Targeting Hydrogen Sulfide Modulates Dexamethasone-Induced Muscle Atrophy and Microvascular Rarefaction, through Inhibition of NOX4 and Induction of MGF, M2 Macrophages and Endothelial Progenitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size Estimation
2.2. Ethical Statement
2.3. Research Design
2.4. Sample Preparation
2.5. Muscle Functional Study
2.5.1. Muscle Force Measurements
2.5.2. Establish Frequency–Force Relationship
2.5.3. Maximum Isometric Tetanic Force
2.5.4. Specific Force Calculations
2.6. Measurement of Muscle Weight and Cross-Sectional Area
2.7. Serum K⁺ and Creatine Kinase-MM (CK-MM) Assay
2.8. Total Antioxidant Capacity (TAC)
2.9. Assay of Lipid Peroxidation Marker Malondialdehyde (MDA) and Antioxidant Reduced Glutathione (GSH) Activity in Muscle Tissues
2.10. RNA Isolation and RT-PCR
2.11. Histopathological Examination of Muscle Tissue
2.12. Immunohistochemical Study
2.13. Morphometric Analysis
2.14. Statistical Analysis
3. Results
3.1. Changes in Soleus Muscle Mass, Length and Cross-Sectional Area
3.2. Changes in Isometric Contractile Properties of Soleus Muscle
3.3. Oxidative Stress Markers, Serum K⁺ Level, and Creatine Kinase-MM (CK-MM)
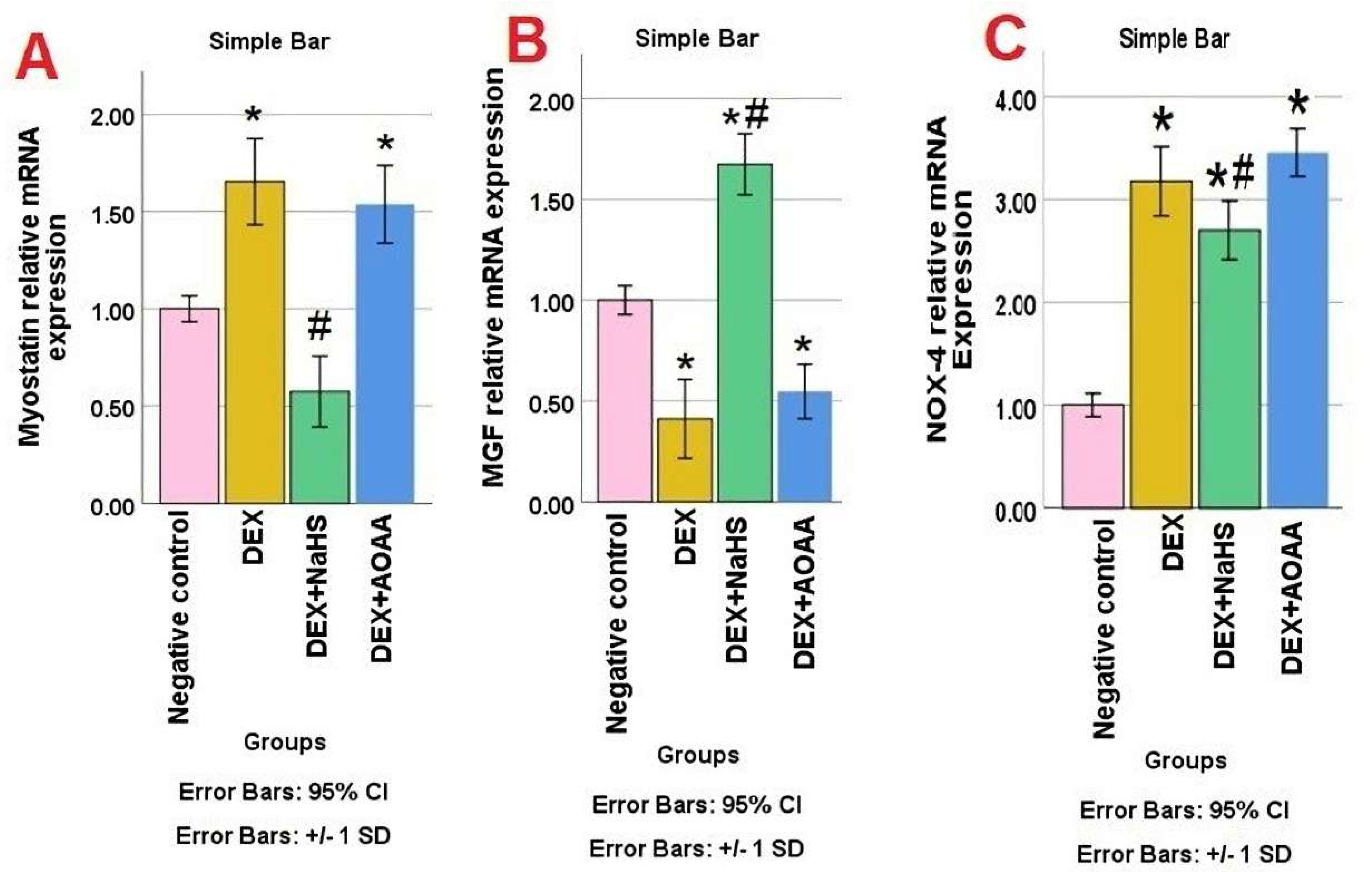
3.4. mRNA Expression of Myostatin, MGF, and NOX-4 in Soleus Muscle Tissues
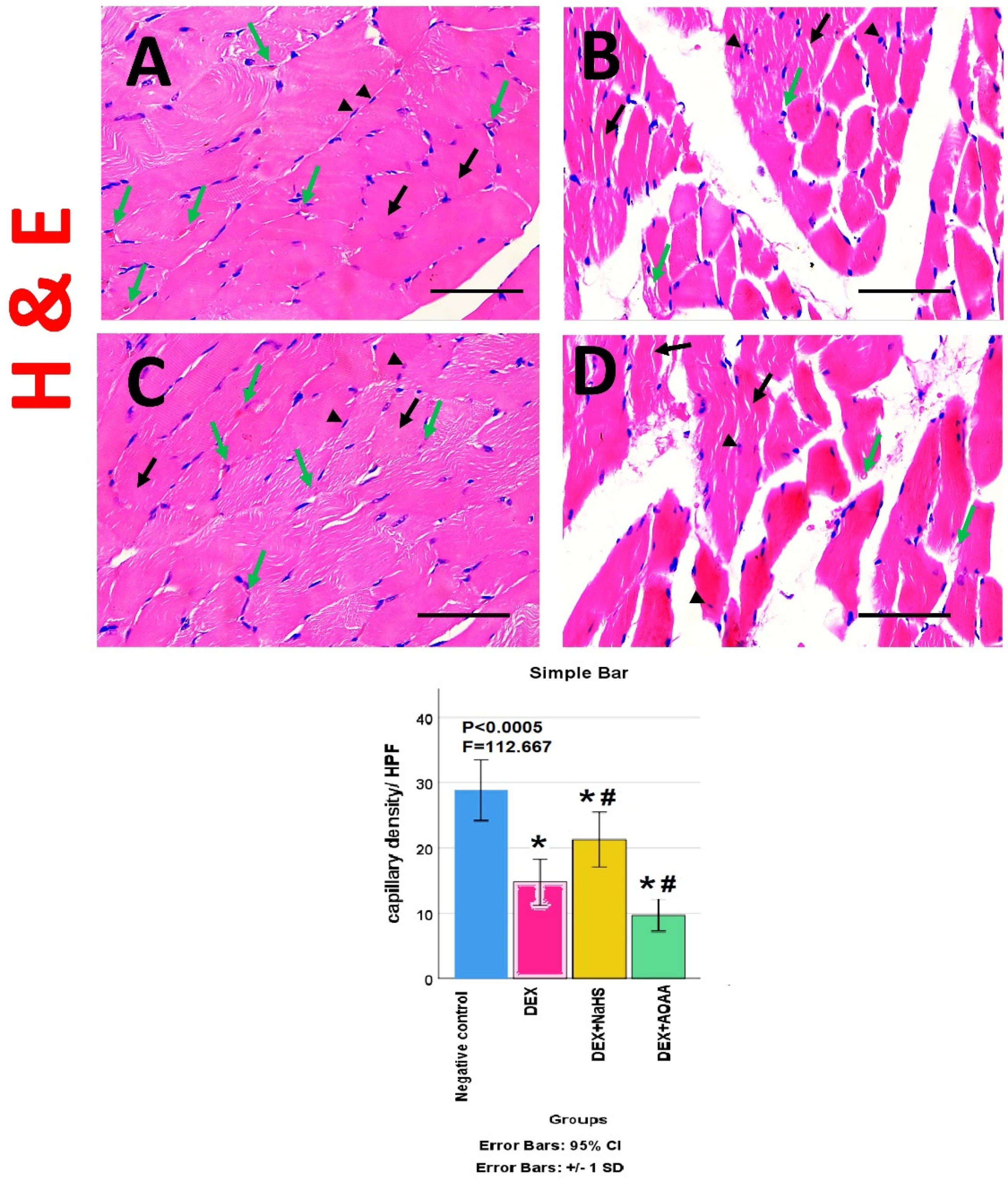
3.5. Results of Haematoxylin and Eosin (HE)- Stained Sections
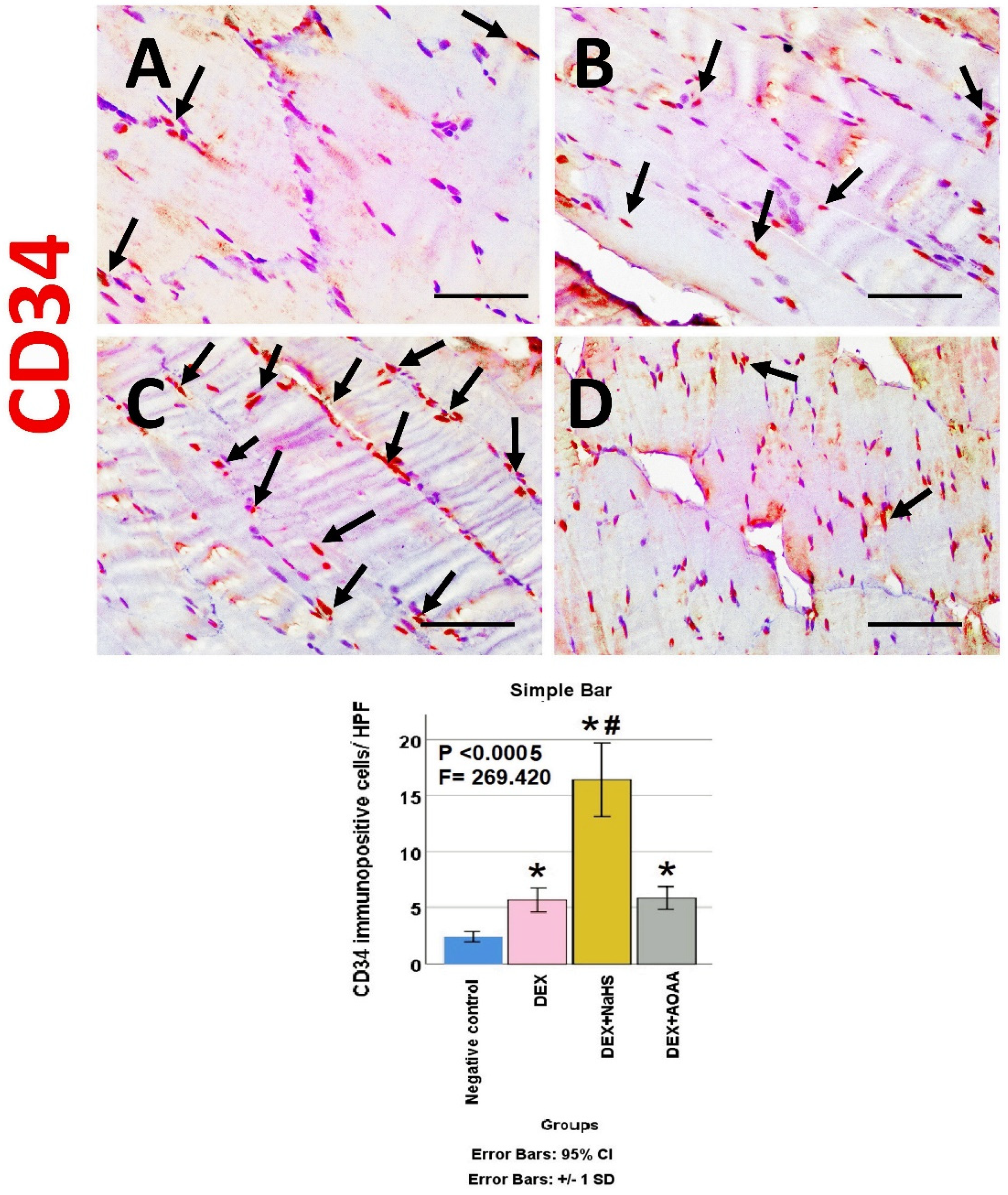
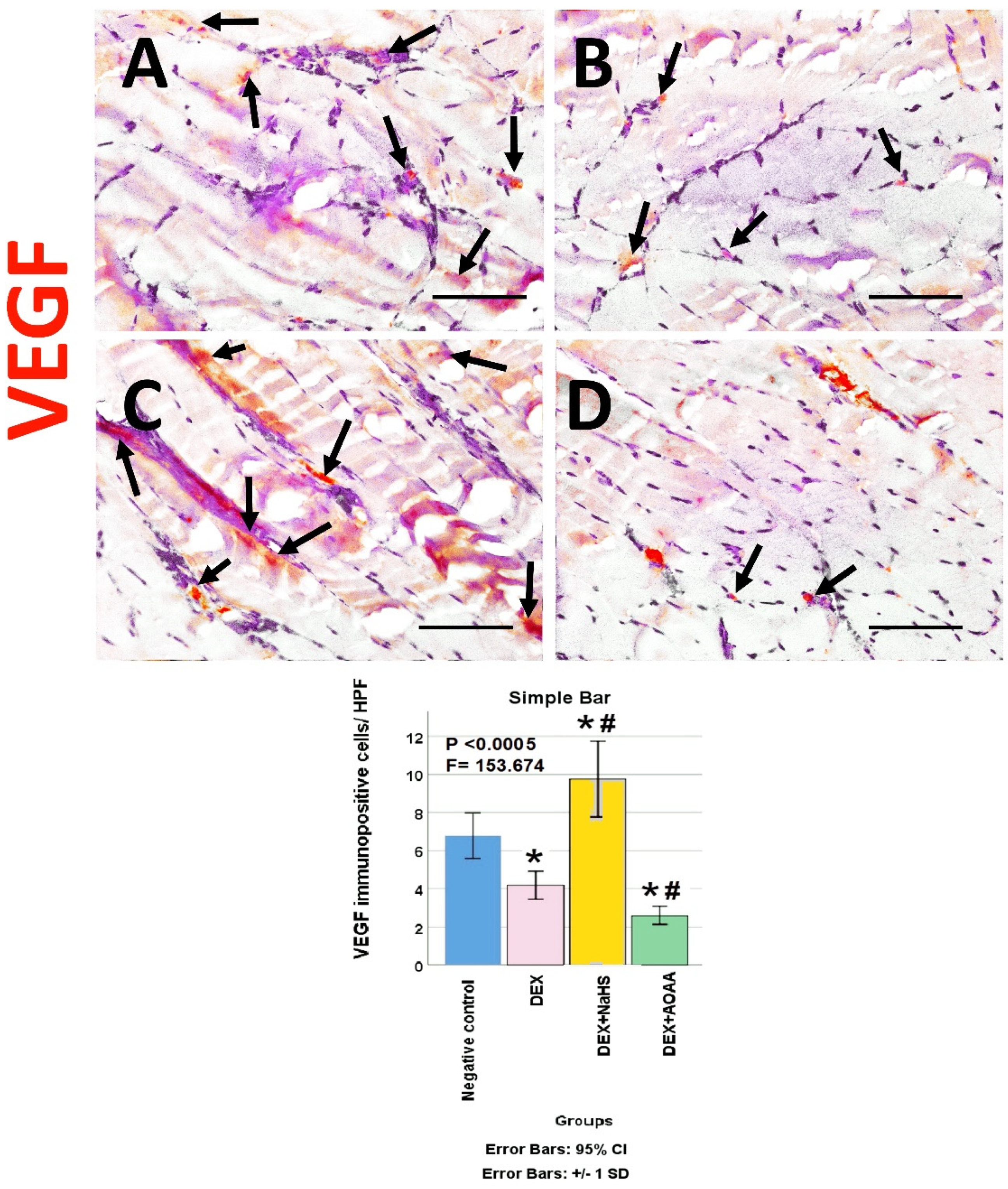
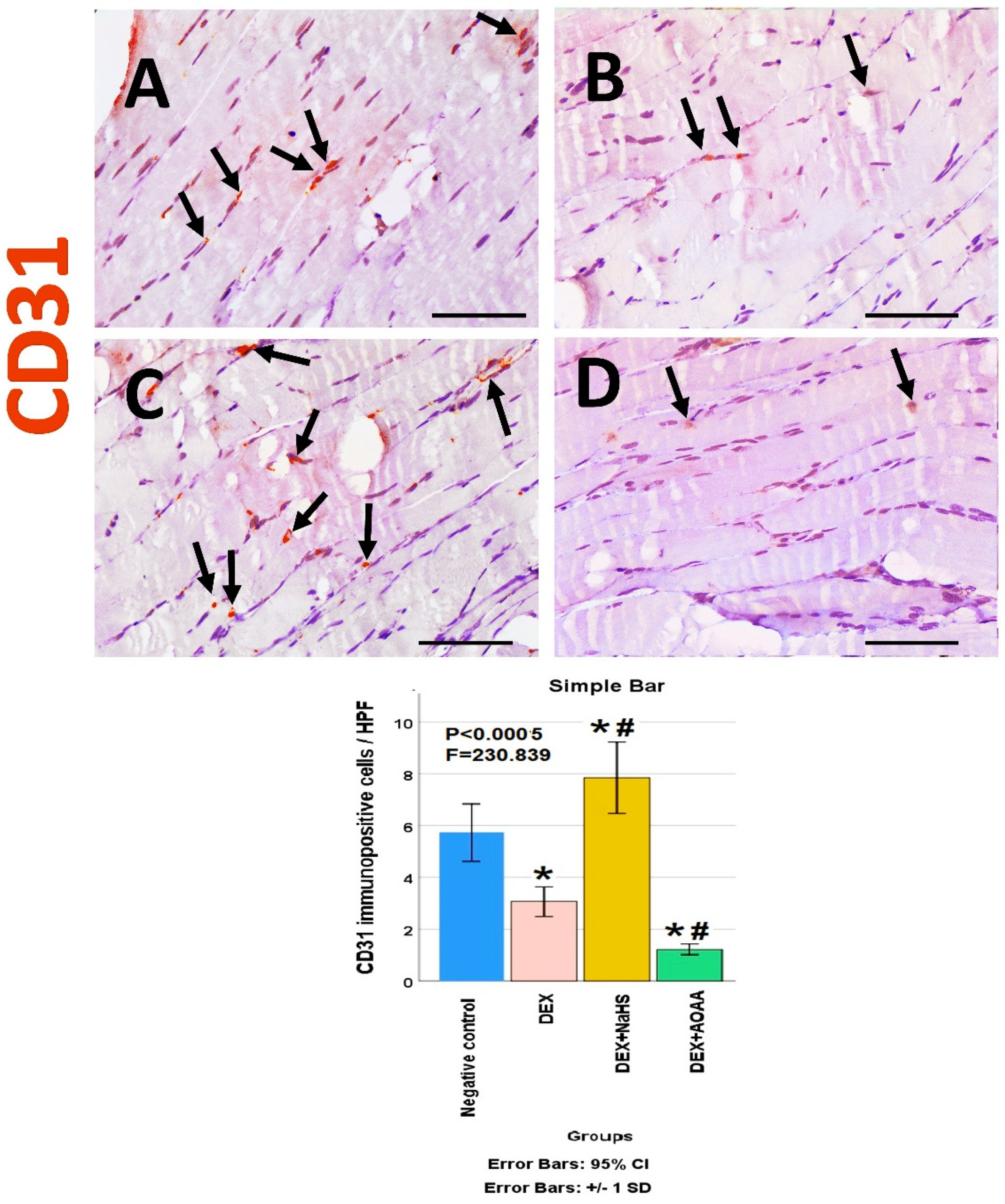
3.6. Immunohistochemical Results
3.7. Results of Morphometric Analysis of Immunohistochemical Studies
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Koike, H.; Ono, T.; Hayashi, S.; Kudo, F.; Kaneda, A.; Kagechika, H.; Manabe, I.; Nakashima, T.; Oishi, Y. Identification of a KLF5-dependent program and drug development for skeletal muscle atrophy. Proc. Natl. Acad. Sci. USA 2021, 118, e2102895118. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.C.; Xu, Q.; Li, H.; Qin, Y.F.; Song, L.C.; Wang, C.G.; Cui, W.H.; Zheng, Z.; Yan, D.W.; Li, Z.J.; et al. NADPH Oxidase Isoforms Are Involved in Glucocorticoid-Induced Preosteoblast Apoptosis. Oxid. Med. Cell Longev. 2019, 2019, 9192413. [Google Scholar] [CrossRef] [PubMed]
