Sex-Dependent Role of Adipose Tissue HDAC9 in Diet-Induced Obesity and Metabolic Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Human Subjects
2.3. Adipose Tissue Fractionation
2.4. Quantitative PCR
2.5. Western Blot
2.6. Locomotor Activity, Food Consumption and Energy Expenditure Measurements
2.7. Body Composition Measurements
2.8. Hepatic Triglyceride Measurement
2.9. Adaptive Thermogenesis
2.10. Histology and Measurement of Adipocyte Cell Size
2.11. Glucose Tolerance Test
2.12. Insulin Tolerance Test
2.13. Statistics
3. Results
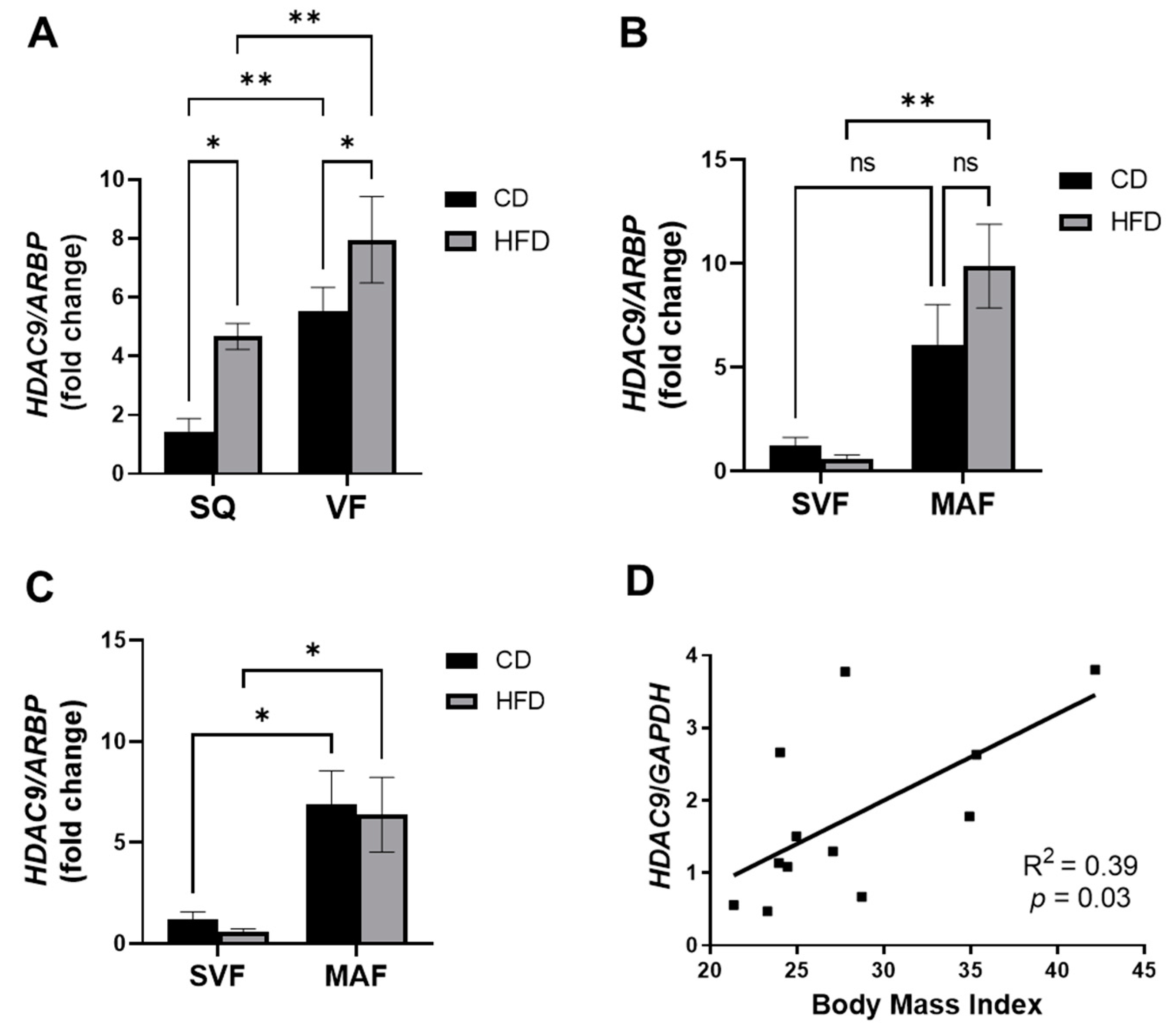
3.1. Elevated Expression of HDAC9 under Obese Conditions
3.2. Generation of Adipocyte-Specific HDAC9 KO Mice
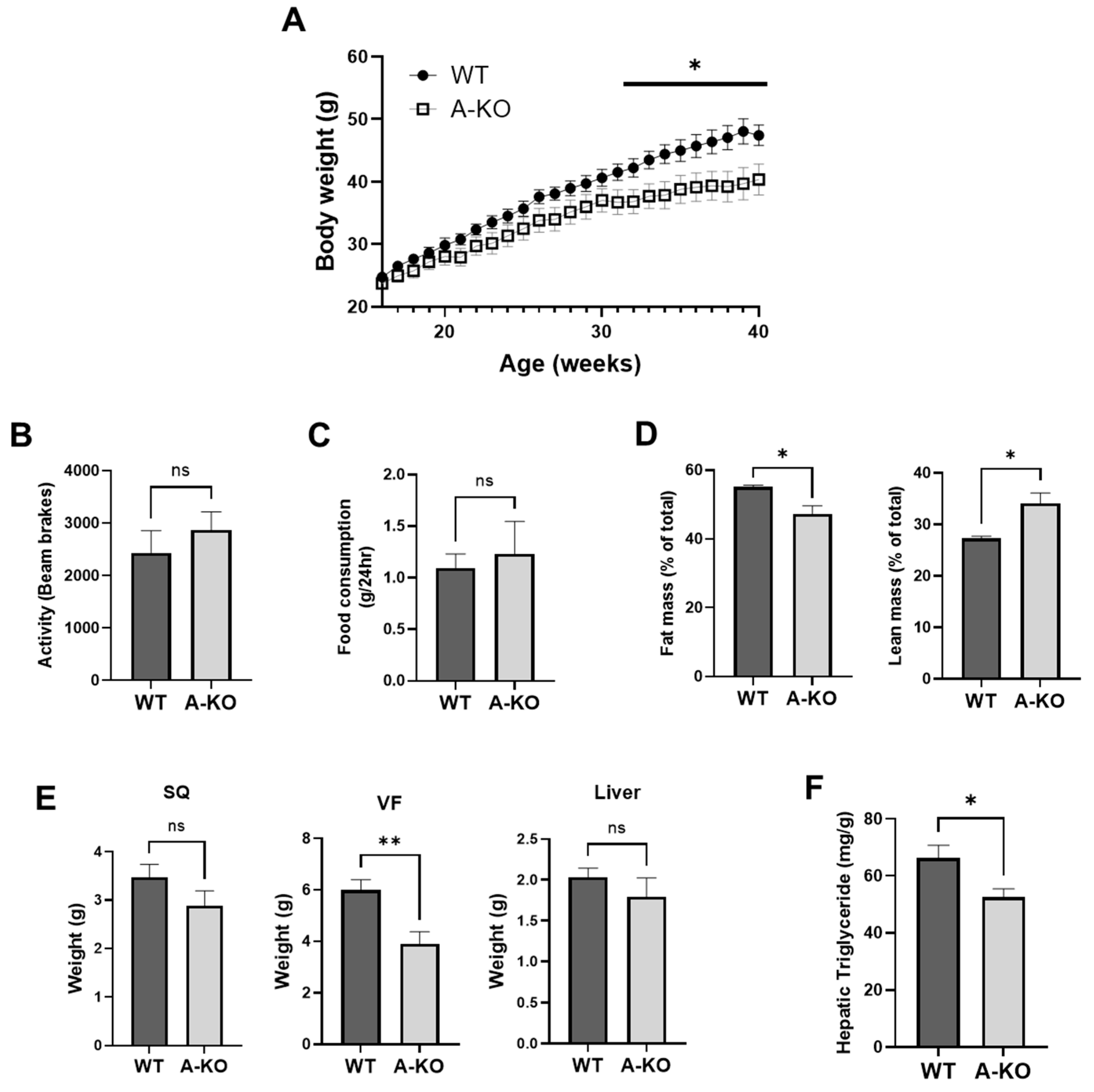
3.3. Adipocyte HDAC9 Gene Deletion Ameliorates HFD-Induced Obesity in Female Mice
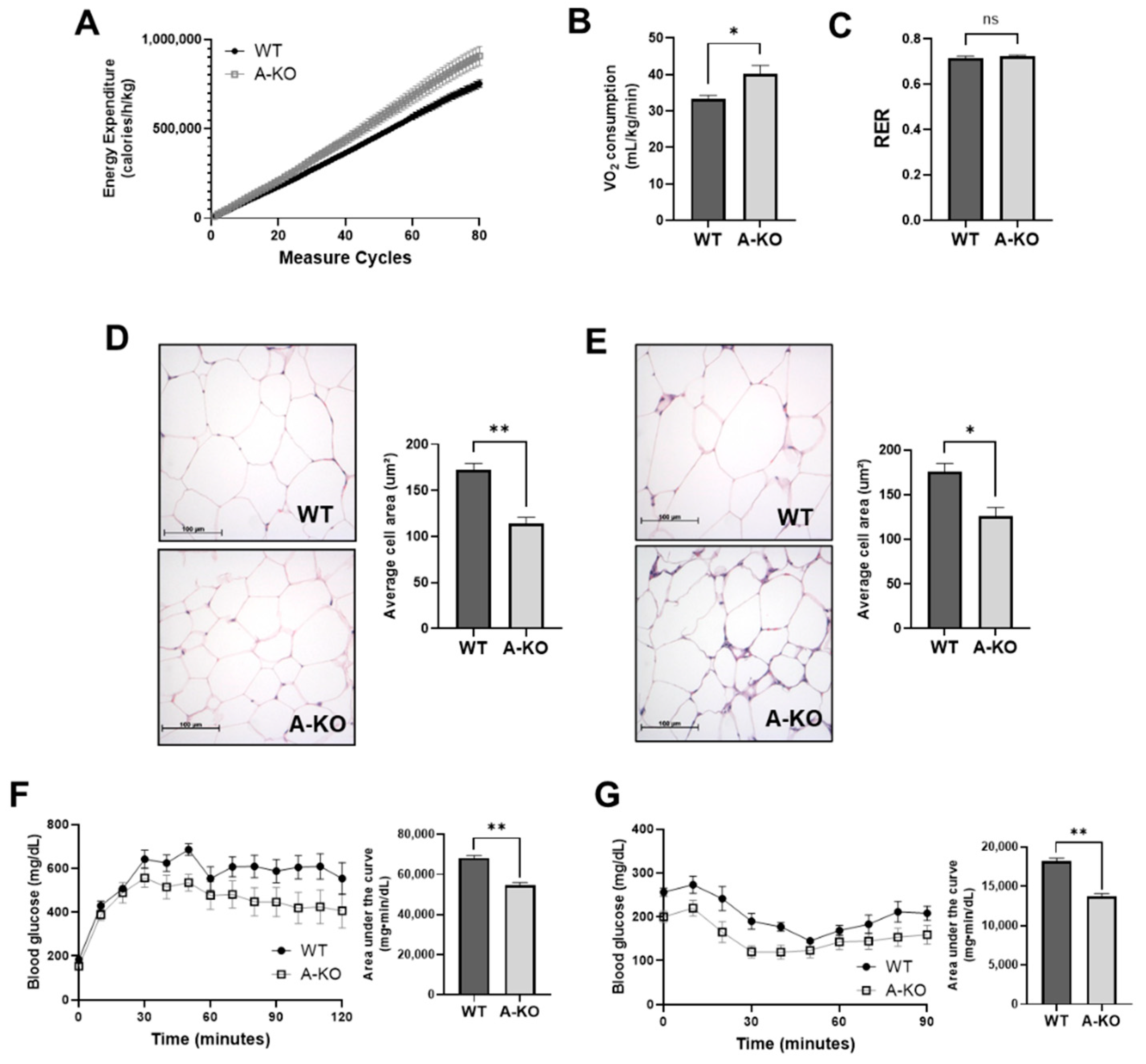
3.4. Adipocyte HDAC9 Regulates Energy Expenditure and Adaptive Thermogenesis
3.5. Adipocyte HDAC9 Gene Deletion Reduces Adipocyte Hypertrophy and Improves Glucose Tolerance and Insulin Sensitivity in HFD-Fed Female Mice
3.6. Body Weight and Metabolic Status Is Not Significantly Altered by Adipocyte HDAC9 Deletion in CD-Fed Mice
3.7. Adipocyte HDAC9 Gene Deletion Improves Adaptive Thermogenesis and Upregulates Expression of Adipogenic, Metabolic and Beiging Genes
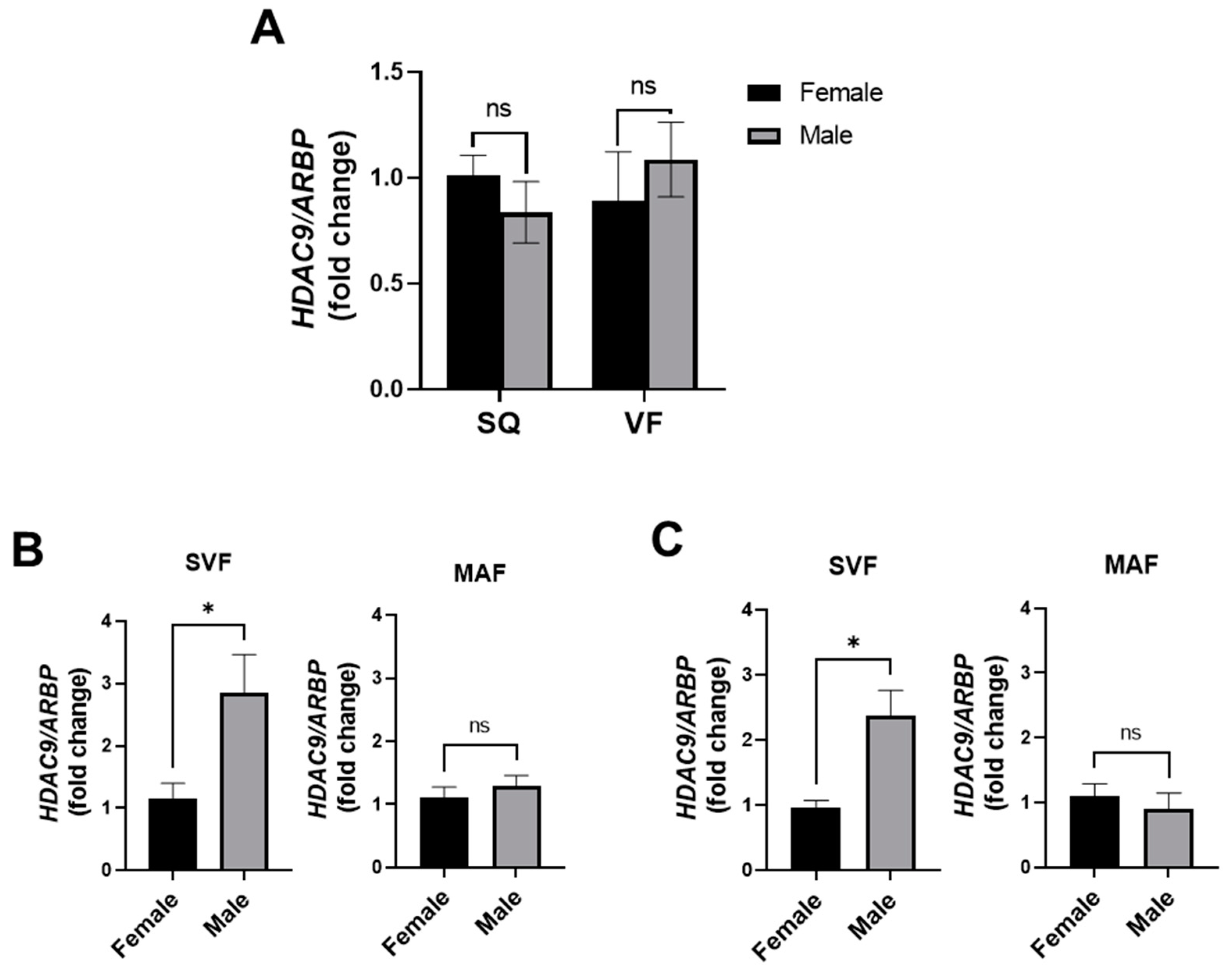
3.8. Adipose Tissue HDAC9 Is Differentially Regulated in a Sex-Dependent Manner
