Excessive Oxalic Acid Secreted by Sparassis latifolia Inhibits the Growth of Mycelia during Its Saprophytic Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain, Media, and Culture Conditions
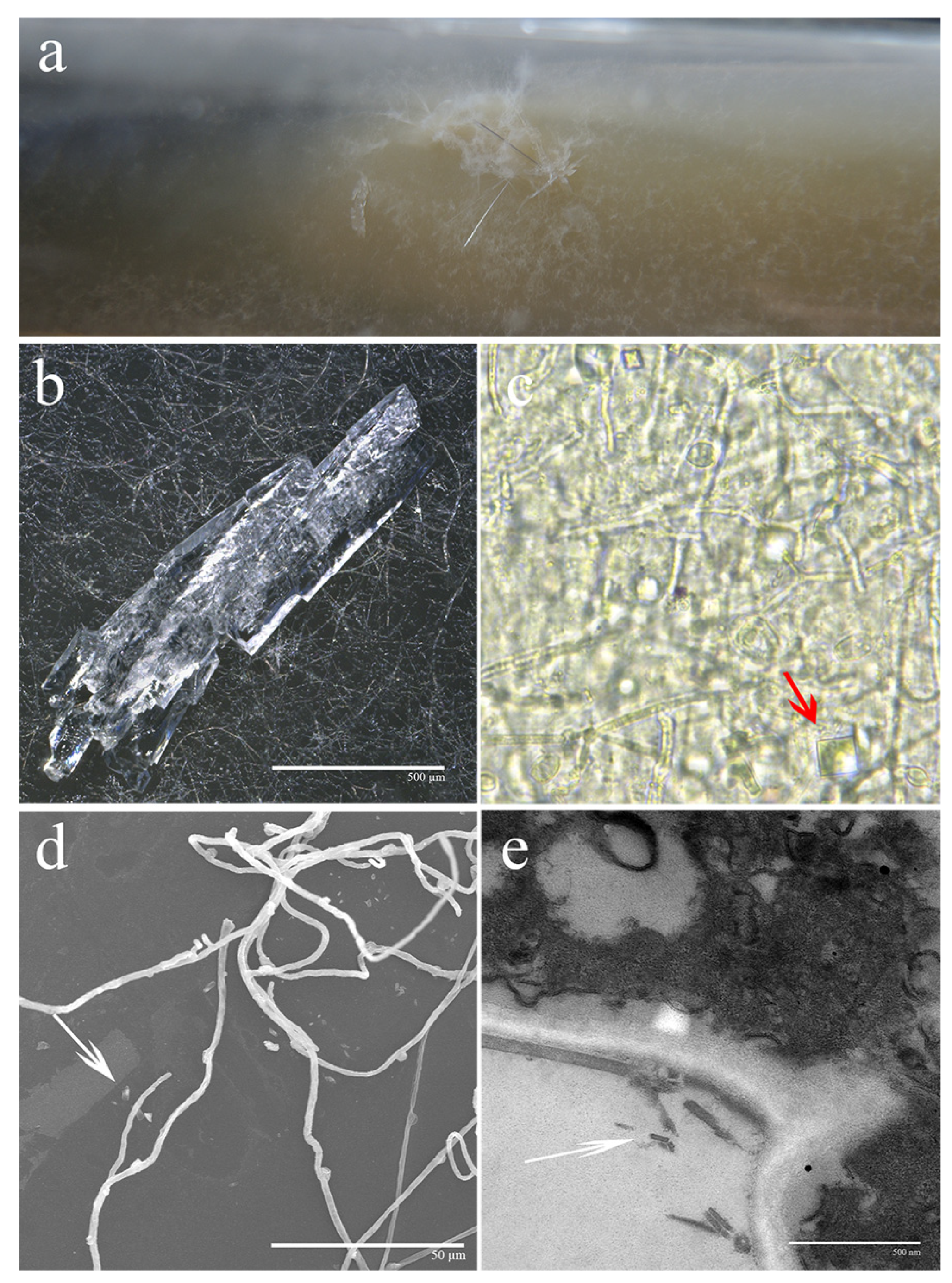
2.2. Microscopic Observations of the Mycelium and the Crystals Formed on Its Surface
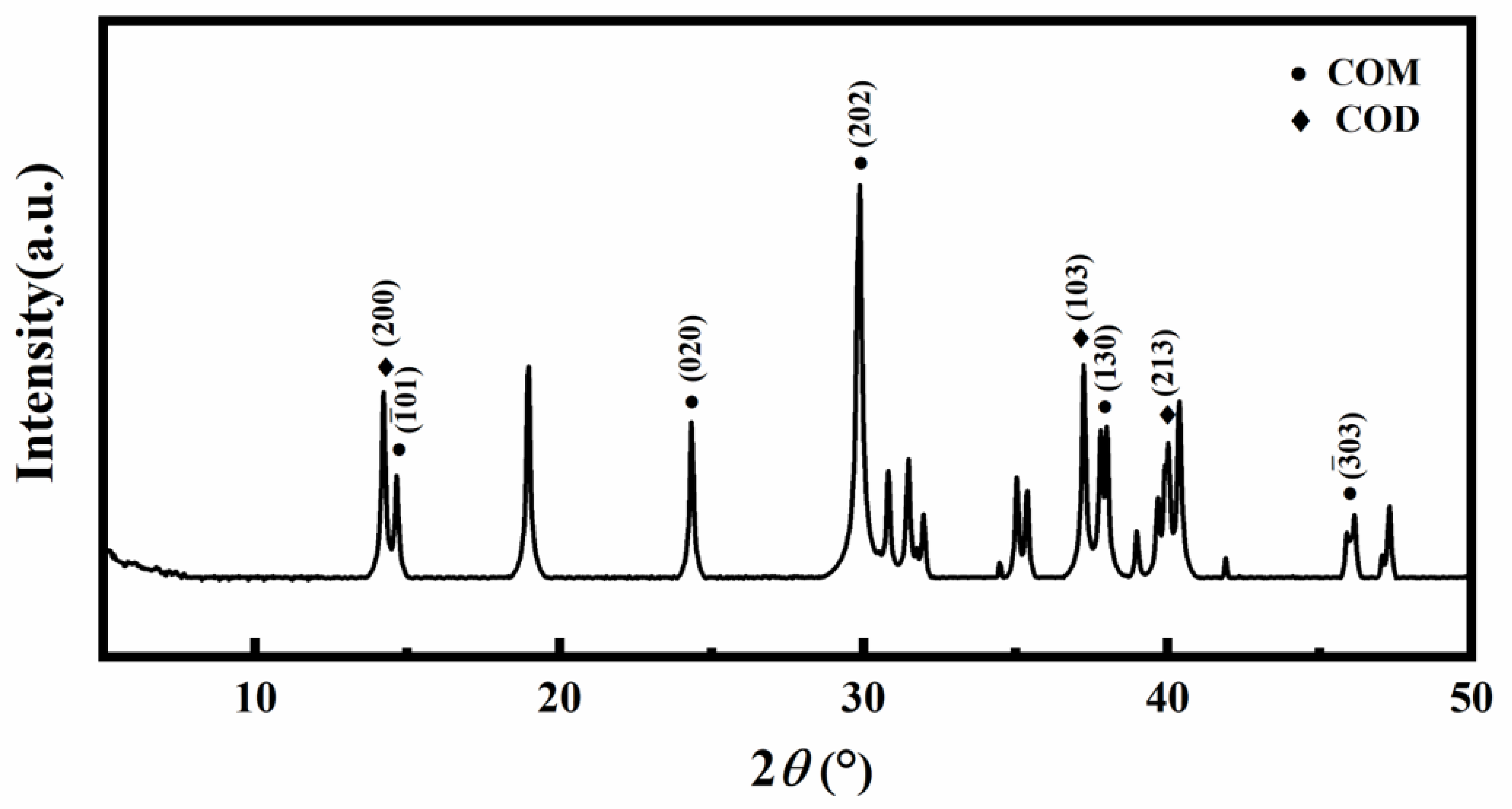
2.3. Identifying Crystal Components Using XRD
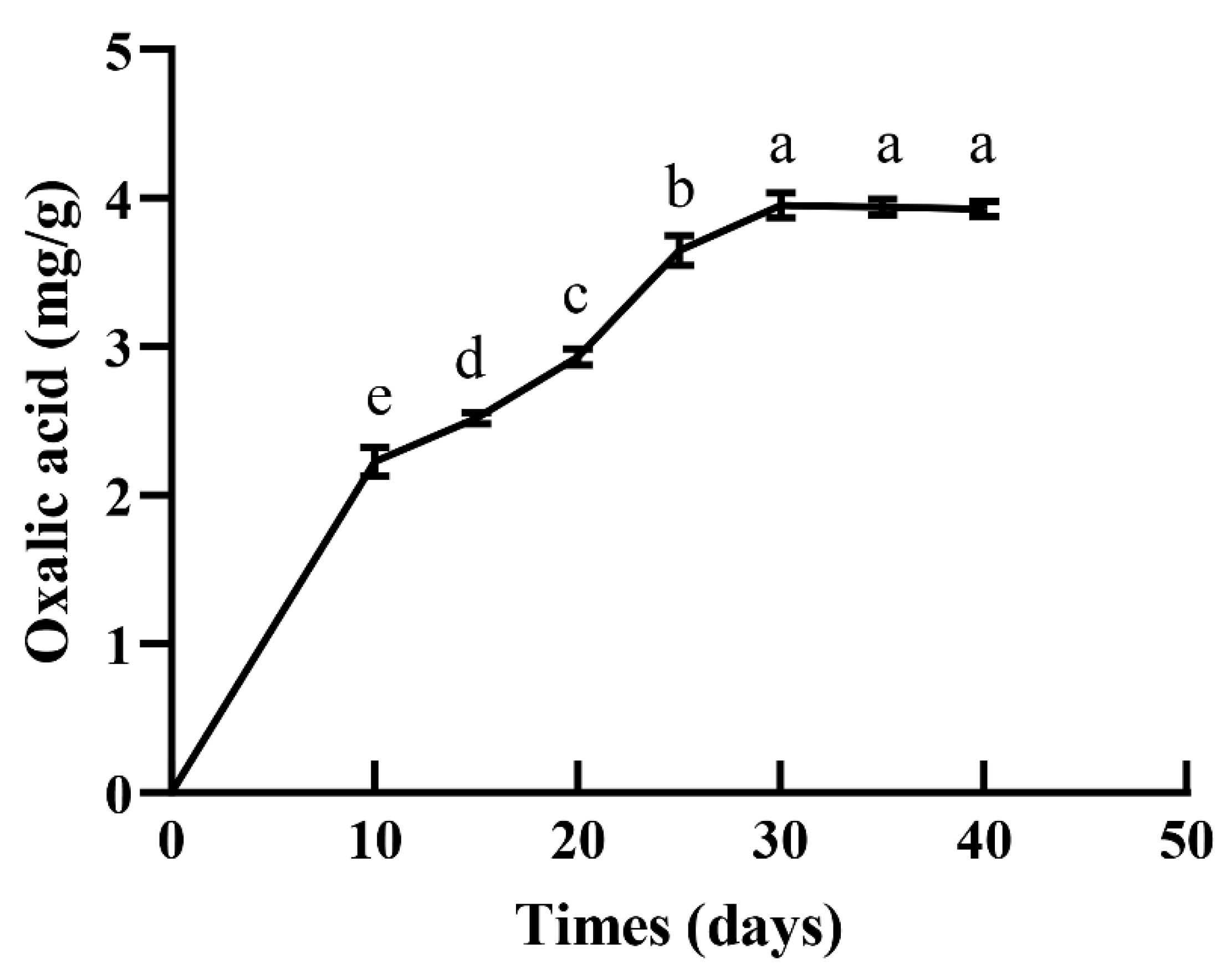
2.4. Quantifying OA Secretion by S. latifolia
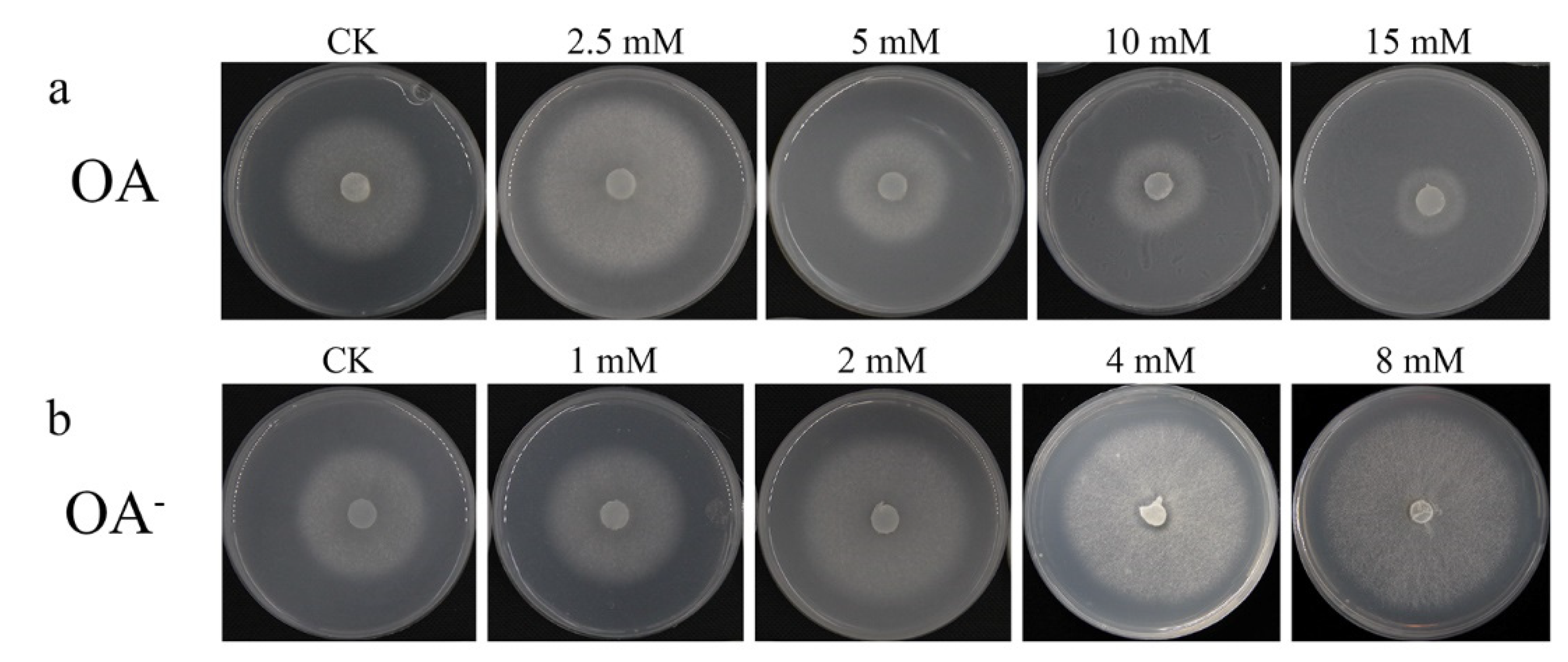
2.5. The Effects of Different OA Concentrations on Mycelial Growth of S. latifolia
2.6. Observation of the Structure of Pine Sawdust under SEM
2.7. Determination of Cellulose, Hemicellulose, and Lignin Content of Pine Sawdust
2.8. Effects of OA on Activities of Extracellular Degradation Enzymes of S. latifolia
2.9. Statistical Analysis
3. Results
3.1. Observation of Crystalline Characterizations under Different Microscopic Magnifications
3.2. XRD Analysis of Crystals
3.3. S. latifolia Mycelia Secreted Large Amounts of OA during Longer Cultivation
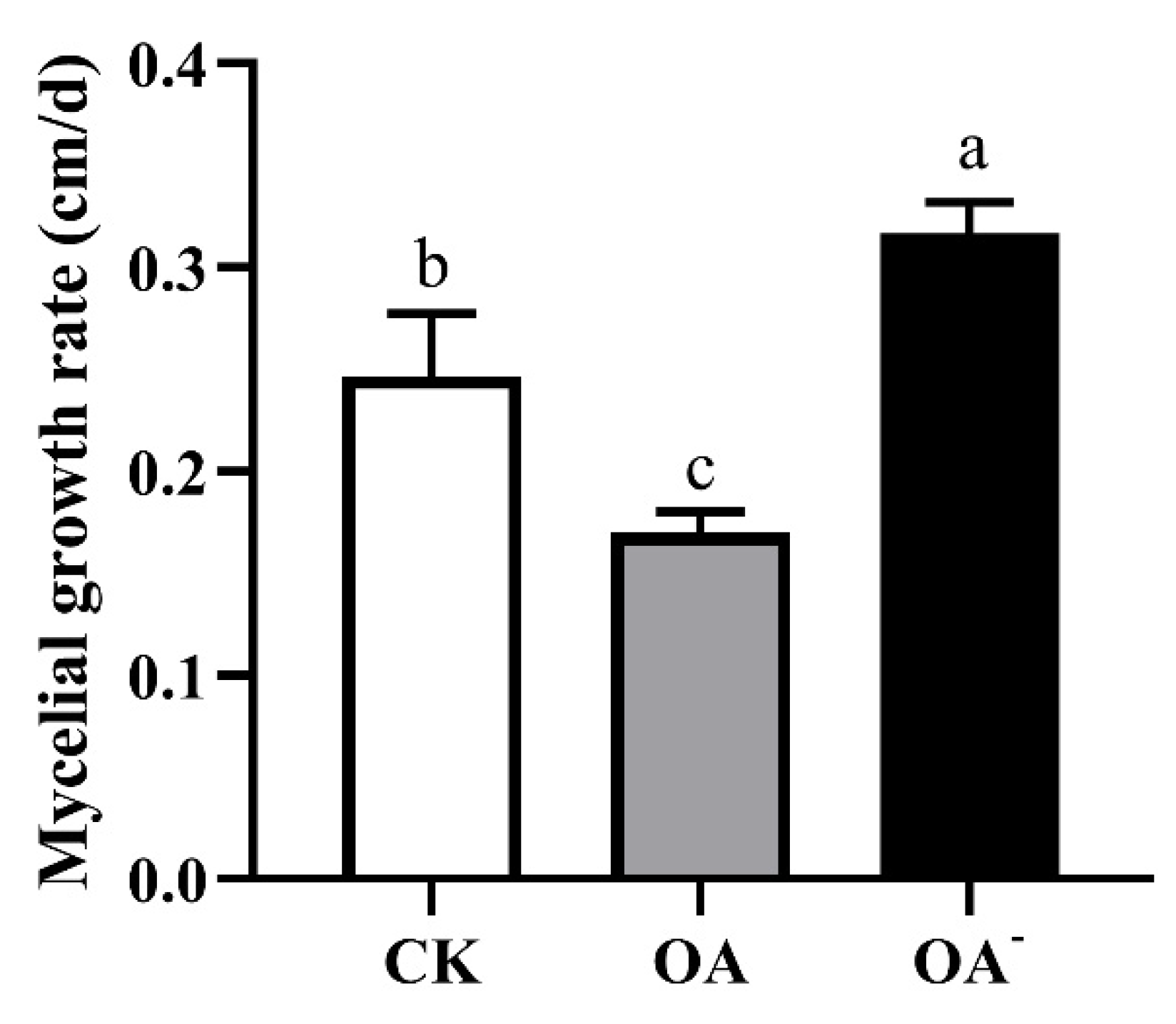
3.4. The Level of OA Affected the Growth of Mycelia in Different Media
3.5. High Concentrations of OA Inhibited the Degradation of Pine Sawdust by S. latifolia
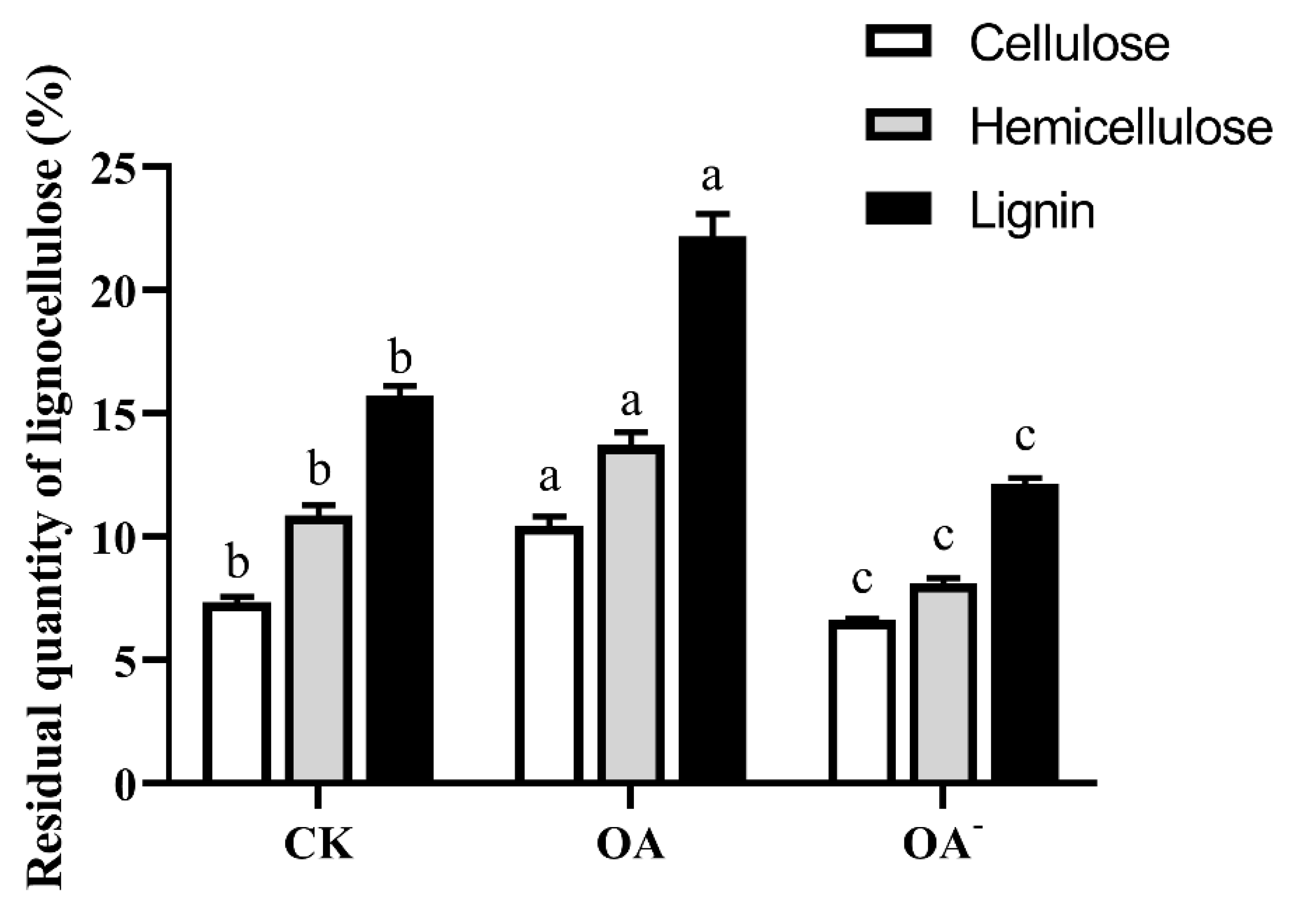
3.6. High Concentration of OA Inhibited the Degradation of Lignocellulose by S. latifolia
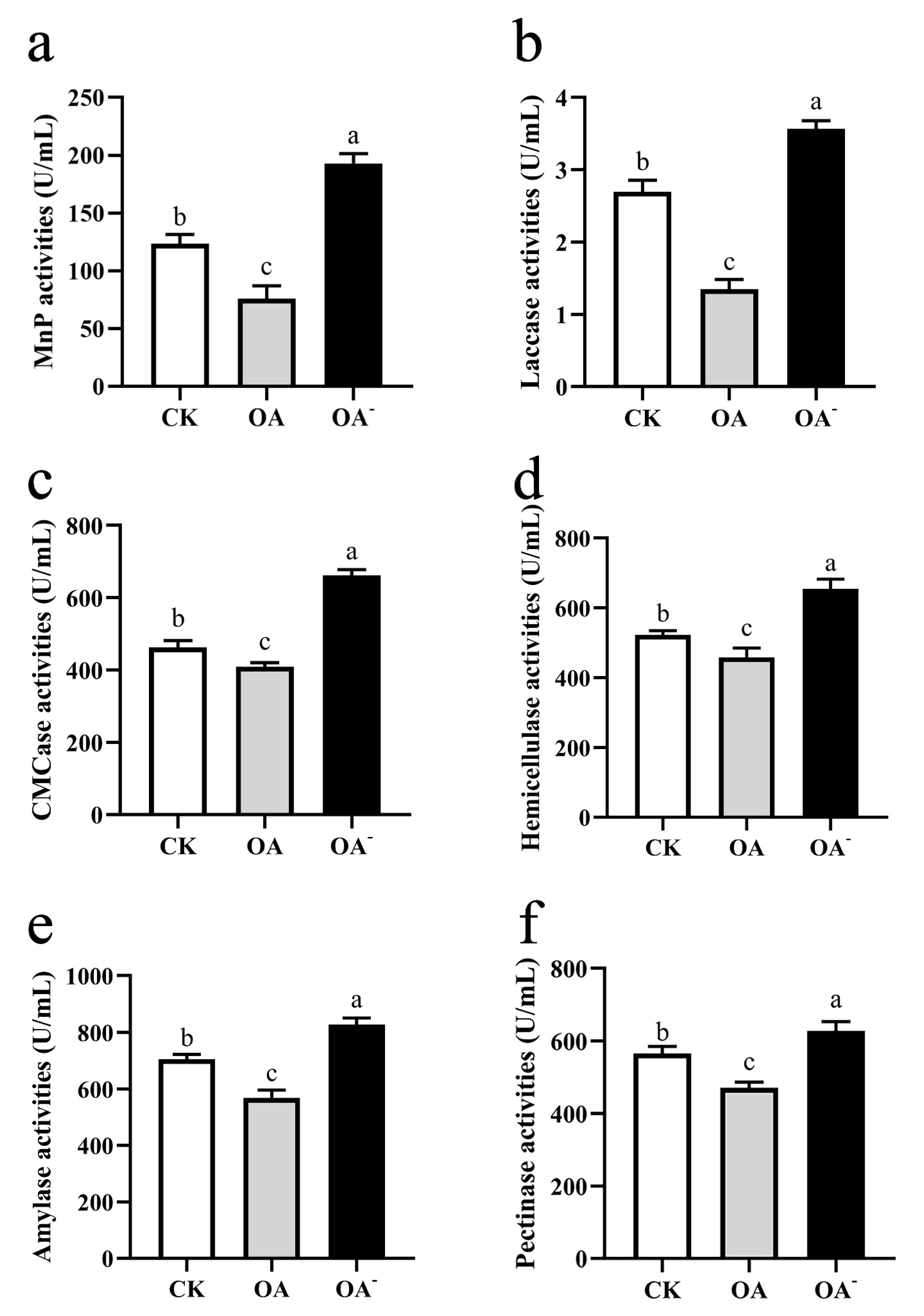
