In Vivo Comparison of Synthetic Macroporous Filamentous and Sponge-like Skin Substitute Matrices Reveals Morphometric Features of the Foreign Body Reaction According to 3D Biomaterial Designs
Abstract
:1. Introduction
2. Materials and Methods
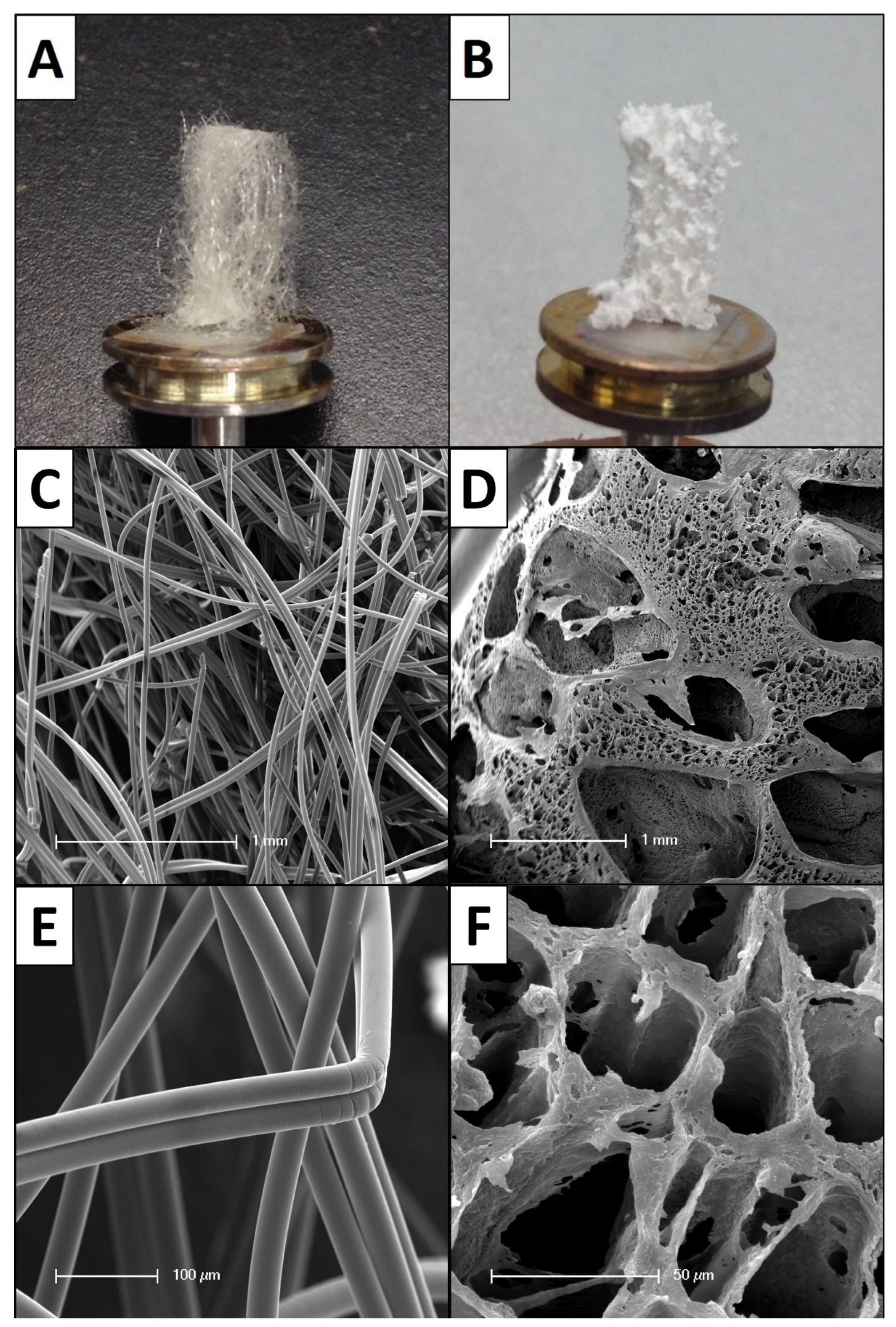
2.1. Biomaterial Matrices
2.2. Study Design and Biomaterial Implantation
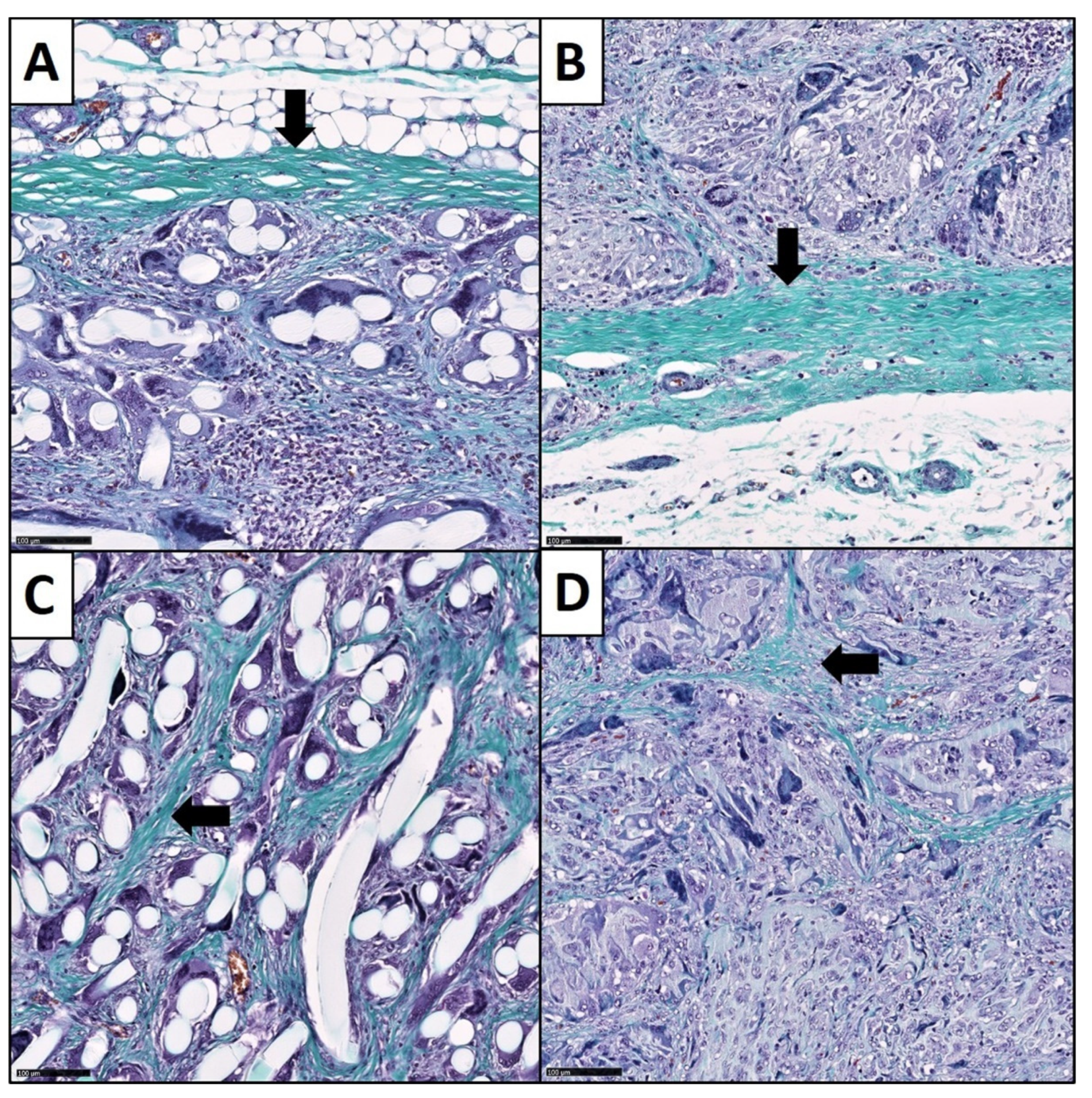
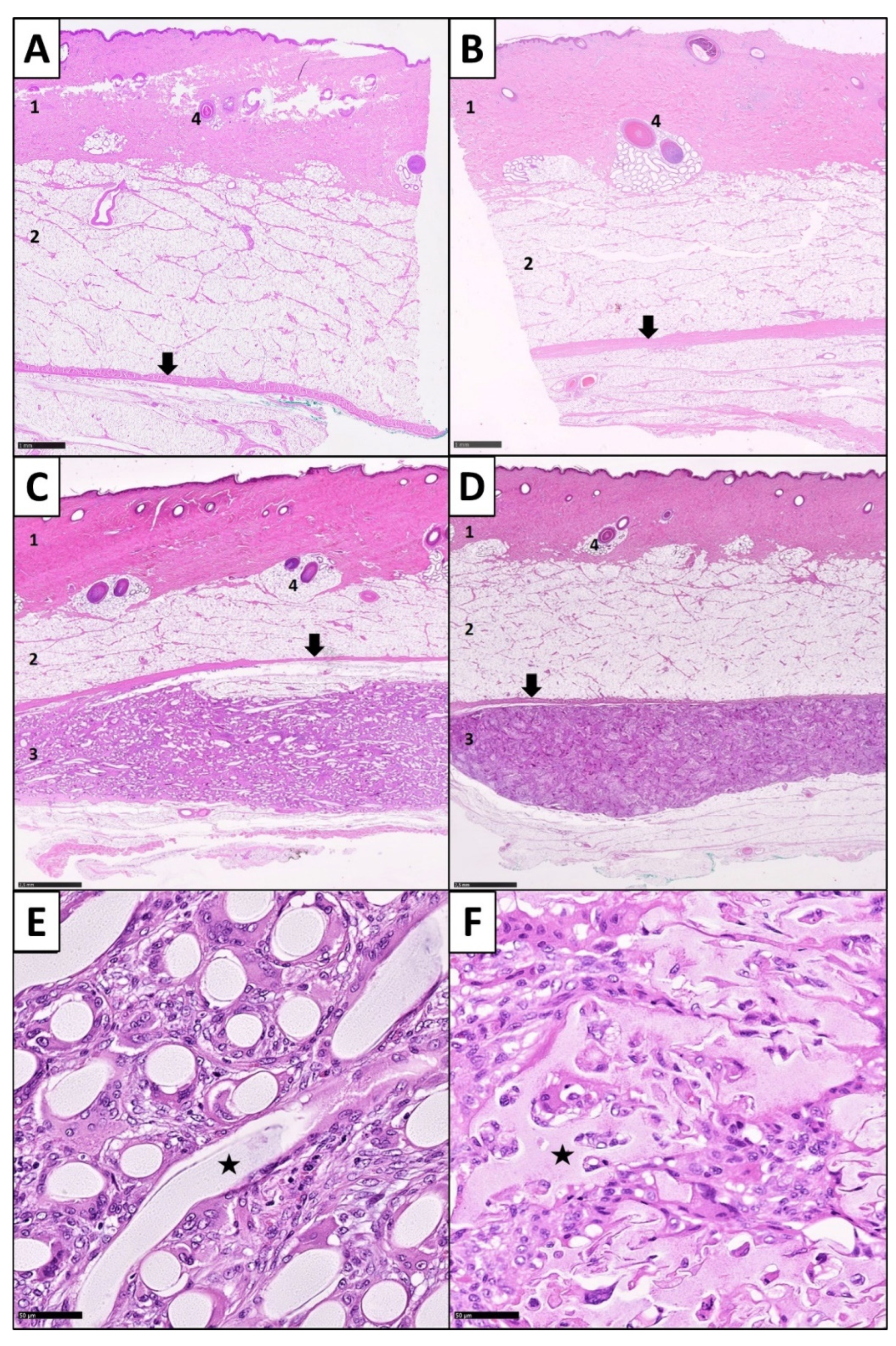
2.3. Sample Processing and Histological Staining
2.4. Histopathological Analysis
2.4.1. Determination of the Inflammatory Response
2.4.2. Determination of Foreign Body Giant Cells
2.4.3. Analysis of the Fibrotic Reactions
2.5. Statistical Analysis
3. Results
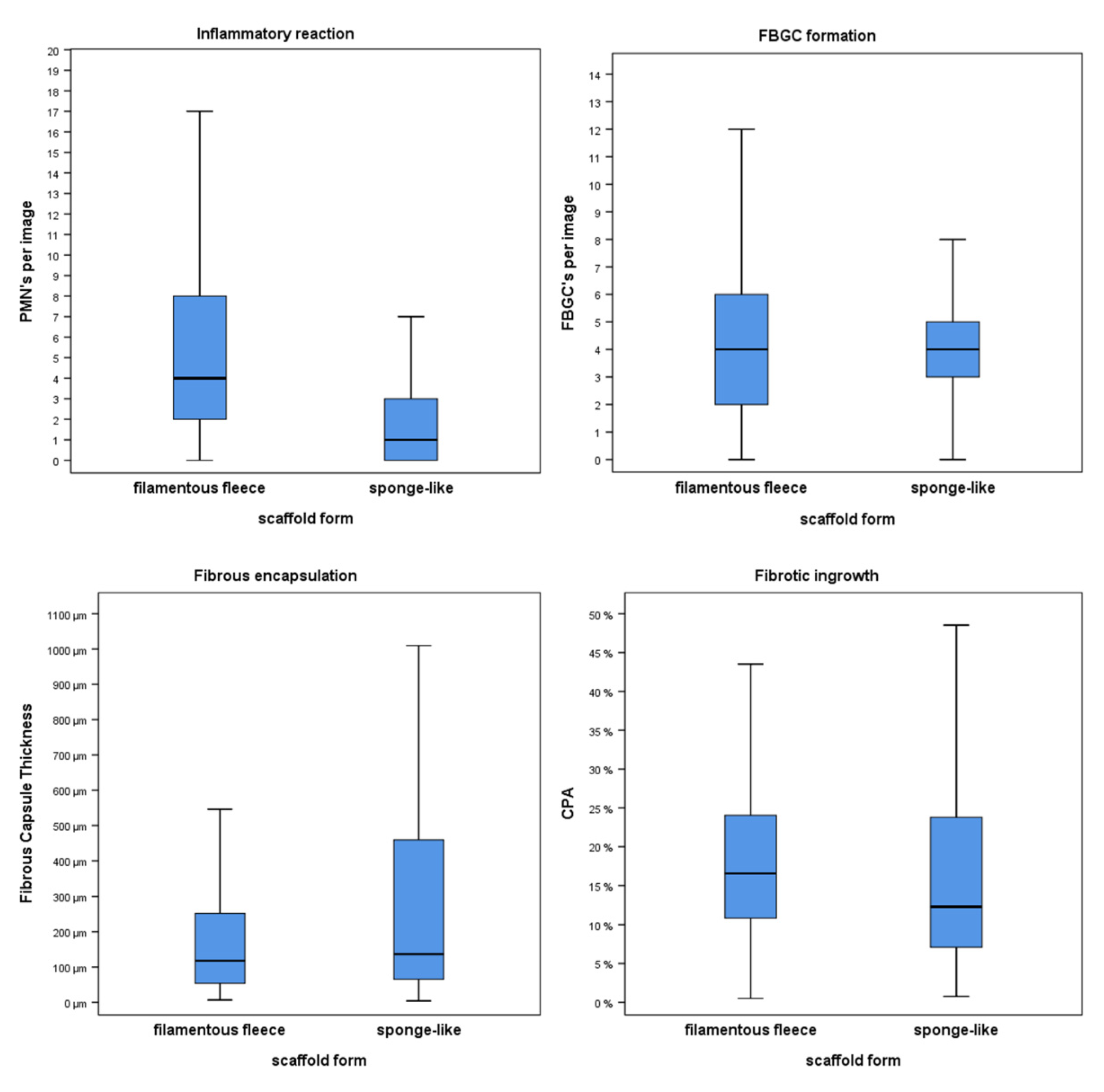
3.1. Inflammatory Response
3.2. Expression of Foreign Body Giant Cells
3.3. Fibrotic Reactions
3.4. Correlations between Variables of the Foreign Body Reaction
4. Discussion
4.1. PMNs and Their Dependence on the Biomaterial Architecture
4.2. Foreign Body Giant Cells in the Context of Synthetic Matrix Designs
4.3. Fibrotic Growth Patterns and Their Dependence on Physical Matrix Parameters
4.4. The Importance of Biomaterial Design for the Use as Skin Substitutes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olson, J.L.; Atala, A.; Yoo, J.J. Tissue engineering: Current strategies and future directions. Chonnam Med. J. 2011, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tartarini, D.; Mele, E. Adult Stem Cell Therapies for Wound Healing: Biomaterials and Computational Models. Front. Bioeng. Biotechnol. 2015, 3, 206. [Google Scholar] [CrossRef] [PubMed]
- Theoret, C. Tissue engineering in wound repair: The three “R” s-repair, replace, regenerate. Vet. Surg. 2009, 38, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Greaves, N.S.; Iqbal, S.A.; Baguneid, M.; Bayat, A. The role of skin substitutes in the management of chronic cutaneous wounds. Wound Repair Regen. Off. Publ. Wound Health Soc. Eur. Tissue Repair Soc. 2013, 21, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Markeson, D.; Pleat, J.M.; Sharpe, J.R.; Harris, A.L.; Seifalian, A.M.; Watt, S.M. Scarring, stem cells, scaffolds and skin repair. J. Tissue Eng. Regen. Med. 2015, 9, 649–668. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Badylak, S.F. Biomaterials for tissue engineering applications. Semin. Pediatr. Surg. 2014, 23, 112–118. [Google Scholar] [CrossRef]
- Kirsner, R.S.; Bohn, G.; Driver, V.R.; Mills, J.L., Sr.; Nanney, L.B.; Williams, M.L.; Wu, S.C. Human acellular dermal wound matrix: Evidence and experience. Int. Wound J. 2015, 12, 646–654. [Google Scholar] [CrossRef]
- Chandika, P.; Ko, S.C.; Jung, W.K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int. J. Biol. Macromol. 2015, 77, 24–35. [Google Scholar] [CrossRef]
- Bi, H.; Jin, Y. Current progress of skin tissue engineering: Seed cells, bioscaffolds, and construction strategies. Burn. Trauma 2013, 1, 63–72. [Google Scholar] [CrossRef]