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Chatterjee, T.K.; Idelman, G.; Blanco, V.; Blomkalns, A.L.; Piegore, M.G., Jr.; Weintraub, D.S.; Kumar, S.; Rajsheker, S.; Manka, D.; Rudich, S.M.; et al. Histone Deacetylase 9 Is a Negative Regulator of Adipogenic Differentiation*. J. Biol. Chem. 2011, 286, 27836–27847. [Google Scholar] [CrossRef] [PubMed]
- Yiew, N.K.H.; Greenway, C.; Zarzour, A.; Ahmadieh, S.; Goo, B.; Kim, D.; Benson, T.W.; Ogbi, M.; Tang, Y.; Chen, W.; et al. Enhancer of zeste homolog 2 (EZH2) regulates adipocyte lipid metabolism independent of adipogenic differentiation: Role of apolipoprotein E. J. Biol. Chem. 2019, 294, 8577–8591. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.K.; Stoll, L.L.; Denning, G.M.; Harrelson, A.; Blomkalns, A.L.; Idelman, G.; Rothenberg, F.G.; Neltner, B.; Romig-Martin, S.A.; Dickson, E.W.; et al. Proinflammatory Phenotype of Perivascular Adipocytes: Influence of high-fat feeding. Circ. Res. 2009, 104, 541–549. [Google Scholar] [CrossRef]
- Yiew, N.K.H.; Chatterjee, T.K.; Tang, Y.; Pellenberg, R.; Stansfield, B.K.; Bagi, Z.; Fulton, D.J.; Stepp, D.W.; Chen, W.; Patel, V.; et al. A novel role for the Wnt inhibitor APCDD1 in adipocyte differentiation: Implications for diet-induced obesity. J. Biol. Chem. 2017, 292, 6312–6324. [Google Scholar] [CrossRef]
- Chatterjee, T.K.; Aronow, B.J.; Tong, W.S.; Manka, D.; Tang, Y.; Bogdanov, V.Y.; Unruh, D.; Blomkalns, A.L.; Piegore, M.G., Jr.; Weintraub, D.S.; et al. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol. Genom. 2013, 45, 697–709. [Google Scholar] [CrossRef]
