Metabolic Pathways in Breast Cancer Reprograming: An Insight to Non-Coding RNAs
Abstract
:1. Introduction
2. Non-Coding RNAs
3. Breast Cancer
4. Non-Coding RNAs as a Regulator of Metabolic Reprograming in BC
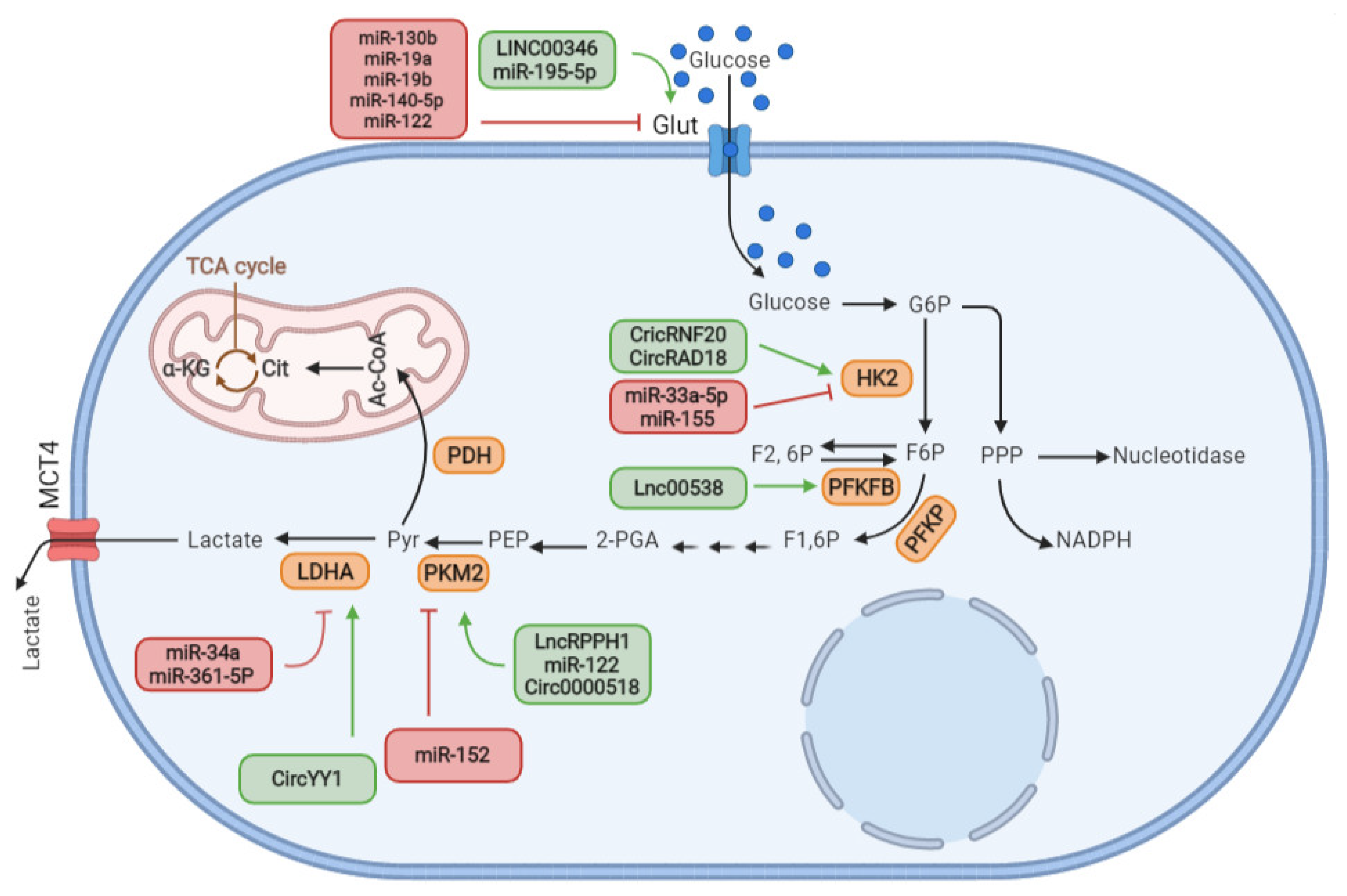
5. ncRNAs Function as a Regulator of Glucose Metabolisms Reprograming in BC
5.1. ncRNAs Regulate GLUT Expression to Control Glucose Cellular Uptake and Metabolism
5.2. ncRNA Relevance with the Key Enzymes of Glycolysis for the Efficient Regulation of Glucose Metabolism
6. ncRNAs Act as an Effective Regulator of Lipid Metabolism in Breast Cancer
7. ncRNAs Modulate Glutamine Reprograming in BC
8. ncRNAs as a Potential Tool for Prognosis Metabolic Pathway Dysregulation in Breast Cancer
9. Non-Coding RNA as Therapeutic Targets for the Regulation of Reprogramed Metabolic Pathways of Breast Cancer
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.M.; Yeung, S.-C.J.; Lee, M.-H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [PubMed]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef] [PubMed]
- Long, J.-P.; Li, X.-N.; Zhang, F. Targeting metabolism in breast cancer: How far we can go? World J. Clin. Oncol. 2016, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Lei, Q.-Y. Perspectives of reprogramming breast cancer metabolism. In Translational Research in Breast Cancer; Springer: Singapore, 2017; pp. 217–232. [Google Scholar]
- de Cedrón, M.G.; de Molina, A.R. Microtargeting cancer metabolism: Opening new therapeutic windows based on lipid metabolism. J. Lipid Res. 2016, 57, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Denkert, C.; Lehtinen, L.; Müller, B.; Brockmöller, S.; Seppänen-Laakso, T.; Budczies, J.; Bucher, E.; Yetukuri, L.; Castillo, S. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011, 71, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.F.; Whitaker-Menezes, D.; Howell, A.; Sotgia, F.; Lisanti, M.P. Mitochondrial biogenesis in epithelial cancer cells promotes breast cancer tumor growth and confers autophagy resistance. Cell Cycle 2012, 11, 4174–4180. [Google Scholar] [CrossRef] [PubMed]
- Blücher, C.; Stadler, S.C. Obesity and Breast Cancer: Current Insights on the Role of Fatty Acids and Lipid Metabolism in Promoting Breast Cancer Growth and Progression. Front. Endocrinol. 2017, 8, 293. [Google Scholar] [CrossRef]
- Shankaraiah, R.C.; Veronese, A.; Sabbioni, S.; Negrini, M. Non-coding RNAs in the reprogramming of glucose metabolism in cancer. Cancer Lett. 2018, 419, 167–174. [Google Scholar] [CrossRef]
- Tang, Y.-T.; Xu, X.-H.; Yang, X.-D.; Hao, J.; Cao, H.; Zhu, W.; Zhang, S.-Y.; Cao, J.-P. Role of non-coding RNAs in pancreatic cancer: The bane of the microworld. World J. Gastroenterol. WJG 2014, 20, 9405. [Google Scholar]
- Ghasabi, M.; Mansoori, B.; Mohammadi, A.; Duijf, P.H.; Shomali, N.; Shirafkan, N.; Mokhtarzadeh, A.; Baradaran, B. MicroRNAs in cancer drug resistance: Basic evidence and clinical applications. J. Cell. Physiol. 2019, 234, 2152–2168. [Google Scholar] [CrossRef]
- Xia, M.; Feng, S.; Chen, Z.; Wen, G.; Zu, X.; Zhong, J. Non-coding RNAs: Key regulators of aerobic glycolysis in breast cancer. Life Sci. 2020, 250, 117579. [Google Scholar] [CrossRef]
- Palazzo, A.F.; Lee, E.S. Non-coding RNA: What is functional and what is junk? Front. Genet. 2015, 6, 2. [Google Scholar] [CrossRef]
- Balihodzic, A.; Barth, D.A.; Prinz, F.; Pichler, M. Involvement of Long Non-Coding RNAs in Glucose Metabolism in Cancer. Cancers 2021, 13, 977. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, Q.; Wang, C.-Q.; Zhu, L.; Bi, C.; Zhang, S.; Wang, X.; Jin, H. LncRNAs regulate metabolism in cancer. Int. J. Biol. Sci. 2020, 16, 1194. [Google Scholar] [CrossRef]
- Guo, Y.; Lv, B.; Liu, R.; Dai, Z.; Zhang, F.; Liang, Y.; Yu, B.; Zeng, D.; Lv, X.-B.; Zhang, Z. Role of LncRNAs in regulating cancer amino acid metabolism. Cancer Cell Int. 2021, 21, 209. [Google Scholar] [CrossRef]
- Wei, L.; Sun, J.; Zhang, N.; Zheng, Y.; Wang, X.; Lv, L.; Liu, J.; Xu, Y.; Shen, Y.; Yang, M. Noncoding RNAs in gastric cancer: Implications for drug resistance. Mol. Cancer 2020, 19, 62. [Google Scholar] [CrossRef]
- Seidi, K.; Eatemadi, A.; Mansoori, B.; Jahanban-Esfahlan, R.; Farajzadeh, D. Nanomagnet-based detoxifying machine: An alternative/complementary approach in HIV therapy. J. AIDS Clin. Res. 2014, 5, 304. [Google Scholar] [CrossRef]
- Li, J.; Sun, D.; Pu, W.; Wang, J.; Peng, Y. Circular RNAs in cancer: Biogenesis, function, and clinical significance. Trends Cancer 2020, 6, 319–336. [Google Scholar] [CrossRef]
- De Fraipont, F.; Gazzeri, S.; Cho, W.C.; Eymin, B. Circular RNAs and RNA splice variants as biomarkers for prognosis and therapeutic response in the liquid biopsies of lung cancer patients. Front. Genet. 2019, 10, 390. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Shirjang, S.; Baradaran, B. Micro-RNAs: The new potential biomarkers in cancer diagnosis, prognosis and cancer therapy. Cell. Mol. Biol. (Noisy-Le-Grand Fr.) 2015, 61, 1–10. [Google Scholar]
- Mansoori, B.; Mohammadi, A.; Shirjang, S.; Baradaran, B. MicroRNAs in the diagnosis and treatment of cancer. Immunol. Investig. 2017, 46, 880–897. [Google Scholar] [CrossRef]