- Zhong, S.P.; Zhang, Y.Z.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. WIREs Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef]
- Del Bakhshayesh, A.R.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Cronin, H.; Goldstein, G. Biologic skin substitutes and their applications in dermatology. Dermatol. Surg. 2013, 39, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, A.D.; Ferguson, M.W. Bioengineering skin using mechanisms of regeneration and repair. Biomaterials 2007, 28, 5100–5113. [Google Scholar] [CrossRef] [PubMed]
- Bottcher-Haberzeth, S.; Biedermann, T.; Reichmann, E. Tissue engineering of skin. Burns 2010, 36, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Bayat, A. Dermal substitute-assisted healing: Enhancing stem cell therapy with novel biomaterial design. Arch. Dermatol. Res. 2011, 303, 301–315. [Google Scholar] [CrossRef]
- Janouskova, O. Synthetic polymer scaffolds for soft tissue engineering. Physiol. Res. 2018, 67, S335–S348. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Brusselaers, N.; Pirayesh, A.; Hoeksema, H.; Richters, C.D.; Verbelen, J.; Beele, H.; Blot, S.I.; Monstrey, S. Skin replacement in burn wounds. J. Trauma 2010, 68, 490–501. [Google Scholar] [CrossRef]
- Ojeh, N.; Pastar, I.; Tomic-Canic, M.; Stojadinovic, O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. Int. J. Mol. Sci. 2015, 16, 25476–25501. [Google Scholar] [CrossRef]
- Hogrebe, N.J.; Reinhardt, J.W.; Gooch, K.J. Biomaterial microarchitecture: A potent regulator of individual cell behavior and multicellular organization. J. Biomed. Mater. Res. Part A 2017, 105, 640–661. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- Yildirimer, L.; Thanh, N.T.; Seifalian, A.M. Skin regeneration scaffolds: A multimodal bottom-up approach. Trends Biotechnol. 2012, 30, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Tenenhaus, M.; Rennekampff, H.O. Current Concepts in Tissue Engineering: Skin and Wound. Plast. Reconstr. Surg. 2016, 138, 42s–50s. [Google Scholar] [CrossRef]
- Nicholas, M.N.; Jeschke, M.G.; Amini-Nik, S. Methodologies in creating skin substitutes. Cell. Mol. Life Sci. 2016, 73, 3453–3472. [Google Scholar] [CrossRef]
- Van der Veen, V.C.; van der Wal, M.B.; van Leeuwen, M.C.; Ulrich, M.M.; Middelkoop, E. Biological background of dermal substitutes. Burns 2010, 36, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Cosson, S.; Otte, E.A.; Hezaveh, H.; Cooper-White, J.J. Concise review: Tailoring bioengineered scaffolds for stem cell applications in tissue engineering and regenerative medicine. Stem Cells Transl. Med. 2015, 4, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Sow, W.T.; Devi, D.; Ng, K.W.; Mandal, B.B.; Cho, N.J. Silk fibroin-keratin based 3D scaffolds as a dermal substitute for skin tissue engineering. Integr. Biol. 2015, 7, 53–63. [Google Scholar] [CrossRef]
- Jimenez, P.A.; Jimenez, S.E. Tissue and cellular approaches to wound repair. Am. J. Surg. 2004, 187, 56s–64s. [Google Scholar] [CrossRef]