- Ulla, A.; Uchida, T.; Miki, Y.; Sugiura, K.; Higashitani, A.; Kobayashi, T.; Ohno, A.; Nakao, R.; Hirasaka, K.; Sakakibara, I.; et al. Morin attenuates dexamethasone-mediated oxidative stress and atrophy in mouse C2C12 skeletal myotubes. Arch. Biochem. Biophys. 2021, 704, 108873. [Google Scholar] [CrossRef] [PubMed]
- Seok, Y.M.; Yoo, J.M.; Nam, Y.; Kim, J.; Kim, J.S.; Son, J.H.; Kim, H.J. Mountain ginseng inhibits skeletal muscle atrophy by decreasing muscle RING finger protein-1 and atrogin1 through forkhead box O3 in L6 myotubes. J. Ethnopharmacol. 2021, 270, 113557. [Google Scholar] [CrossRef]
- Wang, R.; Li, K.; Wang, H.; Jiao, H.; Wang, X.; Zhao, J.; Lin, H. Endogenous CSE/Hydrogen Sulfide System Regulates the Effects of Glucocorticoids and Insulin on Muscle Protein Synthesis. Oxid. Med. Cell Longev. 2019, 2019, 9752698. [Google Scholar] [CrossRef]
- Jesus, I.; Herrera, N.A.; Andreo, J.C.; Santos, C.F.; Amaral, S.L. Training counteracts DEX-induced microvascular rarefaction by improving the balance between apoptotic and angiogenic proteins. Steroids 2020, 156, 108573. [Google Scholar] [CrossRef]
- Langendorf, E.K.; Rommens, P.M.; Drees, P.; Ritz, U. Dexamethasone Inhibits the Pro-Angiogenic Potential of Primary Human Myoblasts. Int. J. Mol. Sci. 2021, 22, 7986. [Google Scholar] [CrossRef]
- Chazaud, B. Inflammation and skeletal muscle regeneration: Leave it to the macrophages! Trends Immunol. 2020, 41, 481–492. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, R.; Wu, L.; Yang, G. Hydrogen sulfide signaling in regulation of cell behaviors. Nitric Oxide 2020, 103, 9–19. [Google Scholar] [CrossRef]
- Yang, R.; Jia, Q.; Li, Y.; Mehmood, S. Protective effect of exogenous hydrogen sulfide on diaphragm muscle fibrosis in streptozotocin-induced diabetic rats. Exp. Biol. Med. 2020, 245, 1280–1289. [Google Scholar] [CrossRef]
- Lu, F.; Lu, B.; Zhang, L.; Wen, J.; Wang, M.; Zhang, S.; Li, Q.; Shu, F.; Sun, Y.; Liu, N. Hydrogen sulphide ameliorating skeletal muscle atrophy in db/db mice via Muscle RING finger 1 S-sulfhydration. J. Cell. Mol. Med. 2020, 24, 9362–9377. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Desgeorges, T.; Caratti, G.; Mounier, R.; Tuckermann, J.; Chazaud, B. Glucocorticoids Shape Macrophage Phenotype for Tissue Repair. Front. Immunol. 2019, 10, 1591. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.; Park, J.; Jun, W. Curcuma longa L. Water Extract Improves Dexamethasone-Induced Sarcopenia by Modulating the Muscle-Related Gene and Oxidative Stress in Mice. Antioxidants 2021, 10, 1000. [Google Scholar] [CrossRef]