3.7. High Concentration of OA Inhibited the Activity of Lignocellulose-Degrading Enzymes of S. latifolia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chandrasekaran, G.; Lee, Y.C.; Park, H.; Wu, Y.; Shin, H.J. Antibacterial and antifungal activities of lectin extracted from fruiting bodies of the Korean cauliflower medicinal mushroom, Sparassis latifolia (Agaricomycetes). Int. J. Med. Mushrooms 2016, 18, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Nowacka-Jechalke, N.; Pietrzak, W.; Gawlik-Dziki, U. A new look at edible and medicinal mushrooms as a source of ergosterol and ergosterol peroxide—UHPLC-MS/MS analysis. Food Chem. 2021, 369, 130927. [Google Scholar]
- Zhang, M.; Lou, B.-Y.; Zhang, Y.-J.; Mei, S.-J.; Gao, L.-Y.; Chen, W.-J. Preparation and characterization of Sparassis latifolia β-glucan microcapsules. Open Chem. 2022, 20, 351–360. [Google Scholar] [CrossRef]
- Ma, L.; Lin, Y.; Yang, C.; Ying, Z.; Jiang, X. Production of liquid spawn of an edible mushroom, Sparassis latifolia by submerged fermentation and mycelial growth on pine wood sawdust. Sci. Hortic. 2016, 209, 22–30. [Google Scholar] [CrossRef]
- Lili, S.; Li, Y. First report of Sparassis latifolia causing basal rot of Pinus koraiensis in China. Plant Disease 2017, 101, 833. [Google Scholar]