- Debels, H.; Hamdi, M.; Abberton, K.; Morrison, W. Dermal matrices and bioengineered skin substitutes: A critical review of current options. Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [Google Scholar] [CrossRef]
- Demirbag, B.; Huri, P.Y.; Kose, G.T.; Buyuksungur, A.; Hasirci, V. Advanced cell therapies with and without scaffolds. Biotechnol. J. 2011, 6, 1437–1453. [Google Scholar] [CrossRef]
- Yu, H.; Peng, J.; Xu, Y.; Chang, J.; Li, H. Bioglass Activated Skin Tissue Engineering Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, C.; Renner, R.; Milkova, L.; Simon, J.C. Regenerative medicine in dermatology: Biomaterials, tissue engineering, stem cells, gene transfer and beyond. Exp. Dermatol. 2010, 19, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Peramo, A.; Marcelo, C.L. Bioengineering the skin-implant interface: The use of regenerative therapies in implanted devices. Ann. Biomed. Eng. 2010, 38, 2013–2031. [Google Scholar] [CrossRef] [PubMed]
- Felder, J.M., 3rd; Goyal, S.S.; Attinger, C.E. A systematic review of skin substitutes for foot ulcers. Plast. Reconstr. Surg. 2012, 130, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.M.; Biswas, S.K.; Wong, S.C. MicroRNA-mediated immune modulation as a therapeutic strategy in host-implant integration. Adv. Drug Deliv. Rev. 2015, 88, 92–107. [Google Scholar] [CrossRef]
- Sides, C.R.; Stenken, J.A. Microdialysis sampling techniques applied to studies of the foreign body reaction. Eur. J. Pharm. Sci. 2014, 57, 74–86. [Google Scholar] [CrossRef]
- Brochhausen, C.; Schmitt, V.H.; Rajab, T.K.; Planck, C.N.E.; Krämer, B.; Tapprich, C.; Wallwiener, M.; Hierlemann, H.; Planck, H.; Kirkpatrick, C.J. Mesothelial morphology and organisation after peritoneal treatment with solid and liquid adhesion barriers—A scanning electron microscopical study. J. Mater. Sci. Mater. Med. 2012, 23, 1931–1939. [Google Scholar] [CrossRef]
- Brochhausen, C.; Schmitt, V.H.; Mamilos, A.; Schmitt, C.; Planck, C.N.E.; Rajab, T.K.; Hierlemann, H.; Kirkpatrick, C.J. Expression of CD68 positive macrophages in the use of different barrier materials to prevent peritoneal adhesions-an animal study. J. Mater. Sci. Mater. Med. 2017, 28, 15. [Google Scholar] [CrossRef]
- Morris, A.H.; Stamer, D.K.; Kyriakides, T.R. The host response to naturally-derived extracellular matrix biomaterials. Semin. Immunol. 2017, 29, 72–91. [Google Scholar] [CrossRef]
- Schmitt, V.H.; Mamilos, A.; Schmitt, C.; Neitzer-Planck, C.N.E.; Rajab, T.K.; Hollemann, D.; Wagner, W.; Krämer, B.; Hierlemann, H.; Kirkpatrick, C.J.; et al. Tissue response to five commercially available peritoneal adhesion barriers—A systematic histological evaluation. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.N.P.V.H.; Planck, C.N.; Kramer, B.; Mamilos, A.; Rajab, T.K.; Hollemann, D.; Wallwiener, C.; Hierlemann, H.; Planck, H.; Kirkpatrick, C.J.; et al. Histological and Immunohistological Evaluation of the Tissue Response of a New Barrier Material Based on D,L-Polylactide, Trimethylene Carbonate and Caprolactone to Prevent Peritoneal Adhesion Formation. J. Tissue Sci. Eng. 2014, 5, 1000138. [Google Scholar] [CrossRef]