- Amorim, M.; Salta, S.; Henrique, R.; Jerónimo, C. Decoding the usefulness of non-coding RNAs as breast cancer markers. J. Transl. Med. 2016, 14, 265. [Google Scholar] [CrossRef]
- Sørlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, L.; Li, H.; Sun, T.; Wen, X.; Li, X.; Meng, Y.; Li, Y.; Liu, M.; Liu, S. Nuclear-encoded lncRNA MALAT1 epigenetically controls metabolic reprogramming in HCC cells through the mitophagy pathway. Mol. Ther. Nucleic Acids 2021, 23, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Qiu, Z.; Guan, Y.; Zhang, X.; Zhang, X.; Chai, D.; Chen, C.; Hu, Q.; Wang, W. STK25 enhances hepatocellular carcinoma progression through the STRN/AMPK/ACC1 pathway. Cancer Cell Int. 2022, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, L.; Yin, Y.; He, J.; Li, Q.; Qian, X.; You, Y.; Lu, Z.; Peiper, S.; Shu, Y. Regulatory circuit of PKM2/NF-κB/miR-148a/152-modulated tumor angiogenesis and cancer progression. Oncogene 2015, 34, 5482–5493. [Google Scholar] [CrossRef] [PubMed]
- Lucantoni, F.; Dussmann, H.; Prehn, J.H. Metabolic targeting of breast cancer cells with the 2-deoxy-D-glucose and the mitochondrial bioenergetics inhibitor MDIVI-1. Front. Cell Dev. Biol. 2018, 6, 113. [Google Scholar] [CrossRef]
- Dar, S.; Chhina, J.; Mert, I.; Chitale, D.; Buekers, T.; Kaur, H.; Giri, S.; Munkarah, A.; Rattan, R. Bioenergetic adaptations in chemoresistant ovarian cancer cells. Sci. Rep. 2017, 7, 8760. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Alfardus, H.; McIntyre, A.; Smith, S. MicroRNA regulation of glycolytic metabolism in glioblastoma. BioMed Res. Int. 2017, 2017, 9157370. [Google Scholar] [CrossRef]
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.; Chin, A.R. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Sniegowski, T.; Korac, K.; Bhutia, Y.D.; Ganapathy, V. SLC6A14 and SLC38A5 Drive the Glutaminolysis and Serine–Glycine–One-Carbon Pathways in Cancer. Pharmaceuticals 2021, 14, 216. [Google Scholar] [CrossRef]
- Szablewski, L. Expression of glucose transporters in cancers. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2013, 1835, 164–169. [Google Scholar] [CrossRef]
- Chen, X.; Lu, P.; Zhou, S.; Zhang, L.; Zhao, J.-H.; Tang, J.-H. Predictive value of glucose transporter-1 and glucose transporter-3 for survival of cancer patients: A meta-analysis. Oncotarget 2017, 8, 13206. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Wang, W.; Yu, X.; Xu, Q. LINC00346 regulates glycolysis by modulation of glucose transporter 1 in breast cancer cells. Mol. Cell. Probes 2020, 54, 101667. [Google Scholar] [CrossRef]
- He, Y.; Deng, F.; Zhao, S.; Zhong, S.; Zhao, J.; Wang, D.; Chen, X.; Zhang, J.; Hou, J.; Zhang, W. Analysis of miRNA–mRNA network reveals miR-140-5p as a suppressor of breast cancer glycolysis via targeting GLUT1. Epigenomics 2019, 11, 1021–1036. [Google Scholar] [CrossRef]
- Li, L.; Liang, Y.; Kang, L.; Liu, Y.; Gao, S.; Chen, S.; Li, Y.; You, W.; Dong, Q.; Hong, T. Transcriptional regulation of the Warburg effect in cancer by SIX1. Cancer Cell 2018, 33, 368–385.e367. [Google Scholar] [CrossRef]
- Feng, Z.; Levine, A.J. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 2010, 20, 427–434. [Google Scholar] [CrossRef]
- Bhaskar, P.T.; Nogueira, V.; Patra, K.C.; Jeon, S.-M.; Park, Y.; Robey, R.B.; Hay, N. mTORC1 hyperactivity inhibits serum deprivation-induced apoptosis via increased hexokinase II and GLUT1 expression, sustained Mcl-1 expression, and glycogen synthase kinase 3β inhibition. Mol. Cell. Biol. 2009, 29, 5136–5147. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Peiris-Pagès, M.; Martinez-Outschoorn, U.E.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer stem cell metabolism. Breast Cancer Res. 2016, 18, 55. [Google Scholar] [CrossRef]
- Clarke, A.R.; Wigley, D.B.; Chia, W.N.; Barstow, D.; Atkinson, T.; Holbrook, J.J. Site-directed mutagenesis reveals role of mobile arginine residue in lactate dehydrogenase catalysis. Nature 1986, 324, 699–702. [Google Scholar] [CrossRef]
- Tomasetti, M.; Amati, M.; Santarelli, L.; Neuzil, J. MicroRNA in metabolic re-programming and their role in tumorigenesis. Int. J. Mol. Sci. 2016, 17, 754. [Google Scholar] [CrossRef]
- Patra, K.C.; Hay, N. Hexokinase 2 as oncotarget. Oncotarget 2013, 4, 1862. [Google Scholar] [CrossRef]
- Cassim, S.; Raymond, V.-A.; Dehbidi-Assadzadeh, L.; Lapierre, P.; Bilodeau, M. Metabolic reprogramming enables hepatocarcinoma cells to efficiently adapt and survive to a nutrient-restricted microenvironment. Cell Cycle 2018, 17, 903–916. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, L.F.; Zhang, H.W.; Hu, S.; Lu, M.H.; Liang, S.; Li, B.; Li, Y.; Li, D.; Wang, E.D. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012, 31, 1985–1998. [Google Scholar] [CrossRef]
