Hypoxia as a Double-Edged Sword to Combat Obesity and Comorbidities
Abstract
1. Introduction
1.1. The Potential Connection between Endogenous Hypoxic or Hyperoxia in Adipose Tissues and Obesity
1.2. Hypoxic Training Ameliorates the Symptoms in Obesity
1.3. Revisit of the Previous Studies and Therapeutic Interventions
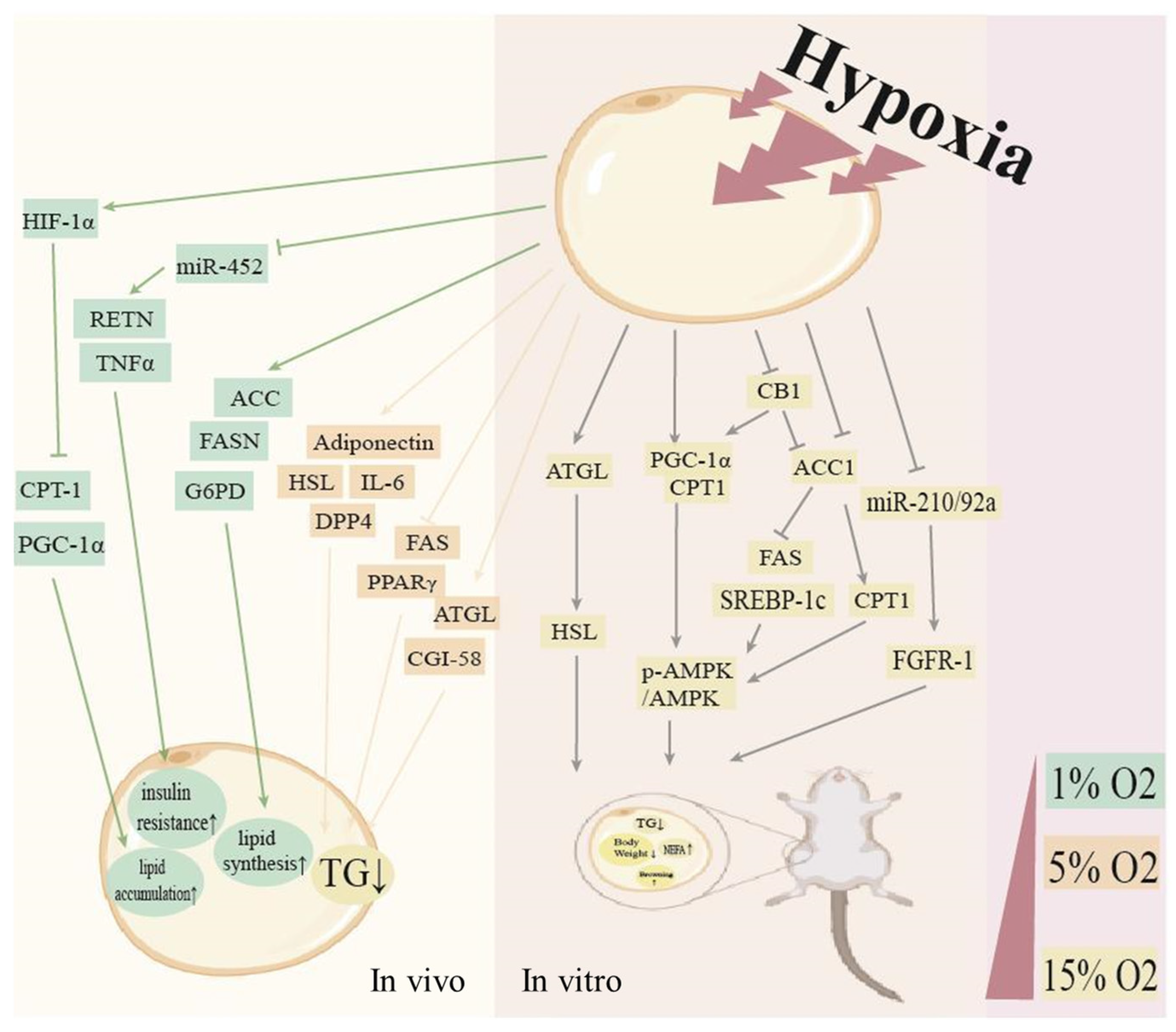
2. Endogenous Hypoxic in Adipose Tissue
2.1. Types of Adipose Tissue and Their Functions
2.2. Induction of the Endogenous Hypoxic Environment in Adipose Tissues and the Molecular Mediators
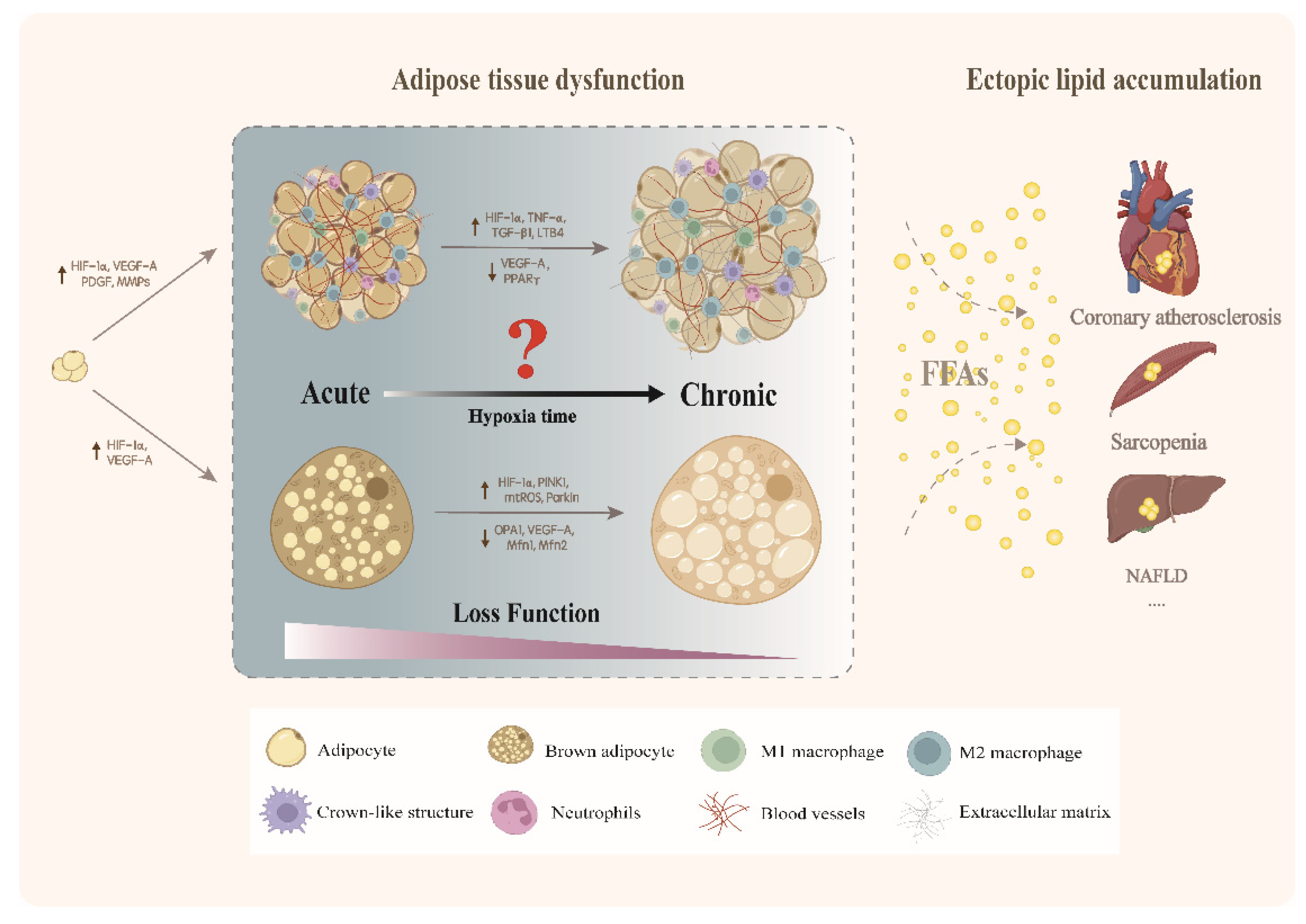
3. Endogenous Hypoxic and Adipose Tissue Dysfunction
3.1. Endogenous Hypoxic Modulates Dysfunction of White Adipose Tissue
3.1.1. Acute Endogenous Hypoxic Induces Adaptive Remodeling of White Adipose Tissue
3.1.2. Adverse Changes Induced by Chronic Endogenous Hypoxic
Aggravation of Inflammation
Fibrosis Contributes to the Dysfunction of White Adipose Tissue Induced by Hypoxic
Hypoxic Causes Adipose Tissue Dysfunction Accompanied with Ectopic Lipid Accumulation
Hypoxic Induces Mitochondrial Physiological Adaptation and Leads to Lipid Accumulation
3.2. Endogenous Hypoxic and Brown Adipose Tissue
4. Targeting the Critical Mediators in Endogenous Hypoxic to Combat Obesity and Comorbidities
4.1. Lysyl Oxidase (LOX) Inhibitors
4.2. Antagonists of LTB4 Receptor
5. Exogenous Hypoxic and Obesity
5.1. The Positive Effect of Exogenous Hypoxic
5.2. Hypoxic Caused Negative Energy Balance
5.3. Adverse Events of Obesity Due to Hypoxic
5.4. Mechanism of Different Hypoxic Concentration on Energy Metabolism
5.4.1. Exogenous Oxygen Concentrations Exceed 10%
5.4.2. The Oxygen Concentration of 5% to 10%
6. Application of Hypoxic Exercise in Treatment of Metabolic Disease
6.1. Obesity
6.2. Diabetes
7. Discussion and Future Direction
7.1. The Distinct Features of Endogenous and Exogenous Hypoxic
- How to define “acute” and “long-term” hypoxic periods;
- Given the complexity in the physiological conditions of organisms, previous experiments may not adequately mimic the endogenous hypoxic in adipose tissue.