- Chatterjee, T.K.; Basford, J.E.; Knoll, E.; Tong, W.S.; Blanco, V.; Blomkalns, A.L.; Rudich, S.; Lentsch, A.B.; Hui, D.Y.; Weintraub, N.L. HDAC9 Knockout Mice Are Protected From Adipose Tissue Dysfunction and Systemic Metabolic Disease During High-Fat Feeding. Diabetes 2013, 63, 176–187. [Google Scholar] [CrossRef]
- Benson, T.W.; Weintraub, D.S.; Crowe, M.; Yiew, N.K.; Popoola, O.; Pillai, A.; Joseph, J.; Archer, K.; Greenway, C.; Chatterjee, T.K.; et al. Deletion of the Duffy antigen receptor for chemokines (DARC) promotes insulin resistance and adipose tissue inflammation during high fat feeding. Mol. Cell. Endocrinol. 2018, 473, 79–88. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, Y.; Ikeda, K.; Chen, Y.; Alba, D.L.; Stifler, D.; Shinoda, K.; Hosono, T.; Maretich, P.; Yang, Y.; Ishigaki, Y.; et al. Repression of Adipose Tissue Fibrosis through a PRDM16-GTF2IRD1 Complex Improves Systemic Glucose Homeostasis. Cell Metab. 2018, 27, 180–194.e6. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diabetes Rep. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Rong, S.; Repa, J.; Clair, R.S.; Parks, J.S.; Mishra, N. Histone Deacetylase 9 Represses Cholesterol Efflux and Alternatively Activated Macrophages in Atherosclerosis Development. Arter. Thromb. Vasc. Biol. 2014, 34, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Lecce, L.; Xu, Y.; V’Gangula, B.; Chandel, N.; Pothula, V.; Caudrillier, A.; Santini, M.P.; D’Escamard, V.; Ceholski, D.K.; Gorski, P.A.; et al. Histone deacetylase 9 promotes endothelial-mesenchymal transition and an unfavorable atherosclerotic plaque phenotype. J. Clin. Investig. 2021, 131, e131178. [Google Scholar] [CrossRef]