- Guo, W.; Qiu, Z.; Wang, Z.; Wang, Q.; Tan, N.; Chen, T.; Chen, Z.; Huang, S.; Gu, J.; Li, J. MiR-199a-5p is negatively associated with malignancies and regulates glycolysis and lactate production by targeting hexokinase 2 in liver cancer. Hepatology 2015, 62, 1132–1144. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.; Jung, J.; Lee, J.W.; Kim, H.J.; Kim, J.; ju Yoo, H.; Lee, H.J.; Chae, S.Y.; Jeon, S.M. microRNA-155 positively regulates glucose metabolism via PIK3R1-FOXO3a-cMYC axis in breast cancer. Oncogene 2018, 37, 2982–2991. [Google Scholar] [CrossRef]
- Cao, L.; Wang, M.; Dong, Y.; Xu, B.; Chen, J.; Ding, Y.; Qiu, S.; Li, L.; Zaharieva, E.K.; Zhou, X. Circular RNA circRNF20 promotes breast cancer tumorigenesis and Warburg effect through miR-487a/HIF-1α/HK2. Cell Death Dis. 2020, 11, 145. [Google Scholar] [CrossRef]
- Zou, Y.; Zheng, S.; Xiao, W.; Xie, X.; Yang, A.; Gao, G.; Xiong, Z.; Xue, Z.; Tang, H.; Xie, X. circRAD18 sponges miR-208a/3164 to promote triple-negative breast cancer progression through regulating IGF1 and FGF2 expression. Carcinogenesis 2019, 40, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Li, Y.; Zhang, X.; Huang, G. Knockdown of circRAD18 Mitigates Breast Cancer Progression through the Regulation of miR-613/HK2 Axis. Cancer Manag. Res. 2020, 12, 3661. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J. 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase and tumor cell glycolysis. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Al Hasawi, N.; Alkandari, M.F.; Luqmani, Y.A. Phosphofructokinase: A mediator of glycolytic flux in cancer progression. Crit. Rev. Oncol. Hematol. 2014, 92, 312–321. [Google Scholar] [CrossRef]
- Ge, X.; Lyu, P.; Cao, Z.; Li, J.; Guo, G.; Xia, W.; Gu, Y. Overexpression of miR-206 suppresses glycolysis, proliferation and migration in breast cancer cells via PFKFB3 targeting. Biochem. Biophys. Res. Commun. 2015, 463, 1115–1121. [Google Scholar] [CrossRef]
- Du, J.-Y.; Wang, L.-F.; Wang, Q.; Yu, L.-D. miR-26b inhibits proliferation, migration, invasion and apoptosis induction via the downregulation of 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase-3 driven glycolysis in osteosarcoma cells. Oncol. Rep. 2015, 33, 1890–1898. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, Y.; Liang, K.; Yan, L.; Xiang, Y.; Li, C.; Hu, Q.; Jin, F.; Putluri, V.; Putluri, N. Expression of long noncoding RNA YIYA promotes glycolysis in breast cancer. Cancer Res. 2018, 78, 4524–4532. [Google Scholar] [CrossRef]
- Yu, H.; Luo, H.; Liu, X. Knockdown of circ_0102273 inhibits the proliferation, metastasis and glycolysis of breast cancer through miR-1236-3p/PFKFB3 axis. Anti-Cancer Drugs 2022, 33, 323–334. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Gupta, V.; Gopinath, P.; Mazurek, S.; Bamezai, R.N. Pyruvate kinase M2 and cancer: An updated assessment. FEBS Lett. 2014, 588, 2685–2692. [Google Scholar] [CrossRef]
- Jurica, M.S.; Mesecar, A.; Heath, P.J.; Shi, W.; Nowak, T.; Stoddard, B.L. The allosteric regulation of pyruvate kinase by fructose-1, 6-bisphosphate. Structure 1998, 6, 195–210. [Google Scholar] [CrossRef]
- Qattan, A. Metabolic reprogramming of triple-negative breast cancer: The role of miRNAs. MicroRNA Diagn. Ther. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Wu, M.; Neilson, A.; Swift, A.L.; Moran, R.; Tamagnine, J.; Parslow, D.; Armistead, S.; Lemire, K.; Orrell, J.; Teich, J. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. -Cell Physiol. 2007, 292, C125–C136. [Google Scholar] [CrossRef] [Green Version]
- Zahra, K.; Dey, T.; Ashish, A.; Pandey, U.; Mishra, S.P. Pyruvate Kinase M2 and Cancer: The role of PKM2 in promoting tumorigenesis. Front. Oncol. 2020, 10, 159. [Google Scholar] [CrossRef]
- Yao, A.; Xiang, Y.; Si, Y.R.; Fan, L.J.; Li, J.P.; Li, H.; Guo, W.; He, H.X.; Liang, X.J.; Tan, Y. PKM2 promotes glucose metabolism through a let-7a-5p/Stat3/hnRNP-A1 regulatory feedback loop in breast cancer cells. J. Cell. Biochem. 2019, 120, 6542–6554. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L. Inhibition of breast cancer cell proliferation and tumorigenesis by long non-coding RNA RPPH1 down-regulation of miR-122 expression. Cancer Cell Int. 2017, 17, 109. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Yang, Z. MiR-122 inhibits cell proliferation and tumorigenesis of breast cancer by targeting IGF1R. PLoS ONE 2012, 7, e47053. [Google Scholar] [CrossRef]
- Jiang, J.; Lin, H.; Shi, S.; Hong, Y.; Bai, X.; Cao, X. Hsa_circRNA _0000518 facilitates breast cancer development via regulation of the miR-326/FGFR1 axis. Thoracic Cancer 2020, 11, 3181–3192. [Google Scholar] [CrossRef]
- He, X.; Xu, T.; Hu, W.; Tan, Y.; Wang, D.; Wang, Y.; Zhao, C.; Yi, Y.; Xiong, M.; Lv, W. Circular RNAs: Their Role in the Pathogenesis and Orchestration of Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 431. [Google Scholar] [CrossRef]
- Liu, G.; Huang, X. Circ_0000518 contributes to breast cancer development depending on the regulation of miR-1258/ZEB1 axis. All Life 2021, 14, 195–208. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487. [Google Scholar] [CrossRef]
