The Adjustment Strategy of Venus Flytrap Photosynthetic Apparatus to UV-A Radiation
Abstract
:1. Introduction
2. Materials and Methods
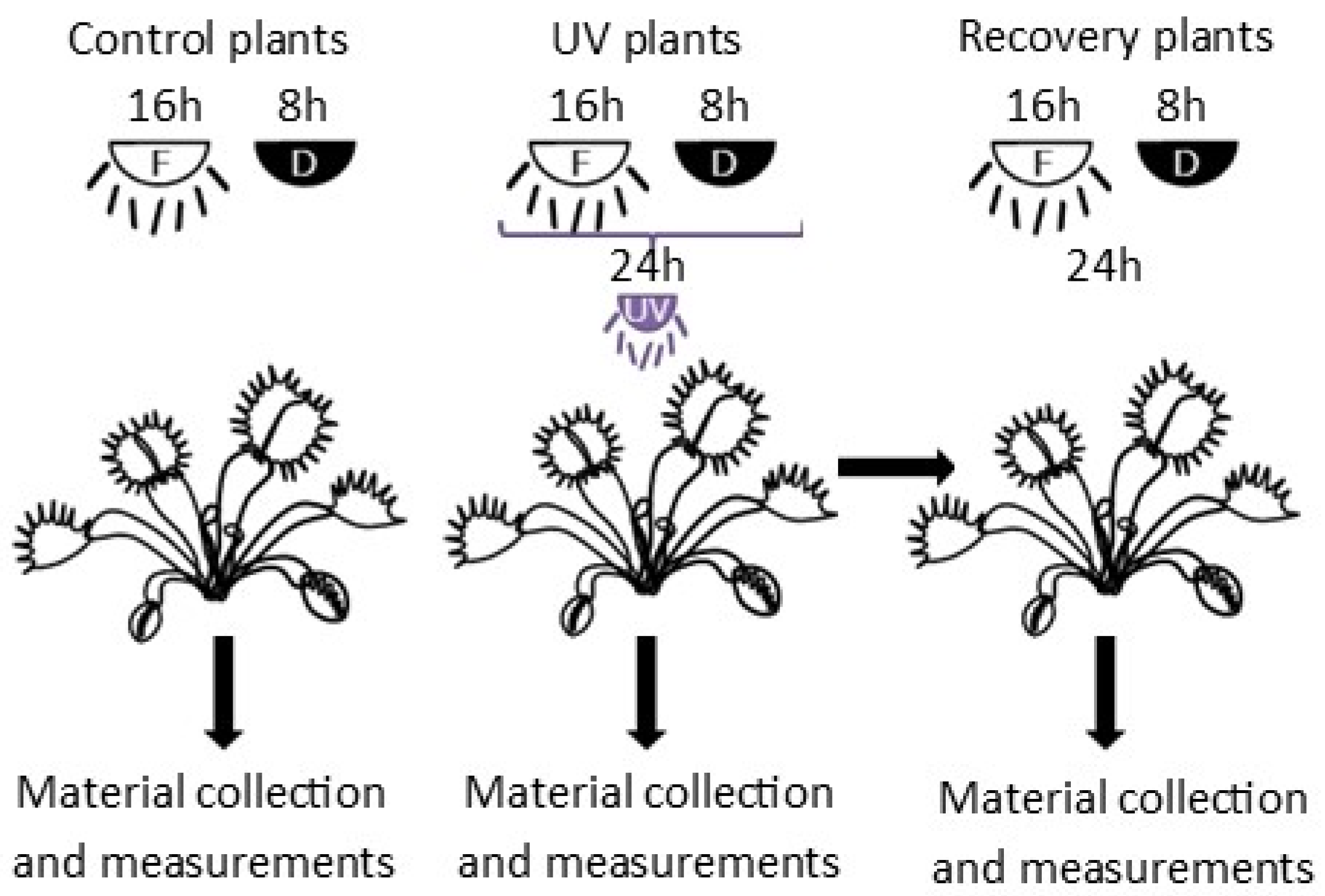
2.1. Plant Material, Acclimatization and Experimental Conditions
2.2. Determination of Dry Weight Content
2.3. Estimation of Photosynthetic Apparatus Response
2.3.1. Photosynthetic Pigments Content Estimation
2.3.2. Chlorophyll a Fluorescence Measurement
2.3.3. Gas-Exchange Measurement
2.3.4. Protein Content Determination
2.4. Estimation of Sugar Content
2.5. Estimation of Lipid Peroxidation Level using Malondialdehyde (MDA) Content
2.6. Estimation of Antioxidant Enzymes Activity
Peroxidase (POD), Superoxide Dismutase (SOD) and Catalase (CAT) Activity Estimation
2.7. Estimation of Low-Molecular-Weight Antioxidant Contents and Activities
2.7.1. Phenolic Compound Content Estimation
Total Phenolic Compounds
Phenolic Compound Groups
Specific Phenolic Compounds
2.7.2. Estimation of Total Glutathione Content
2.8. Statistical Analysis
3. Results
3.1. Growth of D. Muscipula Plants under UV-A treatment
3.2. Photosynthetic Apparatus Performance of D. Muscipula Leaves under UV Treatment
3.3. Sugar Content in D. Muscipula Leaves under UV-A treatment
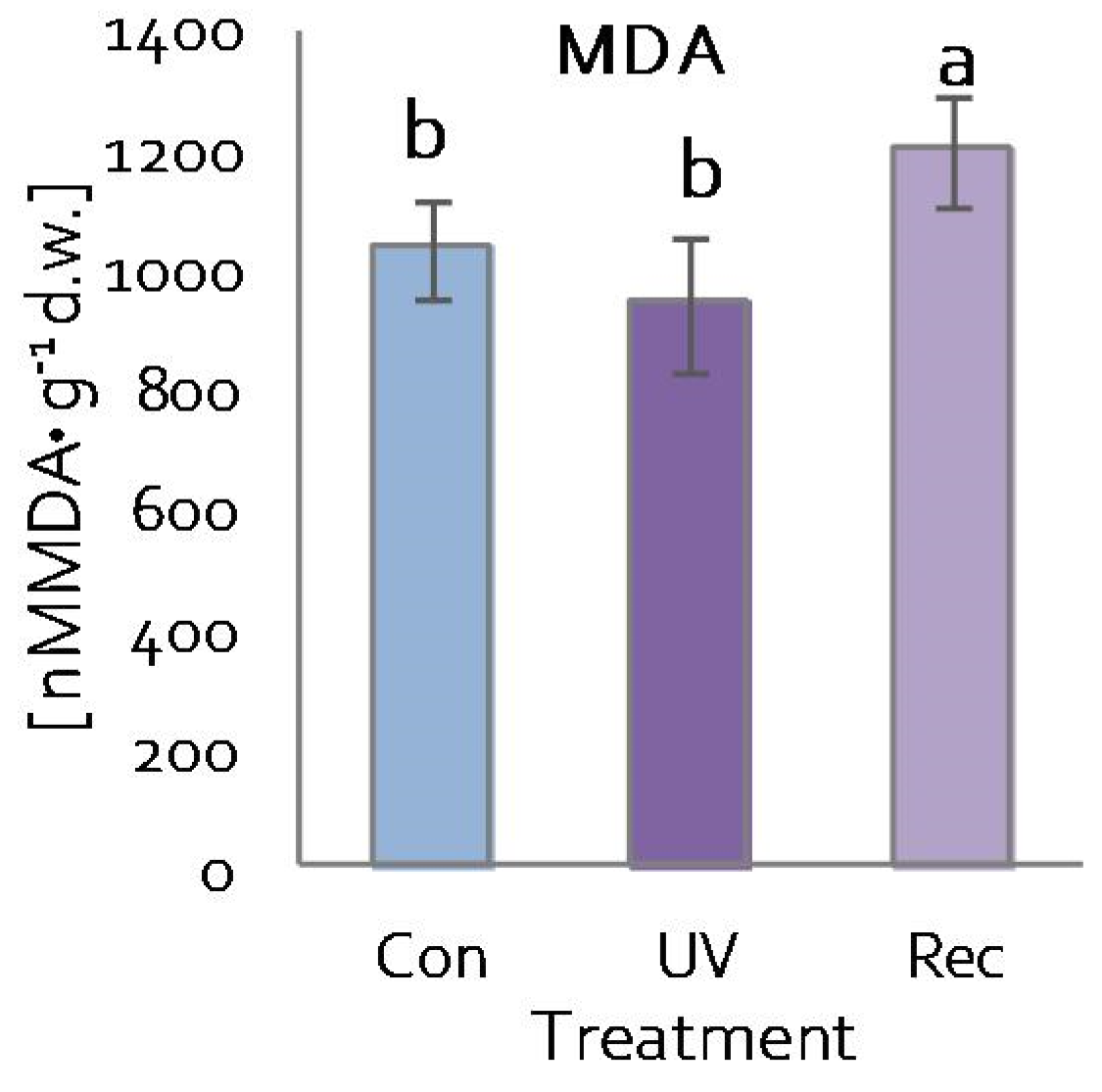
3.4. Plasma Membranes of D. Muscipula Leaves under UV treatment
3.5. Reaction of Antioxidant System of D. Muscipula Leaves to UV-A treatment
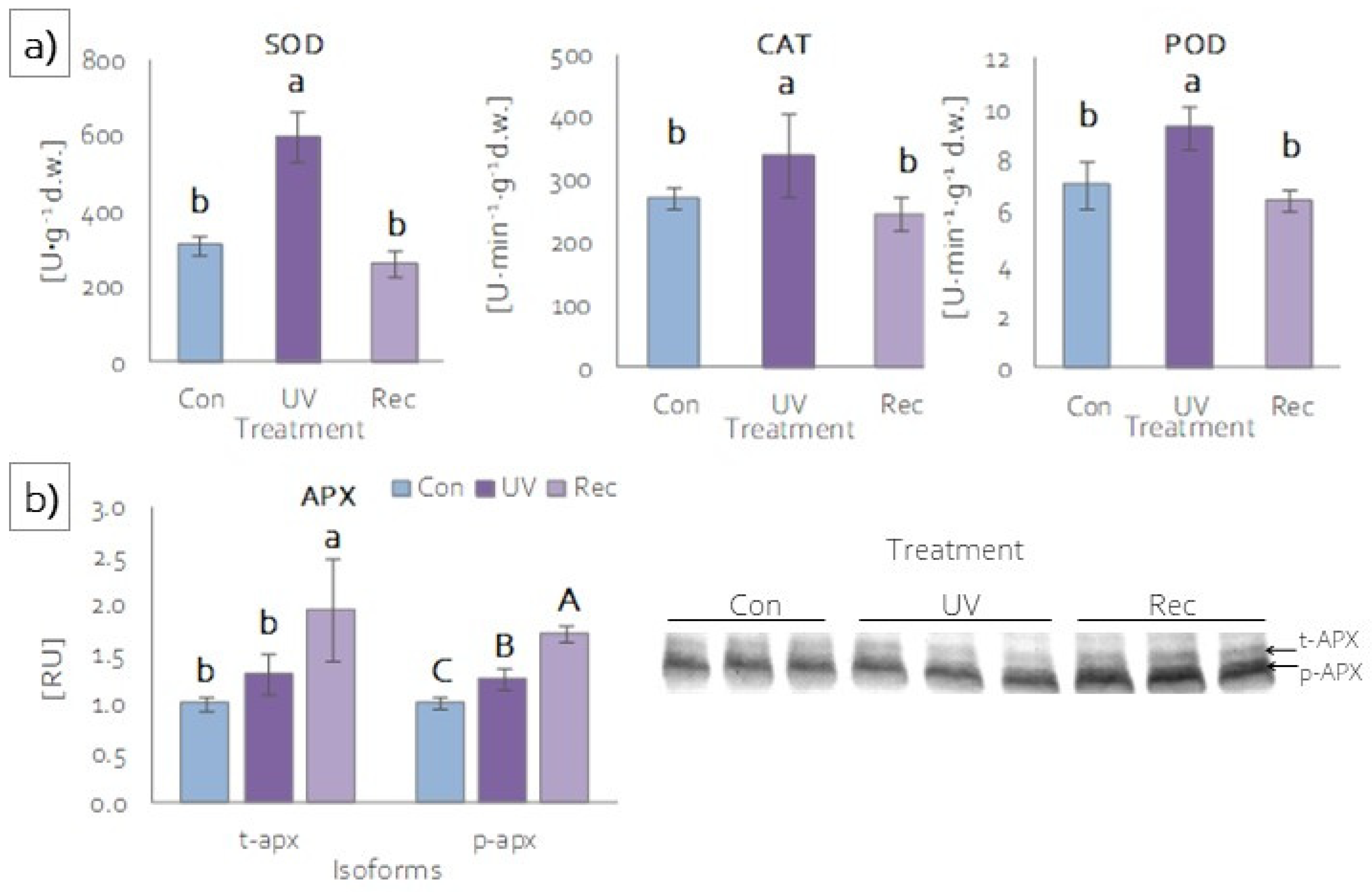
3.5.1. Enzymatic Antioxidants Activity and Content
3.5.2. Low-Molecular-Weight Antioxidant Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Björn, L.O. Ultraviolet-A, B, and C. UV4Plants Bull. 2015, 1, 17–18. [Google Scholar]
- Rai, N.; Morales, L.O.; Aphalo, P. Perception of solar UV radiation by plants: Photoreceptors and mechanisms. Plant Physiol. 2021, 186, 1382–1396. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Aro, E.M.; Millar, A.H. Mechanisms of photodamage and protein turnover in photoinhibition. Trends Plant Sci. 2018, 23, 667–676. [Google Scholar] [CrossRef]