- Wang, M.; Gu, M.; Li, Z.; Sun, B.; Cheng, X.; Dai, Z.; Li, S.; Xiao, L.; Zhao, M.; Wang, Z.; et al. HDAC9 Polymorphisms Predict Susceptibility, Severity, and Short-Term Outcome of Large Artery Atherosclerotic Stroke in Chinese Population. J. Mol. Neurosci. 2018, 67, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Qingxu, G.; Yan, Z.; Jiannan, X.; Yunlong, L. Association Between the Gene Polymorphisms of HDAC9 and the Risk of Atherosclerosis and Ischemic Stroke. Pathol. Oncol. Res. 2015, 22, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Chiou, H.-Y.; Bai, C.-H.; Lien, L.-M.; Hu, C.-J.; Jeng, J.-S.; Tang, S.-C.; Lin, H.-J.; Hsieh, Y.-C. Interactive Effects of a Combination of the HDAC3 and HDAC9 Genes with Diabetes Mellitus on the Risk of Ischemic Stroke. Thromb. Haemost. 2020, 121, 396–404. [Google Scholar] [CrossRef]
- Petr, M.A.; Alfaras, I.; Krawcyzk, M.; Bair, W.-N.; Mitchell, S.J.; Morrell, C.H.; Studenski, S.A.; Price, N.L.; Fishbein, K.W.; Spencer, R.G.; et al. A cross-sectional study of functional and metabolic changes during aging through the lifespan in male mice. eLife 2021, 10, e62952. [Google Scholar] [CrossRef]
- Yu, R.; Cao, X.; Sun, L.; Zhu, J.-Y.; Wasko, B.M.; Liu, W.; Crutcher, E.; Liu, H.; Jo, M.C.; Qin, L.; et al. Inactivating histone deacetylase HDA promotes longevity by mobilizing trehalose metabolism. Nat. Commun. 