenous bumetanide and furosemide in the pure-bred beagle. Toxicol. Appl. Pharmacol. 1979, 48, 157–169. [Google Scholar] [CrossRef]
- Bourke, E. Letter: Frusemide, bumetanide, and ototoxicity. Lancet 1976, 1, 917–918. [Google Scholar] [CrossRef]
- Santos, M.; Marques, C.; Nóbrega Pinto, A.; Fernandes, R.; Coutinho, M.B.; Almeida, E.S.C. Autism spectrum disorders and the amplitude of auditory brainstem response wave I. Autism Res. 2017, 10, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Rodier, P.M.; Ingram, J.L.; Tisdale, B.; Nelson, S.; Romano, J. Embryological origin for autism: Developmental anomalies of the cranial nerve motor nuclei. J. Comp. Neurol. 1996, 370, 247–261. [Google Scholar] [CrossRef]
- Mansour, Y.; Kulesza, R. Three dimensional reconstructions of the superior olivary complex from children with autism spectrum disorder. Hear. Res. 2020, 393, 107974. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.; Reinert, T.; Dietz, B.; Nerlich, J.; Rübsamen, R.; Milenkovic, I. Depolarizing chloride gradient in developing cochlear nucleus neurons: Underlying mechanism and implication for calcium signaling. Neuroscience 2014, 261, 207–222. [Google Scholar] [CrossRef]
- Kotak, V.C.; Korada, S.; Schwartz, I.R.; Sanes, D.H. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J. Neurosci. 1998, 18, 4646–4655. [Google Scholar] [CrossRef] [Green Version]
- Rotschafer, S.E. Auditory Discrimination in Autism Spectrum Disorder. Front. Neurosci. 2021, 15, 651209. [Google Scholar] [CrossRef]
- Wright, E.M. Transport processes in the formation of the cerebrospinal fluid. Rev. Physiol. Biochem. Pharmacol. 1978, 83, 3–34. [Google Scholar]
- Johanson, C.E. Ventricles and Cerebrospinal Fluid. In Neuroscience in Medicine; J. B. Lippincott & Co.: Philadelphia, PA, USA, 1995; pp. 171–196. [Google Scholar]
- Reed, D.J. The effect of furosemide on cerebrospinal fluid flow in rabbits. Arch. Int. Pharmacodyn. Ther. 1969, 178, 324–330. [Google Scholar]
- Domer, F.R. Effects of diuretics on cerebrospinal fluid formation and potassium movement. Exp. Neurol. 1969, 24, 54–64. [Google Scholar] [CrossRef]
- Buhrley, L.E.; Reed, D.J. The effect of furosemide on sodium-22 uptake into cerebrospinal fluid and brain. Exp. Brain Res. 1972, 14, 503–510. [Google Scholar] [CrossRef]
- Keep, R.F.; Xiang, J.; Betz, A.L. Potassium cotransport at the rat choroid plexus. Am. J. Physiol. Cell Physiol. 1994, 267, C1616–C1622. [Google Scholar] [CrossRef]
- Plotkin, M.D.; Kaplan, M.R.; Peterson, L.N.; Gullans, S.R.; Hebert, S.C.; Delpire, E. Expression of the Na+-K+-2Cl− cotransporter BSC2 in the nervous system. Am. J. Physiol. Cell Physiol. 1997, 272, C173–C183. [Google Scholar] [CrossRef] [PubMed]
- Delpire, E.; Gagnon, K.B. Elusive role of the Na-K-2Cl cotransporter in the choroid plexus. Am. J. Physiol. Cell Physiol. 2019, 316, C522–C524. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Leefmans, F.J. CrossTalk proposal: Apical NKCC1 of choroid plexus epithelial cells works in the net inward flux mode under basal conditions, maintaining intracellular Cl− and cell volume. J. Physiol. 2020, 598, 4733–4736. [Google Scholar] [CrossRef] [PubMed]
- Tornheim, P.A.; McLaurin, R.L.; Sawaya, R. Effect of furosemide on experimental traumatic cerebral edema. Neurosurgery 1979, 4, 48–52. [Google Scholar] [CrossRef]
- Wilkinson, H.A.; Rosenfeld, S.R. Furosemide and mannitol in the treatment of acute experimental intracranial hypertension. Neurosurgery 1983, 12, 405–410. [Google Scholar] [CrossRef]