- Takenouchi, T.; Morozumi, T.; Wada, E.; Suzuki, S.; Nishiyama, Y.; Sukegawa, S.; Uenishi, H. Dexamethasone enhances CD163 expression in porcine IPKM immortalized macrophages. Vitro Cell Dev. Biol. Anim. 2021, 57, 10–16. [Google Scholar] [CrossRef]
- Baek, K.W.; Jung, Y.K.; Kim, J.S.; Park, J.S.; Hah, Y.S.; Kim, S.J.; Yoo, J.I. Rodent Model of Muscular Atrophy for Sarcopenia Study. J. Bone. Metab. 2020, 27, 97–110. [Google Scholar] [CrossRef]
- Anderson, L.J.; Liu, H.; Garcia, J.M. Sex Differences in Muscle Wasting. Adv. Exp. Med. Biol. 2017, 1043, 153–197. [Google Scholar] [CrossRef]
- Karnia, M.J.; Korewo, D.; Myslinska, D.; Ciepielewski, Z.M.; Puchalska, M.; Konieczna-Wolska, K.; Kowalski, K.; Kaczor, J.J. The Positive Impact of Vitamin D on Glucocorticoid-Dependent Skeletal Muscle Atrophy. Nutrients 2021, 13, 936. [Google Scholar] [CrossRef]
- Revenko, O.; Pavlovskiy, Y.; Savytska, M.; Yashchenko, A.; Kovalyshyn, V.; Chelpanova, I.; Varyvoda, O.; Zayachkivska, O. Hydrogen Sulfide Prevents Mesenteric Adipose Tissue Damage, Endothelial Dysfunction, and Redox Imbalance From High Fructose Diet-Induced Injury in Aged Rats. Front. Pharmacol. 2021, 12, 693100. [Google Scholar] [CrossRef]
- El-Sayed, S.S.; Shahin, R.M.; Fahmy, A.; Elshazly, S.M. Quercetin ameliorated remote myocardial injury induced by renal ischemia/reperfusion in rats: Role of Rho-kinase and hydrogen sulfide. Life Sci. 2021, 287, 120144. [Google Scholar] [CrossRef]
- Zdero, R.; Borkowski, M.M.; Coirault, C. Measuring the contraction force, velocity, and length of skeletal muscle. In Experimental Methods in Orthopaedic Biomechanics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 363–378. [Google Scholar] [CrossRef]
- Yue, L.; Talukder, M.A.H.; Gurjar, A.; Lee, J.I.; Noble, M.; Dirksen, R.T.; Chakkalakal, J.; Elfar, J.C. 4-Aminopyridine attenuates muscle atrophy after sciatic nerve crush injury in mice. Muscle Nerve 2019, 60, 192–201. [Google Scholar] [CrossRef]