7.2. The Translational Perspective of Combining Therapies to Target Endogenous and Exogenous Hypoxic
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zeng, Q.; Li, N.; Pan, X.F.; Chen, L.; Pan, A. Clinical Management and Treatment of Obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, T.B. Health Implications of Overweight and Obesity in the United States. Ann. Intern. Med. 1985, 103 Pt 2, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, Y.; Yi, C.X.; Tong, Q.; Cai, D. The Hypothalamus for Whole-Body Physiology: From Metabolism to Aging. Protein Cell 2022, 13, 394–421. [Google Scholar] [CrossRef]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sata, M. Roles of Perivascular Adipose Tissue in the Pathogenesis of Atherosclerosis. Front. Physiol. 2018, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Fruhbeck, G.; Gomez-Ambrosi, J.; Muruzabal, F.J.; Burrell, M.A. The Adipocyte: A Model for Integration of Endocrine and Metabolic Signaling in Energy Metabolism Regulation. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E827–E847. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Endocrine and Signalling Role of Adipose Tissue: New Perspectives on Fat. Acta Physiol. Scand. 2005, 184, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Rajala, M.W.; Scherer, P.E. Minireview: The Adipocyte—At the Crossroads of Energy Homeostasis, Inflammation, and Atherosclerosis. Endocrinology 2003, 144, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Gao, Z.; Yin, J.; He, Q. Hypoxia Is a Potential Risk Factor for Chronic Inflammation and Adiponectin Reduction in Adipose Tissue of Ob/Ob and Dietary Obese Mice. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1118–E1128. [Google Scholar] [CrossRef]
- Hosogai, N.; Fukuhara, A.; Oshima, K.; Miyata, Y.; Tanaka, S.; Segawa, K.; Furukawa, S.; Tochino, Y.; Komuro, R.; Matsuda, M.; et al. Adipose Tissue Hypoxia in Obesity and Its Impact on Adipocytokine Dysregulation. Diabetes 2007, 56, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.E.; Weisberg, S.; Vardhana, P.; Tortoriello, D.V. Obesity in C57bl/6j Mice Is Characterized by Adipose Tissue Hypoxia and Cytotoxic T-Cell Infiltration. Int. J. Obes. 2008, 32, 451–463. [Google Scholar] [CrossRef]
- Wood, I.S.; de Heredia, F.P.; Wang, B.; Trayhurn, P. Cellular Hypoxia and Adipose Tissue Dysfunction in Obesity. Proc. Nutr. Soc. 2009, 68, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and Adipocyte Physiology: Implications for Adipose Tissue Dysfunction in Obesity. Annu. Rev. Nutr. 2014, 34, 207–236. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Hypoxia and Adipose Tissue Function and Dysfunction in Obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef]
- Vink, R.G.; Roumans, N.J.; Cajlakovic, M.; Cleutjens, J.P.M.; Boekschoten, M.V.; Fazelzadeh, P.; Vogel, M.A.A.; Blaak, E.E.; Mariman, E.C.; van Baak, M.A.; et al. Diet-Induced Weight Loss Decreases Adipose Tissue Oxygen Tension with Parallel Changes in Adipose Tissue Phenotype and Insulin Sensitivity in Overweight Humans. Int. J. Obes. 2017, 41, 722–728. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-Inducible Factors and the Response to Hypoxic Stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef]
- Pang, C.; Gao, Z.; Yin, J.; Zhang, J.; Jia, W.; Ye, J. Macrophage Infiltration into Adipose Tissue May Promote Angiogenesis for Adipose Tissue Remodeling in Obesity. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E313–E322. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of Hif-Alpha to the Von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Keophiphath, M.; Achard, V.; Henegar, C.; Rouault, C.; Clément, K.; Lacasa, D. Macrophage-Secreted Factors Promote a Profibrotic Phenotype in Human Preadipocytes. Mol. Endocrinol. 2009, 23, 11–24. [Google Scholar] [CrossRef]
- de Heredia, F.P.; Gomez-Martinez, S.; Marcos, A. Obesity, Inflammation and the Immune System. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Marcelin, G.; Gautier, E.L.; Clément, K. Adipose Tissue Fibrosis in Obesity: Etiology and Challenges. Annu Rev Physiol. 2022, 84, 135–155. [Google Scholar] [CrossRef]
- Sakers, A.; de Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-Tissue Plasticity in Health and Disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.S.; Chiu, W.T.; Hsu, P.L.; Lin, S.C.; Peng, I.C.; Wang, C.Y.; Tsai, S.J. Pathophysiological Implications of Hypoxia in Human Diseases. J. Biomed. Sci. 2020, 27, 63. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; del Buono, M.G.; Ozemek, C.; Lavie, C.J. Obesity, Risk of Diabetes and Role of Physical Activity, Exercise Training and Cardiorespiratory Fitness. Prog. Cardiovasc. Dis. 2019, 62, 327–333. [Google Scholar] [CrossRef]
- Gubert, C.; Hannan, A.J. Exercise Mimetics: Harnessing the Therapeutic Effects of Physical Activity. Nat. Rev. Drug. Discov. 2021, 20, 862–879. [Google Scholar] [CrossRef]
- Hobbins, L.; Hunter, S.; Gaoua, N.; Girard, O. Normobaric Hypoxic Conditioning to Maximize Weight Loss and Ameliorate Cardio-Metabolic Health in Obese Populations: A Systematic Review. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R251–R264. [Google Scholar] [CrossRef]
- Hamad, N.; Travis, S.P. Weight Loss at High Altitude: Pathophysiology and Practical Implications. Eur. J. Gastroenterol. Hepatol. 2006, 18, 5–10. [Google Scholar] [CrossRef]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.C.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the Cardiovascular System: Clinical Science and Cardiovascular Outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Serebrovskaya, T.V.; Manukhina, E.B.; Smith, M.L.; Downey, H.F.; Mallet, R.T. Intermittent Hypoxia: Cause of or Therapy for Systemic Hypertension? Exp. Biol. Med. 2008, 233, 627–650. [Google Scholar] [CrossRef]
- Netzer, N.C.; Chytra, R.; Kupper, T. Low Intense Physical Exercise in Normobaric Hypoxia Leads to More Weight Loss in Obese People Than Low Intense Physical Exercise in Normobaric Sham Hypoxia. Sleep Breath 2008, 12, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Wiesner, S.; Engeli, S.; Luft, F.C.; Jordan, J. Influences of Normobaric Hypoxia Training on Metabolic Risk Markers in Human Subjects. Med. Sci. Sports Exerc. 2008, 40, 1939–1944. [Google Scholar] [CrossRef]
- Liang, T.; Xu, Q.; Zhang, H.; Wang, S.; Diekwisch, T.G.H.; Qin, C.; Lu, Y. Enamel Defects Associated with Dentin Sialophosphoprotein Mutation in Mice. Front. Physiol. 2021, 12, 724098. [Google Scholar] [CrossRef]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood, J.C.; Burk, D.H.; Smith, S.R. Reduced Adipose Tissue Oxygenation in Human Obesity: Evidence for Rarefaction, Macrophage Chemotaxis, and Inflammation without an Angiogenic Response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef]
- Lawler, H.M.; Underkofler, C.M.; Kern, P.A.; Erickson, C.; Bredbeck, B.; Rasouli, N. Adipose Tissue Hypoxia, Inflammation, and Fibrosis in Obese Insulin-Sensitive and Obese Insulin-Resistant Subjects. J. Clin. Endocrinol. Metab. 2016, 101, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Cifarelli, V.; Beeman, S.C.; Smith, G.I.; Yoshino, J.; Morozov, D.; Beals, J.W.; Kayser, B.D.; Watrous, J.D.; Jain, M.; Patterson, B.W.; et al. Decreased Adipose Tissue Oxygenation Associates with Insulin Resistance in Individuals with Obesity. J. Clin. Investig. 2020, 130, 6688–6699. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G.H.; Bizzarri, A.; Venteclef, N.; Essers, Y.; Cleutjens, J.P.; Konings, E.; Jocken, J.W.; Cajlakovic, M.; Ribitsch, V.; Clément, K.; et al. Increased Adipose Tissue Oxygen Tension in Obese Compared with Lean Men Is Accompanied by Insulin Resistance, Impaired Adipose Tissue Capillarization, and Inflammation. Circulation 2011, 124, 67–76. [Google Scholar] [CrossRef]
- Hodson, L.; Humphreys, S.M.; Karpe, F.; Frayn, K.N. Metabolic Signatures of Human Adipose Tissue Hypoxia in Obesity. Diabetes 2013, 62, 1417–1425. [Google Scholar] [CrossRef]
- Kumari, M.; Heeren, J.; Scheja, L. Regulation of Immunometabolism in Adipose Tissue. Semin. Immunopathol. 2018, 40, 189–202. [Google Scholar] [CrossRef]
- Cat, A.N.; Briones, A.M. Isolation of Mature Adipocytes from White Adipose Tissue and Gene Expression Studies by Real-Time Quantitative Rt-Pcr. Methods Mol. Biol. 2017, 1527, 283–295. [Google Scholar]
- Zhu, Q.; An, Y.A.; Scherer, P.E. Mitochondrial Regulation and White Adipose Tissue Homeostasis. Trends Cell Biol. 2022, 32, 351–364. [Google Scholar] [CrossRef]
- Rui, L. Brown and Beige Adipose Tissues in Health and Disease. Compr. Physiol. 2017, 7, 1281–1306. [Google Scholar]
