Thyroid Hormone Induces Oral Cancer Growth via the PD-L1-Dependent Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Confocal Microscopy
2.3. Transfection of Short Hairpin (sh)RNA
2.4. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.5. Western Blot Analysis
2.6. Cell Viability Assay
2.7. Statistical Analysis
3. Results
3.1. Thyroxine Induces PD-L1 and β-Catenin Expressions in Human Oral Cancer Cells
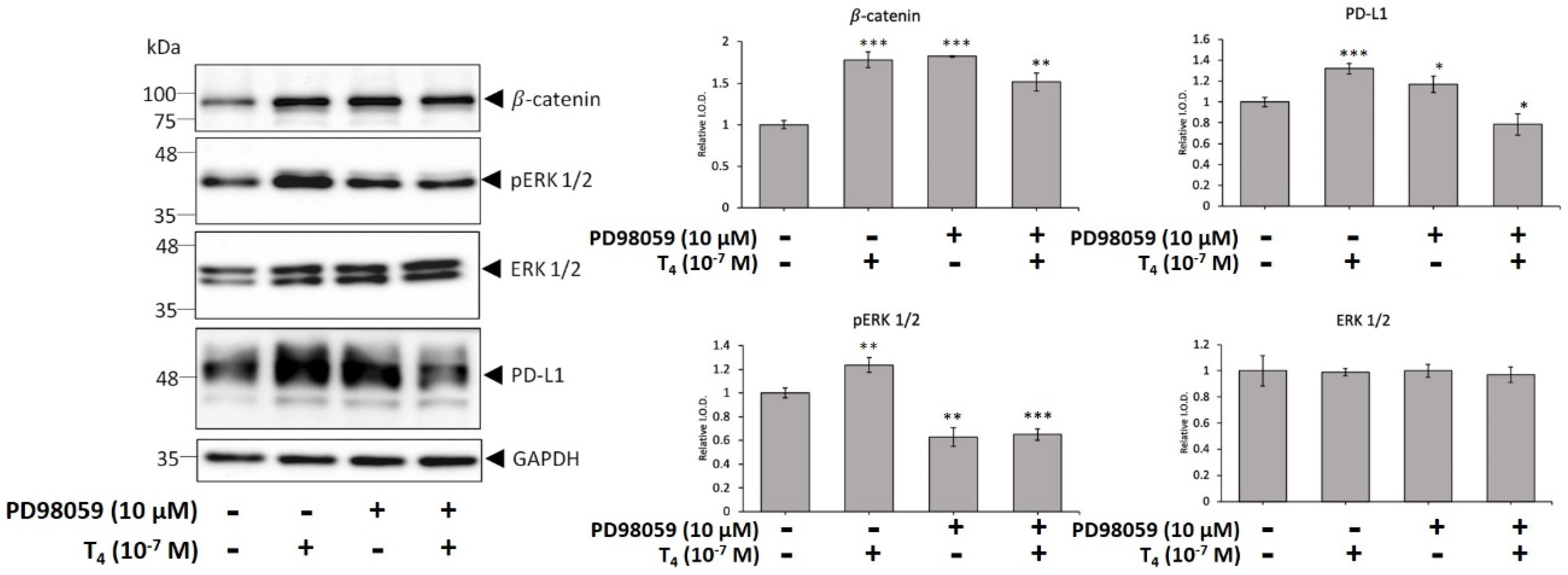
3.2. Activation of ERK1/2 and STAT3 Involve in Thyroid Hormone-Induced Accumulation of PD-L1 and β-Catenin and Proliferation in Oral Cancer Cells
3.3. Thyroid Hormone Induced PD-L1 Expression
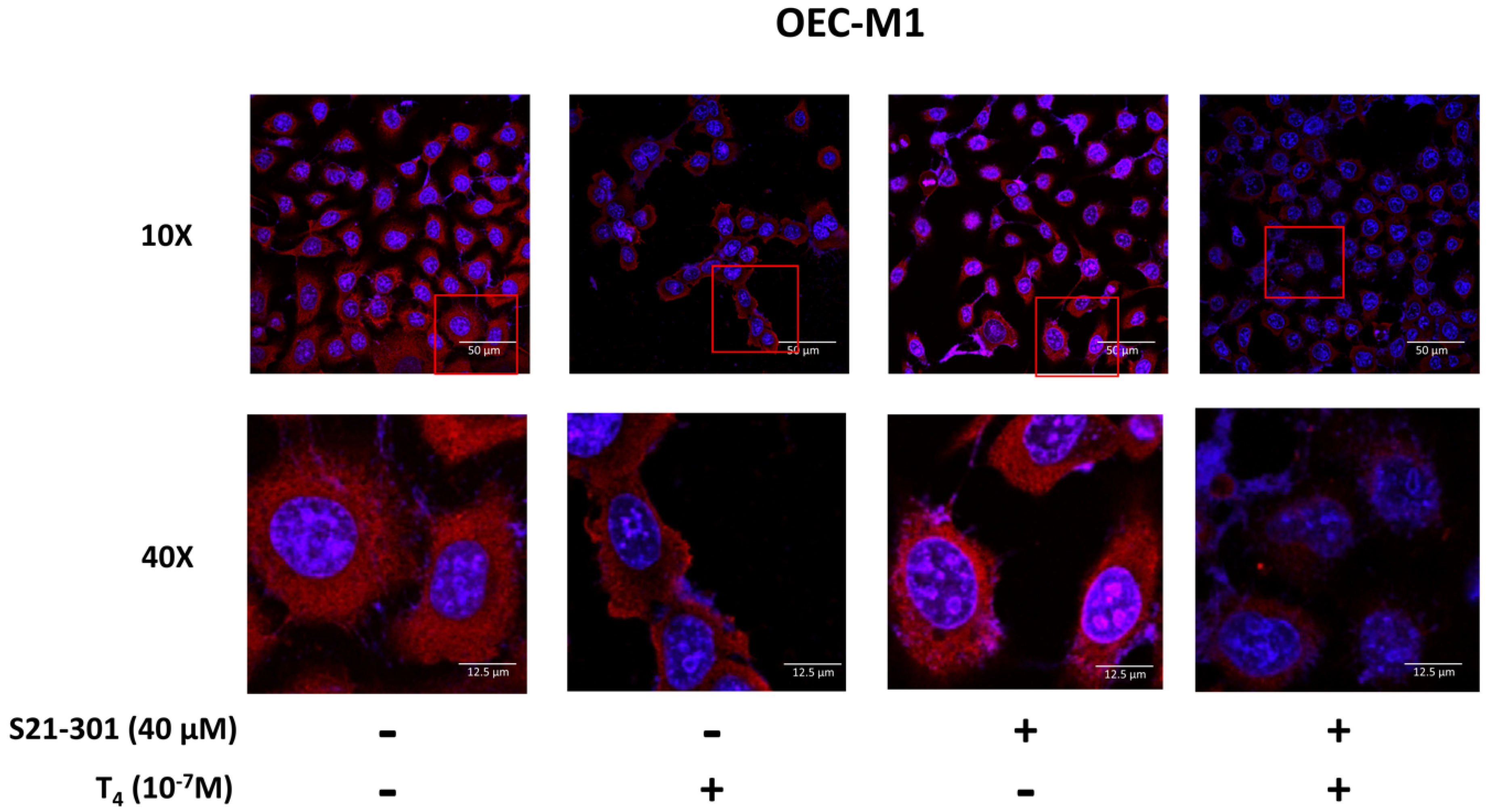
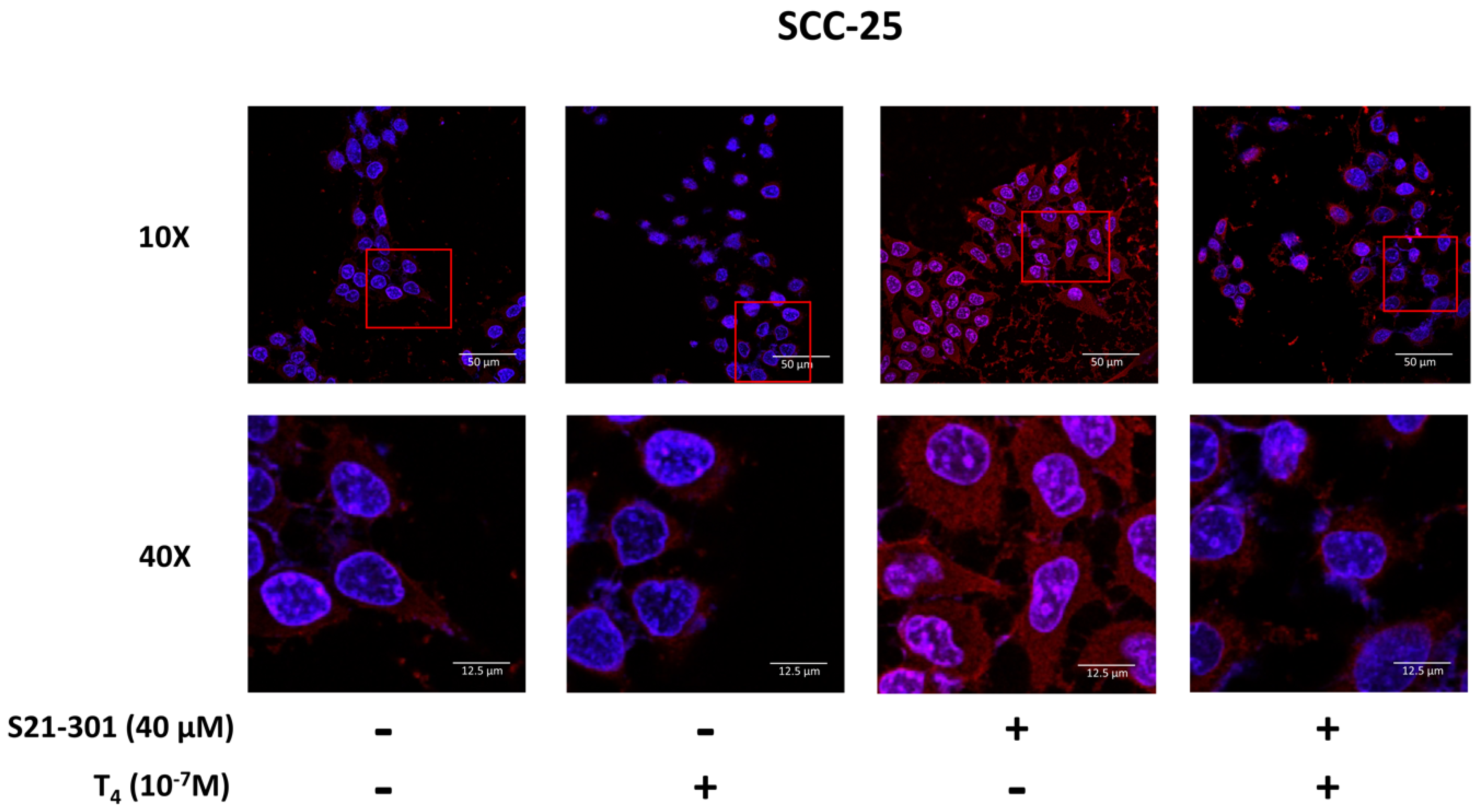
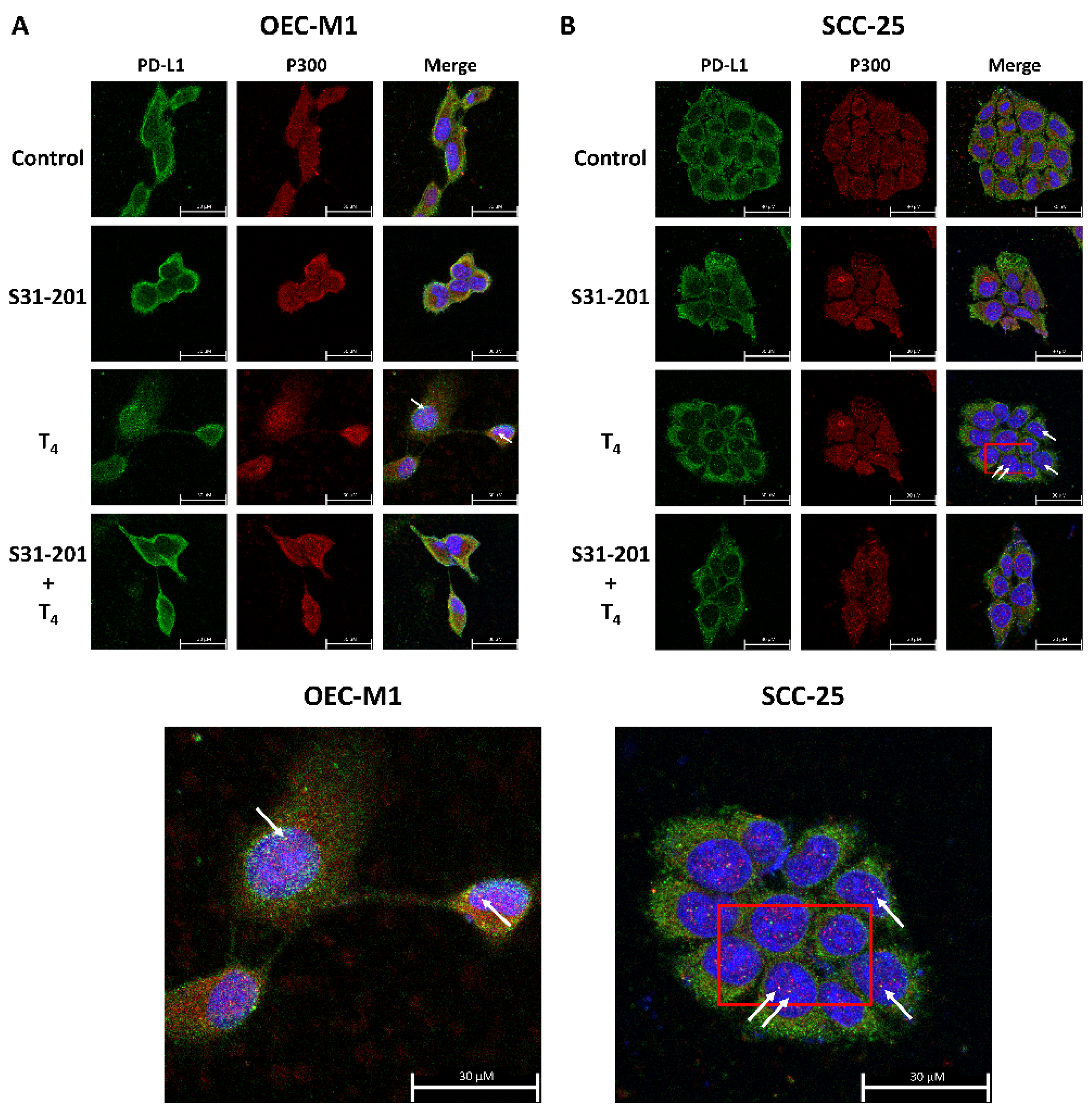
3.4. Inhibition of PD-L1 Signaling Disrupts the Thyroid Hormone-Induced Nuclear Protein Complex in Human Oral Cells
3.5. Inhibition of PD-L1 Expression Disrupts Thyroid Hormone-Induced Gene Expressions in Human Oral Cancer Cells
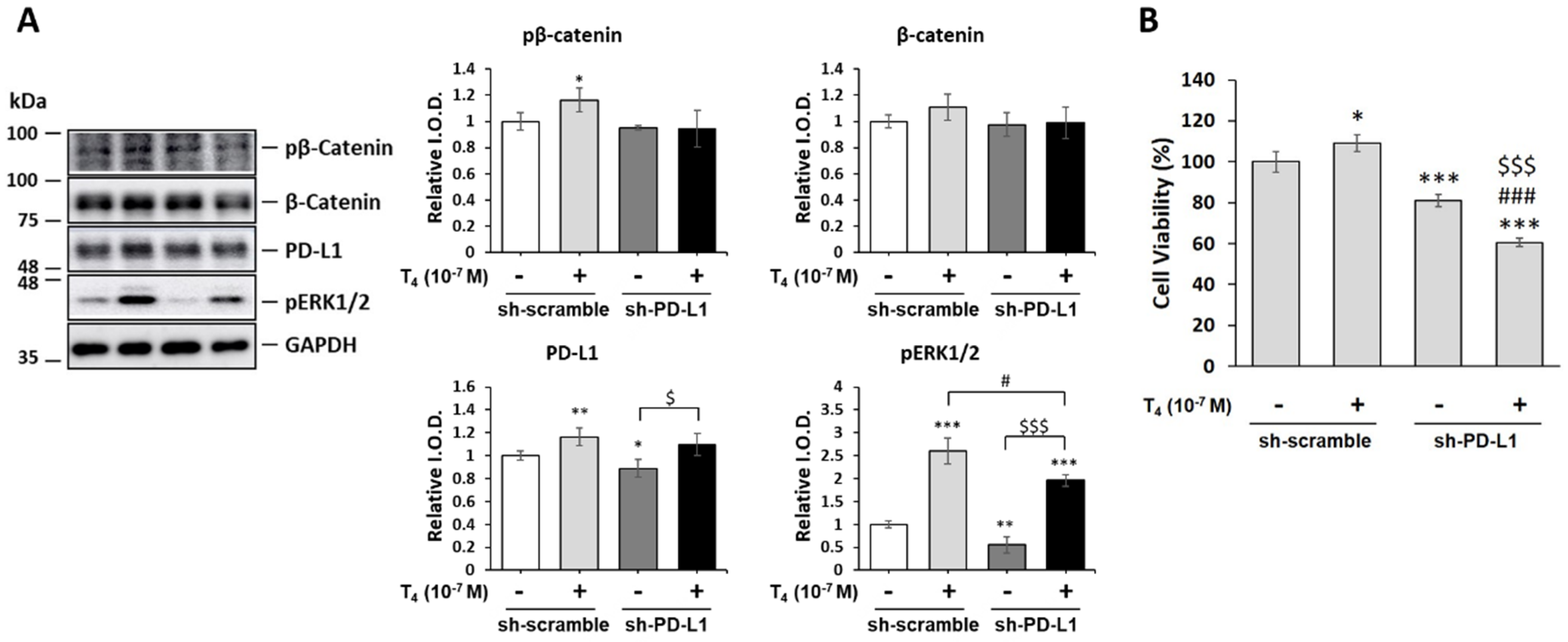
3.6. Thyroid Hormone (T4)-Induced PD-L1-Dependent Protein Expression and Cell Proliferation in Oral Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kao, S.Y.; Lim, E. An overview of detection and screening of oral cancer in Taiwan. Chin. J. Dent. Res. 2015, 18, 7–12. [Google Scholar] [PubMed]
- Tseng, C.H. Oral cancer in Taiwan: Is diabetes a risk factor? Clin. Oral Investig. 