- Turcsânyi, E.; Vass, I. Inhibition of photosynthetic electron transport by UV-A radiation targets the photosystem II complex. Photochem. Photobiol. 2000, 72, 513–520. [Google Scholar] [CrossRef]
- Kale, R.; Hebert, A.E.; Frankel, L.K.; Sallans, L.; Bricker, T.M.; Pospíšil, P. Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of Photosystem II. Proc. Natl. Acad. Sci. USA 2017, 114, 2988–2993. [Google Scholar] [CrossRef]
- Apostolova, E.L.; Dobrikova, A.G. Effect of High Temperature and UV-A Radiation on the Photosystem II. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 577–591. [Google Scholar]
- Verdaguer, D.; Jansen, M.A.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- de Paula Bernado, W.; Baroni, D.F.; Ruas, K.F.; Santos, A.R.; de Souza, S.B.; Passos, L.C.; Façanha, A.R.; Ramalho, J.C.; Campostrini, E.; Rakocevic, M.; et al. Ultraviolet radiation underlies metabolic energy reprograming in Coffea arabica and Coffea canephora genotypes. Sci. Hortic. 2022, 295, 110881. [Google Scholar] [CrossRef]
- Pfündel, E.E.; Pan, R.S.; Diley, R.A. Inhibition of violaxantin deep oxidation by ultraviolet-B radiation in isolated chloroplats and intact leaves. Plant Physiol. 1992, 98, 1372–1380. [Google Scholar] [CrossRef]
- Guidi, L.; Brunetti, C.; Fini, A.; Agati, G.; Ferrini, F.; Gori, A.; Tattini, M. UV radiation promotes flavonoid biosynthesis, while negatively affecting the biosynthesis and the de-epoxidation of xanthophylls: Consequence for photoprotection? Environ. Exp. Bot. 2016, 127, 14–25. [Google Scholar] [CrossRef]
- Grossweiner, L.I.; Grossweiner, J.B.; Gerald Rogers, B.H. Photochemical damage to biological systems. In The Science of Phototherapy: An Introduction; Jones, L.R., Ed.; Springer: Dordrecht, Germany, 2005; pp. 171–195. [Google Scholar]
- Jordan, B.R. Molecular response of plant cells to UV-B stress. Funct. Plant Biol. 2002, 29, 909–916. [Google Scholar] [CrossRef]