- Deng, X.; Wang, Q.; Cheng, M.; Chen, Y.; Yan, X.; Guo, R.; Sun, L.; Li, Y.; Liu, Y. Pyruvate dehydrogenase kinase 1 interferes with glucose metabolism reprogramming and mitochondrial quality control to aggravate stress damage in cancer. J. Cancer 2020, 11, 962. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rosales-Corral, S.; Acuna-Castroviejo, D.; Escames, G. Inhibition of mitochondrial pyruvate dehydrogenase kinase: A proposed mechanism by which melatonin causes cancer cells to overcome cytosolic glycolysis, reduce tumor biomass and reverse insensitivity to chemotherapy. Melatonin Res. 2019, 2, 105–119. [Google Scholar] [CrossRef]
- Sun, R.C.; Fadia, M.; Dahlstrom, J.E.; Parish, C.R.; Board, P.G.; Blackburn, A.C. Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Res. Treat. 2010, 120, 253–260. [Google Scholar] [CrossRef]
- Jeoung, N.H. Pyruvate dehydrogenase kinases: Therapeutic targets for diabetes and cancers. Diabetes Metab. J. 2015, 39, 188. [Google Scholar] [CrossRef]
- Eastlack, S.C.; Dong, S.; Ivan, C.; Alahari, S.K. Suppression of PDHX by microRNA-27b deregulates cell metabolism and promotes growth in breast cancer. Mol. Cancer 2018, 17, 100. [Google Scholar] [CrossRef]
- Guda, M.R.; Asuthkar, S.; Labak, C.M.; Tsung, A.J.; Alexandrov, I.; Mackenzie, M.J.; Prasad, D.V.; Velpula, K.K. Targeting PDK4 inhibits breast cancer metabolism. Am. J. Cancer Res. 2018, 8, 1725. [Google Scholar]
- Peng, F.; Wang, J.-H.; Fan, W.-J.; Meng, Y.-T.; Li, M.-M.; Li, T.-T.; Cui, B.; Wang, H.-F.; Zhao, Y.; An, F. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene 2018, 37, 1062–1074. [Google Scholar] [CrossRef]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef]
- Zhang, D.-G.; Zheng, J.-N.; Pei, D.-S. P53/microRNA-34-induced metabolic regulation: New opportunities in anticancer therapy. Mol. Cancer 2014, 13, 115. [Google Scholar] [CrossRef]
- Oshima, N.; Ishida, R.; Kishimoto, S.; Beebe, K.; Brender, J.R.; Yamamoto, K.; Urban, D.; Rai, G.; Johnson, M.S.; Benavides, G. Dynamic imaging of LDH inhibition in tumors reveals rapid in vivo metabolic rewiring and vulnerability to combination therapy. Cell Rep. 2020, 30, 1798–1810.e1794. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Zhou, X.; Song, L. Inhibition of lactate dehydrogenase A by microRNA-34a resensitizes colon cancer cells to 5-fluorouracil. Mol. Med. Rep. 2015, 11, 577–582. [Google Scholar] [CrossRef]
- Tamura, K.; Matsushita, Y.; Watanabe, H.; Motoyama, D.; Ito, T.; Sugiyama, T.; Otsuka, A.; Miyake, H. Feasibility of the ACL (albumin, C-reactive protein and lactate dehydrogenase) model as a novel prognostic tool in patients with metastatic renal cell carcinoma previously receiving first-line targeted therapy. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 6.e9–6.e16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fu, Y.; Guo, H. c-Myc-induced long non-coding RNA small nucleolar RNA host gene 7 regulates glycolysis in breast cancer. J. Breast Cancer 2019, 22, 533. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Huang, X.; Ye, F.; Chen, B.; Song, C.; Wen, J.; Zhang, Z.; Zheng, G.; Tang, H.; Xie, X. The miR-34a-LDHA axis regulates glucose metabolism and tumor growth in breast cancer. Sci. Rep. 2016, 6, 21735. [Google Scholar] [CrossRef] [PubMed]
- Hitosugi, T.; Fan, J.; Chung, T.-W.; Lythgoe, K.; Wang, X.; Xie, J.; Ge, Q.; Gu, T.-L.; Polakiewicz, R.D.; Roesel, J.L. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol. Cell 2011, 44, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhang, L.; Ma, L.; Zhang, Y.; Zhang, J.; Guo, B. MiR-361-5p inhibits glycolytic metabolism, proliferation and invasion of breast cancer by targeting FGFR1 and MMP-1. J. Exp. Clin. Cancer Res. 2017, 36, 158. [Google Scholar] [CrossRef]
- Sazgarnia, A.; Montazerabadi, A.R.; Bahreyni-Toosi, M.H.; Ahmadi, A.; Aledavood, A. In vitro survival of MCF-7 breast cancer cells following combined treatment with ionizing radiation and mitoxantrone-mediated photodynamic therapy. Photodiagnosis Photodyn. Ther. 2013, 10, 72–78. [Google Scholar] [CrossRef]
- Lei, K.; Du, W.; Lin, S.; Yang, L.; Xu, Y.; Gao, Y.; Xu, B.; Tan, S.; Xu, Y.; Qian, X. 3B, a novel photosensitizer, inhibits glycolysis and inflammation via miR-155-5p and breaks the JAK/STAT3/SOCS1 feedback loop in human breast cancer cells. Biomed. Pharmacother. 2016, 82, 141–150. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Feng, Q. CircRNA circYY1 (hsa_circ_0101187) Modulates Cell Glycolysis and Malignancy Through Regulating YY1 Expression by Sponging miR-769-3p in Breast Cancer. Cancer Manag. Res. 2021, 13, 1145. [Google Scholar] [CrossRef]
- Wang, K.; Yang, S.; Gao, Y.; Zhang, C.; Sui, Q. Erratum: MicroRNA-769-3p inhibits tumor progression in glioma by suppressing ZEB2 and inhibiting the Wnt/β-catenin signaling pathway. Oncol. Lett. 2021, 21, 228. [Google Scholar] [CrossRef]
- Prebil, M.; Jensen, J.; Zorec, R.; Kreft, M. Astrocytes and energy metabolism. Arch. Physiol. Biochem. 2011, 117, 64–69. [Google Scholar] [CrossRef]
- Feron, O. Pyruvate into lactate and back: From the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. 2009, 92, 329–333. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Tepper, S.A. Influence of medium-chain triglyceride (MCT) on cholesterol metabolism in rats. J. Nutr. 1965, 86, 67–72. [Google Scholar] [CrossRef]