- Zhang, Y.; Chua, S., Jr. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar] [PubMed]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine Dysregulation and Adipose Tissue Inflammation in Human Obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Alves, C.R.R.; Stanford, K.I.; Middelbeek, R.J.W.; Nigro, P.; Ryan, R.E.; Xue, R.; Sakaguchi, M.; Lynes, M.D.; So, K.; et al. Tgf-Β2 Is an Exercise-Induced Adipokine That Regulates Glucose and Fatty Acid Metabolism. Nat. Metab. 2019, 1, 291–303. [Google Scholar] [CrossRef]
- Yu, Z.; Han, S.; Cao, X.; Zhu, C.; Wang, X.; Guo, X. Genetic Polymorphisms in Adipokine Genes and the Risk of Obesity: A Systematic Review and Meta-Analysis. Obesity (Silver Spring) 2012, 20, 396–406. [Google Scholar] [CrossRef]
- Romacho, T.; Elsen, M.; Röhrborn, D.; Eckel, J. Adipose Tissue and Its Role in Organ Crosstalk. Acta Physiol. 2014, 210, 733–753. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Dos Santos, A.R.; de Oliveira Zanuso, B.; Miola, V.F.B.; Barbalho, S.M.; Bueno, P.C.S.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef]
- Lepper, C.; Fan, C.M. Inducible Lineage Tracing of Pax7-Descendant Cells Reveals Embryonic Origin of Adult Satellite Cells. Genesis 2010, 48, 424–436. [Google Scholar] [CrossRef]
- Sanchez-Gurmaches, J.; Hung, C.M.; Sparks, C.A.; Tang, Y.; Li, H.; Guertin, D.A. Pten Loss in the Myf5 Lineage Redistributes Body Fat and Reveals Subsets of White Adipocytes That Arise from Myf5 Precursors. Cell Metab. 2012, 16, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Origins and Early Development of the Concept That Brown Adipose Tissue Thermogenesis Is Linked to Energy Balance and Obesity. Biochimie 2017, 134, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Peirce, V.; Carobbio, S.; Vidal-Puig, A. The Different Shades of Fat. Nature 2014, 510, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Baruch, A.; Wong, C.; Chinn, L.W.; Vaze, A.; Sonoda, J.; Gelzleichter, T.; Chen, S.; Lewin-Koh, N.; Morrow, L.; Dheerendra, S.; et al. Antibody-Mediated Activation of the Fgfr1/Klothoβ Complex Corrects Metabolic Dysfunction and Alters Food Preference in Obese Humans. Proc. Natl. Acad. Sci. USA 2020, 117, 28992–29000. [Google Scholar] [CrossRef]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose Tissue as an Endocrine Organ. Mol. Cell Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown Adipose Tissue as a Secretory Organ. Nat. Rev. Endocrinol. 2017, 13, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown Adipose Tissue Activity Controls Triglyceride Clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef]
- Grefhorst, A.; van den Beukel, J.C.; van Houten, E.L.; Steenbergen, J.; Visser, J.A.; Themmen, A.P. Estrogens Increase Expression of Bone Morphogenetic Protein 8b in Brown Adipose Tissue of Mice. Biol. Sex Differ. 2015, 6, 7. [Google Scholar] [CrossRef]
- Gavaldà-Navarro, A.; Villarroya, J.; Cereijo, R.; Giralt, M.; Villarroya, F. The Endocrine Role of Brown Adipose Tissue: An Update on Actors and Actions. Rev. Endocr. Metab. Disord. 2022, 23, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Miller, D.S. Energy Balance Following Sympathetic Denervation of Brown Adipose Tissue. Can. J. Physiol. Pharmacol. 1984, 62, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Kumar, D.; Gupta, S.; Varshney, S.; Rajan, S.; Srivastava, A.; Gupta, A.; Gupta, A.P.; Vishwakarma, A.L.; Gayen, J.R.; et al. Role of Brown Adipose Tissue in Modulating Adipose Tissue Inflammation and Insulin Resistance in High-Fat Diet Fed Mice. Eur. J. Pharmacol. 2019, 854, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Takx, R.A.; Ishai, A.; Truong, Q.A.; MacNabb, M.H.; Scherrer-Crosbie, M.; Tawakol, A. Supraclavicular Brown Adipose Tissue 18f-Fdg Uptake and Cardiovascular Disease. J. Nucl. Med. 2016, 57, 1221–1225. [Google Scholar] [CrossRef]
- Franssens, B.T.; Hoogduin, H.; Leiner, T.; van der Graaf, Y.; Visseren, F.L.J. Relation between Brown Adipose Tissue and Measures of Obesity and Metabolic Dysfunction in Patients with Cardiovascular Disease. J. Magn. Reson Imaging 2017, 46, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. Prdm16 Controls a Brown Fat/Skeletal Muscle Switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Wang, Q.A.; Song, A.; Vishvanath, L.; Busbuso, N.C.; Scherer, P.E.; Gupta, R.K. Cellular Origins of Beige Fat Cells Revisited. Diabetes 2019, 68, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shin, S.; Liu, L.; Jahan, I.; Ong, S.G.; Xu, P.; Berry, D.C.; Jiang, Y. Progenitor-Like Characteristics in a Subgroup of Ucp1+ Cells within White Adipose Tissue. Dev. Cell 2021, 56, 985–999.e4. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J Clin Invest 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Shao, M.; Hepler, C.; Zhang, Q.; Shan, B.; Vishvanath, L.; Henry, G.H.; Zhao, S.; An, Y.A.; Wu, Y.; Strand, D.W.; et al. Pathologic Hif1α Signaling Drives Adipose Progenitor Dysfunction in Obesity. Cell Stem Cell 2021, 28, 685–701.e7. [Google Scholar] [CrossRef]
- O’Rourke, R.W. Adipose Tissue and the Physiologic Underpinnings of Metabolic Disease. Surg. Obes. Relat. Dis. 2018, 14, 1755–1763. [Google Scholar] [CrossRef]
- Scarpace, P.J.; Matheny, M. Leptin Induction of Ucp1 Gene Expression Is Dependent on Sympathetic Innervation. Am. J. Physiol. 1998, 275, E259–E264. [Google Scholar] [CrossRef]
- Shen, J.; Tanida, M.; Niijima, A.; Nagai, K. In Vivo Effects of Leptin on Autonomic Nerve Activity and Lipolysis in Rats. Neurosci. Lett. 2007, 416, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Poekes, L.; Lanthier, N.; Leclercq, I.A. Brown Adipose Tissue: A Potential Target in the Fight against Obesity and the Metabolic Syndrome. Clin. Sci. 2015, 129, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Böhm, A.; Keuper, M.; Meile, T.; Zdichavsky, M.; Fritsche, A.; Häring, H.U.; de Angelis, M.H.; Staiger, H.; Franko, A. Increased Mitochondrial Respiration of Adipocytes from Metabolically Unhealthy Obese Compared to Healthy Obese Individuals. Sci. Rep. 2020, 10, 12407. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barrios, A.; Dirakvand, G.; Pervin, S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells 2021, 10, 3030. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, E.; Kurz, A.; Niedermayr, M.; Schebesta, K.; Kimberger, O.; Sessler, D.I.; Kabon, B.; Prager, G. Tissue Oxygenation in Obese and Non-Obese Patients During Laparoscopy. Obes. Surg. 2005, 15, 813–819. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Pouyssegur, J. Oxygen, a Source of Life and Stress. FEBS Lett. 2007, 581, 3582–3591. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, J.W.; Osborne, O.; Oh, D.Y.; Sasik, R.; Schenk, S.; Chen, A.; Chung, H.; Murphy, A.; Watkins, S.M.; et al. Increased Adipocyte O2 Consumption Triggers Hif-1α, Causing Inflammation and Insulin Resistance in Obesity. Cell 2014, 157, 1339–1352. [Google Scholar] [CrossRef]
- Choe, S.S.; Kim, J.B. Hypoxia-Inducible Factors: New Strategies for Treatment of Obesity-Induced Metabolic Diseases. Postgrad. Med. J. 2020, 96, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, Y.; Usui, I.; Ishizuka, K.; Bukhari, A.; Fujisaka, S.; Urakaze, M.; Haruta, T.; Kishimoto, T.; Naka, T.; Kobayashi, M. Effects of Pioglitazone on Suppressor of Cytokine Signaling 3 Expression: Potential Mechanisms for Its Effects on Insulin Sensitivity and Adiponectin Expression. Diabetes 2007, 56, 795–803. [Google Scholar] [CrossRef]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. Hif-1-Mediated Expression of Pyruvate Dehydrogenase Kinase: A Metabolic Switch Required for Cellular Adaptation to Hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Krishnan, J.; Danzer, C.; Simka, T.; Ukropec, J.; Walter, K.M.; Kumpf, S.; Mirtschink, P.; Ukropcova, B.; Gasperikova, D.; Pedrazzini, T.; et al. Dietary Obesity-Associated Hif1α Activation in Adipocytes Restricts Fatty Acid Oxidation and Energy Expenditure Via Suppression of the Sirt2-Nad+ System. Genes Dev. 2012, 26, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Poblete, J.M.S.; Ballinger, M.N.; Bao, S.; Alghothani, M.; Nevado, J.B., Jr.; Eubank, T.D.; Christman, J.W.; Magalang, U.J. Macrophage Hif-1α Mediates Obesity-Related Adipose Tissue Dysfunction Via Interleukin-1 Receptor-Associated Kinase M. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E689–E700. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Xie, C.; Jiang, C. The Role of Hypoxia-Inducible Factors in Metabolic Diseases. Nat. Rev. Endocrinol. 2018, 15, 21–32. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Kotzbeck, P.; Giordano, A.; Mondini, E.; Murano, I.; Severi, I.; Venema, W.; Cecchini, M.P.; Kershaw, E.E.; Barbatelli, G.; Haemmerle, G.; et al. Brown Adipose Tissue Whitening Leads to Brown Adipocyte Death and Adipose Tissue Inflammation. J. Lipid Res. 2018, 59, 784–794. [Google Scholar] [CrossRef]