- Kim, S.R.; Kang, H.W.; Ro, H.S. Generation and evaluation of high β-glucan producing mutant strains of Sparassis crispa. Mycobiology 2013, 41, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Sou, H.-D.; Ryoo, R.; Ka, K.-H.; Park, H. The mycelial growth and ligninolytic enzyme activity of cauliflower mushroom (Sparassis latifolia ). For. Sci. Technol. 2017, 13, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Dutton, M.; Evans, C. Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment. Can. J. Microbiol. 2011, 42, 881–895. [Google Scholar] [CrossRef]
- Gonzalez, J.; Costa, M.D.; Silva, I.; César, J.; Barros, N.; Borges, A. Accumulation of oxalic acid and calcium crystals in eucalypt ectomycorrhizas.: I—oxalic acid production and nutrient concentration in fine lateral roots colonized with ectomicorrhizal fungi. Rev. Bras. Cienc. Solo 2009, 33, 541–553. [Google Scholar] [CrossRef]
- Mou, H.Y.; Wu, X.; Huang, J.; Liu, Y.; Fan, H. Eucalyptus lignin modification for dynamic adsorption with lignocellulose-degradation enzymes dependent on pH values. Ind. Crops Prod. 2021, 169, 113650. [Google Scholar] [CrossRef]
- Osińska-Jaroszuk, M.; Wlizło, K.; Szałapata, K.; Jarosz-Wilkołazka, A. Correlation between the production of exopolysaccharides and oxalic acid secretion by Ganoderma applanatum and Tyromyces palustris. World J. Microbiol. Biot. 2014, 30, 3065–3074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, K.; Yuan, J.; Liao, H.; Banga, S.; Kumar, R.; Ding, Y.; Qian, W. Host-induced gene silencing reveals the role of Sclerotinia sclerotiorum oxaloacetate acetylhydrolase gene in fungal oxalic acid accumulation and virulence. Microbiol. Res. 2022, 258, 126981. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.; Dashek, W.; Highley, T. Detection and quantification of oxalic acid from the brown-rot decay fungus, Postia placenta. Holzforschung 1996, 50, 312–318. [Google Scholar] [CrossRef]
- Li, T.; Cui, L.; Song, X.; Cui, X.; Wei, Y.; Tang, L.; Mu, Y.; Xu, Z. Wood decay fungi: An analysis of worldwide research. J. Soil. Sediment. 2022, 22, 1688–1702. [Google Scholar] [CrossRef]
- Guggiari, M.; Bloque, R.; Aragno, M.; Verrecchia, E.; Job, D.; Junier, P. Experimental calcium-oxalate crystal production and dissolution by selected wood-rot fungi. Int. Biodeter. Biodegr. 2011, 65, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Ma, L.; Xiao, D.; Ying, Z.; Jiang, X.; Lin, Y. Integration of ATAC-Seq and RNA-Seq identifies key genes in light-induced primordia formation of Sparassis latifolia. Int. J. Mol. Sci. 2019, 21, 185. [Google Scholar] [CrossRef] [Green Version]
- Meng, D.; Betchem, G.; Ren, Y.; Yao, M.; Xia, X.; Li, M.; Wang, Y. Antifungal activity of chitosan against Aspergillus ochraceus and its possible mechanisms of action. Int. J. Biol. Macromol. 2020, 158, 1063–1070. [Google Scholar] [CrossRef]
- Werner, H.; Bapat, S.; Schobesberger, M.; Segets, D.; Schwaminger, S.P. Calcium oxalate crystallization: Influence of pH, energy input, and supersaturation ratio on the synthesis of artificial kidney stones. ACS Omega 2021, 6, 26566–26574. [Google Scholar] [CrossRef]