- Puri, S.; Juvale, K. Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: A review with structure-activity relationship insights. Eur. J. Med. Chem. 2020, 199, 112393. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, X.; Zhang, S.; Zhu, A.; Yuan, Y.; Xu, H.; Lei, J.; Yan, C. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 2021, 184, 370–383.e313. [Google Scholar] [CrossRef]
- Romero-Cordoba, S.L.; Rodriguez-Cuevas, S.; Bautista-Pina, V.; Maffuz-Aziz, A.; D’Ippolito, E.; Cosentino, G.; Baroni, S.; Iorio, M.V.; Hidalgo-Miranda, A. Loss of function of miR-342-3p results in MCT1 over-expression and contributes to oncogenic metabolic reprogramming in triple negative breast cancer. Sci. Rep. 2018, 8, 12252. [Google Scholar] [CrossRef]
- Liu, W.; Kang, L.; Han, J.; Wang, Y.; Shen, C.; Yan, Z.; Tai, Y.; Zhao, C. miR-342-3p suppresses hepatocellular carcinoma proliferation through inhibition of IGF-1R-mediated Warburg effect. OncoTargets Ther. 2018, 11, 1643. [Google Scholar] [CrossRef]
- Hou, L.; Zhao, Y.; Song, G.-Q.; Ma, Y.-H.; Jin, X.-H.; Jin, S.-L.; Fang, Y.-H.; Chen, Y.-C. Interfering cellular lactate homeostasis overcomes Taxol resistance of breast cancer cells through the microRNA-124-mediated lactate transporter (MCT1) inhibition. Cancer Cell Int. 2019, 19, 193. [Google Scholar] [CrossRef]
- Liu, T.; Ye, P.; Ye, Y.; Han, B. MicroRNA-216b targets HK2 to potentiate autophagy and apoptosis of breast cancer cells via the mTOR signaling pathway. Int. J. Biol. Sci. 2021, 17, 2970. [Google Scholar] [CrossRef]
- Saumet, A.; Vetter, G.; Bouttier, M.; Antoine, E.; Roubert, C.; Orsetti, B.; Theillet, C.; Lecellier, C.-H. Estrogen and retinoic acid antagonistically regulate several microRNA genes to control aerobic glycolysis in breast cancer cells. Mol. BioSyst. 2012, 8, 3242–3253. [Google Scholar] [CrossRef]
- Li, L.; Kang, L.; Zhao, W.; Feng, Y.; Liu, W.; Wang, T.; Mai, H.; Huang, J.; Chen, S.; Liang, Y. miR-30a-5p suppresses breast tumor growth and metastasis through inhibition of LDHA-mediated Warburg effect. Cancer Lett. 2017, 400, 89–98. [Google Scholar] [CrossRef]
- Ding, M.; Fu, Y.; Guo, F.; Chen, H.; Fu, X.; Tan, W.; Zhang, H. Long non-coding RNA MAFG-AS1 knockdown blocks malignant progression in breast cancer cells by inactivating JAK2/STAT3 signaling pathway via MAFG-AS1/miR-3196/TFAP2A axis. Int. J. Clin. Exp. Pathol. 2020, 13, 2455. [Google Scholar]
- Mota, M.S.; Jackson, W.P.; Bailey, S.K.; Vayalil, P.; Landar, A.; Rostas III, J.W.; Mulekar, M.S.; Samant, R.S.; Shevde, L.A. Deficiency of tumor suppressor Merlin facilitates metabolic adaptation by co-operative engagement of SMAD-Hippo signaling in breast cancer. Carcinogenesis 2018, 39, 1165–1175. [Google Scholar] [CrossRef]
- Xing, Z.; Park, P.K.; Lin, C.; Yang, L. LncRNA BCAR4 wires up signaling transduction in breast cancer. RNA Biol. 2015, 12, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Fang, K.; Xu, Z.-J.; Jiang, S.-X.; Tang, D.-S.; Yan, C.-S.; Deng, Y.-Y.; Zhao, F.-Y. lncRNA FGD5-AS1 promotes breast cancer progression by regulating the hsa-miR-195-5p/NUAK2 axis. Mol. Med. Rep. 2021, 23, 460. [Google Scholar] [CrossRef]
- Du, Y.; Wei, N.; Ma, R.; Jiang, S.-H.; Song, D. Long Noncoding RNA MIR210HG promotes the Warburg effect and tumor growth by enhancing HIF-1α translation in triple-negative breast cancer. Front. Oncol. 2020, 10, 580176. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, R.; Deng, C.; Zhou, Z. Circle RNA circABCB10 modulates PFN2 to promote breast cancer progression, as well as aggravate radioresistance through facilitating glycolytic metabolism via miR-223-3p. Cancer Biother. Radiopharm. 2020, 36, 477–490. [Google Scholar] [CrossRef]
- Ma, F.; Liu, X.; Zhou, S.; Li, W.; Liu, C.; Chadwick, M.; Qian, C. Long non-coding RNA FGF13-AS1 inhibits glycolysis and stemness properties of breast cancer cells through FGF13-AS1/IGF2BPs/Myc feedback loop. Cancer Lett. 2019, 450, 63–75. [Google Scholar] [CrossRef]
- Ren, S.; Liu, J.; Feng, Y.; Li, Z.; He, L.; Li, L.; Cao, X.; Wang, Z.; Zhang, Y. Knockdown of circDENND4C inhibits glycolysis, migration and invasion by up-regulating miR-200b/c in breast cancer under hypoxia. J. Exp. Clin. Cancer Res. 2019, 38, 388. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, X.; Liu, J.; Zhang, M.; Feng, K.; Wang, X. Hsa_circ_0069094 accelerates cell malignancy and glycolysis through regulating the miR-591/HK2 axis in breast cancer. Cell. Signal. 2021, 79, 109878. [Google Scholar] [CrossRef]
- Ni, J.; Xi, X.; Xiao, S.; Xiao, X. Silencing of circHIPK3 Sensitizes Paclitaxel-Resistant Breast Cancer Cells to Chemotherapy by Regulating HK2 Through Targeting miR-1286. Cancer Manag. Res. 2021, 13, 5573. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Freter, C. Lipid metabolism, apoptosis and cancer therapy. Int. J. Mol. Sci. 2015, 16, 924–949. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Guo, D.D.; Lee, H.G.; Choi, Y.J.; Kang, J.S.; Jo, K.; Eom, J.M.; Yun, C.H.; Cho, C.S. Alpha-eleostearic acid suppresses proliferation of MCF-7 breast cancer cells via activation of PPARγ and inhibition of ERK 1/2. Cancer Sci. 2010, 101, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, W.W.; Kurokawa, M. Lipid metabolic reprogramming as an emerging mechanism of resistance to kinase inhibitors in breast cancer. Cancer Drug Resist. 2020, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yin, L.; Wei, J.; Yang, Z.; Jiang, G. ATP citrate lyase is increased in human breast cancer, depletion of which promotes apoptosis. Tumor Biol. 2017, 39, 1010428317698338. [Google Scholar] [CrossRef]