- Blüher, M. Adipose Tissue Dysfunction Contributes to Obesity Related Metabolic Diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; An, Y.A.; Scherer, P.E. The Ominous Triad of Adipose Tissue Dysfunction: Inflammation, Fibrosis, and Impaired Angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef]
- Boudina, S.; Graham, T.E. Mitochondrial Function/Dysfunction in White Adipose Tissue. Exp. Physiol. 2014, 99, 1168–1178. [Google Scholar] [CrossRef]
- Ko, M.S.; Yun, J.Y.; Baek, I.J.; Jang, J.E.; Hwang, J.J.; Lee, S.E.; Heo, S.H.; Bader, D.A.; Lee, C.H.; Han, J.; et al. Mitophagy Deficiency Increases Nlrp3 to Induce Brown Fat Dysfunction in Mice. Autophagy 2021, 17, 1205–1221. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, Z.; Khazaei, M. The Relationship between Inflammatory Markers, Angiogenesis, and Obesity. ARYA Atheroscler. 2013, 9, 247–253. [Google Scholar]
- Gealekman, O.; Gurav, K.; Chouinard, M.; Straubhaar, J.; Thompson, M.; Malkani, S.; Hartigan, C.; Corvera, S. Control of Adipose Tissue Expandability in Response to High Fat Diet by the Insulin-Like Growth Factor-Binding Protein-4. J. Biol. Chem. 2014, 289, 18327–18338. [Google Scholar] [CrossRef]
- Herold, J.; Kalucka, J. Angiogenesis in Adipose Tissue: The Interplay between Adipose and Endothelial Cells. Front. Physiol. 2020, 11, 624903. [Google Scholar] [CrossRef] [PubMed]
- Onogi, Y.; Wada, T.; Kamiya, C.; Inata, K.; Matsuzawa, T.; Inaba, Y.; Kimura, K.; Inoue, H.; Yamamoto, S.; Ishii, Y.; et al. Pdgfrβ Regulates Adipose Tissue Expansion and Glucose Metabolism Via Vascular Remodeling in Diet-Induced Obesity. Diabetes 2017, 66, 1008–1021. [Google Scholar] [CrossRef]
- Akama, T.; Chun, T.H. Transcription Factor 21 (Tcf21) Promotes Proinflammatory Interleukin 6 Expression and Extracellular Matrix Remodeling in Visceral Adipose Stem Cells. J. Biol. Chem. 2018, 293, 6603–6610. [Google Scholar] [CrossRef]
- Babaei, R.; Schuster, M.; Meln, I.; Lerch, S.; Ghandour, R.A.; Pisani, D.F.; Bayindir-Buchhalter, I.; Marx, J.; Wu, S.; Schoiswohl, G.; et al. Jak-Tgfβ Cross-Talk Links Transient Adipose Tissue Inflammation to Beige Adipogenesis. Sci. Signal. 2018, 11, eaai7838. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Y.; Chen, C.; Yang, L.; Lee, H.H.; Wang, Z.; Zhang, N.; Kolonin, M.G.; An, Z.; Ge, X.; et al. Critical Role of Matrix Metalloproteinase 14 in Adipose Tissue Remodeling During Obesity. Mol. Cell Biol. 2020, 40, e00564-19. [Google Scholar] [CrossRef]
- de Jong, J.M.; Larsson, O.; Cannon, B.; Nedergaard, J. A Stringent Validation of Mouse Adipose Tissue Identity Markers. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1085–E1105. [Google Scholar] [CrossRef]
- Sam, S.; Mazzone, T. Adipose Tissue Changes in Obesity and the Impact on Metabolic Function. Transl. Res. 2014, 164, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, A.; Mahmood, A.; Nawaz, A.; Kado, T.; Okabe, K.; Yamamoto, S.; Aminuddin, A.; Senda, S.; Tsuneyama, K.; Ikutani, M.; et al. Hif-1α in Myeloid Cells Promotes Adipose Tissue Remodeling toward Insulin Resistance. Diabetes 2016, 65, 3649–3659. [Google Scholar] [CrossRef]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome with Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity (Silver Spring) 2011, 19, 1109–1117. [Google Scholar] [CrossRef]
- Regazzetti, C.; Peraldi, P.; Grémeaux, T.; Najem-Lendom, R.; Ben-Sahra, I.; Cormont, M.; Bost, F.; le Marchand-Brustel, Y.; Tanti, J.F.; Giorgetti-Peraldi, S. Hypoxia Decreases Insulin Signaling Pathways in Adipocytes. Diabetes 2009, 58, 95–103. [Google Scholar] [CrossRef]
- Escribese, M.M.; Casas, M.; Corbí, A.L. Influence of Low Oxygen Tensions on Macrophage Polarization. Immunobiology 2012, 217, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.; de Souza, C.N.; Câmara, N.O.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2015, 6, 637. [Google Scholar] [CrossRef]
- Xu, X.; Grijalva, A.; Skowronski, A.; van Eijk, M.; Serlie, M.J.; Ferrante, A.W., Jr. Obesity Activates a Program of Lysosomal-Dependent Lipid Metabolism in Adipose Tissue Macrophages Independently of Classic Activation. Cell Metab. 2013, 18, 816–830. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Budavari, A.; Murray, D.; Spiegelman, B.M. Reduced Tyrosine Kinase Activity of the Insulin Receptor in Obesity-Diabetes. Central Role of Tumor Necrosis Factor-Alpha. J. Clin. Investig. 1994, 94, 1543–1549. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased Adipose Tissue Expression of Tumor Necrosis Factor-Alpha in Human Obesity and Insulin Resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Gual, P.; le Marchand-Brustel, Y.; Tanti, J.F. Positive and Negative Regulation of Insulin Signaling through Irs-1 Phosphorylation. Biochimie 2005, 87, 99–109. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Domínguez, A.P.; Drägert, K.; Humar, R.; Haas, E.; Battegay, E.J. Hypoxia Potentiates Tumor Necrosis Factor-A Induced Expression of Inducible Nitric Oxide Synthase and Cyclooxygenase-2 in White and Brown Adipocytes. Biochem. Biophys. Res. Commun. 2015, 461, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, J.I.; Ricardo-Gonzalez, R.R.; Goforth, M.H.; Morel, C.R.; Subramanian, V.; Mukundan, L.; Eagle, A.R.; Vats, D.; Brombacher, F.; Ferrante, A.W.; et al. Macrophage-Specific Ppargamma Controls Alternative Activation and Improves Insulin Resistance. Nature 2007, 447, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Usui, I.; Bukhari, A.; Ikutani, M.; Oya, T.; Kanatani, Y.; Tsuneyama, K.; Nagai, Y.; Takatsu, K.; Urakaze, M.; et al. Regulatory Mechanisms for Adipose Tissue M1 and M2 Macrophages in Diet-Induced Obese Mice. Diabetes 2009, 58, 2574–2582. [Google Scholar] [CrossRef]
- Ferreira, A.E.; Sisti, F.; Sônego, F.; Wang, S.; Filgueiras, L.R.; Brandt, S.; Serezani, A.P.; Du, H.; Cunha, F.Q.; Alves-Filho, J.C.; et al. Ppar-Γ/Il-10 Axis Inhibits Myd88 Expression and Ameliorates Murine Polymicrobial Sepsis. J. Immunol. 2014, 192, 2357–2365. [Google Scholar] [CrossRef]
- Gustafson, B.; Smith, U. Cytokines Promote Wnt Signaling and Inflammation and Impair the Normal Differentiation and Lipid Accumulation in 3t3-L1 Preadipocytes. J. Biol. Chem. 2006, 281, 9507–9516. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and Inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Segerstrom, S.C. Stress, Energy, and Immunity: An Ecological View. Curr. Dir. Psychol. Sci. 2007, 16, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, M.M.; Wang, K.; Adler, A.J.; Vella, A.T.; Zhou, B. Macrophage Polarization and Meta-Inflammation. Transl. Res. 2018, 191, 29–44. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal Muscle Inflammation and Insulin Resistance in Obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Ye, J. Regulation of Ppargamma Function by Tnf-Alpha. Biochem. Biophys. Res. Commun. 2008, 374, 405–408. [Google Scholar] [CrossRef]
- Liu, B.; Tan, P. Ppar Γ/Tlr4/Tgf-Β1 Axis Mediates the Protection Effect of Erythropoietin on Cyclosporin a-Induced Chronic Nephropathy in Rat. Ren. Fail. 2020, 42, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.C.; Kumari, M.; Taleb, S.; Tenen, D.; Jacobs, C.; Lyubetskaya, A.; Tsai, L.T.; Rosen, E.D. Adipocytes Fail to Maintain Cellular Identity During Obesity Due to Reduced Pparγ Activity and Elevated Tgfβ-Smad Signaling. Mol. Metab. 2020, 42, 101086. [Google Scholar] [CrossRef] [PubMed]
- Stanek, A.; Brozyna-Tkaczyk, K.; Myslinski, W. The Role of Obesity-Induced Perivascular Adipose Tissue (Pvat) Dysfunction in Vascular Homeostasis. Nutrients 2021, 13, 3843. [Google Scholar] [CrossRef] [PubMed]
- Famulla, S.; Horrighs, A.; Cramer, A.; Sell, H.; Eckel, J. Hypoxia Reduces the Response of Human Adipocytes Towards Tnfalpha Resulting in Reduced Nf-Kappab Signaling and Mcp-1 Secretion. Int. J. Obes. 2012, 36, 986–992. [Google Scholar] [CrossRef]
- Vogel, M.A.A.; Jocken, J.W.E.; Sell, H.; Hoebers, N.; Essers, Y.; Rouschop, K.M.A.; Cajlakovic, M.; Blaak, E.E.; Goossens, G.H. Differences in Upper and Lower Body Adipose Tissue Oxygen Tension Contribute to the Adipose Tissue Phenotype in Humans. J. Clin. Endocrinol. Metab. 2018, 103, 3688–3697. [Google Scholar] [CrossRef]
- Famulla, S.; Schlich, R.; Sell, H.; Eckel, J. Differentiation of Human Adipocytes at Physiological Oxygen Levels Results in Increased Adiponectin Secretion and Isoproterenol-Stimulated Lipolysis. Adipocyte 2012, 1, 132–181. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and Adipose Tissue Dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Marcelin, G.; Silveira, A.L.M.; Martins, L.B.; Ferreira, A.V.; Clément, K. Deciphering the Cellular Interplays Underlying Obesity-Induced Adipose Tissue Fibrosis. J. Clin. Investig. 2019, 129, 4032–4040. [Google Scholar] [CrossRef]