- Garcia, R.A.; Vanelli, C.P.; Pereira, O.d.S., Jr.; Corrêa, J.O.d.A. Comparative analysis for strength serum sodium and potassium in three different methods: Flame photometry, ion-selective electrode (ISE) and colorimetric enzymatic. J. Clin. Lab. Anal. 2018, 32, e22594. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Elhadidy, M.G.; Elmasry, A.; Elsayed, H.R.H.; El-Nablaway, M.; Hamed, S.; Elalfy, M.M.; Rabei, M.R. Modulation of COX-2 and NADPH oxidase-4 by alpha-lipoic acid ameliorates busulfan-induced pulmonary injury in rats. Heliyon 2021, 7, e08171. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Bisen, P.S. Laboratory Protocols in Applied Life Sciences; Taylor & Francis: Abingdon, UK, 2014. [Google Scholar] [CrossRef]
- Elsayed, H.R.H.; Anbar, H.S.; Rabei, M.R.; Adel, M.; El-Gamal, R. Eicosapentaenoic and docosahexaenoic acids attenuate methotrexate-induced apoptosis and suppression of splenic T, B-Lymphocytes and macrophages with modulation of expression of CD3, CD20 and CD68. Tissue Cell 2021, 72, 101533. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef]
- Yamamoto, D.; Maki, T.; Herningtyas, E.H.; Ikeshita, N.; Shibahara, H.; Sugiyama, Y.; Nakanishi, S.; Iida, K.; Iguchi, G.; Takahashi, Y. Branched-chain amino acids protect against dexamethasone-induced soleus muscle atrophy in rats. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2010, 41, 819–827. [Google Scholar] [CrossRef]
- Sun, H.; Gong, Y.; Qiu, J.; Chen, Y.; Ding, F.; Zhao, Q. TRAF6 inhibition rescues dexamethasone-induced muscle atrophy. Int. J. Mol. Sci. 2014, 15, 11126–11141. [Google Scholar] [CrossRef]
- Noh, K.K.; Chung, K.W.; Choi, Y.J.; Park, M.H.; Jang, E.J.; Park, C.H.; Yoon, C.; Kim, N.D.; Kim, M.K.; Chung, H.Y. β–Hydroxy β–Methylbutyrate improves dexamethasone-induced muscle atrophy by modulating the muscle degradation pathway in SD rat. PLoS ONE 2014, 9, e102947. [Google Scholar] [CrossRef] [PubMed]
- Canepari, M.; Agoni, V.; Brocca, L.; Ghigo, E.; Gnesi, M.; Minetto, M.; Bottinelli, R. Structural and molecular adaptations to dexamethasone and unacylated ghrelin administration in skeletal muscle of the mice. J. Physiol. Pharmacol. 2018, 69, 283–296. [Google Scholar] [CrossRef]
- Baehr, L.M.; Furlow, J.D.; Bodine, S.C. Muscle sparing in muscle RING finger 1 null mice: Response to synthetic glucocorticoids. J. Physiol. 2011, 589, 4759–4776. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ashida, Y.; Tatebayashi, D.; Himori, K. Myofibrillar function differs markedly between denervated and dexamethasone-treated rat skeletal muscles: Role of mechanical load. PLoS ONE 2019, 14, e0223551. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.R.; Steiner, J.L.; Rossetti, M.L.; Kimball, S.R.; Gordon, B.S. A clinically relevant decrease in contractile force differentially regulates control of glucocorticoid receptor translocation in mouse skeletal muscle. J. Appl. Physiol. 2021, 130, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.C.C.; Rêgo-Monteiro, I.C.d.; Louzada, R.A.; Ortenzi, V.H.; Aguiar, A.P.d.; Abreu, E.S.d.; Cavalcanti-de-Albuquerque, J.P.A.; Hecht, F.; Oliveira, A.C.d.; Ceccatto, V.M. Differential expression of NADPH oxidases depends on skeletal muscle fiber type in rats. Oxidative Med. Cell. Longev. 2016, 2016, 6738701. [Google Scholar] [CrossRef] [PubMed]
- Osório Alves, J.; Matta Pereira, L.; Cabral Coutinho do Rêgo Monteiro, I.; Pontes dos Santos, L.H.; Soares Marreiros Ferraz, A.; Carneiro Loureiro, A.C.; Calado Lima, C.; Leal-Cardoso, J.H.; Pires Carvalho, D.; Soares Fortunato, R. Strenuous acute exercise induces slow and fast twitch-dependent NADPH oxidase expression in rat skeletal muscle. Antioxidants 2020, 9, 57. [Google Scholar] [CrossRef]
- Mostafa, A.F.; Samir, S.M. Ferulic Acid Promotes Growth of Both Fast Glycolytic and Slow Oxidative Skeletal Muscles in Corticosteroid-Induced Rat Myopathy. Med. J. Cairo Univ. 2019, 87, 1703–1715. [Google Scholar] [CrossRef]
- Bitar, M.S.; Nader, J.; Al-Ali, W.; Al Madhoun, A.; Arefanian, H.; Al-Mulla, F. Hydrogen Sulfide Donor NaHS Improves Metabolism and Reduces Muscle Atrophy in Type 2 Diabetes: Implication for Understanding Sarcopenic Pathophysiology. Oxid. Med. Cell Longev. 2018, 2018, 6825452. [Google Scholar] [CrossRef]
- Ichinoseki-Sekine, N.; Smuder, A.J.; Morton, A.B.; Hinkley, J.M.; Mor Huertas, A.; Powers, S.K. Hydrogen sulfide donor protects against mechanical ventilation-induced atrophy and contractile dysfunction in the rat diaphragm. Clin. Transl. Sci. 2021, 14, 2139–2145. [Google Scholar] [CrossRef]
- Wang, X.L.; Pan, L.L.; Long, F.; Wu, W.J.; Yan, D.; Xu, P.; Liu, S.Y.; Qin, M.; Jia, W.W.; Liu, X.H.; et al. Endogenous Hydrogen Sulfide Ameliorates NOX4 Induced Oxidative Stress in LPS-Stimulated Macrophages and Mice. Cell Physiol. Biochem. 2018, 47, 458–474. [Google Scholar] [CrossRef]
- Yi, J.; Yuan, Y.; Zheng, J.; Zhao, T. Hydrogen sulfide alleviates uranium-induced rat hepatocyte cytotoxicity via inhibiting Nox4/ROS/p38 MAPK pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22255. [Google Scholar] [CrossRef]