- Hatzivassiliou, G.; Zhao, F.; Bauer, D.E.; Andreadis, C.; Shaw, A.N.; Dhanak, D.; Hingorani, S.R.; Tuveson, D.A.; Thompson, C.B. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 2005, 8, 311–321. [Google Scholar] [CrossRef]
- Migita, T.; Narita, T.; Nomura, K.; Miyagi, E.; Inazuka, F.; Matsuura, M.; Ushijima, M.; Mashima, T.; Seimiya, H.; Satoh, Y. ATP citrate lyase: Activation and therapeutic implications in non–small cell lung cancer. Cancer Res. 2008, 68, 8547–8554. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Ye, T. MiR-22 down-regulates the proto-oncogene ATP citrate lyase to inhibit the growth and metastasis of breast cancer. Am. J. Transl. Res. 2018, 10, 659. [Google Scholar]
- Singh, R.; Yadav, V.; Saini, N. MicroRNA-195 inhibits proliferation, invasion and metastasis in breast cancer cells by targeting FASN, HMGCR, ACACA and CYP27B1. Sci. Rep. 2015, 5, 17454. [Google Scholar] [CrossRef]
- Cheng, C.-s.; Wang, Z.; Chen, J. Targeting FASN in breast cancer and the discovery of promising inhibitors from natural products derived from traditional Chinese medicine. Evid.-Based Complementary Altern. Med. 2014, 2014, 232946. [Google Scholar] [CrossRef]
- Wu, H.; Xu, J.; Gong, G.; Zhang, Y.; Wu, S. CircARL8B Contributes to the Development of Breast Cancer Via Regulating miR-653-5p/HMGA2 Axis. Biochem. Genet. 2021, 59, 1648–1665. [Google Scholar] [CrossRef]
- Abedi Gaballu, F.; Cho, W.C.-S.; Dehghan, G.; Zarebkohan, A.; Baradaran, B.; Mansoori, B.; Abbaspour-Ravasjani, S.; Mohammadi, A.; Sheibani, N.; Aghanejad, A. Silencing of HMGA2 by siRNA loaded methotrexate functionalized polyamidoamine dendrimer for human breast cancer cell therapy. Genes 2021, 12, 1102. [Google Scholar] [CrossRef]
- Wu, H.; Liu, B.; Chen, Z.; Li, G.; Zhang, Z. MSC-induced lncRNA HCP5 drove fatty acid oxidation through miR-3619-5p/AMPK/PGC1α/CEBPB axis to promote stemness and chemo-resistance of gastric cancer. Cell Death Dis. 2020, 11, 233. [Google Scholar] [CrossRef]
- Tan, Z.; Zou, Y.; Zhu, M.; Luo, Z.; Wu, T.; Zheng, C.; Xie, A.; Wang, H.; Fang, S.; Liu, S. Carnitine palmitoyl transferase 1A is a novel diagnostic and predictive biomarker for breast cancer. BMC Cancer 2021, 21, 409. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Song, Y.; Yan, Z. Inhibition of carnitine palmitoyl transferase 1A-induced fatty acid oxidation suppresses cell progression in gastric cancer. Arch. Biochem. Biophys. 2020, 696, 108664. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Z.; Li, Z.; Wang, S.; Shen, N.; Xin, Y.; Huang, T. Long non-coding RNA nuclear paraspeckle assembly transcript 1 interacts with microRNA-107 to modulate breast cancer growth and metastasis by targeting carnitine palmitoyltransferase-1. Int. J. Oncol. 2019, 55, 1125–1136. [Google Scholar] [CrossRef]
- Zeng, F.; Yao, M.; Wang, Y.; Zheng, W.; Liu, S.; Hou, Z.; Cheng, X.; Sun, S.; Li, T.; Zhao, H. Fatty acid β-oxidation promotes breast cancer stemness and metastasis via the miRNA-328-3p-CPT1A pathway. Cancer Gene Ther. 2021, 29, 383–395. [Google Scholar] [CrossRef]
- Baek, A.E.; Krawczynska, N.; Das Gupta, A.; Dvoretskiy, S.V.; You, S.; Park, J.; Deng, Y.-H.; Sorrells, J.E.; Smith, B.P.; Ma, L. The Cholesterol Metabolite 27HC Increases Secretion of Extracellular Vesicles Which Promote Breast Cancer Progression. Endocrinology 2021, 162, bqab095. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Singh, H.; Trinidad, C.M.; Albarracin, C.T.; Hunt, K.K.; Bedrosian, I. The isomiR-140-3p-regulated mevalonic acid pathway as a potential target for prevention of triple negative breast cancer. Breast Cancer Res. 2018, 20, 150. [Google Scholar] [CrossRef]
- Qin, Y.; Hou, Y.; Liu, S.; Zhu, P.; Wan, X.; Zhao, M.; Peng, M.; Zeng, H.; Li, Q.; Jin, T. A Novel Long Non-Coding RNA lnc030 Maintains Breast Cancer Stem Cell Stemness by Stabilizing SQLE mRNA and Increasing Cholesterol Synthesis. Adv. Sci. 2021, 8, 2002232. [Google Scholar] [CrossRef]
- Koufaris, C.; Valbuena, G.; Pomyen, Y.; Tredwell, G.; Nevedomskaya, E.; Lau, C.-H.; Yang, T.; Benito, A.; Ellis, J.; Keun, H. Systematic integration of molecular profiles identifies miR-22 as a regulator of lipid and folate metabolism in breast cancer cells. Oncogene 2016, 35, 2766–2776. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Shi, J.; Cao, P.; Wan, M.; Zhang, Q.; Wang, Y.; Kridel, S.J.; Liu, W.; Xu, J. Fatty acid synthase is a primary target of MiR-15a and MiR-16-1 in breast cancer. Oncotarget 2016, 7, 78566. [Google Scholar] [CrossRef]