- Datta, R.; Podolsky, M.J.; Atabai, K. Fat Fibrosis: Friend or Foe? JCI Insight 2018, 3, e122289. [Google Scholar] [CrossRef]
- McMahon, M.; Ye, S.; Pedrina, J.; Dlugolenski, D.; Stambas, J. Extracellular Matrix Enzymes and Immune Cell Biology. Front. Mol. Biosci. 2021, 8, 703868. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug. Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Martins, V.; de los Santos, F.G.; Wu, Z.; Capelozzi, V.; Phan, S.H.; Liu, T. Fizz1-Induced Myofibroblast Transdifferentiation from Adipocytes and Its Potential Role in Dermal Fibrosis and Lipoatrophy. Am. J. Pathol. 2015, 185, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-Inducible Factor 1alpha Induces Fibrosis and Insulin Resistance in White Adipose Tissue. Mol. Cell Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, R.; Nakamura, K.; MacLauchlan, S.; Ngo, D.T.; Shimizu, I.; Fuster, J.J.; Katanasaka, Y.; Yoshida, S.; Qiu, Y.; Yamaguchi, T.P.; et al. An Antiangiogenic Isoform of Vegf-a Contributes to Impaired Vascularization in Peripheral Artery Disease. Nat. Med. 2014, 20, 1464–1471. [Google Scholar] [CrossRef]
- Karki, S.; Ngo, D.T.M.; Farb, M.G.; Park, S.Y.; Saggese, S.M.; Hamburg, N.M.; Carmine, B.; Hess, D.T.; Walsh, K.; Gokce, N. Wnt5a Regulates Adipose Tissue Angiogenesis Via Antiangiogenic Vegf-a(165)B in Obese Humans. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H200–H206. [Google Scholar] [CrossRef]
- Bai, W.; Zhou, J.; Zhou, N.; Liu, Q.; Cui, J.; Zou, W.; Zhang, W. Hypoxia-Increased Rage Expression Regulates Chemotaxis and Pro-Inflammatory Cytokines Release through Nuclear Translocation of Nf-Κ B and Hif1α in Thp-1 Cells. Biochem. Biophys. Res. Commun. 2018, 495, 2282–2288. [Google Scholar] [CrossRef]
- Unger, R.H.; Clark, G.O.; Scherer, P.E.; Orci, L. Lipid Homeostasis, Lipotoxicity and the Metabolic Syndrome. Biochim. Biophys. Acta 2010, 1801, 209–214. [Google Scholar] [CrossRef]
- Suganami, T.; Tanaka, M.; Ogawa, Y. Adipose Tissue Inflammation and Ectopic Lipid Accumulation. Endocr. J. 2012, 59, 849–857. [Google Scholar] [CrossRef]
- Tanaka, M.; Ikeda, K.; Suganami, T.; Komiya, C.; Ochi, K.; Shirakawa, I.; Hamaguchi, M.; Nishimura, S.; Manabe, I.; Matsuda, T.; et al. Macrophage-Inducible C-Type Lectin Underlies Obesity-Induced Adipose Tissue Fibrosis. Nat. Commun. 2014, 5, 4982. [Google Scholar] [CrossRef] [PubMed]
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of Hif Genes. Int. J. Mol. Sci. 2019, 20, 6140. [Google Scholar] [CrossRef]
- Mammoto, A.; Connor, K.M.; Mammoto, T.; Yung, C.W.; Huh, D.; Aderman, C.M.; Mostoslavsky, G.; Smith, L.E.; Ingber, D.E. A Mechanosensitive Transcriptional Mechanism That Controls Angiogenesis. Nature 2009, 457, 1103–1108. [Google Scholar] [CrossRef]
- Hunyenyiwa, T.; Hendee, K.; Matus, K.; Kyi, P.; Mammoto, T.; Mammoto, A. Obesity Inhibits Angiogenesis through Twist1-Slit2 Signaling. Front. Cell Dev. Biol. 2021, 9, 693410. [Google Scholar] [CrossRef]
- Fattet, L.; Jung, H.Y.; Matsumoto, M.W.; Aubol, B.E.; Kumar, A.; Adams, J.A.; Chen, A.C.; Sah, R.L.; Engler, A.J.; Pasquale, E.B.; et al. Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis Via a Mechanoresponsive Epha2/Lyn Complex. Dev. Cell 2020, 54, 302–316.e7. [Google Scholar] [CrossRef]
- Wang, Y.C.; Xie, H.; Zhang, Y.C.; Meng, Q.H.; Xiong, M.M.; Jia, M.W.; Peng, F.; Tang, D.L. Exosomal Mir-107 Antagonizes Profibrotic Phenotypes of Pericytes by Targeting a Pathway Involving Hif-1α/Notch1/Pdgfrβ/Yap1/Twist1 Axis in Vitro. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H520–H534. [Google Scholar] [CrossRef]
- Wierzbicki, A.S.; Oben, J. Nonalcoholic Fatty Liver Disease and Lipids. Curr. Opin. Lipidol. 2012, 23, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Berk, P.D.; Verna, E.C. Nonalcoholic Fatty Liver Disease: Lipids and Insulin Resistance. Clin. Liver Dis. 2016, 20, 245–262. [Google Scholar] [CrossRef]
- Chandel, N.S.; McClintock, D.S.; Feliciano, C.E.; Wood, T.M.; Melendez, J.A.; Rodriguez, A.M.; Schumacker, P.T. Reactive Oxygen Species Generated at Mitochondrial Complex Iii Stabilize Hypoxia-Inducible Factor-1alpha During Hypoxia: A Mechanism of O2 Sensing. J. Biol. Chem. 2000, 275, 25130–25138. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, E.; van de Velde, S.; Matsumura, S.; Hao, E.; LeLay, J.; Kaestner, K.; Montminy, M. Feedback Inhibition of Creb Signaling Promotes Beta Cell Dysfunction in Insulin Resistance. Cell Rep. 2015, 10, 1149–1157. [Google Scholar] [CrossRef]
- Woo, C.Y.; Jang, J.E.; Lee, S.E.; Koh, E.H.; Lee, K.U. Mitochondrial Dysfunction in Adipocytes as a Primary Cause of Adipose Tissue Inflammation. Diabetes Metab. J. 2019, 43, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.S.; Stezhka, T.; Trayhurn, P. Modulation of Adipokine Production, Glucose Uptake and Lactate Release in Human Adipocytes by Small Changes in Oxygen Tension. Pflugers Arch. 2011, 462, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, R.; Hu, Z.; Tian, Y.; Zhu, Y.; Gu, L.; Zhou, L. Pi3k and Erk-Induced Rac1 Activation Mediates Hypoxia-Induced Hif-1α Expression in Mcf-7 Breast Cancer Cells. PLoS ONE 2011, 6, e25213. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Heredia, F.; Wood, I.S.; Trayhurn, P. Hypoxia Stimulates Lactate Release and Modulates Monocarboxylate Transporter (Mct1, Mct2, and Mct4) Expression in Human Adipocytes. Pflugers Arch. 2010, 459, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Qvisth, V.; Hagström-Toft, E.; Moberg, E.; Sjöberg, S.; Bolinder, J. Lactate Release from Adipose Tissue and Skeletal Muscle in Vivo: Defective Insulin Regulation in Insulin-Resistant Obese Women. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E709–E714. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of Glycolysis by the Hypoxia-Inducible Factor (Hif): Implications for Cellular Physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef]
- Cheng, L.; Qin, T.; Ma, J.; Duan, W.; Xu, Q.; Li, X.; Han, L.; Li, W.; Wang, Z.; Zhang, D.; et al. Hypoxia-Inducible Factor-1α Mediates Hyperglycemia-Induced Pancreatic Cancer Glycolysis. Anticancer Agents Med. Chem. 2019, 19, 1503–1512. [Google Scholar] [CrossRef]
- Ahmed, K.; Tunaru, S.; Tang, C.; Müller, M.; Gille, A.; Sassmann, A.; Hanson, J.; Offermanns, S. An Autocrine Lactate Loop Mediates Insulin-Dependent Inhibition of Lipolysis through Gpr81. Cell Metab. 2010, 11, 311–319. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, L.; Sun, J.; Qu, Y.; Chen, M. The Role of Camp-Pka Pathway in Lactate-Induced Intramuscular Triglyceride Accumulation and Mitochondria Content Increase in Mice. Front. Physiol. 2021, 12, 709135. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate as a Fulcrum of Metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef]
- Cadoudal, T.; Blouin, J.M.; Collinet, M.; Fouque, F.; Tan, G.D.; Loizon, E.; Beale, E.G.; Frayn, K.N.; Karpe, F.; Vidal, H.; et al. Acute and Selective Regulation of Glyceroneogenesis and Cytosolic Phosphoenolpyruvate Carboxykinase in Adipose Tissue by Thiazolidinediones in Type 2 Diabetes. Diabetologia 2007, 50, 666–675. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Obesity and the Risk of Myocardial Infarction in 27,000 Participants from 52 Countries: A Case-Control Study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Britton, K.A.; Massaro, J.M.; Murabito, J.M.; Kreger, B.E.; Hoffmann, U.; Fox, C.S. Body Fat Distribution, Incident Cardiovascular Disease, Cancer, and All-Cause Mortality. J. Am. Coll. Cardiol. 2013, 62, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, W.Y.; Bergstrom, R.W.; Boyko, E.J.; Chen, K.W.; Leonetti, D.L.; Newell-Morris, L.; Shofer, J.B.; Wahl, P.W. Visceral Adiposity and Incident Coronary Heart Disease in Japanese-American Men. The 10-Year Follow-up Results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care 1999, 22, 1808–1812. [Google Scholar] [CrossRef]
- Engin, A.B. What Is Lipotoxicity? Adv. Exp. Med. Biol. 2017, 960, 197–220. [Google Scholar] [PubMed]
- Shimizu, I.; Walsh, K. The Whitening of Brown Fat and Its Implications for Weight Management in Obesity. Curr. Obes. Rep. 2015, 4, 224–229. [Google Scholar] [CrossRef]
- Shimizu, I.; Aprahamian, T.; Kikuchi, R.; Shimizu, A.; Papanicolaou, K.N.; MacLauchlan, S.; Maruyama, S.; Walsh, K. Vascular Rarefaction Mediates Whitening of Brown Fat in Obesity. J. Clin. Investig. 2014, 124, 2099–2112. [Google Scholar] [CrossRef]
- Fredriksson, J.M.; Lindquist, J.M.; Bronnikov, G.E.; Nedergaard, J. Norepinephrine Induces Vascular Endothelial Growth Factor Gene Expression in Brown Adipocytes through a Beta -Adrenoreceptor/Camp/Protein Kinase a Pathway Involving Src but Independently of Erk1/2. J. Biol. Chem. 2000, 275, 13802–13811. [Google Scholar] [CrossRef]
