ToBRFV Infects the Reproductive Tissues of Tomato Plants but Is Not Transmitted to the Progenies by Pollination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Inoculation Using Infected Seeds or Pollen as Inoculum
2.3. Plant Material
2.4. Viral Detection by RT-PCR
2.5. Fluorescent In Situ Hybridization (FISH)
2.6. 4′,6-Diamidino-2-phenylindole Dihydrochloride (DAPI) Staining
2.7. Plant Pollination
2.8. Germination of Pollen Grains
2.9. Seed Harvesting
2.10. Statistical Analyses
3. Results
3.1. ToBRFV Can Penetrate Tomato’s Reproductive Tissues
3.2. Cross-Pollination of Virus-Free Mother Plants with ToBRFV-Infected Pollen
3.3. ToBRFV’s Effect on Pollen Germination
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanssen, M.I.; Lapidot, M.; Thomma, P.H.J.B. Emerging viral diseases of tomato crops. Mol. Plant-Microbe Interact. 2010, 23, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.W.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A.; et al. A New Israeli Tobamovirus Isolate Infects Tomato Plants Harboring Tm-22 Resistance Genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef] [PubMed]
- Maayan, Y.; Pandaranayaka, E.; Srivastava, D.A.; Lapidot, M.; Levin, I.; Dombrovsky, A.; Harel, A. Using genomic analysis to identify tomato Tm-2 resistance breaking mutations and their underlined evolutionary path in a new and emerging tobamovirus. Arch. Virol. 2018, 163, 1863–1875. [Google Scholar] [CrossRef]
- Alfaro-Fernández, A.; Castillo, P.; Sanahuja, E.; Rodríguez-Salido, M.C.; Font, M.I. First report of tomato brown rugose fruit virus in tomato in Spain. Plant Dis. 2021, 105, 515. [Google Scholar] [CrossRef] [PubMed]
- Panno, S.; Caruso, A.G.; Davino, S. First report of tomato brown fruit rugose virus on tomato crops in Italy. Plant Dis. 2019, 103, 1443. [Google Scholar] [CrossRef]
- Beris, D.; Malandraki, I.; Kektsidou, O.; Theologidis, I.; Vassilakos, N.; Varveri, C. First report of tomato brown rugose fruit virus infecting tomato in Greece. Plant Dis. 2021, 104, 2035. [Google Scholar] [CrossRef]
- Amer, M.A.; Mahmoud, S.Y. First report of tomato brown rugose fruit virus on tomato in Egypt. New Dis. Rep. 2020, 41, 24. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, H.; Han, S.; Geng, C.; Tian, Y.; Li, X. First report of Tomato brown rugose fruit virus infecting tomato in China. Plant Disease. 2019, 103. [Google Scholar] [CrossRef]
- Fidan, H.; Sarikaya, P.; Calis, O. First report of tomato brown rugose fruit virus on tomato in Turkey. New Dis. Rep. 2019, 39, 18. [Google Scholar] [CrossRef] [Green Version]
- Ling, K.-S.; Tian, T.; Gurung, S.; Salati, R.; Gilliard, A. First report of tomato brown fruit rugose virus infecting greenhouse tomato in the United States. Plant Disease 2019, 103, 1439. [Google Scholar] [CrossRef]
- Davidson, K. Tomato Brown Rugose Fruit Virus Identified in Ontario. 2019. Available online: http://thegrower.org/news/tomato-brown-rugose-fruit-virus-identified-ontario (accessed on 14 August 2022).