- Gao, K.; Wu, Y.; Li, G.; Wu, H.; Villafane, V.E.; Helbling, E.W. Solar UV radiation drives CO2 fixation in marine phytoplankton: A double-edged sword. Plant Physiol. 2007, 144, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Gao, K. Use of UV-A energy for photosynthesis in the Red Macroalga Gracilaria lemaneiformis. Photochem. Photobiol. 2010, 86, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.H.; Gitz, D.C.; Peek, M.S.; McElrone, A.J. Response of three eastern tree species to supplemental UV-B radiation: Leaf chemistry and gas exchange. Agric. For. Meteorol. 2003, 120, 219–228. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N. Intraspecific variations in growth, yield and photosynthesis of sorghum varieties to ambient UV (280–400 nm) radiation. Plant Sci. 2012, 196, 85–92. [Google Scholar] [CrossRef]
- Turnbull, T.L.; Barlow, A.M.; Adams, M.A. Photosynthetic benefits of ultraviolet-A to Pimelea ligustrina, a woody shrub of sub-alpine Australia. Oecologia 2013, 173, 375–385. [Google Scholar] [CrossRef]
- Tokarz, K.; Piwowarczyk, B.; Wysocka, A.; Wójtowicz, T.; Makowski, W.; Golemiec, E. Response of grass pea (Lathyrus sativus L.) photosynthetic apparatus to short-term intensive UV-A: Red radiation. Acta Physiol. Plant. 2019, 41, 168. [Google Scholar] [CrossRef]
- Johnson, G.A.; Day, T.A. Enhancement of photosynthesis in Sorghum bicolor by ultraviolet radiation. Physiol. Plant. 2002, 116, 554–562. [Google Scholar] [CrossRef]
- Mantha, S.V.; Johnson, G.A.; Day, T.A. Evidence from Action and Fluorescence Spectra that UV-Induced Violet–Blue–Green Fluorescence Enhances Leaf Photosynthesis. Photochem. Photobiol. 2001, 73, 249–256. [Google Scholar]
- Chen, C.; Xiao, Y.G.; Li, X.; Ni, M. Light-regulated stomatal aperture in Arabidopsis. Mol. Plant 2012, 5, 566–572. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic Compounds: Introduction. In Handbook of Natural Products; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 50, pp. 1544–1580. [Google Scholar]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Makowski, W.; Tokarz, B.; Banasiuk, R.; Królicka, A.; Dziurka, M.; Wojciechowska, R.; Tokarz, K. Is a blue-red light a good elicitor of phenolic compounds in the family Droseraceae? A comparative study. J. Photochem. Photobiol. B 2019, 201, 111679. [Google Scholar] [CrossRef]