- Lowell, B.B.; Susulic, V.S.; Hamann, A.; Lawitts, J.A.; Himms-Hagen, J.; Boyer, B.B.; Kozak, L.P.; Flier, J.S. Development of Obesity in Transgenic Mice after Genetic Ablation of Brown Adipose Tissue. Nature 1993, 366, 740–742. [Google Scholar] [CrossRef]
- Roberts-Toler, C.; O’Neill, B.T.; Cypess, A.M. Diet-Induced Obesity Causes Insulin Resistance in Mouse Brown Adipose Tissue. Obesity (Silver Spring) 2015, 23, 1765–1770. [Google Scholar] [CrossRef]
- Ahmad, B.; Vohra, M.S.; Saleemi, M.A.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Brown/Beige Adipose Tissues and the Emerging Role of Their Secretory Factors in Improving Metabolic Health: The Batokines. Biochimie 2021, 184, 26–39. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. New Powers of Brown Fat: Fighting the Metabolic Syndrome. Cell Metab. 2011, 13, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Wang, Q.; Tajima, K.; Matsushita, M.; Maki, H.; Igarashi, K.; Dai, Z.; White, P.J.; McGarrah, R.W.; Ilkayeva, O.R.; et al. Bcaa Catabolism in Brown Fat Controls Energy Homeostasis through Slc25a44. Nature 2019, 572, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Guzy, R.D.; Hoyos, B.; Robin, E.; Chen, H.; Liu, L.; Mansfield, K.D.; Simon, M.C.; Hammerling, U.; Schumacker, P.T. Mitochondrial Complex Iii Is Required for Hypoxia-Induced Ros Production and Cellular Oxygen Sensing. Cell Metab. 2005, 1, 401–408. [Google Scholar] [CrossRef] [PubMed]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial Dysfunction in Obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef]
- Sebastián, D.; Hernández-Alvarez, M.I.; Segalés, J.; Sorianello, E.; Muñoz, J.P.; Sala, D.; Waget, A.; Liesa, M.; Paz, J.C.; Gopalacharyulu, P.; et al. Mitofusin 2 (Mfn2) Links Mitochondrial and Endoplasmic Reticulum Function with Insulin Signaling and Is Essential for Normal Glucose Homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 5523–5528. [Google Scholar] [CrossRef]
- Quirós, P.M.; Ramsay, A.J.; Sala, D.; Fernández-Vizarra, E.; Rodríguez, F.; Peinado, J.R.; Fernández-García, M.S.; Vega, J.A.; Enríquez, J.A.; Zorzano, A.; et al. Loss of Mitochondrial Protease Oma1 Alters Processing of the Gtpase Opa1 and Causes Obesity and Defective Thermogenesis in Mice. Embo J. 2012, 31, 2117–2133. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Turner, N. Mitochondrial Dysfunction and Insulin Resistance: An Update. Endocr. Connect. 2015, 4, R1–R15. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.H.; Kang, Y.H.; Kim, J.S.; Yun, S.C.; Kang, S.W.; Song, Y. Suppression of Brown Adipocyte Autophagy Improves Energy Metabolism by Regulating Mitochondrial Turnover. Int. J. Mol. Sci. 2019, 20, 3520. [Google Scholar] [CrossRef]
- Reiser, K.; McCormick, R.J.; Rucker, R.B. Enzymatic and Nonenzymatic Cross-Linking of Collagen and Elastin. FASEB J. 1992, 6, 2439–2449. [Google Scholar] [CrossRef]
- Robins, S.P. Biochemistry and Functional Significance of Collagen Cross-Linking. Biochem. Soc. Trans. 2007, 35 Pt 5, 849–852. [Google Scholar] [CrossRef]
- Chaudhari, N.; Findlay, A.D.; Stevenson, A.W.; Clemons, T.D.; Yao, Y.; Joshi, A.; Sayar, S.; Wallace, G.; Rea, S.; Toshniwal, P.; et al. Topical Application of an Irreversible Small Molecule Inhibitor of Lysyl Oxidases Ameliorates Skin Scarring and Fibrosis. Nat. Commun. 2022, 13, 5555. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Findlay, A.; Stolp, J.; Rayner, B.; Ask, K.; Jarolimek, W. Pan-Lysyl Oxidase Inhibitor Pxs-5505 Ameliorates Multiple-Organ Fibrosis by Inhibiting Collagen Crosslinks in Rodent Models of Systemic Sclerosis. Int. J. Mol. Sci. 2022, 23, 5533. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, T.; Izumi, T.; Shimizu, T. Leukotriene B4: Metabolism and Signal Transduction. Arch Biochem Biophys 2001, 385, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Shimizu, T. Recent Advances in Function and Structure of Two Leukotriene B(4) Receptors: Blt1 and Blt2. Biochem. Pharmacol. 2022, 203, 115178. [Google Scholar] [CrossRef]
- Michaelian, N.; Sadybekov, A.; Besserer-Offroy, É.; Han, G.W.; Krishnamurthy, H.; Zamlynny, B.A.; Fradera, X.; Siliphaivanh, P.; Presland, J.; Spencer, K.B.; et al. Structural Insights on Ligand Recognition at the Human Leukotriene B4 Receptor 1. Nat. Commun. 2021, 12, 2971. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.; Wang, L.; Kong, L.; Hou, S.; Wan, Y.; Ma, C.; Chen, J.; Xing, X.; Xing, C.; et al. Hepatocyte Leukotriene B4 Receptor 1 Promotes Nafld Development in Obesity. Hepatology, 2022; Online ahead of print. [Google Scholar]
- Sezin, T.; Murthy, S.; Attah, C.; Seutter, M.; Holtsche, M.M.; Hammers, C.M.; Schmidt, E.; Meshrkey, F.; Mousavi, S.; Zillikens, D.; et al. Dual Inhibition of Complement Factor 5 and Leukotriene B4 Synergistically Suppresses Murine Pemphigoid Disease. JCI Insight 2019, 4, e128239. [Google Scholar] [CrossRef]
- Moro, M.G.; Oliveira, M.D.S.; Santana, M.M.; de Jesus, F.N.; Feitosa, K.; Teixeira, S.A.; Franco, G.C.N.; Spolidorio, L.C.; Muscará, M.N.; Holzhausen, M. Leukotriene Receptor Antagonist Reduces Inflammation and Alveolar Bone Loss in a Rat Model of Experimental Periodontitis. J. Periodontol. 2021, 92, e84–e93. [Google Scholar] [CrossRef]
- Dunnwald, T.; Gatterer, H.; Faulhaber, M.; Arvandi, M.; Schobersberger, W. Body Composition and Body Weight Changes at Different Altitude Levels: A Systematic Review and Meta-Analysis. Front. Physiol. 2019, 10, 430. [Google Scholar] [CrossRef]
- Kayser, B.; Verges, S. Hypoxia, Energy Balance, and Obesity: An Update. Obes. Rev. 2021, 22 (Suppl. 2), e13192. [Google Scholar] [CrossRef]
- Voss, J.D.; Masuoka, P.; Webber, B.J.; Scher, A.I.; Atkinson, R.L. Association of Elevation, Urbanization and Ambient Temperature with Obesity Prevalence in the United States. Int. J. Obes. 2013, 37, 1407–1412. [Google Scholar] [CrossRef]
- Lopez-Pascual, A.; Arevalo, J.; Martinez, J.A.; Gonzalez-Muniesa, P. Inverse Association between Metabolic Syndrome and Altitude: A Cross-Sectional Study in an Adult Population of Ecuador. Front. Endocrinol. 2018, 9, 658. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pascual, A.; Bes-Rastrollo, M.; Sayon-Orea, C.; Perez-Cornago, A.; Diaz-Gutierrez, J.; Pons, J.J.; Martinez-Gonzalez, M.A.; Gonzalez-Muniesa, P.; Martinez, J.A. Living at a Geographically Higher Elevation Is Associated with Lower Risk of Metabolic Syndrome: Prospective Analysis of the Sun Cohort. Front. Physiol. 2016, 7, 658. [Google Scholar] [CrossRef] [PubMed]
- Sherpa, L.Y.; Stigum, D.H.; Chongsuvivatwong, V.; Thelle, D.S.; Bjertness, E. Obesity in Tibetans Aged 30-70 Living at Different Altitudes under the North and South Faces of Mt. Everest. Int. J. Environ. Res. Public Health 2010, 7, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Voss, J.D.; Allison, D.B.; Webber, B.J.; Otto, J.L.; Clark, L.L. Lower Obesity Rate During Residence at High Altitude among a Military Population with Frequent Migration: A Quasi Experimental Model for Investigating Spatial Causation. PLoS ONE 2014, 9, e93493. [Google Scholar] [CrossRef]
- Lippl, F.J.; Neubauer, S.; Schipfer, S.; Lichter, N.; Tufman, A.; Otto, B.; Fischer, R. Hypobaric Hypoxia Causes Body Weight Reduction in Obese Subjects. Obesity (Silver Spring) 2010, 18, 675–681. [Google Scholar] [CrossRef]
- Workman, C.; Basset, F.A. Post-Metabolic Response to Passive Normobaric Hypoxic Exposure in Sedendary Overweight Males: A Pilot Study. Nutr. Metab. 2012, 9, 103. [Google Scholar] [CrossRef]
- Costalat, G.; Lemaitre, F.; Tobin, B.; Renshaw, G. Intermittent Hypoxia Revisited: A Promising Non-Pharmaceutical Strategy to Reduce Cardio-Metabolic Risk Factors? Sleep Breath 2018, 22, 267–271. [Google Scholar] [CrossRef]
- Marlatt, K.L.; Greenway, F.L.; Schwab, J.K.; Ravussin, E. Two Weeks of Moderate Hypoxia Improves Glucose Tolerance in Individuals with Type 2 Diabetes. Int. J. Obes. 2020, 44, 744–747. [Google Scholar] [CrossRef]
- Ioja, S.; Singamsetty, S.; Corey, C.; Guo, L.; Shah, F.; Jurczak, M.J.; McVerry, B.J.; Shiva, S.; O’Donnell, C.P. Nocturnal Hypoxia Improves Glucose Disposal, Decreases Mitochondrial Efficiency, and Increases Reactive Oxygen Species in the Muscle and Liver of C57bl/6j Mice Independent of Weight Change. Oxid. Med. Cell. Longev. 2018, 2018, 9649608. [Google Scholar] [CrossRef]
- Diaz-Gutierrez, J.; Martinez-Gonzalez, M.A.; Izquierdo, J.J.P.; Gonzalez-Muniesa, P.; Martinez, J.A.; Bes-Rastrollo, M. Living at Higher Altitude and Incidence of Overweight/Obesity: Prospective Analysis of the Sun Cohort. PLoS ONE 2016, 11, e0164483. [Google Scholar] [CrossRef]
- O’Brien, K.A.; Atkinson, R.A.; Richardson, L.; Koulman, A.; Murray, A.J.; Harridge, S.D.R.; Martin, D.S.; Levett, D.Z.H.; Mitchell, K.; Mythen, M.G.; et al. Metabolomic and Lipidomic Plasma Profile Changes in Human Participants Ascending to Everest Base Camp. Sci. Rep. 2019, 9, 2297. [Google Scholar] [CrossRef] [PubMed]