- Scheid, S.; Goeller, M.; Baar, W.; Wollborn, J.; Buerkle, H.; Schlunck, G.; Lagreze, W.; Goebel, U.; Ulbrich, F. Hydrogen Sulfide Reduces Ischemia and Reperfusion Injury in Neuronal Cells in a Dose- and Time-Dependent Manner. Int. J. Mol. Sci. 2021, 22, 10099. [Google Scholar] [CrossRef]
- Tang, J.J.; Podratz, J.L.; Lange, M.; Scrable, H.J.; Jang, M.-H.; Windebank, A.J. Mechano growth factor, a splice variant of IGF-1, promotes neurogenesis in the aging mouse brain. Mol. Brain 2017, 10, 23. [Google Scholar] [CrossRef]
- Jing, X.; Ye, Y.; Bao, Y.; Zhang, J.; Huang, J.; Wang, R.; Guo, J.; Guo, F. Mechano-growth factor protects against mechanical overload induced damage and promotes migration of growth plate chondrocytes through RhoA/YAP pathway. Exp. Cell Res. 2018, 366, 81–91. [Google Scholar] [CrossRef]
- Abdelmonem, M.; Shahin, N.N.; Rashed, L.A.; Amin, H.A.A.; Shamaa, A.A.; Shaheen, A.A. Hydrogen sulfide enhances the effectiveness of mesenchymal stem cell therapy in rats with heart failure: In vitro preconditioning versus in vivo co-delivery. Biomed. Pharmacother. 2019, 112, 108584. [Google Scholar] [CrossRef]
- Sha, Y.; Yang, L.; Lv, Y. MGF E peptide improves anterior cruciate ligament repair by inhibiting hypoxia-induced cell apoptosis and accelerating angiogenesis. J. Cell. Physiol. 2019, 234, 8846–8861. [Google Scholar] [CrossRef]
- Cheng, Z.; Kishore, R. Potential role of hydrogen sulfide in diabetes-impaired angiogenesis and ischemic tissue repair. Redox Biol. 2020, 37, 101704. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Zhang, J.; Dong, G.; Xiao, W.; Xu, X. Hydrogen Sulfide Alleviates Skeletal Muscle Fibrosis via Attenuating Inflammation and Oxidative Stress. Front. Physiol. 2020, 11, 533690. [Google Scholar] [CrossRef]
- di Villa Bianca, R.d.E.; Mitidieri, E.; Donnarumma, E.; Tramontano, T.; Brancaleone, V.; Cirino, G.; Bucci, M.; Sorrentino, R. Hydrogen sulfide is involved in dexamethasone-induced hypertension in rat. Nitric Oxide 2015, 46, 80–86. [Google Scholar] [CrossRef]
- Micheli, L.; Mitidieri, E.; Turnaturi, C.; Vanacore, D.; Ciampi, C.; Lucarini, E.; Cirino, G.; Ghelardini, C.; Sorrentino, R.; Di Cesare Mannelli, L.; et al. Beneficial Effect of H2S-Releasing Molecules in an In Vitro Model of Sarcopenia: Relevance of Glucoraphanin. Int. J. Mol. Sci. 2022, 23, 5955. [Google Scholar] [CrossRef] [PubMed]