- Wildhalm, J.R.; Rhodes, D. Biosynthesis and molecular actions of specialized 1,4-naphthoquinone natural products produced by horticultural plants. Hortic. Res. 2016, 3, 16046. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, K.; Makowski, W.; Banasiuk, R.; Królicka, A.; Piwowarczyk, B. Response of Dionaea muscipula J. Ellis to light stress in in vitro: Physiological study. Plant Cell Tissue Organ Cult. 2018, 134, 65–77. [Google Scholar] [CrossRef]
- Barnes, P.W.; Kersting, A.R.; Flint, S.D.; Beyschlag, W.; Ryel, R.J. Adjustments in epidermal UV-transmittance of leaves in sun-shade transitions. Physiol. Plant. 2013, 149, 200–213. [Google Scholar] [PubMed]
- Gaascht, F.; Dicato, M.; Diederich, M. Venus Flytrap (Dionaea muscipula Solander ex Ellis) Contains Powerful Compounds that Prevent and Cure Cancer. Front. Oncol. 2013, 3, 202. [Google Scholar] [CrossRef]
- Keller, H. Pharmaceutical containing the digestive juices of carnivorous plants for treatment of malignant and chronic diseases. Ger. Offen DE 1987, 3, 619. [Google Scholar]
- Kukułczanka, K.; Budzianowski, J. Dionaea muscipula Ellis (Venus Flytrap): In Vitro Cultures and In Vitro Production of Secondary Metabolites. In Medicinal and Aromatic Plants XII. Biotechnology in Agriculture and Forestry; Nagata, T., Ebizuka, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 51, pp. 50–74. [Google Scholar]
- Królicka, A.; Szpitter, A.; Gilgenast, E.; Romanik, G.; Kamiński, M.; Łojkowska, E. Stimulation of antibacterial naphthoquinones and flavonoids accumulation in carnivorous plants grown in vitro by addition of elicitors. Enzym. Microb. Technol. 2008, 42, 216–221. [Google Scholar] [CrossRef]
- Makowski, W.; Tokarz, K.M.; Tokarz, B.; Banasiuk, R.; Witek, K.; Królicka, A. Elicitation-based method for increasing production of antioxidant and bactericidal phenolic compounds in Dionaea muscipula J. Ellis tissue. Molecules 2020, 25, 1794. [Google Scholar] [CrossRef]
- Królicka, A.; Szpitter, A.; Maciąg, M.; Biskup, E.; Gilgenast, E.; Romanik, G.; Kamiński, M.; Wegrzyn, G. Antibacterial and antioxidant activity of the secondary metabolites from in vitro cultures of the Alice Sundew (Drosera alicie). Biotechnol. Appl. Biochem. 2009, 53, 157–184. [Google Scholar]