2013, 17, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhao, R.; Xia, W.; Chang, C.W.; You, Y.; Hsu, J.M.; Nie, L.; Chen, Y.; Wang, Y.C.; Liu, C.; et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020, 22, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chin, Y.T.; Shih, Y.J.; Chen, Y.R.; Chung, Y.Y.; Lin, C.Y.; Hsiung, C.N.; Whang-Peng, J.; Lee, S.Y.; Lin, H.Y.; et al. Resveratrol antagonizes thyroid hormone-induced expression of checkpoint and proliferative genes in oral cancer cells. J. Dent. Sci. 2019, 14, 255–262. [Google Scholar] [CrossRef]
- Brown, A.R.; Simmen, R.C.; Simmen, F.A. The role of thyroid hormone signaling in the prevention of digestive system cancers. Int. J. Mol. Sci. 2013, 14, 16240–16257. [Google Scholar] [CrossRef]
- Vonlaufen, A.; Wiedle, G.; Borisch, B.; Birrer, S.; Luder, P.; Imhof, B.A. Integrin alpha(v)beta(3) expression in colon carcinoma correlates with survival. Mod. Pathol. 2001, 14, 1126–1132. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Chen, L.; Han, X. Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. J. Clin. Investig. 2015, 125, 3384–3391. [Google Scholar] [CrossRef]
- Page, D.B.; Postow, M.A.; Callahan, M.K.; Allison, J.P.; Wolchok, J.D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 2014, 65, 185–202. [Google Scholar] [CrossRef]
- Schmidt, L.H.; Kummel, A.; Gorlich, D.; Mohr, M.; Brockling, S.; Mikesch, J.H.; Grunewald, I.; Marra, A.; Schultheis, A.M.; Wardelmann, E.; et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS ONE 2015, 10, e0136023. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tang, H.Y.; Keating, T.; Wu, Y.H.; Shih, A.; Hammond, D.; Sun, M.; Hercbergs, A.; Davis, F.B.; Davis, P.J. Resveratrol is pro-apoptotic and thyroid hormone is anti-apoptotic in glioma cells: Both actions are integrin and ERK mediated. Carcinogenesis 2008, 29, 62–69. [Google Scholar] [CrossRef]