| Studied Parameter | Reference | Means | Standard Deviations | Effect Sizes | Sample Sizes |
|---|---|---|---|---|---|
| CD31 | [2] | 35, 60, 70 and 80 | 4 | 4.1810 | 8 |
| NOX4 | [6] | 1, 1.15, 1.3 and 1.65 | 1.15 | 1.6073 | 12 |
| CD163 | [13] | 2, 3, 25 and 37 | 3 | 4.9575 | 8 |
| Myostatin | [14] | 1, 1.2, 1.2 and 4 | 0.3 | 4.1466 | 8 |
| VEGF | [15] | 90, 80, 100 and 100 | 4 | 1.0308 | 24 |
| Gene | Sequence |
|---|---|
| Myostatin | Forward primer: TGCTGTAACCTTCCCAGGACCA Reverse primer: GTGAGGGGGTAGCGACAGCAC |
| NOX-4 | Forward primer: TGTTGGGCCTAGGATTGTGT Reverse primer: CTTCTGTGATCCGCGAAGGT |
| MGF | Forward primer: GGAGGCTGGAGATGTACTGTGCT Reverse primer: TCCTTTGCAGCTTCCTTTTCTTG |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Forward primer: AGGTCGGTGTGAACGGATTTG Reverse primer: TGTAGACCATGTAGTTGAGGTCA |
| Name | Cat. Number | Source and Clonality | Dilution |
|---|---|---|---|
| Caspase-3 | Servicebio GB11532 | Rabbit polyclonal | 1/500 |
| CD34 | Servicebio GB111693 | Rabbit polyclonal | 1/500 |
| VEGF | Servicebio GB14165 | Mouse monoclonal | 1/200 |
| CD31 | Dako M0823 | Mouse monoclonal | 1/200 |
| CD163 | Servicebio GB11340-1 | Rabbit polyclonal | 1/500 |
| Saline Control | Dex | Dex + H2SDonor (NaHS) | Dex + H2S Blocker (AOAA) | F Value | p Value | |
|---|---|---|---|---|---|---|
| Muscle mass (mg) | 127.8 ± 13.02 | 98.35 ± 7.027 * | 122.4 ± 14.40 # | 93.58 ± 10.24 * | 13.161 | <0.0005 |
| Optimal muscle length (mm) | 12.56 ± 0.615 | 10.27 ± 0.603 * | 11.72 ± 1.345 # | 8.983 ± 0.693 * | 19.784 | <0.0005 |
| Cross-sectional area (μm2) | 3497 ± 418.5 | 2470 ± 438.4 * | 3229 ± 520.3 # | 2060 ± 205.2 * | 15.578 | <0.0005 |
| Control Sal. | Dex | Dex + H2S Donor (NaHS) | Dex + H2S Blocker (AOAA) | F Value | p Value | |
|---|---|---|---|---|---|---|
| Time to peak twitch (ms) | 45.19 ± 07.223 | 25.54 ± 3.252 * | 35.62 ± 4.909 #* | 23.64 ± 4.656 * | 21.941 | <0.0005 |
| Half relaxation time (ms) | 53.84 ± 10.61 | 38.20 ± 4.218 * | 51.88 ± 2.940 # | 35.41 ± 4.335 * | 13.368 | <0.0005 |
| Max. isometric twitch force (g) | 23.77 ± 4.464 | 5.28 ± 0.992 * | 20.56 ± 3.862 # | 3.26 ± 0.611 * | 72.376 | <0.0005 |
| Specific force (gm/m2) | 63.96 ± 12.01 | 34.00 ± 6.385 * | 57.45 ± 10.79 # | 22.42 ± 0.421 * | 28.673 | <0.0005 |
| Force after tetanic contraction (g) | 5.437 ± 0.819 | 3.863 ± 0.569 * | 5.23 ± 0.78 # | 3.00 ± 0.56 * | 16.710 | <0.0005 |