- Taraszkiewicz, A.; Jafra, S.; Skrzypczak, A.; Kamiński, M.; Królicka, A. Antibacterial activity of secondary metabolites from in vitro culture of Drosera gigantea against the plant pathogenic bacteria Pseudomonas syringae pv. syringae and P. syringae pv. morsprunorum. J. Plant Pathol. 2012, 94, 63–68. [Google Scholar]
- Checker, R.; Sharma, D.; Sandur, S.K.; Khanam, S.; Poduval, T.B. Anti-inflammatory effects of plumbagin are mediated by inhibition of NF-kappaB activation in lymphocytes. Int. Immunopharmacol. 2009, 9, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; Tangmouo, J.G.; Lontsi, D.; Etoa, F.X.; Lohoue, P.J. In vitro antifungal activity of extract and plumbagin from the stem bark of Diospyros crassiflora Hiern (Ebenaceae). Phytother. Res. 2007, 21, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.; Dandawate, P.; Yusufi, M.; Ahmad, A.; Sarkar, F.H. Perspectives on medicinal properties of plumbaginy and its analogs. Med. Res. Rev. 2010, 32, 1131–1158. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Advances in Photosynthesis and Respiration; Papageorgiou, G., Govindjee, Eds.; Springer: Dordrecht, Germany, 2004; Volume 19, pp. 321–336. [Google Scholar]
- Jiang, H.X.; Chen, L.S.; Zheng, J.G.; Han, S.; Tang, N.; Smith, B.R. Aluminum-induced effects on photosystem II photochemistry in Citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol. 2008, 28, 1863–1871. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Govindjee Bosa, K.; Kościelniak, J.; Zuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Laureau, C.; De Paepe, R.; Latouche, G.; Moreno-Chacon, M.; Finazzi, G.; Kuntz, M.; Cornic, G.; Streb, P. Plastid terminal oxidase (PTOX) has the potential to act as a safety valve for excess excitation energy in the alpine plant species Ranunculus glacialis L. Plant Cell Environ. 2013, 36, 1296–1310. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Dhindsa, R.H.; Plumb-Dhindsa, R.; Thorpe, T.A. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Miszalski, Z.; Ślesak, I.; Niewiadomska, E.; Baczek-Kwinta, R.; Lüttge, U.; Ratajczak, R. Subcellular localization and stress responses of superoxide dismutase isoforms from leaves in the C3-CAM intermediate halophyte Mesembryanthemum crystallinum L. Plant Cell Environ. 