- Li, A.; Dearman, B.L.; Crompton, K.E.; Moore, T.G.; Greenwood, J.E. Evaluation of a novel biodegradable polymer for the generation of a dermal matrix. J. Burn. Care Res. 2009, 30, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Gibon, E.; Cordova, L.A.; Lu, L.; Lin, T.H.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The biological response to orthopedic implants for joint replacement. II: Polyethylene, ceramics, PMMA, and the foreign body reaction. J. Biomed. Mater. Research. Part B Appl. Biomater. 2016, 105, 1685–1691. [Google Scholar] [CrossRef]
- Ogle, M.E.; Segar, C.E.; Sridhar, S.; Botchwey, E.A. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp. Biol. Med. 2016, 241, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, V.C.; Boekema, B.K.; Ulrich, M.M.; Middelkoop, E. New dermal substitutes. Wound Repair Regen. 2011, 19 (Suppl. S1), s59–s65. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Rahmanian-Schwarz, A.; Werner, J.O.; Schiefer, J.; Rothenberger, J.; Hubner, G.; Schaller, H.E.; Held, M. The use of collagen-based matrices in the treatment of full-thickness wounds. Burns 2016, 42, 1257–1264. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, W.; Tang, L. Non-invasive Characterization of Immune Responses to Biomedical Implants. Ann. Biomed. Eng. 2016, 44, 693–704. [Google Scholar] [CrossRef]
- Marcos-Garces, V.; Harvat, M.; Aguilar, P.M.; Izquierdo, A.F.; Ruiz-Sauri, A. Comparative measurement of collagen bundle orientation by Fourier analysis and semiquantitative evaluation: Reliability and agreement in Masson’s trichrome, Picrosirius red and confocal microscopy techniques. J. Microsc. 2017, 267, 130–142. [Google Scholar] [CrossRef]
- Vega-Estrada, A.; Silvestre-Albero, J.; Rodriguez, A.E.; Rodriguez-Reinoso, F.; Gomez-Tejedor, J.A.; Antolinos-Turpin, C.M.; Bataille, L.; Alio, J.L. Biocompatibility and Biomechanical Effect of Single Wall Carbon Nanotubes Implanted in the Corneal Stroma: A Proof of Concept Investigation. J. Ophthalmol. 2016, 2016, 4041767. [Google Scholar] [CrossRef] [Green Version]
- Schipke, J.; Brandenberger, C.; Rajces, A.; Manninger, M.; Alogna, A.; Post, H.; Muhlfeld, C. Assessment of cardiac fibrosis: A morphometric method comparison for collagen quantification. J. Appl. Physiol. 2017, 122, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Y.; Tai, D.C.S.; Wang, S.; Cheng, C.L.; Peng, Q.; Yan, J.; Chen, Y.; Sun, J.; Liang, X.; et al. qFibrosis: A fully-quantitative innovative method incorporating histological features to facilitate accurate fibrosis scoring in animal model and chronic hepatitis B patients. J. Hepatol. 2014, 61, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Restellini, S.; Goossens, N.; Clement, S.; Lanthier, N.; Negro, F.; Rubbia-Brandt, L.; Spahr, L. Collagen proportionate area correlates to hepatic venous pressure gradient in non-abstinent cirrhotic patients with alcoholic liver disease. World J. Hepatol. 2018, 10, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Graca, Y.L.; Opolski, A.C.; Barboza, B.E.; Erbano, B.O.; Mazzaro, C.C.; Klostermann, F.C.; Sucharski, E.E.; Kubrusly, L.F. Biocompatibility of Ricinus communis polymer with addition of calcium carbonate compared to titanium. Experimental study in guinea pigs. Rev. Bras. Cir. Cardiovasc. 2014, 29, 272–278. [Google Scholar]