| Negative Control | Dex | Dex + NaHS | Dex + AOAA | F Value | p Value | |
|---|---|---|---|---|---|---|
| MDA (nmol/mg) | 194.8 ± 32.31 | 345.2 ± 38.36 * | 239.6 ± 36.39 # | 410.6 ± 93.59 * | 18.386 | <0.0005 |
| GSH (nmol/mg) | 4.931 ± 0.158 | 3.908 ± 0.299 * | 4.619 ± 0.318 #* | 3.611 ± 0.098 * | 39.864 | <0.0005 |
| TAC (nmol Trolox Eq/mL) | 1.192 ± 0.189 | 0.745 ± 0.116 * | 1.051 ± 0.232 # | 0.544 ± 0.074 * | 19.016 | <0.0005 |
| Serum K⁺ level (mg/dL) | 4.513 ± 0.172 | 4.071 ± 0.125 * | 4.353 ± 0.142 # | 3.789 ± 0.157 #* | 27.146 | <0.0005 |
| Creatine kinase-MM (CK-MM) (ng/mL) | 17.09 ± 1.485 | 27.47 ± 4.392 * | 19.67 ± 2.299 # | 41.21 ± 7.159 *# | 36.120 | <0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adel, M.; Elsayed, H.R.H.; El-Nablaway, M.; Hamed, S.; Eladl, A.; Fouad, S.; El Nashar, E.M.; Al-Otaibi, M.L.; Rabei, M.R. Targeting Hydrogen Sulfide Modulates Dexamethasone-Induced Muscle Atrophy and Microvascular Rarefaction, through Inhibition of NOX4 and Induction of MGF, M2 Macrophages and Endothelial Progenitors. Cells 2022, 11, 2500. https://doi.org/10.3390/cells11162500
Adel M, Elsayed HRH, El-Nablaway M, Hamed S, Eladl A, Fouad S, El Nashar EM, Al-Otaibi ML, Rabei MR. Targeting Hydrogen Sulfide Modulates Dexamethasone-Induced Muscle Atrophy and Microvascular Rarefaction, through Inhibition of NOX4 and Induction of MGF, M2 Macrophages and Endothelial Progenitors. Cells. 2022; 11(16):2500. https://doi.org/10.3390/cells11162500
Chicago/Turabian StyleAdel, Mohamed, Hassan Reda Hassan Elsayed, Mohammad El-Nablaway, Shereen Hamed, Amira Eladl, Samah Fouad, Eman Mohamad El Nashar, Mohammed Lafi Al-Otaibi, and Mohammed R. Rabei. 2022. "Targeting Hydrogen Sulfide Modulates Dexamethasone-Induced Muscle Atrophy and Microvascular Rarefaction, through Inhibition of NOX4 and Induction of MGF, M2 Macrophages and Endothelial Progenitors" Cells 11, no. 16: 2500. https://doi.org/10.3390/cells11162500
APA StyleAdel, M., Elsayed, H. R. H., El-Nablaway, M., Hamed, S., Eladl, A., Fouad, S., El Nashar, E. M., Al-Otaibi, M. L., & Rabei, M. R. (2022). Targeting Hydrogen Sulfide Modulates Dexamethasone-Induced Muscle Atrophy and Microvascular Rarefaction, through Inhibition of NOX4 and Induction of MGF, M2 Macrophages and Endothelial Progenitors. Cells, 11(16), 2500. https://doi.org/10.3390/cells11162500