- Han, J.; Qu, H.; Han, M.; Ding, Y.; Xie, M.; Hu, J.; Chen, Y.; Dong, H. MSC-induced lncRNA AGAP2-AS1 promotes stemness and trastuzumab resistance through regulating CPT1 expression and fatty acid oxidation in breast cancer. Oncogene 2021, 40, 833–847. [Google Scholar] [CrossRef]
- Cha, Y.J.; Kim, E.-S.; Koo, J.S. Amino acid transporters and glutamine metabolism in breast cancer. Int. J. Mol. Sci. 2018, 19, 907. [Google Scholar] [CrossRef] [Green Version]
- Ulaner, G.A.; Schuster, D.M. Amino acid metabolism as a target for breast cancer imaging. PET Clin. 2018, 13, 437. [Google Scholar] [CrossRef]
- Becker, R.M.; Wu, G.; Galanko, J.A.; Chen, W.; Maynor, A.R.; Bose, C.L.; Rhoads, J.M. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J. Pediatrics 2000, 137, 785–793. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Sayed, N.; Ditsworth, D.; Thompson, C.B. Brick by brick: Metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 2008, 18, 54–61. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619. [Google Scholar] [CrossRef]
- Fischer, W.-N.; André, B.; Rentsch, D.; Krolkiewicz, S.; Tegeder, M.; Breitkreuz, K.; Frommer, W.B. Amino acid transport in plants. Trends Plant Sci. 1998, 3, 188–195. [Google Scholar] [CrossRef]
- Lampa, M.; Arlt, H.; He, T.; Ospina, B.; Reeves, J.; Zhang, B.; Murtie, J.; Deng, G.; Barberis, C.; Hoffmann, D. Glutaminase is essential for the growth of triple-negative breast cancer cells with a deregulated glutamine metabolism pathway and its suppression synergizes with mTOR inhibition. PLoS ONE 2017, 12, e0185092. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Xu, P.; Huang, M.; Chen, X.; Zeng, S.; Wang, Q.; Chen, J.; Li, K.; Gao, W.; Liu, R. The heterocyclic compound Tempol inhibits the growth of cancer cells by interfering with glutamine metabolism. Cell Death Dis. 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Delgir, S.; Ilkhani, K.; Safi, A.; Seif, F.; Bastami, M.; Alivand, M.-R. The Expression of miR-513c and miR-3163 was Downregulated in Tumor Tissues Compared with Margin Tissues of Patients With Breast Cancer. BMC Med. Genom. 2021, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, Q.; Bhutkar, A.; Galván, J.A.; Karamitopoulou, E.; Noordermeer, D.; Peng, M.-W.; Piersigilli, A.; Perren, A.; Zlobec, I. GKAP acts as a genetic modulator of NMDAR signaling to govern invasive tumor growth. Cancer Cell 2018, 33, 736–751.e735. [Google Scholar] [CrossRef]
- Yin, J.; Tu, G.; Peng, M.; Zeng, H.; Wan, X.; Qiao, Y.; Qin, Y.; Liu, M.; Luo, H. GPER-regulated lncRNA-Glu promotes glutamate secretion to enhance cellular invasion and metastasis in triple-negative breast cancer. FASEB J. 2020, 34, 4557–4572. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Tamba, M.; Ishii, T.; Bannai, S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 1999, 274, 11455–11458. [Google Scholar] [CrossRef]
- Liu, X.-X.; Li, X.-J.; Zhang, B.; Liang, Y.-J.; Zhou, C.-X.; Cao, D.-X.; He, M.; Chen, G.-Q.; He, J.-R.; Zhao, Q. MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett. 2011, 585, 1363–1367. [Google Scholar] [CrossRef]
- Pilati, P.; Mocellin, S.; Bertazza, L.; Galdi, F.; Briarava, M.; Mammano, E.; Tessari, E.; Zavagno, G.; Nitti, D. Prognostic value of putative circulating cancer stem cells in patients undergoing hepatic resection for colorectal liver metastasis. Ann. Surg. Oncol. 2012, 19, 402–408. [Google Scholar] [CrossRef]
- Salles, G.; de Jong, D.; Xie, W.; Rosenwald, A.; Chhanabhai, M.; Gaulard, P.; Klapper, W.; Calaminici, M.; Sander, B.; Thorns, C. Prognostic significance of immunohistochemical biomarkers in diffuse large B-cell lymphoma: A study from the Lunenburg Lymphoma Biomarker Consortium. Blood J. Am. Soc. Hematol. 2011, 117, 7070–7078. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Andorfer, C.A.; Necela, B.M.; Thompson, E.A.; Perez, E.A. MicroRNA signatures: Clinical biomarkers for the diagnosis and treatment of breast cancer. Trends Mol. Med. 2011, 17, 313–319. [Google Scholar] [CrossRef]
- Shah, N.R.; Chen, H. MicroRNAs in pathogenesis of breast cancer: Implications in diagnosis and treatment. World J. Clin. Oncol. 2014, 5, 48. [Google Scholar] [CrossRef]
- Chen, B.; Tang, H.; Liu, X.; Liu, P.; Yang, L.; Xie, X.; Ye, F.; Song, C.; Xie, X.; Wei, W. miR-22 as a prognostic factor targets glucose transporter protein type 1 in breast cancer. Cancer Lett. 2015, 356, 410–417. [Google Scholar] [CrossRef]
- Beltrán-Anaya, F.O.; Cedro-Tanda, A.; Hidalgo-Miranda, A.; Romero-Cordoba, S.L. Insights into the regulatory role of non-coding RNAs in cancer metabolism. Front. Physiol. 2016, 7, 342. [Google Scholar] [CrossRef]
- Kim, Y.; Ko, J.Y.; Lee, S.-B.; Oh, S.; Park, J.W.; Kang, H.-G.; Kim, D.-H.; Chung, D.; Lim, S.; Kong, H. Reduced miR-371b-5p expression drives tumor progression via CSDE1/RAC1 regulation in triple-negative breast cancer. Oncogene 2022, 41, 3151–3161. [Google Scholar] [CrossRef]
- Petkova, V.; Marinova, D.; Kyurkchiyan, S.; Stancheva, G.; Mekov, E.; Kachakova-Yordanova, D.; Slavova, Y.; Kostadinov, D.; Mitev, V.; Kaneva, R. MiRNA expression profiling in adenocarcinoma and squamous cell lung carcinoma reveals both common and specific deregulated microRNAs. Medicine 2022, 101, e30027. [Google Scholar] [CrossRef]