- Sheldrick, G. A Short History of ShelX. Acta crystallographica. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, K.; Liu, Y.; Xie, Z.; Zhang, C. Oxalic acid from Sesbania rostrata seed exudates mediates the chemotactic response of Azorhizobium caulinodans ORS571 using multiple strategies. Front. Microbiol. 2019, 10, 2727. [Google Scholar] [CrossRef] [Green Version]
- Joosten, H.J.; Han, Y.; Niu, W.; Vervoort, J.; Mariano, P.; Schaap, P. Oxaloacetate hydrolase, the C-C bond lyase of oxalate secreting fungi. J. Biol. Chem. 2007, 282, 9581–9590. [Google Scholar]
- Trevorah, R.; Huynh, T.; Brkljača, R.; Maazuza, O. Structural and morphological analysis of cellulose pulp produced from the fractionation of Eucalyptus obliqua sawdust using γ-valerolactone. ACS Omega 2021, 6, 4126–4136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Sun, X.; Wang, Y.; Liu, Z. Separation and characterization of biomass components (cellulose, hemicellulose, and lignin) from corn stalk. BioResources 2021, 16, 7205–7219. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, W.; Wang, H.; Ziya, N.; Luo, Y.; Jia, P.; Zhang, G.; Ng, T. Purification and properties of a laccase from the mushroom Agaricus sinodeliciosus. Biotech. Appl. Biochem. 2020, 68, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Nuryana, I.; Ilmiah, Z.; Andriani, A.; Yopi, Y. Laccase and manganese Peroxidase (MnP) activities in the white-rot fungus Trametes hirsuta in response to aromatic compounds. Ann. Bogo. 2020, 23, 66. [Google Scholar] [CrossRef]