- Camacho-Beltran, E.; Perez-Villarreal, A.; Leyva-Lopez, N.E.; Rodriguez-Negrete, E.A.; Ceniceros-Ojeda, E.A.; Mendez-Lozano, J. Occurrence of Tomato brown rugose fruit virus infecting tomato crops in Mexico. Plant Dis. 2019, 103, 1440. [Google Scholar] [CrossRef]
- Lewandowski, D.J. Tobamoviruses. In Encyclopedia of Virology; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press Inc.: New York, NY, USA, 2008; pp. 68–72. [Google Scholar]
- Broadbent, L. Epidemiology and control of tomato mosaic virus. Annu. Rev. Phytopathol. 1976, 14, 75–96. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Smith, E. Seed transmission of tobamoviruses: Aspects of global disease distribution. In Seed Biology; Jimenez-Lopez, J.C., Ed.; IntechOpen Limited: London, UK, 2017; pp. 234–260. [Google Scholar]
- Dombrovsky, A.; Tran-Nguyen, L.T.T.; Jones, R.C.A. Cucumber green mottle mosaic virus: Rapidly increasing global distribution, etiology, epidemiology, and management. Annu. Rev. Phytopathol. 2017, 55, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Davino, S.; Caruso, A.G.; Bertacca, S.; Barone, S.; Panno, S. Tomato Brown Rugose Fruit Virus: Seed Transmission Rate and Efficacy of Different Seed Disinfection Treatments. Plants 2020, 9, 1615. [Google Scholar] [CrossRef]
- Klap, C.; Luria, N.; Smith, E.; Bakelman, E.; Belausov, E.; Laskar, O.; Lachman, O.; Gal-On, A.; Dombrovsky, A. The Potential Risk of Plant-Virus Disease Initiation by Infected Tomatoes. Plants 2020, 9, 623. [Google Scholar] [CrossRef]
- Samarah, N.; Sulaiman, A.; Salem, N.M.; Turina, M. Disinfection treatments eliminated tomato brown rugose fruit virus in tomato seeds. Eur. J. Plant Pathol. 2020. [Google Scholar] [CrossRef]
- Chanda, B.; Shamimuzzaman, M.D.; Gilliard, A.; Ling, K.-S. Effectiveness of disinfectants against the spread of tobamoviruses: Tomato brown rugose fruit virus and Cucumber green mottle mosaic virus. Virol. J. 2021, 18. [Google Scholar] [CrossRef]
- Salem, N.M.; Sulaiman, A.; Samarah, N.; Turina, M.; Vallino, M. Localization and mechanical transmission of tomato brown rugose fruit virus in tomato seeds. Plant Dis. 2021, 22. [Google Scholar] [CrossRef]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef]
- Lapidot, M.; Levin, I. Genetic resistance to viruses in tomato. In Achieving Sustainable Cultivation of Tomatoes; Mattoo, A., Handa, A., Eds.; Burleigh Dodds Science Publishing: London, UK, 2017; pp. 381–400. [Google Scholar]
- Zinger, A.; Lapidot, M.; Harel, A.; Doron-Faigenboim, A.; Gelbart, D.; Levin, I. Identification and mapping of tomato genome loci controlling tolerance and resistance to tomato brown rugose fruit virus. Plants 2021, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Hak, H.; Spiegelman, Z. The tomato brown rugose fruit virus movement protein overcomes Tm-22 resistance in tomato while attenuating viral transport. Mol. Plant-Microbe Interact. 2021, 34, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Avni, B.; Gelbart, D.; Sufrin-Ringwald, T.; Zinger, A.; Chen, L.; Machbash, Z.; Bekelman, I.; Segoli, M.; Dombrovsky, A.; Kamenetsky, R.; et al. Tomato genetic resistance to tobamoviruses is compromised. Acta Hortic. 2021, 1316, 89–98. [Google Scholar] [CrossRef]
- USDA Federal Order. 2019. Available online: https://www.aphis.usda.gov/aphis/ourfocus/planthealth/import-information/federal-import-orders/tobrfv/tomato-brown-rugose-fruit-virus (accessed on 17 January 2022).
- EU Commission Implementing Regulation (EU) 2020/1191 of 11 August 2020. Establishing Measures to Prevent the Introduction into and the Spread within the Union of Tomato Brown Rugose Fruit Virus (ToBRFV) and Repealing Implementing Decision (EU) 2019/1615 C/2020/5453. Available online: http://data.europa.eu/eli/reg_impl/2020/1191/oj (accessed on 2 May 2022).