- Gaither, K.A.; Watson, C.J.; Madarampalli, B.; Lazarus, P. Expression of activating transcription factor 5 (ATF5) is mediated by microRNA-520b-3p under diverse cellular stress in cancer cells. PLoS ONE 2020, 15, e0225044. [Google Scholar] [CrossRef]
- Roy, B.; Ghose, S.; Biswas, S. Therapeutic strategies for miRNA delivery to reduce hepatocellular carcinoma. Semin. Cell Dev. Biol. 2022, 124, 134–144. [Google Scholar] [CrossRef]
- Wang, H.; Chirshev, E.; Hojo, N.; Suzuki, T.; Bertucci, A.; Pierce, M.; Perry, C.; Wang, R.; Zink, J.; Glackin, C.A. The Epithelial–Mesenchymal Transcription Factor SNAI1 Represses Transcription of the Tumor Suppressor miRNA let-7 in Cancer. Cancers 2021, 13, 1469. [Google Scholar] [CrossRef]
- Kardani, A.; Yaghoobi, H.; Alibakhshi, A.; Khatami, M. Inhibition of miR-155 in MCF-7 breast cancer cell line by gold nanoparticles functionalized with antagomir and AS1411 aptamer. J. Cell. Physiol. 2020, 235, 6887–6895. [Google Scholar] [CrossRef]
- Abtahi, N.A.; Haghiralsadat, F.; Siyadatpanah, A.; Reza, J.Z.; Nissapatorn, V.; Mahboob, T.; Pereira, M.D.L.; Wilairatana, P.; Hossain, R.; Islam, M.T. The Novel Method for Delivery miRNA-34a: A New Cationic PEGylated Niosomal Formulation for the Treatment of Breast Cancer. Nanotechnol. Res. 2021. [Google Scholar] [CrossRef]
- Yadav, A.; Biswas, T.; Praveen, A.; Ateeq, B. MALAT1 ablation dismantles homologous recombination repair machinery and sensitizes castrate resistant prostate cancer cells to PARP inhibitor. Cancer Res. 2022, 82, 1542. [Google Scholar] [CrossRef]
- Sun, X.; Xin, S.; Zhang, Y.; Jin, L.; Liu, X.; Zhang, J.; Mei, W.; Zhang, B.; Ma, W.; Ye, L. Long non-coding RNA CASC11 interacts with YBX1 to promote prostate cancer progression by suppressing the p53 pathway. Int. J. Oncol. 2022, 61, 110. [Google Scholar] [CrossRef]
- Catuogno, S.; Di Martino, M.T.; Nuzzo, S.; Esposito, C.L.; Tassone, P.; de Franciscis, V. An anti-BCMA RNA aptamer for miRNA intracellular delivery. Mol. Ther. Nucleic Acids 2019, 18, 981–990. [Google Scholar] [CrossRef]
- Khan, A.; Nosheen, F.; Tabassum, B.; Adeyinka, O.S.; Shehzad, K.; Shahid, N.; Khan, A.M.; Nasir, I.A. Comparative Silencing Effect of Different siRNA Fragments on Potato Virus X Coat Protein in Transient Transfection Assays. Pak. J. Zool. 2022, 54, 1–501. [Google Scholar] [CrossRef]
- Han, D.; Li, J.; Wang, H.; Su, X.; Hou, J.; Gu, Y.; Qian, C.; Lin, Y.; Liu, X.; Huang, M. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology 2017, 66, 1151–1164. [Google Scholar] [CrossRef] [Green Version]

| Non-Coding RNAs | Target Enzyme | Function | Ref. |
|---|---|---|---|
| miR-206 | PFKFB3 | Downregulated | [56] |
| miR-Let-7a-5p | PFKM2 | Downregulated | [65] |
| MiR216b | HK2 | Downregulated | [100] |
| miR-23a/24/210 | LDHA, LDHB | Downregulated | [101] |
| miR-30a-5p | LDHA | Downregulated | [102] |
| IncRNA MAFG-AS1 | HK2 | Upregulated | [103] |
| lncRNA UCA1 | HK2 | Upregulated | [104] |
| lncRNA BCAR4 | PFKFB3 | Upregulated | [105] |
| lncRNA FGD5-AS1 | PKM2 | Upregulated | [106] |
| lnc RNA MIR210HG | LDHA | Upregulated | [107] |
| lncRNA HISLA | LDHA | Upregulated | [108] |
| lncRNA FGF13-AS1 | PDK1 | Downregulated | [109] |
| Circle RNA circABCB10 | LDHA | Upregulated | [108] |
| CircRNA DENND4C | HK2 | Upregulated | [110] |
| miR-Let-7a | PKM2 | Downregulated | [111] |
| Hsa_circ_0069094 | HK2 | Upregulated | [111] |
| circHIPK3 | HK2 | Upregulated | [112] |
| Non-Coding RNA | Target Enzyme | Metabolic Pathway | Function | Ref |
|---|---|---|---|---|
| miR-22 | ACL | Upregulated | Fatty acid metabolism | [133] |
| miR15-1a, miR16-1a | FASN | downregulated | Fatty acid metabolism | [134] |
| lncRNA AGAP2-AS1 | CPT1 | Upregulated | Fatty acid oxidation | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedi-Gaballu, F.; Kamal Kazemi, E.; Salehzadeh, S.A.; Mansoori, B.; Eslami, F.; Emami, A.; Dehghan, G.; Baradaran, B.; Mansoori, B.; Cho, W.C. Metabolic Pathways in Breast Cancer Reprograming: An Insight to Non-Coding RNAs. Cells 2022, 11, 2973. https://doi.org/10.3390/cells11192973
Abedi-Gaballu F, Kamal Kazemi E, Salehzadeh SA, Mansoori B, Eslami F, Emami A, Dehghan G, Baradaran B, Mansoori B, Cho WC. Metabolic Pathways in Breast Cancer Reprograming: An Insight to Non-Coding RNAs. Cells. 2022; 11(19):2973. https://doi.org/10.3390/cells11192973
Chicago/Turabian StyleAbedi-Gaballu, Fereydoon, Elham Kamal Kazemi, Seyed Ahmad Salehzadeh, Behnaz Mansoori, Farhad Eslami, Ali Emami, Gholamreza Dehghan, Behzad Baradaran, Behzad Mansoori, and William C. Cho. 2022. "Metabolic Pathways in Breast Cancer Reprograming: An Insight to Non-Coding RNAs" Cells 11, no. 19: 2973. https://doi.org/10.3390/cells11192973
APA StyleAbedi-Gaballu, F., Kamal Kazemi, E., Salehzadeh, S. A., Mansoori, B., Eslami, F., Emami, A., Dehghan, G., Baradaran, B., Mansoori, B., & Cho, W. C. (2022). Metabolic Pathways in Breast Cancer Reprograming: An Insight to Non-Coding RNAs. Cells, 11(19), 2973. https://doi.org/10.3390/cells11192973