- Fen, L.; Xuwei, Z.; Nanyi, L.; Puyu, Z.; Shuang, Z.; Xue, Z.; Pengju, L.; Qichao, Z.; Haiping, L. Screening of lignocellulose-degrading superior mushroom strains and determination of their CMCase and laccase activity. Sci. World J. 2014, 2014, 763108. [Google Scholar] [CrossRef] [Green Version]
- Ofongo, R.; Ohimain, E.; Iyayi, E. Cellulase and hemicellulase activity under submerged fermentation of rice mill feed by fungi. Int. J. Environ. Agric. Biotech. 2019, 4, 233–239. [Google Scholar]
- Bedade, D.; Deska, J.; Bankar, S.; Bejar, S.; Singhal, R.; Shamekh, S. Fermentative production of extracellular amylase from novel amylase producer, Tuber maculatum mycelium, and its characterization. Prep. Biochem. Biotech. 2018, 48, 549–555. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Cai, W.; Song, T.; Fan, L.; Lv, G. Targeted identification of antioxidant compounds from Sparassis latifolia extracts and their antioxidant activities. J. Food Process. Preserv. 2021, 45, e16068. [Google Scholar] [CrossRef]
- Rimmer, S. Sclerotinia sclerotiorum: When “to be or not to be” a pathogen? FEMS Microbiol. Lett. 2005, 251, 177–184. [Google Scholar]
- Akhtar, K.; Haq, I. Chemical modulation of crystalline state of calcium oxalate with nickel Ions. Clin. Chim. Acta 2013, 418, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Green, F.; Clausen, C.; Kuster, T.; Highley, T. Induction of polygalacturonase and the formation of oxalic acid by pectin in brown-rot fungi. World J. Microbiol. Biotechnol. 