- Levin, I.; Gilboa, N. Direct PCR using tomato pollen grain suspension. BioTechniques 1997, 23, 986–990. [Google Scholar] [CrossRef]
- Shargil, D.; Zemach, H.; Belausov, E.; Lachman, O.; Kamenetsky, R.; Dombrovsky, A. Development of a fluorescent in situ hybridization (FISH) technique for visualizing CGMMV in plant tissues. J. Virol. Methods 2015, 223, 55–60. [Google Scholar] [CrossRef]
- Zin, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef]
- Rieu, I.; Twell, D.; Firon, N. Pollen Development at High Temperature: From Acclimation to Collapse. Plant Physiol. 2017, 173, 1967–1976. [Google Scholar] [CrossRef]
- Okada, K.; Kusakari, S.-I.; Kawaratani, M.; Negoro, J.-I.; Ohki, S.T.; Osaki, T. Tobacco mosaic virus is transmissible from tomato to tomato by pollinating bumblebees. J. Gen. Plant Pathol. 2000, 66, 71–74. [Google Scholar] [CrossRef]
- Darzi, E.; Smith, E.; Shargil, D.; Lachman, O.; Ganot, L.; Dombrovsky, A. The honeybee Apis mellifera contributes to Cucumber green mottle mosaic virus spread via pollination. Plant Pathol. 2018, 67, 244–251. [Google Scholar] [CrossRef]