- Tin’kov, A.N.; Aksenov, V.A. Effects of Intermittent Hypobaric Hypoxia on Blood Lipid Concentrations in Male Coronary Heart Disease Patients. High Alt. Med. Biol. 2002, 3, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Ascent to Altitude as a Weight Loss Method: The Good and Bad of Hypoxia Inducible Factor Activation. Obesity (Silver Spring) 2014, 22, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Fothergill, E.; Guo, J.; Howard, L.; Kerns, J.C.; Knuth, N.D.; Brychta, R.; Chen, K.Y.; Skarulis, M.C.; Walter, M.; Walter, P.J.; et al. Persistent Metabolic Adaptation 6 Years after “the Biggest Loser” Competition. Obesity (Silver Spring) 2016, 24, 1612–1619. [Google Scholar] [CrossRef]
- Macena, M.L.; da Silva Junior, D.P.A.E.; Praxedes, D.R.S.; de Melo, I.P.I.S.V.; and Bueno, N.B. Estimates of Resting Energy Expenditure and Total Energy Expenditure Using Predictive Equations in Adults with Overweight and Obesity: A Systematic Review with Meta-Analysis. Nutr. Rev. 2022, 80, 2113–2135. [Google Scholar] [CrossRef]
- Wing-Gaia, S.L. Nutritional Strategies for the Preservation of Fat Free Mass at High Altitude. Nutrients 2014, 6, 665–681. [Google Scholar] [CrossRef]
- Oltmanns, K.M.; Gehring, H.; Rudolf, S.; Schultes, B.; Schweiger, U.; Born, J.; Fehm, H.L.; Peters, A. Persistent Suppression of Resting Energy Expenditure after Acute Hypoxia. Metabolism 2006, 55, 669–675. [Google Scholar] [CrossRef]
- Westerterp, K.R.; Meijer, E.P.; Rubbens, M.; Robach, P.; Richalet, J.P. Operation Everest Iii: Energy and Water Balance. Pflugers Arch. 2000, 439, 483–488. [Google Scholar] [CrossRef]
- Westerterp, K.R.; Kayser, B.; Brouns, F.; Herry, J.P.; Saris, W.H. Energy Expenditure Climbing Mt. Everest. J. Appl. Physiol. (1985) 1992, 73, 1815–1819. [Google Scholar] [CrossRef]
- Kelly, L.P.; Basset, F.A. Acute Normobaric Hypoxia Increases Post-Exercise Lipid Oxidation in Healthy Males. Front. Physiol. 2017, 8, 293. [Google Scholar] [CrossRef]
- Mashaqi, S.; Kallamadi, R.; Matta, A.; Quan, S.F.; Patel, S.I.; Combs, D.; Estep, L.; Lee-Iannotti, J.; Smith, C.; Parthasarathy, S.; et al. Obstructive Sleep Apnea as a Risk Factor for Covid-19 Severity-the Gut Microbiome as a Common Player Mediating Systemic Inflammation Via Gut Barrier Dysfunction. Cells 2022, 11, 1569. [Google Scholar] [CrossRef] [PubMed]
- Borel, A.L. Sleep Apnea and Sleep Habits: Relationships with Metabolic Syndrome. Nutrients 2019, 11, 2628. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; Martinez-Garcia, M.A.; Farre, R.; Gozal, D. Obesity, Sleep Apnea, and Cancer. Int. J. Obes. 2020, 44, 1653–1667. [Google Scholar] [CrossRef] [PubMed]
- Anvari, G.; Bellas, E. Hypoxia Induces Stress Fiber Formation in Adipocytes in the Early Stage of Obesity. Sci. Rep. 2021, 11, 21473. [Google Scholar] [CrossRef]
- Arias-Loste, M.T.; Fabrega, E.; Lopez-Hoyos, M.; Crespo, J. The Crosstalk between Hypoxia and Innate Immunity in the Development of Obesity-Related Nonalcoholic Fatty Liver Disease. Biomed. Res. Int. 2015, 2015, 319745. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Li, J.; Reinke, C.; Bevans-Fonti, S.; Jun, J.C.; Polotsky, V.Y. Intermittent Hypoxia Exacerbates Metabolic Effects of Diet-Induced Obesity. Obesity (Silver Spring) 2011, 19, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.H.; Kim, I.K.; Lee, H.I.; Joo, H.; Lim, J.U.; Lee, J.; Lee, S.H.; Moon, H.S. Chronic Intermittent Hypoxia Induces Liver Fibrosis in Mice with Diet-Induced Obesity Via Tlr4/Myd88/Mapk/Nf-Kb Signaling Pathways. Biochem. Biophys. Res. Commun. 2017, 490, 349–355. [Google Scholar] [CrossRef]
- Ozeke, O.; Ozer, C.; Gungor, M.; Celenk, M.K.; Dincer, H.; Ilicin, G. Chronic Intermittent Hypoxia Caused by Obstructive Sleep Apnea May Play an Important Role in Explaining the Morbidity-Mortality Paradox of Obesity. Med. Hypotheses 2011, 76, 61–63. [Google Scholar] [CrossRef]
- Maatta, J.; Sissala, N.; Dimova, E.Y.; Serpi, R.; Moore, L.G.; Koivunen, P. Hypoxia Causes Reductions in Birth Weight by Altering Maternal Glucose and Lipid Metabolism. Sci. Rep. 2018, 8, 13583. [Google Scholar] [CrossRef]
- Liang, H.; Yan, J.; Song, K. Comprehensive Lipidomic Analysis Reveals Regulation of Glyceride Metabolism in Rat Visceral Adipose Tissue by High-Altitude Chronic Hypoxia. PLoS ONE 2022, 17, e0267513. [Google Scholar] [CrossRef]
- Dusabimana, T.; Park, E.J.; Je, J.; Jeong, K.; Yun, S.P.; Kim, H.J.; Kim, H.; Park, S.W. P2Y2R Deficiency Ameliorates Hepatic Steatosis by Reducing Lipogenesis and Enhancing Fatty Acid β-Oxidation through AMPK and PGC-1α Induction in High-Fat Diet-Fed Mice. Int J Mol Sci. 2021, 22, 5528. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Zhang, Y.; Ga, Q.; Bai, Z.; Ge, R.L. Increased Insulin Sensitivity by High-Altitude Hypoxia in Mice with High-Fat Diet-Induced Obesity Is Associated with Activated Ampk Signaling and Subsequently Enhanced Mitochondrial Biogenesis in Skeletal Muscles. Obes. Facts 2020, 13, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, K.; Qi, G.; Yan, R.; Yang, Y.; Li, Y.; Wang, S.; Bai, Z.; Ge, R.L. Adipose-Derived Exosomal Mir-210/92a Cluster Inhibits Adipose Browning Via the Fgfr-1 Signaling Pathway in High-Altitude Hypoxia. Sci. Rep. 2020, 10, 14390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lee, H.; Zhu, Z.; Minhas, J.K.; Jin, Y. Enrichment of Selective Mirnas in Exosomes and Delivery of Exosomal Mirnas in Vitro and in Vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L110–L121. [Google Scholar] [CrossRef]
- Nader, J.; Meuth, V.M.; Maitrias, P.; Humbert, J.R.; Brigant, B.; Tribouilloy, C.; Metzinger, L.; Caus, T. Mir-92a: A Novel Potential Biomarker of Rapid Aortic Valve Calcification. J. Heart Valve Dis. 2017, 26, 327–333. [Google Scholar]
- Kong, X.; Yao, T.; Zhou, P.; Kazak, L.; Tenen, D.; Lyubetskaya, A.; Dawes, B.A.; Tsai, L.; Kahn, B.B.; Spiegelman, B.M.; et al. Brown Adipose Tissue Controls Skeletal Muscle Function Via the Secretion of Myostatin. Cell Metab. 2018, 28, 631–643.e3. [Google Scholar] [CrossRef]
- Gong, L.J.; Fu, P.Y.; Zhu, R.X.; Wang, L.; Hu, Y. Effects and Mechanism of Hypoxia Exposure on Related Genes in Brown Fat Tissue of Obese Mice Based on Mrna Expression Profile Microarray. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2018, 34, 88–92. [Google Scholar]
- Song, K.; Zhang, Y.; Ga, Q.; Bai, Z.; Ge, R.L. High-Altitude Chronic Hypoxia Ameliorates Obesity-Induced Non-Alcoholic Fatty Liver Disease in Mice by Regulating Mitochondrial and Ampk Signaling. Life Sci. 2020, 252, 117633. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, Q.; Zou, J.; Fan, J.; Li, Y.; Luo, Z. Chronic Intermittent Hypoxia Exposure Alternative to Exercise Alleviates High-Fat-Diet-Induced Obesity and Fatty Liver. Int. J. Mol. Sci. 2022, 23, 5209. [Google Scholar] [CrossRef]
- Drager, L.F.; Yao, Q.; Hernandez, K.L.; Shin, M.K.; Bevans-Fonti, S.; Gay, J.; Sussan, T.E.; Jun, J.C.; Myers, A.C.; Olivecrona, G.; et al. Chronic Intermittent Hypoxia Induces Atherosclerosis Via Activation of Adipose Angiopoietin-Like 4. Am. J. Respir Crit. Care Med. 2013, 188, 240–248. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Han, T.; Cui, X.; Lu, X. Chronic Intermittent Hypoxia Aggravates Skeletal Muscle Aging by Down-Regulating Klc1/Grx1 Expression Via Wnt/Beta-Catenin Pathway. Arch. Gerontol. Geriatr. 2021, 96, 104460. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Vogt, M. Muscle Tissue Adaptations to Hypoxia. J. Exp. Biol. 2001, 204 Pt 18, 3133–3139. [Google Scholar] [CrossRef]
- McNair, B.D.; Marcello, N.A.; Smith, D.T.; Schmitt, E.E.; Bruns, D.R. Changes in Muscle Mass and Composition by Exercise and Hypoxia as Assessed by Dexa in Mice. Medicina 2020, 56, 446. [Google Scholar] [CrossRef]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a Prescription for Patients with Various Diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef]
- Zhu, X.; Yao, T.; Wang, R.; Guo, S.; Wang, X.; Zhou, Z.; Zhang, Y.; Zhuo, X.; Wang, R.; Li, J.Z.; et al. Irf4 in Skeletal Muscle Regulates Exercise Capacity Via Ptg/Glycogen Pathway. Adv. Sci. 2020, 7, 2001502. [Google Scholar] [CrossRef]