1995, 11, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Kaneta, T.; Baba, T.; Ohtsubo, T.; Ikeda, F. Varietal differences in oxalic acid concentration and enzyme activity related to oxalic acid biosynthesis in carambola (Averrhoa carambola L.). Hortic. Res. 2004, 3, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Schilling, J.S.; Jellison, J. Oxalate regulation by two brown rot fungi decaying oxalate-amended and non-amended wood. Holzforschung 2005, 59, 681–688. [Google Scholar] [CrossRef]
- Hatakka, A.; Hammel, K. Fungal Biodegradation of Lignocelluloses; Springer: Berlin/Heidelberg, Germany, 2010; Volume 10, pp. 319–340. [Google Scholar]
- El-Argawy, E. Oxalic acid production by Sclerotinia sclerotiorum and its relation to pathogenicity. J. Plant Protect. Pathol. 2012, 3, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.; Son, S.; Yoon, S.; Lee, Y.; Lee, T.; Lee, S.; Lee, K.; Lee, M. The optimal factors for the mycelial growth of Sparassis crispa. Korean J. Mycol. 1998, 26, 39–46. [Google Scholar]
- Shimada, M. A proposed role of oxalic acid in wood decay systems of wood-rotting basidiomycetes. FEMS Microbiol. Rev. 1994, 13, 285–295. [Google Scholar] [CrossRef]
- Gibson, D.; King, B.; Hayes, M.; Bergstrom, G. Plant pathogens as a source of diverse enzymes for lignocellulose digestion. Curr. Opin. Microbiol. 2011, 14, 264–270. [Google Scholar] [CrossRef]