- Mink, G.I. Pollen- and seed-transmitted viruses and viroids. Annu. Rev. Phytopathol. 1993, 31, 375–402. [Google Scholar] [CrossRef]
- Liu, H.W.; Luo, L.X.; Li, J.Q.; Liu, P.F.; Chen, X.Y.; Hao, J.J. Pollen and seed transmission of cucumber green mottle mosaic virus in cucumber. Plant Pathol. 2014, 63, 72–77. [Google Scholar] [CrossRef]

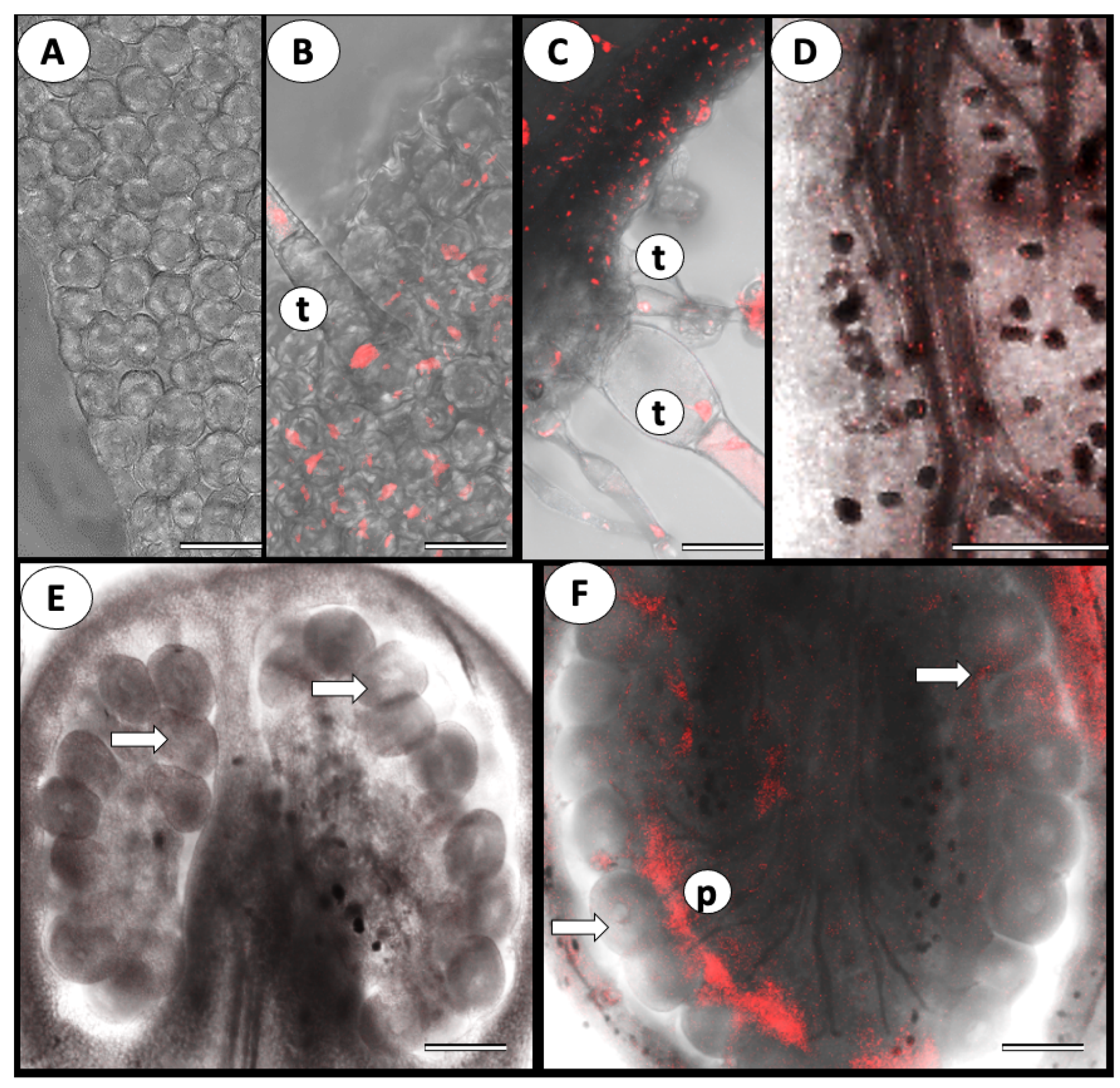
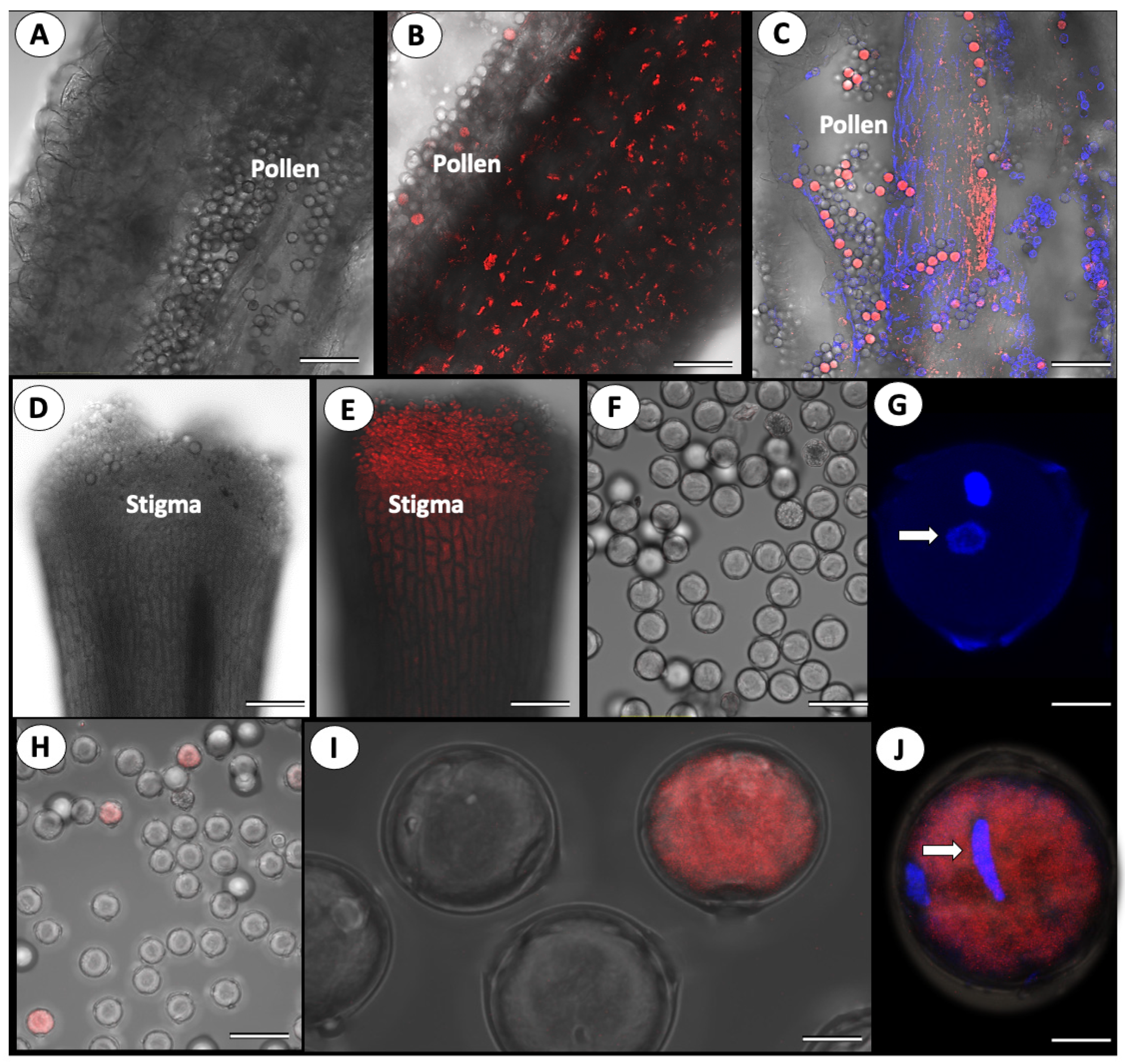
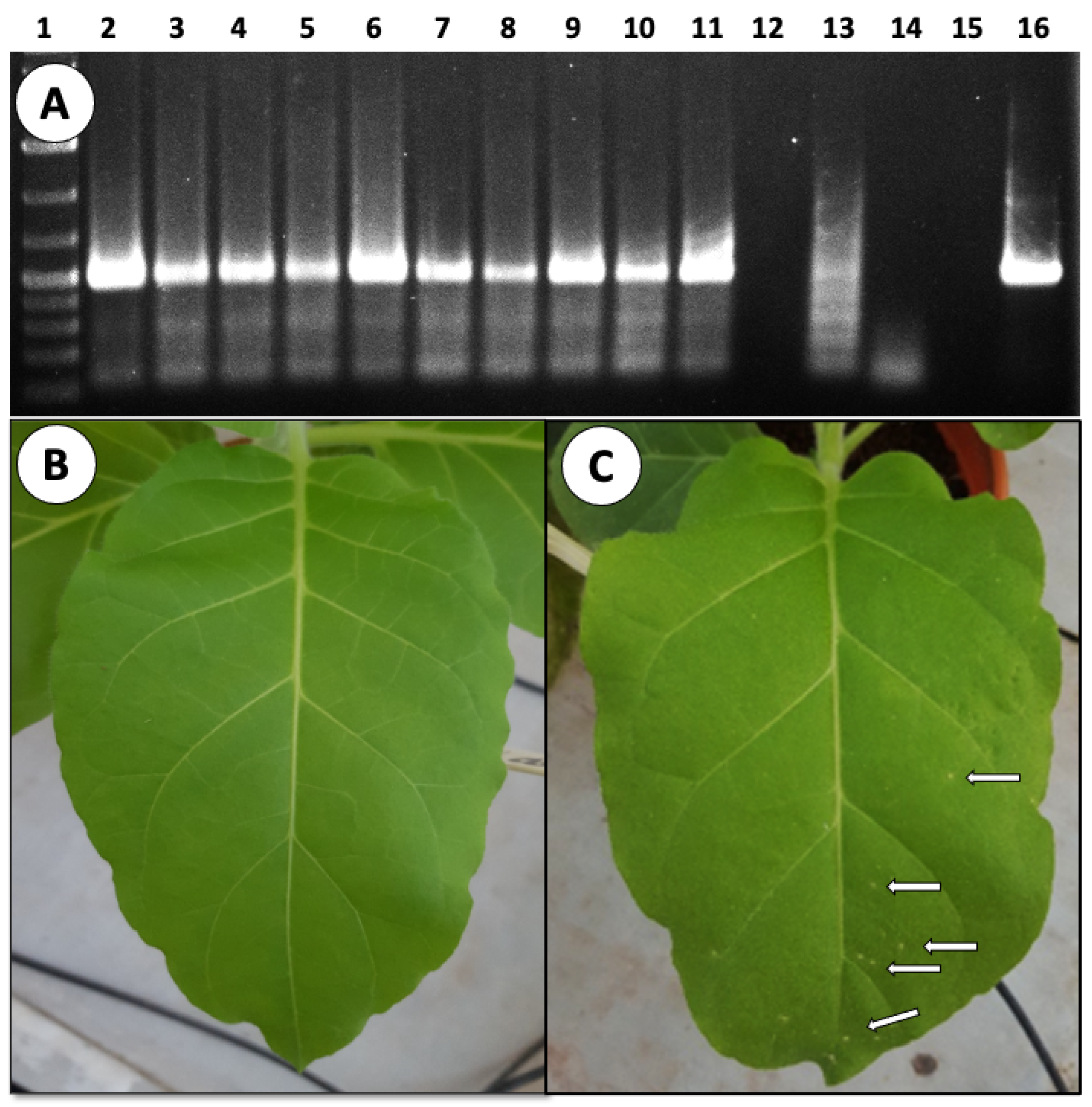

| Ex. | No. of Mother Plants | No. of Fruits | No. of Seeds * | Infection (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Infected ** | Not infected | Infected | Not infected | Infected | Not infected | Fruit | Seeds | |
| I | 7 | 20 | 1 | 39 | 3 | 95.2 | 92.8 | |
| 14 | 0 | 42 | 0 | 96 | 0.0 | 0.0 | ||
| II | 0 | |||||||
| 21 | 0 | 62 | 0 | 930 | 0.0 | 0.0 | ||
| Infected Plants | Non-Infected Plants | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of Pollen Grains | No. of Pollen Grains | |||||||