- Daunoravicius, D.; Besusparis, J.; Zurauskas, E.; Laurinaviciene, A.; Bironaite, D.; Pankuweit, S.; Plancoulaine, B.; Herlin, P.; Bogomolovas, J.; Grabauskiene, V.; et al. Quantification of myocardial fibrosis by digital image analysis and interactive stereology. Diagn. Pathol. 2014, 9, 114. [Google Scholar] [CrossRef]
- Vasiljevic, J.D.; Popovic, Z.B.; Otasevic, P.; Popovic, Z.V.; Vidakovic, R.; Miric, M.; Neskovic, A.N. Myocardial fibrosis assessment by semiquantitative, point-counting and computer-based methods in patients with heart muscle disease: A comparative study. Histopathology 2001, 38, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Marx, S.K.; Laue, C.; Hyder, A.; Juergensen, A.; Bickel, M.; Schrezenmeir, J. HOE 077 reduces fibrotic overgrowth around the barium alginate microcapsules. Transplant. Proc. 2000, 32, 206–209. [Google Scholar] [CrossRef]
- Zhang, W.J.; Laue, C.; Hyder, A.; Schrezenmeir, J. Purity of alginate affects the viability and fibrotic overgrowth of encapsulated porcine islet xenografts. Transplant. Proc. 2001, 33, 3517–3519. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.; Wang, W.; Ma, X. Quantitative analysis of fibrosis formation on the microcapsule surface with the use of picro-sirius red staining, polarized light microscopy, and digital image analysis. J. Biomed. Mater. Res. Part A 2006, 76, 120–125. [Google Scholar] [CrossRef]
- Madabhushi, A.; Lee, G. Image analysis and machine learning in digital pathology: Challenges and opportunities. Med. Image Anal. 2016, 33, 170–175. [Google Scholar] [CrossRef]
- Tang, L.; Eaton, J.W. Natural responses to unnatural materials: A molecular mechanism for foreign body reactions. Mol. Med. 1999, 5, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socarras, T.O.; Vasconcelos, A.C.; Campos, P.P.; Pereira, N.B.; Souza, J.P.; Andrade, S.P. Foreign body response to subcutaneous implants in diabetic rats. PLoS ONE 2014, 9, e110945. [Google Scholar] [CrossRef]
- Al-Maawi, S.; Orlowska, A.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. In vivo cellular reactions to different biomaterials-Physiological and pathological aspects and their consequences. Semin. Immunol. 2017, 29, 49–61. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. Part A 2017, 105, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Barsch, F.; Mamilos, A.; Babel, M.; Wagner, W.L.; Winther, H.B.; Schmitt, V.H.; Hierlemann, H.; Teufel, A.; Brochhausen, C. Semiautomated quantification of the fibrous tissue response to complex three-dimensional filamentous scaffolds using digital image analysis. J. Biomed. Mater. Res. 2021, 110, 353–364. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Foster, M.J.; Keeney, G.E.; Tsai, A.; Giachelli, C.M.; Clark-Lewis, I.; Rollins, B.J.; Bornstein, P. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am. J. Pathol. 2004, 165, 2157–2166. [Google Scholar] [CrossRef]