- Punja, Z.; Huang, J.S.; Jenkins, S.F. Relationship of mycelial growth and production of oxalic acid and cell wall degrading enzymes to virulence in Sclerotium Rolfsii. Can. J. Plant Pathol. 1985, 7, 109–117. [Google Scholar] [CrossRef]
- Shah, F.; Mali, T.; Lundell, T. Polyporales brown rot species Fomitopsis pinicola: Enzyme activity profiles, oxalic acid production, and Fe3+-reducing metabolite secretion. Appl. Environ. Microbiol. 2018, 84, e02662-17. [Google Scholar] [CrossRef] [Green Version]
- Schilling, J.; Jellison, J. High-performance liquid chromatographic analysis of soluble and total oxalate in Ca- and Mg-amended liquid cultures of three wood decay fungi. Holzforschung 2004, 58, 682–687. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, L.; Wang, M.; Wang, S.; Li, Y.; Xu, H.; Qiu, Z.; Li, T. Excessive Oxalic Acid Secreted by Sparassis latifolia Inhibits the Growth of Mycelia during Its Saprophytic Process. Cells 2022, 11, 2423. https://doi.org/10.3390/cells11152423
Shu L, Wang M, Wang S, Li Y, Xu H, Qiu Z, Li T. Excessive Oxalic Acid Secreted by Sparassis latifolia Inhibits the Growth of Mycelia during Its Saprophytic Process. Cells. 2022; 11(15):2423. https://doi.org/10.3390/cells11152423
Chicago/Turabian StyleShu, Lili, Miaoyue Wang, Shuang Wang, Yu Li, Hui Xu, Zhiheng Qiu, and Tianlai Li. 2022. "Excessive Oxalic Acid Secreted by Sparassis latifolia Inhibits the Growth of Mycelia during Its Saprophytic Process" Cells 11, no. 15: 2423. https://doi.org/10.3390/cells11152423