| Plant No. | Germinated | Not Germinated | Total | Germination (%) | Germinated | Not Germinated | Total | Germination (%) |
| 1 | 299 | 279 | 578 | 51.7 | 646 | 247 | 893 | 72.3 |
| 2 | 816 | 533 | 1349 | 60.5 | 931 | 461 | 1392 | 66.9 |
| 3 | 243 | 270 | 513 | 47.4 | 567 | 139 | 706 | 80.3 |
| 4 | 383 | 358 | 741 | 51.7 | 648 | 230 | 878 | 73.8 |
| 5 | 359 | 381 | 740 | 48.5 | 534 | 240 | 774 | 69.0 |
| 6 | 470 | 329 | 799 | 58.8 | 316 | 167 | 483 | 65.4 |
| 7 | 693 | 470 | 1163 | 59.6 | 548 | 335 | 883 | 62.1 |
| 8 | 899 | 972 | 1871 | 48.0 | 880 | 233 | 1113 | 79.1 |
| 9 | 158 | 232 | 390 | 40.5 | 612 | 95 | 707 | 86.6 |
| 10 | 174 | 183 | 357 | 48.7 | 584 | 176 | 760 | 76.8 |
| 11 | 581 | 557 | 1138 | 51.1 | 254 | 131 | 385 | 66.0 |
| 12 | 618 | 679 | 1297 | 47.6 | 1021 | 306 | 1327 | 76.9 |
| 13 | 192 | 233 | 425 | 45.2 | 338 | 129 | 467 | 72.4 |
| 14 | 107 | 150 | 257 | 41.6 | 579 | 164 | 743 | 77.9 |
| 15 | 212 | 470 | 682 | 31.1 | 504 | 193 | 697 | 72.3 |
| Total | 6204 | 6096 | 12,300 | 8962 | 3246 | 12,208 | ||