1998, 21, 169–179. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Lin, H.W.; Chern, R.H.; Lo, H.F.; Li, L. Reduced susceptibility to waterlogging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regul. 1999, 27, 167–172. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Muszyńska, E.; Kołton, A.; Kamińska, I.; Hanus-Fajerska, E. In vitro acclimation to prolonged metallic stress is associated with modulation of antioxidant responses in a woody shrub Daphne jasminea. Plant Cell Tissue Organ Cult. 2019, 139, 339–357. [Google Scholar] [CrossRef] [Green Version]
- Lűck, H. Methoden der Enzymatischen Analyse; Verlag Chemie GmbH: Weinheim, Germany, 1962. [Google Scholar]
- Bartosz, G. Another Side of Oxygen. Free Radicals in Nature; PWN: Warsaw, Poland, 2006. (In Polish) [Google Scholar]
- Hillis, W.E.; Swain, T. The phenolic constituents of Prunus domestica. II.—The analysis of tissues of the Victoria plum tree. J. Sci. Food Agric. 1959, 10, 135–144. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assesing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Queval, G.; Noctor, G. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox profiling during Arabidopsis rosette development. Anal. Biochem. 2007, 363, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AOB Plants 2017, 9, plx009. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Verma, S.K. Salinity Stress Responses and Adaptive Mechanisms in Major Glycophytic Crops: The Story So Far. In Salinity Responses and Tolerance in Plants; Kumar, V., Wani, S., Suprasanna, P., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 1–39. [Google Scholar]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.I.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Yocum, C. Structure and function of photosystems I and II. Annu. Rev. Plant Biol. 2006, 57, 521–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevela, D.; Bjorn, L.O.; Govindjee, G. (Eds.) Basics of Photosynthesis: Light-Dependent Reaction. In Photosynthesis. Solar Energy for Life; World Scientific: New Jersey, NY, USA, 2018; pp. 29–58. [Google Scholar]