- Nana, A.W.; Chin, Y.T.; Lin, C.Y.; Ho, Y.; Bennett, J.A.; Shih, Y.J.; Chen, Y.R.; Changou, C.A.; Pedersen, J.Z.; Incerpi, S.; et al. Tetrac downregulates beta-catenin and HMGA2 to promote the effect of resveratrol in colon cancer. Endocr. Relat. Cancer 2018, 25, 279–293. [Google Scholar] [CrossRef]
- Davis, F.B.; Tang, H.Y.; Shih, A.; Keating, T.; Lansing, L.; Hercbergs, A.; Fenstermaker, R.A.; Mousa, A.; Mousa, S.A.; Davis, P.J.; et al. Acting via a cell surface receptor, thyroid hormone is a growth factor for glioma cells. Cancer Res. 2006, 66, 7270–7275. [Google Scholar] [CrossRef]
- Kogashiwa, Y.; Yasuda, M.; Sakurai, H.; Nakahira, M.; Sano, Y.; Gonda, K.; Ikeda, T.; Inoue, H.; Kuba, K.; Oba, S.; et al. PD-L1 Expression Confers Better Prognosis in Locally Advanced Oral Squamous Cell Carcinoma. Anticancer Res. 2017, 37, 1417–1424. [Google Scholar] [CrossRef]
- Mincione, G.; Di Marcantonio, M.C.; Tarantelli, C.; D’Inzeo, S.; Nicolussi, A.; Nardi, F.; Donini, C.F.; Coppa, A. EGF and TGF-beta1 Effects on Thyroid Function. J. Thyroid Res. 2011, 2011, 431718. [Google Scholar] [CrossRef]
- Atsaves, V.; Tsesmetzis, N.; Chioureas, D.; Kis, L.; Leventaki, V.; Drakos, E.; Panaretakis, T.; Grander, D.; Medeiros, L.J.; Young, K.H.; et al. PD-L1 is commonly expressed and transcriptionally regulated by STAT3 and MYC in ALK-negative anaplastic large-cell lymphoma. Leukemia 2017, 31, 1633–1637. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, J.; Giobbie-Hurder, A.; Wargo, J.; Hodi, F.S. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin. Cancer Res. 2013, 19, 598–609. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Huang, T.Y.; Chang, T.C.; Chin, Y.T.; Pan, Y.S.; Chang, W.J.; Liu, F.C.; Hastuti, E.D.; Chiu, S.J.; Wang, S.H.; Changou, C.A.; et al. NDAT Targets PI3K-Mediated PD-L1 Upregulation to Reduce Proliferation in Gefitinib-Resistant Colorectal Cancer. Cells 2020, 9, 1830. [Google Scholar] [CrossRef]
- Bu, L.L.; Yu, G.T.; Wu, L.; Mao, L.; Deng, W.W.; Liu, J.F.; Kulkarni, A.B.; Zhang, W.F.; Zhang, L.; Sun, Z.J. STAT3 Induces Immunosuppression by Upregulating PD-1/PD-L1 in HNSCC. J. Dent. Res. 2017, 96, 1027–1034. [Google Scholar] [CrossRef]
- Chang, T.C.; Chin, Y.T.; Nana, A.W.; Wang, S.H.; Liao, Y.M.; Chen, Y.R.; Shih, Y.J.; Changou, C.A.; Yang, Y.S.; Wang, K.; et al. Enhancement by Nano-Diamino-Tetrac of Antiproliferative Action of Gefitinib on Colorectal Cancer Cells: Mediation by EGFR Sialylation and PI3K Activation. Horm. Cancer 2018, 9, 420–432. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chin, Y.T.; Nana, A.W.; Shih, Y.J.; Lai, H.Y.; Tang, H.Y.; Leinung, M.; Mousa, S.A.; Davis, P.J. Actions of l-thyroxine and Nano-diamino-tetrac (Nanotetrac) on PD-L1 in cancer cells. Steroids 2016, 114, 59–67. [Google Scholar] [CrossRef]
- Du, L.; Lee, J.H.; Jiang, H.; Wang, C.; Wang, S.; Zheng, Z.; Shao, F.; Xu, D.; Xia, Y.; Li, J.; et al. β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J. Exp. Med. 2020, 217, e20191115. [Google Scholar] [CrossRef]
- Huber, A.H.; Weis, W.I. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell 2001, 105, 391–402. [Google Scholar] [CrossRef]
- Lee, Y.S.; Chin, Y.T.; Shih, Y.J.; Nana, A.W.; Chen, Y.R.; Wu, H.C.; Yang, Y.S.H.; Lin, H.Y.; Davis, P.J. Thyroid Hormone Promotes beta-Catenin Activation and Cell Proliferation in Colorectal Cancer. Horm. Cancer 2018, 9, 156–165. [Google Scholar] [CrossRef]