- Yasuda, T. Selected Methods of Resistance Training for Prevention and Treatment of Sarcopenia. Cells 2022, 11, 1389. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.; Maxwell, N.; Castle, P.; Brickley, G.; Watt, P. Acute Hypoxia and Exercise Improve Insulin Sensitivity (S(I) (2*)) in Individuals with Type 2 Diabetes. Diabetes Metab. Res. Rev. 2011, 27, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Groote, E.D.E.; Britto, F.A.; Bullock, L.; Francois, M.; Buck, D.E.; Nielens, C.H.; Deldicque, L. Hypoxic Training Improves Normoxic Glucose Tolerance in Adolescents with Obesity. Med. Sci. Sports Exerc. 2018, 50, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Urdampilleta, A.; Gonzalez-Muniesa, P.; Portillo, M.P.; Martinez, J.A. Usefulness of Combining Intermittent Hypoxia and Physical Exercise in the Treatment of Obesity. J. Physiol. Biochem. 2012, 68, 289–304. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lee, S.D.; Kuo, C.H.; Ho, L.T. The Effects of Altitude Training on the Ampk-Related Glucose Transport Pathway in the Red Skeletal Muscle of Both Lean and Obese Zucker Rats. High Alt. Med. Biol. 2011, 12, 371–378. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Ye, J.; Zhang, Y.; Zhang, Y. Pparalpha Protein Expression Was Increased by Four Weeks of Intermittent Hypoxic Training Via Ampkalpha2-Dependent Manner in Mouse Skeletal Muscle. PLoS ONE 2015, 10, e0122593. [Google Scholar]
- Wang, R.; Guo, S.; Tian, H.; Huang, Y.; Yang, Q.; Zhao, K.; Kuo, C.H.; Hong, S.; Chen, P.; Liu, T. Hypoxic Training in Obese Mice Improves Metabolic Disorder. Front. Endocrinol. 2019, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Sun, S.; Liu, W.; Liu, Q.; Wang, J. Hypoxia Training Improves Hepatic Steatosis Partly by Downregulation of Cb1 Receptor in Obese Mice. Biochem. Biophys. Res. Commun. 2020, 525, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Fryk, E.; Olausson, J.; Mossberg, K.; Strindberg, L.; Schmelz, M.; Brogren, H.; Gan, L.M.; Piazza, S.; Provenzani, A.; Becattini, B.; et al. Hyperinsulinemia and Insulin Resistance in the Obese May Develop as Part of a Homeostatic Response to Elevated Free Fatty Acids: A Mechanistic Case-Control and a Population-Based Cohort Study. EBioMedicine 2021, 65, 103264. [Google Scholar] [CrossRef] [PubMed]
- van Meijel, R.L.J.; Vogel, M.A.A.; Jocken, J.W.E.; Vliex, L.M.M.; Smeets, J.S.J.; Hoebers, N.; Hoeks, J.; Essers, Y.; Schoffelen, P.F.M.; Sell, H.; et al. Mild Intermittent Hypoxia Exposure Induces Metabolic and Molecular Adaptations in Men with Obesity. Mol. Metab. 2021, 53, 101287. [Google Scholar] [CrossRef] [PubMed]
- Lecoultre, V.; Peterson, C.M.; Covington, J.D.; Ebenezer, P.J.; Frost, E.A.; Schwarz, J.M.; Ravussin, E. Ten Nights of Moderate Hypoxia Improves Insulin Sensitivity in Obese Humans. Diabetes Care 2013, 36, e197–e198. [Google Scholar] [CrossRef]
- Wilkinson, D.; Nolting, M.; Mahadi, M.K.; Chapman, I.; Heilbronn, L. Hyperbaric Oxygen Therapy Increases Insulin Sensitivity in Overweight Men with and without Type 2 Diabetes. Diving Hyperb. Med. 2015, 45, 30–36. [Google Scholar]
- Wilkinson, D.; Chapman, I.M.; Heilbronn, L.K. Hyperbaric Oxygen Therapy Improves Peripheral Insulin Sensitivity in Humans. Diabet Med. 2012, 29, 986–989. [Google Scholar] [CrossRef]
- Sarabhai, T.; Mastrototaro, L.; Kahl, S.; Bonhof, G.J.; Jonuscheit, M.; Bobrov, P.; Katsuyama, H.; Guthoff, R.; Wolkersdorfer, M.; Herder, C.; et al. Hyperbaric Oxygen Rapidly Improves Tissue-Specific Insulin Sensitivity and Mitochondrial Capacity in Humans with Type 2 Diabetes: A Randomised Placebo-Controlled Crossover Trial. Diabetologia, 2022; Online ahead of print. [Google Scholar]
- van Hulten, V.; van Meijel, R.L.J.; Goossens, G.H. The Impact of Hypoxia Exposure on Glucose Homeostasis in Metabolically Compromised Humans: A Systematic Review. Rev. Endocr. Metab. Disord. 2021, 22, 471–483. [Google Scholar] [CrossRef]
- De Groote, E.; Britto, F.A.; Balan, E.; Warnier, G.; Thissen, J.P.; Nielens, H.; Sylow, L.; Deldicque, L. Effect of Hypoxic Exercise on Glucose Tolerance in Healthy and Prediabetic Adults. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E43–E54. [Google Scholar] [CrossRef]
- Mackenzie, R.; Elliott, B.; Maxwell, N.; Brickley, G.; Watt, P. The Effect of Hypoxia and Work Intensity on Insulin Resistance in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lempesis, I.G.; van Meijel, R.L.J.; Manolopoulos, K.N.; Goossens, G.H. Oxygenation of Adipose Tissue: A Human Perspective. Acta Physiol. 2020, 228, e13298. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic Potential of Intermittent Hypoxia: A Matter of Dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef] [PubMed]
- Hopfl, G.; Ogunshola, O.; Gassmann, M. Hifs and Tumors--Causes and Consequences. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R608–R623. [Google Scholar] [CrossRef]
- Amine, Z.E.; Mauger, J.F.; Imbeault, P. Cyp1a1, Vegfa and Adipokine Responses of Human Adipocytes Co-Exposed to Pcb126 and Hypoxia. Cells 2022, 11, 2282. [Google Scholar] [CrossRef]
- Conway, B.; Rene, A. Obesity as a Disease: No Lightweight Matter. Obes. Rev. 2004, 5, 145–151. [Google Scholar] [CrossRef]
- Brooks, G.A.; Butterfield, G.E.; Wolfe, R.R.; Groves, B.M.; Mazzeo, R.S.; Sutton, J.R.; Wolfel, E.E.; Reeves, J.T. Increased Dependence on Blood Glucose after Acclimatization to 4,300 M. J. Appl. Physiol. (1985) 1991, 70, 919–927. [Google Scholar] [CrossRef]
- Duennwald, T.; Gatterer, H.; Groop, P.H.; Burtscher, M.; Bernardi, L. Effects of a Single Bout of Interval Hypoxia on Cardiorespiratory Control and Blood Glucose in Patients with Type 2 Diabetes. Diabetes Care 2013, 36, 2183–2189. [Google Scholar] [CrossRef]
| Subject | Country | Age | FiO2 | Hypoxic Mode | Exercise Method | Result | Reference |
|---|---|---|---|---|---|---|---|
| 23 obese Individuals | France | 52 ± 12 year | 13% | LoHi | 8 weeks, 3 day/week, 75% VO2 max | DBP↓, body composition no change | [184] |
| 24 Korean obese men | Korean | 66.5 ± 0.8 year | 14.40% | 12 weeks, 3 day/week, 30 min, aerobic exercise + resistance exercise 30–40 min | Body weight↓, BMI↓, Fat mass↑, Lean mass↑ | [186] | |
| 32 Korean obesity women | Korean | 47.5 ± 7.5 year | 14.50% | 12 weeks, 3 day/week, 50 min/d, Pilates | DBP↓, TC↓, TG↓ | [187] | |
| 20 sedentary subjects | Japan | 30 ± 2 year | 15% | 4 weeks, 3 day/week,1 h/d, 55% VO2 max | Glucose tolerance↑, Body Weight and abdominal fat area no change, | [189] | |
| 14 obese adolescents | Belgium | 12–15 year | 15% | 6 weeks, 3 day/week, 50–60 min/d, endurance and resistance exercises | TG↓, glucose levels↓, AUC of insulin↓ | [179] | |
| Overweight to obese | Germany | 42.2 ± 1.2 year | 15% | 4 weeks, 3 day/weeks, 65% VO2 max | Fat-free mass↑, Waist circumference, Fasting insulin↓, Fat mass↓ | [193] | |
| 59 overweight/obese women | Uruguay | - | 17.20% | 12 weeks, 3 day/week, High intensity exercise | Waist circumference↓, percentage of trunk fat mass↓, | [195] | |
| 49 obesity male Individuals | Spain | 20–50 year | 13.7–16.7% | IHT | 8 weeks, 2 day/week, 1 h/d,50% aerobic,50% strength | Body weight↓, BMI↓, waist circumference↓ | [185] |
| 14 obese adolescents | Belgium | 12–15 year | 15% | 30 weeks, 1 day/weeks, 50–60 min/d, 12 min bicycle and strength training of the abdominal, quadriceps and biceps muscles | Body weight↓, Fat mass↓, | [190] | |
| 23 obese men | Germany | 52.3–62.5 year | 15% | 6 weeks, 3 day/week, 1 h/d, 60% VO2 max | BMI↓, Fat mass↓, Lean mass↓, HDL↑, TG↓ | [191] | |
| 32 obese people | Germany | mean age 45.5 year | 15% | 8 weeks, 3 day/week, 90 min low intense physical exercise | Body weight↓, BMI↓ | [19] | |
| 82 obese women | Uruguay | - | 17.20% | 12 weeks, 3 day/week, repeated sprint training (130% VO2 max 30 s, 55–65% VO2 max 3 min) | Absolute and relative maximal oxygen uptake↑, absolute and relative VO2 max↑ | [196] | |
| 35 obesity adolescents | China | 12–16 year | 14.70% | HiLo | 4 weeks, 6 day/week, 2 h/d, Aerobic exercise | Body weight↓, BMI↓, Lean mass↑ | [188] |
| 19 overweight or obese females | China | 19.30 ± 1.92 year | 15% | 4 weeks, 3 day/week, 2 h/d | Body weight↓, Fat mass↓, TC↓, adiponectin↓, HDL-C↑ | [192] | |
| 14 metabolic syndromes | Italy | mean age 55.8 year | 16.30% | HiHiLo | 2 weeks, 4 day/week, 3 h/d, 55–65% VO2 max | TC↓, LDL↓, adiponectin↓, TG↓, | [194] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Sun, Q.; Wu, X.; Zhang, Y.; Xing, X.; Lin, K.; Feng, Y.; Wang, M.; Wang, Y.; Wang, R. Hypoxia as a Double-Edged Sword to Combat Obesity and Comorbidities. Cells 2022, 11, 3735. https://doi.org/10.3390/cells11233735
Wang R, Sun Q, Wu X, Zhang Y, Xing X, Lin K, Feng Y, Wang M, Wang Y, Wang R. Hypoxia as a Double-Edged Sword to Combat Obesity and Comorbidities. Cells. 2022; 11(23):3735. https://doi.org/10.3390/cells11233735
Chicago/Turabian StyleWang, Ruwen, Qin Sun, Xianmin Wu, Yiyin Zhang, Xiaorui Xing, Kaiqing Lin, Yue Feng, Mingqi Wang, Yibing Wang, and Ru Wang. 2022. "Hypoxia as a Double-Edged Sword to Combat Obesity and Comorbidities" Cells 11, no. 23: 3735. https://doi.org/10.3390/cells11233735
APA StyleWang, R., Sun, Q., Wu, X., Zhang, Y., Xing, X., Lin, K., Feng, Y., Wang, M., Wang, Y., & Wang, R. (2022). Hypoxia as a Double-Edged Sword to Combat Obesity and Comorbidities. Cells, 11(23), 3735. https://doi.org/10.3390/cells11233735