- Greene, C.S., Jr.; Lorenzo, A.V.; Hornig, G.; Welch, K. The lowering of cerebral spinal fluid and brain interstitial pressure of preterm and term rabbits by furosemide. Z. Kinderchir. 1985, 40 (Suppl. S1), 5–8. [Google Scholar] [CrossRef]
- Lorenzo, A.V.; Hornig, G.; Zavala, L.M.; Boss, V.; Welch, K. Furosemide lowers intracranial pressure by inhibiting CSF production. Z. Kinderchir. 1986, 41 (Suppl. S1), 10–12. [Google Scholar] [CrossRef]
- Sklar, F.H.; Beyer, C.W., Jr.; Ramanathan, M.; Cooper, P.R.; Clark, W.K. Cerebrospinal fluid dynamics in patients with pseudotumor cerebri. Neurosurgery 1979, 5, 208–216. [Google Scholar] [CrossRef]
- Sklar, F.H.; Beyer, C.W., Jr.; Ramanathan, M.; Clark, W.K. The Effects of Furosemide on CSF Dynamics in Patients with Pseudotumor Cerebri. In Intracranial Pressure IV; Shulman, K., Marmarou, A., Miller, J.D., Becker, D.P., Hochwald, G.M., Brock, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1980; pp. 660–663. [Google Scholar]
- Akar, F.; Skinner, E.; Klein, J.D.; Jena, M.; Paul, R.J.; O’Neill, W.C. Vasoconstrictors and nitrovasodilators reciprocally regulate the Na+-K+-2Cl− cotransporter in rat aorta. Am. J. Physiol. 1999, 276, C1383–C1390. [Google Scholar] [CrossRef]
- Wall, S.M.; Knepper, M.A.; Hassell, K.A.; Fischer, M.P.; Shodeinde, A.; Shin, W.; Pham, T.D.; Meyer, J.W.; Lorenz, J.N.; Beierwaltes, W.H.; et al. Hypotension in NKCC1 null mice: Role of the kidneys. Am. J. Physiol. Ren. Physiol. 2006, 290, F409–F416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotton, R.; Suarez, S.; Reese, J. Unexpected extra-renal effects of loop diuretics in the preterm neonate. Acta Paediatr. 2012, 101, 835–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simard, J.M.; Kahle, K.T.; Gerzanich, V. Molecular mechanisms of microvascular failure in central nervous system injury--synergistic roles of NKCC1 and SUR1/TRPM4. J. Neurosurg. 2010, 113, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Menegazzi, R.; Busetto, S.; Dri, P.; Cramer, R.; Patriarca, P. Chloride ion efflux regulates adherence, spreading, and respiratory burst of neutrophils stimulated by tumor necrosis factor-alpha (TNF) on biologic surfaces. J. Cell Biol. 1996, 135, 511–522. [Google Scholar] [CrossRef] [Green Version]
- Karimy, J.K.; Zhang, J.; Kurland, D.B.; Theriault, B.C.; Duran, D.; Stokum, J.A.; Furey, C.G.; Zhou, X.; Mansuri, M.S.; Montejo, J.; et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat. Med. 2017, 23, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wu, M.; Shen, J.; Tang, J.; Li, J.; Xu, J.; Dang, B.; Chen, G. Inhibition of the NKCC1/NF-κB Signaling Pathway Decreases Inflammation and Improves Brain Edema and Nerve Cell Apoptosis in an SBI Rat Model. Front. Mol. Neurosci. 2021, 14, 641993. [Google Scholar] [CrossRef]
- Patterson, P.H. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav. Brain Res. 2009, 204, 313–321. [Google Scholar] [CrossRef]
- Atladóttir, H.; Henriksen, T.B.; Schendel, D.E.; Parner, E.T. Autism after infection, febrile episodes, and antibiotic use during pregnancy: An exploratory study. Pediatrics 2012, 130, e1447–e1454. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, E.Y.; McBride, S.W.; Chow, J.; Mazmanian, S.K.; Patterson, P.H. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. USA 2012, 109, 12776–12781. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [Green Version]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malkova, N.V.; Yu, C.Z.; Hsiao, E.Y.; Moore, M.J.; Patterson, P.H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 2012, 26, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corradini, I.; Focchi, E.; Rasile, M.; Morini, R.; Desiato, G.; Tomasoni, R.; Lizier, M.; Ghirardini, E.; Fesce, R.; Morone, D.; et al. Maternal Immune Activation Delays Excitatory-to-Inhibitory Gamma-Aminobutyric Acid Switch in Offspring. Biol. Psychiatry 2018, 83, 680–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, L.; Neitzel, K.; Ji, T.; Ren, W.; Qu, M.H. Na+-K+-2Cl− symporter contributes to γ-aminobutyric acid-evoked excitation in rat enteric neurons. Sheng Li Xue Bao 2020, 72, 263–273. [Google Scholar] [PubMed]
- Gameiro, A.; Reimann, F.; Habib, A.M.; O’Malley, D.; Williams, L.; Simpson, A.K.; Gribble, F.M. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J. Physiol. 2005, 569, 761–772. [Google Scholar] [CrossRef]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Hsiao, E.Y. Immune dysregulation in autism spectrum disorder. Int. Rev. Neurobiol. 2013, 113, 269–302. [Google Scholar] [CrossRef]
- Iglesias-Vázquez, L.; Van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Ellis, S.E.; Ashar, F.N.; Moes, A.; Bader, J.S.; Zhan, J.; West, A.B.; Arking, D.E. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 2014, 5, 5748. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef] [Green Version]
- Zemkova, H.W.; Bjelobaba, I.; Tomic, M.; Zemkova, H.; Stojilkovic, S.S. Molecular, pharmacological and functional properties of GABA(A) receptors in anterior pituitary cells. J. Physiol. 2008, 586, 3097–3111. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Matsuzaki, H.; Miyachi, T.; Shimmura, C.; Suda, S.; Tsuchiya, K.J.; Matsumoto, K.; Suzuki, K.; Iwata, Y.; Nakamura, K.; et al. Investigation of the serum levels of anterior pituitary hormones in male children with autism. Mol. Autism 2011, 2, 16. [Google Scholar] [CrossRef] [Green Version]

| Tissue | Cell Type and Polarity | Function | References |
|---|---|---|---|
| Inner ear | stria vascularis | hearing, balance | [7,8] |
| Lung | alveolar basolateral | fluid secretion hydration | [9] |
| Stomach | epithelium basolateral | fluid secretion | [10] |
| Intestine | epithelium basolateral | Cl− and fluid secretion | [11] |
| Kidney | Glomerulus, A-type intercalated, IMCD | acid and fluid secretion | [12,13] |
| Salivary gland | epithelium basolateral | saliva secretion | [14,15] |
| Sweat/lacrimal gland | epithelium basolateral | sweat and tear secretion | |
| Skeletal muscle | myocyte | facilitates depolarization, Ca2+ entry and contraction | [16] |
| Vasculature | Smooth mucle cell | facilitates depolarization, Ca2+ entry and contraction | [17,18] |
| Immune cells | Macrophages, Microglia | activation | [19,20,21] |
| Testis | spermatogonia | sperm production | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delpire, E.; Ben-Ari, Y. A Wholistic View of How Bumetanide Attenuates Autism Spectrum Disorders. Cells 2022, 11, 2419. https://doi.org/10.3390/cells11152419
Delpire E, Ben-Ari Y. A Wholistic View of How Bumetanide Attenuates Autism Spectrum Disorders. Cells. 2022; 11(15):2419. https://doi.org/10.3390/cells11152419
Chicago/Turabian StyleDelpire, Eric, and Yehezkel Ben-Ari. 2022. "A Wholistic View of How Bumetanide Attenuates Autism Spectrum Disorders" Cells 11, no. 15: 2419. https://doi.org/10.3390/cells11152419
APA StyleDelpire, E., & Ben-Ari, Y. (2022). A Wholistic View of How Bumetanide Attenuates Autism Spectrum Disorders. Cells, 11(15), 2419. https://doi.org/10.3390/cells11152419