- Pavlides, M.; Birks, J.; Fryer, E.; Delaney, D.; Sarania, N.; Banerjee, R.; Neubauer, S.; Barnes, E.; Fleming, K.A.; Wang, L.M. Interobserver Variability in Histologic Evaluation of Liver Fibrosis Using Categorical and Quantitative Scores. Am. J. Clin. Pathol. 2017, 147, 364–369. [Google Scholar] [CrossRef]
- Hadi, A.M.; Mouchaers, K.T.; Schalij, I.; Grunberg, K.; Meijer, G.A.; Vonk-Noordegraaf, A.; van der Laarse, W.J.; Belien, J.A. Rapid quantification of myocardial fibrosis: A new macro-based automated analysis. Cell. Oncol. 2011, 34, 343–354. [Google Scholar] [CrossRef]
- Leite, S.N.; Junior, A.A.J.; Andrade, T.A.; Ddos, S.M.; Frade, M.A. Experimental models of malnutrition and its effect on skin trophism. An. Bras. Dermatol. 2011, 86, 681–688. [Google Scholar] [CrossRef]
- Caetano, G.F.; Frade, M.A.; Andrade, T.A.; Leite, M.N.; Bueno, C.Z.; Moraes, A.M.; Ribeiro-Paes, J.T. Chitosan-alginate membranes accelerate wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1013–1022. [Google Scholar] [CrossRef]
- Caetano, G.F.; Fronza, M.; Leite, M.N.; Gomes, A.; Frade, M.A. Comparison of collagen content in skin wounds evaluated by biochemical assay and by computer-aided histomorphometric analysis. Pharm. Biol. 2016, 54, 2555–2559. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jin, X.; Fan, Z.; Li, S.; Lu, Z. In vivo degradation of copolymers prepared from L-lactide, 1,3-trimethylene carbonate and glycolide as coronary stent materials. J. Mater. Sci. Mater. Med. 2015, 26, 139. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Triffitt, J.T. A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1–R9. [Google Scholar] [CrossRef] [PubMed]
- Lanao, R.P.F.; Jonker, A.M.; Wolke, J.G.; Jansen, J.A.; van Hest, J.C.; Leeuwenburgh, S.C. Physicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng. Part B Rev. 2013, 19, 380–390. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. In vivo biocompatibility of PLGA-polyhexylthiophene nanofiber scaffolds in a rat model. BioMed Res. Int. 2013, 2013, 390518. [Google Scholar] [CrossRef]
- DiEgidio, P.; Friedman, H.I.; Gourdie, R.G.; Riley, A.E.; Yost, M.J.; Goodwin, R.L. Biomedical implant capsule formation: Lessons learned and the road ahead. Ann. Plast. Surg. 2014, 73, 451–460. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Bygd, H.C.; Forsmark, K.D.; Bratlie, K.M. The significance of macrophage phenotype in cancer and biomaterials. Clin. Transl. Med. 2014, 3, 62. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Lambertz, A.M.; Reznikov, L.R.; Ofori-Amanfo, G.K.; Karp, P.H.; McCray, P.B.; Welsh, M.J.; Stoltz, D.A. Immunohistochemical Detection of Markers for Translational Studies of Lung Disease in Pigs and Humans. Toxicol. Pathol. 2016, 44, 434–441. [Google Scholar] [CrossRef]