| Av: 48.8 ± 2.0 * | Av: 73.2 ± 1.7 * | |||||||
| No. of Pollen Grains | ||||
|---|---|---|---|---|
| Experiment | Field/Slide No. | Total | Infected | Infectivity (%) |
| I | 1 | 32 | 1 | 3.1% |
| 2 | 31 | 2 | 6.5% | |
| 3 | 240 | 6 | 2.5% | |
| 4 | 238 | 8 | 3.4% | |
| 5 | 188 | 6 | 3.2% | |
| 6 | 28 | 1 | 3.6% | |
| 7 | 51 | 3 | 5.9% | |
| 8 | 93 | 5 | 5.4% | |
| 9 | 73 | 1 | 1.4% | |
| 10 | 78 | 1 | 1.3% | |
| 11 | 76 | 1 | 1.3% | |
| II | 1 | 103 | 3 | 2.9% |
| 2 | 54 | 5 | 9.3% | |
| 3 | 56 | 4 | 7.1% | |
| 4 | 223 | 2 | 0.9% | |
| 5 | 16 | 1 | 6.3% | |
| III | 1 | 43 | 1 | 2.3% |
| 2 | 151 | 2 | 1.3% | |
| 3 | 74 | 1 | 1.4% | |
| 4 | 49 | 1 | 2.0% | |
| 5 | 28 | 1 | 3.6% | |
| 6 | 106 | 1 | 0.9% | |
| 7 | 109 | 1 | 0.9% | |
| 8 | 117 | 2 | 1.7% | |
| IV | 1 | 403 | 1 | 0.2% |
| 2 | 171 | 2 | 1.2% | |
| Total: | 26 | 2865 | 68 | |
| Av: 3.1% | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avni, B.; Gelbart, D.; Sufrin-Ringwald, T.; Zemach, H.; Belausov, E.; Kamenetsky-Goldstein, R.; Lapidot, M. ToBRFV Infects the Reproductive Tissues of Tomato Plants but Is Not Transmitted to the Progenies by Pollination. Cells 2022, 11, 2864. https://doi.org/10.3390/cells11182864
Avni B, Gelbart D, Sufrin-Ringwald T, Zemach H, Belausov E, Kamenetsky-Goldstein R, Lapidot M. ToBRFV Infects the Reproductive Tissues of Tomato Plants but Is Not Transmitted to the Progenies by Pollination. Cells. 2022; 11(18):2864. https://doi.org/10.3390/cells11182864
Chicago/Turabian StyleAvni, Ben, Dana Gelbart, Tali Sufrin-Ringwald, Hanita Zemach, Eduard Belausov, Rina Kamenetsky-Goldstein, and Moshe Lapidot. 2022. "ToBRFV Infects the Reproductive Tissues of Tomato Plants but Is Not Transmitted to the Progenies by Pollination" Cells 11, no. 18: 2864. https://doi.org/10.3390/cells11182864
APA StyleAvni, B., Gelbart, D., Sufrin-Ringwald, T., Zemach, H., Belausov, E., Kamenetsky-Goldstein, R., & Lapidot, M. (2022). ToBRFV Infects the Reproductive Tissues of Tomato Plants but Is Not Transmitted to the Progenies by Pollination. Cells, 11(18), 2864. https://doi.org/10.3390/cells11182864