2021, 12, 1981. [Google Scholar] [CrossRef]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and Class II HDACs Control Neurogenic Muscle Atrophy by Inducing E3 Ubiquitin Ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, R.L.; Daniels, E.G.; Molenaars, M.; Houtkooper, R.H.; Janssens, G.E. From molecular promise to preclinical results: HDAC inhibitors in the race for healthy aging drugs. EMBO Mol. Med. 2019, 11, e9854. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.B.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef]
- Fuente-Martín, E.; Argente-Arizón, P.; Ros, P.; Argente, J.; Chowen, J.A. Sex Differences in Adipose Tissue: It Is Not Only a Question of Quantity and Distribution. Adipocyte 2013, 2, 128–134. [Google Scholar] [CrossRef]
- Nickelson, K.J.; Stromsdorfer, K.L.; Pickering, R.; Liu, T.-W.; Ortinau, L.C.; Keating, A.F.; Perfield, J.W. A Comparison of Inflammatory and Oxidative Stress Markers in Adipose Tissue from Weight-Matched Obese Male and Female Mice. Exp. Diabetes Res. 2012, 2012, 859395. [Google Scholar] [CrossRef]
- Estrany, M.E.; Proenza, A.M.; Lladó, I.; Gianotti, M. Isocaloric intake of a high-fat diet modifies adiposity and lipid handling in a sex dependent manner in rats. Lipids Heal. Dis. 2011, 10, 52. [Google Scholar] [CrossRef]
- Estrany, M.E.; Proenza, A.M.; Gianotti, M.; Lladó, I. High-fat diet feeding induces sex-dependent changes in inflammatory and insulin sensitivity profiles of rat adipose tissue. Cell Biochem. Funct. 2012, 31, 504–510. [Google Scholar] [CrossRef]
- Vasconcelos, R.P.; Peixoto, M.S.; De Oliveira, K.A.; Ferreira, A.C.F.; Coelho-De-Souza, A.N.; Carvalho, D.; Oliveira, A.; Fortunato, R.S. Sex differences in subcutaneous adipose tissue redox homeostasis and inflammation markers in control and high-fat diet fed rats. Appl. Physiol. Nutr. Metab. 2019, 44, 720–726. [Google Scholar] [CrossRef]