- Anderson, J.M.; McNally, A.K. Biocompatibility of implants: Lymphocyte/macrophage interactions. Semin. Immunopathol. 2011, 33, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Serin, G.; Baylancicek, S.; Aksoy, E.; Polat, S.; Saglican, Y.; Inanli, S. Evaluation of tissue response to Gore-Tex (expanded polytetrafluoroethylene) implantation. J. Craniofacial Surg. 2013, 24, 1428–1430. [Google Scholar] [CrossRef] [PubMed]
- Christo, S.N.; Diener, K.R.; Bachhuka, A.; Vasilev, K.; Hayball, J.D. Innate Immunity and Biomaterials at the Nexus: Friends or Foes. BioMed Res. Int. 2015, 2015, 342304. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.N. Some aspects of macrophage behavior at the implant interface. J. Biomed. Mater. Res. 1984, 18, 395–401. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Liu, C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef]
- Feng, B.; Jinkang, Z.; Zhen, W.; Jianxi, L.; Jiang, C.; Jian, L.; Guolin, M.; Xin, D. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecture in vivo. Biomed. Mater. 2011, 6, 015007. [Google Scholar] [CrossRef]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef]
- Ward, W.K.; Slobodzian, E.P.; Tiekotter, K.L.; Wood, M.D. The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants. Biomaterials 2002, 23, 4185–4192. [Google Scholar] [CrossRef]
- Hilborn, J.; Bjursten, L.M. A new and evolving paradigm for biocompatibility. J. Tissue Eng. Regen. Med. 2007, 1, 110–119. [Google Scholar] [CrossRef]
- Blackwood, K.A.; McKean, R.; Canton, I.; Freeman, C.O.; Franklin, K.L.; Cole, D.; Brook, I.; Farthing, P.; Rimmer, S.; Haycock, J.W.; et al. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials 2008, 29, 3091–3104. [Google Scholar] [CrossRef]
- Venugopal, J.; Ramakrishna, S. Biocompatible nanofiber matrices for the engineering of a dermal substitute for skin regeneration. Tissue Eng. 2005, 11, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.; Ashwin, H.; Chen, R.; Smart, N.J.; Bayon, Y.; Wohlert, S.; Hunt, J.A. Evaluation of six synthetic surgical meshes implanted subcutaneously in a rat model. J. Tissue Eng. Regen. Med. 2016, 10, E305–E315. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; McHugh, K.; Chew, S.Y.; Anderson, J.M. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J. Biomed. Mater. Res. Part A 2010, 93, 1151–1159. [Google Scholar] [CrossRef]
- Kempf, M.; Miyamura, Y.; Liu, P.Y.; Chen, A.C.; Nakamura, H.; Shimizu, H.; Tabata, Y.; Kimble, R.M.; McMillan, J.R. A denatured collagen microfiber scaffold seeded with human fibroblasts and keratinocytes for skin grafting. Biomaterials 2011, 32, 4782–4792. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Romanova, O.A.; Grigor’ev, T.E.; Goncharov, M.E.; Rudyak, S.G.; Solov’yova, E.V.; Krasheninnikov, S.T.; Saprykin, V.P.; Sytina, E.V.; Chvalun, S.N.; Pal’tsev, M.A.; et al. Chitosan as a Modifying Component of Artificial Scaffold for Human Skin Tissue Engineering. Bull. Exp. Biol. Med. 2015, 159, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Barbeck, M.; Detsch, R.; Deisinger, U.; Hilbig, U.; Rausch, V.; Sader, R.; Unger, R.E.; Ziegler, G.; Kirkpatrick, C.J. The chemical composition of synthetic bone substitutes influences tissue reactions in vivo: Histological and histomorphometrical analysis of the cellular inflammatory response to hydroxyapatite, beta-tricalcium phosphate and biphasic calcium phosphate ceramics. Biomed. Mater. 2012, 7, 015005. [Google Scholar] [CrossRef] [PubMed]
- Nyame, T.T.; Chiang, H.A.; Leavitt, T.; Ozambela, M.; Orgill, D.P. Tissue-Engineered Skin Substitutes. Plast. Reconstr. Surg. 2015, 136, 1379–1388. [Google Scholar] [CrossRef]
- Major, M.R.; Wong, V.W.; Nelson, E.R.; Longaker, M.T.; Gurtner, G.C. The foreign body response: At the interface of surgery and bioengineering. Plast. Reconstr. Surg. 2015, 135, 1489–1498. [Google Scholar] [CrossRef]



| Biomaterial Group | Correlations | Spearman’s r | Significance (2-Tailed) |
|---|---|---|---|
| Filamentous fleeces | PMN & FBGC PMN & FCT PMN & CPA FBGC & FCT FBGC & CPA FCT & CPA | (−)0.551 0.221 (−)0.290 (−)0.250 0.044 0.472 | 0.001 * 0.224 0.873 0.893 0.810 0.006 * |
| Sponge-like form | PMN & FBGC PMN & FCT PMN & CPA FBGC & FCT FBGC & CPA FCT & CPA | 0.380 0.021 0.065 0.208 0.161 0.738 | 0.110 0.932 0.792 0.408 0.523 0.000 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barsch, F.; Mamilos, A.; Schmitt, V.H.; Babel, M.; Winter, L.; Wagner, W.; Winther, H.; Ottomann, C.; Niedermair, T.; Schreml, S.; et al. In Vivo Comparison of Synthetic Macroporous Filamentous and Sponge-like Skin Substitute Matrices Reveals Morphometric Features of the Foreign Body Reaction According to 3D Biomaterial Designs. Cells 2022, 11, 2834. https://doi.org/10.3390/cells11182834
Barsch F, Mamilos A, Schmitt VH, Babel M, Winter L, Wagner W, Winther H, Ottomann C, Niedermair T, Schreml S, et al. In Vivo Comparison of Synthetic Macroporous Filamentous and Sponge-like Skin Substitute Matrices Reveals Morphometric Features of the Foreign Body Reaction According to 3D Biomaterial Designs. Cells. 2022; 11(18):2834. https://doi.org/10.3390/cells11182834
Chicago/Turabian StyleBarsch, Friedrich, Andreas Mamilos, Volker H. Schmitt, Maximilian Babel, Lina Winter, Willi Wagner, Hinrich Winther, Christian Ottomann, Tanja Niedermair, Stephan Schreml, and et al. 2022. "In Vivo Comparison of Synthetic Macroporous Filamentous and Sponge-like Skin Substitute Matrices Reveals Morphometric Features of the Foreign Body Reaction According to 3D Biomaterial Designs" Cells 11, no. 18: 2834. https://doi.org/10.3390/cells11182834
APA StyleBarsch, F., Mamilos, A., Schmitt, V. H., Babel, M., Winter, L., Wagner, W., Winther, H., Ottomann, C., Niedermair, T., Schreml, S., Hierlemann, H., & Brochhausen, C. (2022). In Vivo Comparison of Synthetic Macroporous Filamentous and Sponge-like Skin Substitute Matrices Reveals Morphometric Features of the Foreign Body Reaction According to 3D Biomaterial Designs. Cells, 11(18), 2834. https://doi.org/10.3390/cells11182834