- Schweizer, L.; Varmus, H. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 2003, 4, 4. [Google Scholar] [CrossRef][Green Version]
- Gorka, J.; Marona, P.; Kwapisz, O.; Waligorska, A.; Pospiech, E.; Dobrucki, J.W.; Rys, J.; Jura, J.; Miekus, K. MCPIP1 inhibits Wnt/beta-catenin signaling pathway activity and modulates epithelial-mesenchymal transition during clear cell renal cell carcinoma progression by targeting miRNAs. Oncogene 2021, 40, 6720–6735. [Google Scholar] [CrossRef]
- Wend, P.; Runke, S.; Wend, K.; Anchondo, B.; Yesayan, M.; Jardon, M.; Hardie, N.; Loddenkemper, C.; Ulasov, I.; Lesniak, M.S.; et al. WNT10B/beta-catenin signalling induces HMGA2 and proliferation in metastatic triple-negative breast cancer. EMBO Mol. Med. 2013, 5, 264–279. [Google Scholar] [CrossRef]
- Zha, L.; Zhang, J.; Tang, W.; Zhang, N.; He, M.; Guo, Y.; Wang, Z. HMGA2 elicits EMT by activating the Wnt/beta-catenin pathway in gastric cancer. Dig. Dis. Sci. 2013, 58, 724–733. [Google Scholar] [CrossRef]
- Cho, J.; Ahn, S.; Yoo, K.H.; Kim, J.H.; Choi, S.H.; Jang, K.T.; Lee, J. Treatment outcome of PD-1 immune checkpoint inhibitor in Asian metastatic melanoma patients: Correlative analysis with PD-L1 immunohistochemistry. Investig. New Drugs 2016, 34, 677–684. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Liu, F.; Zhao, Y.; Liu, J.; Hua, Y.; Liu, J.; Huang, J.; Ge, C. A blockade of PD-L1 produced antitumor and antimetastatic effects in an orthotopic mouse pancreatic cancer model via the PI3K/Akt/mTOR signaling pathway. OncoTargets Ther. 2017, 10, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chen, D.; Lu, B.; Wang, C.; Zhang, J.; Huang, L.; Wang, X.; Timmons, C.L.; Hu, J.; Liu, B.; et al. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS ONE 2013, 8, e65821. [Google Scholar] [CrossRef] [PubMed]
- Okita, R.; Maeda, A.; Shimizu, K.; Nojima, Y.; Saisho, S.; Nakata, M. PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol. Immunother. 2017, 66, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Foy, J.P.; Bertolus, C.; Michallet, M.C.; Deneuve, S.; Incitti, R.; Bendriss-Vermare, N.; Albaret, M.A.; Ortiz-Cuaran, S.; Thomas, E.; Colombe, A.; et al. The immune microenvironment of HPV-negative oral squamous cell carcinoma from never-smokers and never-drinkers patients suggests higher clinical benefit of IDO1 and PD1/PD-L1 blockade. Ann. Oncol. 2017, 28, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Yang, K. Inhibitors of the PD-1/PD-L1 axis for the treatment of head and neck cancer: Current status and future perspectives. Drug Des. Dev. Ther. 2017, 11, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Chen, Y.S.; Chin, Y.T.; Li, Z.L.; Shih, Y.J.; Yang, Y.S.H.; ChangOu, C.A.; Su, P.Y.; Wang, S.H.; Wu, Y.H.; et al. Thyroid hormone-induced expression of inflammatory cytokines interfere with resveratrol-induced anti-proliferation of oral cancer cells. Food Chem. Toxicol. 2019, 132, 110693. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Leoni, V.; Barboni, C.; Sanapo, M.; Zaghini, A.; Malatesta, P.; Campadelli-Fiume, G.; Gianni, T. alphavbeta3-integrin regulates PD-L1 expression and is involved in cancer immune evasion. Proc. Natl. Acad. Sci. USA 2019, 116, 20141–20150. [Google Scholar] [CrossRef] [PubMed]