- Hauner, H. Secretory factors from human adipose tissue and their functional role. Proc. Nutr. Soc. 2005, 64, 163–169. [Google Scholar] [CrossRef]
- Joe, A.W.; Yi, L.; Even, Y.; Vogl, A.W.; Rossi, F.M. Depot-Specific Differences in Adipogenic Progenitor Abundance and Proliferative Response to High-Fat Diet. Stem Cells 2009, 27, 2563–2570. [Google Scholar] [CrossRef]
- Newell-Fugate, A.E. The role of sex steroids in white adipose tissue adipocyte function. Reproduction 2017, 153, R133–R149. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.; Lee, S.; Bowles, A.; Semon, J.; Bunnell, B.; Wu, X.; Gimble, J. Gender and age-related cell compositional differences in C57BL/6 murine adipose tissue stromal vascular fraction. Adipocyte 2018, 7, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Caër, C.; Rouault, C.; Le Roy, T.; Poitou, C.; Aron-Wisnewsky, J.; Torcivia, A.; Bichet, J.-C.; Clément, K.; Guerre-Millo, M.; André, S. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci. Rep. 2017, 7, 3000. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandari, P.; Arneson, D.; Hart, S.K.; Ahn, I.S.; Diamante, G.; Santos, L.C.; Zaghari, N.; Feng, A.-C.; Thomas, B.J.; Vergnes, L.; et al. Single cell analysis reveals immune cell–adipocyte crosstalk regulating the transcription of thermogenic adipocytes. eLife 2019, 8, e49501. [Google Scholar] [CrossRef]