- Salama, H.M.; Al Watban, A.A.; Al-Fughom, A.T. Effect of ultraviolet radiation on chlorophyll, carotenoid, protein and proline contents of some annual desert plants. Saudi J. Biol. Sci. 2011, 18, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Häder, D.P. Impact of UV radiation on rice-field cyanobacteria: Role of photoprotective compounds. In Environmental UV Radiation: Impact on Ecosystems and Human Health and Predictive Models; Ghetti, F., Checcucci, G., Bornman, J.F., Eds.; Springer: Dordrecht, Germany, 2006; Volume 57, pp. 217–230. [Google Scholar]
- Sá, M.R.D.A.E.; André, F.; de Brito André, P.S. Classroom fundamentals: Measuring the Planck constant. Sci. Sch. 2014, 28, 28–32. [Google Scholar]
- Vass, I.; Turcsányi, E.; Touloupakis, E.; Ghanotakis, D.; Petrouleas, V. The mechanism of UV-A radiation-induced inhibition of photosystem II electron transport studied by EPR and chlorophyll fluorescence. Biochemistry 2002, 41, 10200–10208. [Google Scholar] [CrossRef] [PubMed]
- Tyystjärvi, E. Photoinhibition of photosystem II and photodamage of the oxygen evolving manganese cluster. Coord. Chem. Rev. 2008, 252, 361–376. [Google Scholar] [CrossRef]
- Stirbet, A.; Riznichenko, G.Y.; Rubin, A.B. Modeling chlorophyll a fluorescence transient: Relation to photosynthesis. Biochem. 2014, 79, 291–323. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Piwowarczyk, B.; Tokarz, K.; Muszyńska, E.; Makowski, W.; Jędrzejczyk, R.; Gajewski, Z.; Hanus-Fajerska, E. The acclimatization strategies of kidney vetch (Anthyllis vulneraria L.) to Pb toxicity. Environ. Sci. Pollut. Res. 2018, 25, 19739–19752. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, K.M.; Makowski, W.; Tokarz, B.; Hanula, M.; Sitek, E.; Muszyńska, E.; Jedrzejczyk, R.; Banasiuk, R.; Chajec, Ł.; Mazur, S. Can ceylon leadwort (Plumbago zeylanica L.) acclimate to lead toxicity?—Studies of photosynthetic apparatus efficiency. Int. J. Mol. Sci. 2020, 21, 1866. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, K.M.; Wesołowski, W.; Tokarz, B.; Makowski, W.; Wysocka, A.; Jędrzejczyk, R.J.; Chrabaszcz, K.; Malek, K.; Kostecka-Gugała, A. Stem photosynthesis—A key element of grass pea (Lathyrus sativus L.) acclimatisation to salinity. Int. J. Mol. Sci. 2021, 22, 685. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Kramer, D.M.; Fisher, N.; Fu, X. Flexibility in the energy balancing network of photosynthesis enables safe operation under changing environmental conditions. Plants 2020, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Tóth, S.Z.; Nagy, V.; Puthur, J.T.; Kovács, L.; Garab, G. The physiological role of ascorbate as photosystem II electron donor: Protection against photoinacivation in heat-stressed leaves. Plant Physiol. 2011, 156, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Garstka, M. Structural background of photosynthetic light reaction. Adv. Cell Biol. 2007, 34, 445–476. (In Polish) [Google Scholar]
- Hura, T.; Hura, K.; Grzesiak, S. Possible contribution of cell-wall-bound ferulic acid in drought resistance and recovery in triticale seedlings. J. Plant Physiol. 2009, 166, 1720–1733. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miernicka, K.; Tokarz, B.; Makowski, W.; Mazur, S.; Banasiuk, R.; Tokarz, K.M. The Adjustment Strategy of Venus Flytrap Photosynthetic Apparatus to UV-A Radiation. Cells 2022, 11, 3030. https://doi.org/10.3390/cells11193030
Miernicka K, Tokarz B, Makowski W, Mazur S, Banasiuk R, Tokarz KM. The Adjustment Strategy of Venus Flytrap Photosynthetic Apparatus to UV-A Radiation. Cells. 2022; 11(19):3030. https://doi.org/10.3390/cells11193030
Chicago/Turabian StyleMiernicka, Karolina, Barbara Tokarz, Wojciech Makowski, Stanisław Mazur, Rafał Banasiuk, and Krzysztof M. Tokarz. 2022. "The Adjustment Strategy of Venus Flytrap Photosynthetic Apparatus to UV-A Radiation" Cells 11, no. 19: 3030. https://doi.org/10.3390/cells11193030
APA StyleMiernicka, K., Tokarz, B., Makowski, W., Mazur, S., Banasiuk, R., & Tokarz, K. M. (2022). The Adjustment Strategy of Venus Flytrap Photosynthetic Apparatus to UV-A Radiation. Cells, 11(19), 3030. https://doi.org/10.3390/cells11193030