- Nana, A.W.; Wu, S.Y.; Yang, Y.S.; Chin, Y.T.; Cheng, T.M.; Ho, Y.; Li, W.S.; Liao, Y.M.; Chen, Y.R.; Shih, Y.J.; et al. Nano-Diamino-Tetrac (NDAT) Enhances Resveratrol-Induced Antiproliferation by Action on the RRM2 Pathway in Colorectal Cancers. Horm. Cancer 2018, 9, 349–360. [Google Scholar] [CrossRef]
- Konopleva, M.Y.; Walter, R.B.; Faderl, S.H.; Jabbour, E.J.; Zeng, Z.; Borthakur, G.; Huang, X.; Kadia, T.M.; Ruvolo, P.P.; Feliu, J.B.; et al. Preclinical and early clinical evaluation of the oral AKT inhibitor, MK-2206, for the treatment of acute myelogenous leukemia. Clin. Cancer Res. 2014, 20, 2226–2235. [Google Scholar] [CrossRef]
- Hu, T.; Li, C. Convergence between Wnt-beta-catenin and EGFR signaling in cancer. Mol. Cancer 2010, 9, 236. [Google Scholar] [CrossRef]
- Paul, I.; Bhattacharya, S.; Chatterjee, A.; Ghosh, M.K. Current Understanding on EGFR and Wnt/beta-Catenin Signaling in Glioma and Their Possible Crosstalk. Genes Cancer 2013, 4, 427–446. [Google Scholar] [CrossRef]
- Gkouveris, I.; Nikitakis, N.; Sklavounou, A. p38 Expression and Modulation of STAT3 Signaling in Oral Cancer. Pathol. Oncol. Res. 2020, 26, 183–192. [Google Scholar] [CrossRef]
- Leelahavanichkul, K.; Amornphimoltham, P.; Molinolo, A.A.; Basile, J.R.; Koontongkaew, S.; Gutkind, J.S. A role for p38 MAPK in head and neck cancer cell growth and tumor-induced angiogenesis and lymphangiogenesis. Mol. Oncol. 2014, 8, 105–118. [Google Scholar] [CrossRef]
- Gao, Y.; Nihira, N.T.; Bu, X.; Chu, C.; Zhang, J.; Kolodziejczyk, A.; Fan, Y.; Chan, N.T.; Ma, L.; Liu, J.; et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat. Cell Biol. 2020, 22, 1064–1075. [Google Scholar] [CrossRef]