- Macdougall, C.E.; Wood, E.G.; Loschko, J.; Scagliotti, V.; Cassidy, F.C.; Robinson, M.E.; Feldhahn, N.; Castellano, L.; Voisin, M.-B.; Marelli-Berg, F.; et al. Visceral Adipose Tissue Immune Homeostasis Is Regulated by the Crosstalk between Adipocytes and Dendritic Cell Subsets. Cell Metab. 2018, 27, 588–601.e4. [Google Scholar] [CrossRef]
- Chazenbalk, G.; Bertolotto, C.; Heneidi, S.; Jumabay, M.; Trivax, B.; Aronowitz, J.; Yoshimura, K.; Simmons, C.F.; Dumesic, D.A.; Azziz, R. Novel Pathway of Adipogenesis through Cross-Talk between Adipose Tissue Macrophages, Adipose Stem Cells and Adipocytes: Evidence of Cell Plasticity. PLoS ONE 2011, 6, e17834. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goo, B.; Ahmadieh, S.; Zarzour, A.; Yiew, N.K.H.; Kim, D.; Shi, H.; Greenway, J.; Cave, S.; Nguyen, J.; Aribindi, S.; et al. Sex-Dependent Role of Adipose Tissue HDAC9 in Diet-Induced Obesity and Metabolic Dysfunction. Cells 2022, 11, 2698. https://doi.org/10.3390/cells11172698
Goo B, Ahmadieh S, Zarzour A, Yiew NKH, Kim D, Shi H, Greenway J, Cave S, Nguyen J, Aribindi S, et al. Sex-Dependent Role of Adipose Tissue HDAC9 in Diet-Induced Obesity and Metabolic Dysfunction. Cells. 2022; 11(17):2698. https://doi.org/10.3390/cells11172698
Chicago/Turabian StyleGoo, Brandee, Samah Ahmadieh, Abdalrahman Zarzour, Nicole K. H. Yiew, David Kim, Hong Shi, Jacob Greenway, Stephen Cave, Jenny Nguyen, Swetha Aribindi, and et al. 2022. "Sex-Dependent Role of Adipose Tissue HDAC9 in Diet-Induced Obesity and Metabolic Dysfunction" Cells 11, no. 17: 2698. https://doi.org/10.3390/cells11172698