- Lin, H.Y.; Su, Y.F.; Hsieh, M.T.; Lin, S.; Meng, R.; London, D.; Lin, C.; Tang, H.Y.; Hwang, J.; Davis, F.B.; et al. Nuclear monomeric integrin alphav in cancer cells is a coactivator regulated by thyroid hormone. FASEB J. 2013, 27, 3209–3216. [Google Scholar] [CrossRef]
- Cabodevilla, A.; Sánchez, L.; Nintou, E.; Boiadjieva, V.; Picatoste, F.; Gubern, A.; Claro, E. Cell Survival during Complete Nutrient Deprivation Depends on Lipid Droplet-fueled -Oxidation of Fatty Acids. J. Biol. Chem. 2013, 288, 27777. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef]


| Forward | Reverse | |
|---|---|---|
| PD-L1 | 5′-GTTGAAGGACCAGCTCTCCC-3′ | 5′-ACCCCTGCATCCTGCAATTT-3′ |
| β-catenin | 5′-CTGGTCCTTTTTGGTCGAGGA-3‘ | 5′-GCAAGGCTAGGGTTTGATAAAT-3′ |
| CCND1 | 5′-CAAGGCCTGAACCTGAGGAG-3′ | 5′-GATCACTCTGGAGAGGAAGCG-3′ |
| PCNA | 5′-TCTGAGGGCTTCGACACCTA-3′ | 5′-TCATTGCCGGCGCATTTTAG-3′ |
| p21 | 5′-CTGGGGATGTCCGTCAGAAC-3′ | 5′-CATTAGCGCATCACAGTCGC-3′ |
| BAD | 5′-CTTTAAGAAGGGACTTCCTCGCC-3′ | 5′-AAGTTCCGATCCCACCAGGA-3′ |
| 18S | 5’-GTAACCCGTTGAACCCCATT-3’ | 5’-CCATCCAATCGGTAGTAGCG-3’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, K.-W.; Lin, H.-Y.; Chiu, H.-C.; Shen, S.-Y.; ChangOu, C.A.; Crawford, D.R.; Yang, Y.-C.S.H.; Shih, Y.-J.; Li, Z.-L.; Huang, H.-M.; et al. Thyroid Hormone Induces Oral Cancer Growth via the PD-L1-Dependent Signaling Pathway. Cells 2022, 11, 3050. https://doi.org/10.3390/cells11193050
Su K-W, Lin H-Y, Chiu H-C, Shen S-Y, ChangOu CA, Crawford DR, Yang Y-CSH, Shih Y-J, Li Z-L, Huang H-M, et al. Thyroid Hormone Induces Oral Cancer Growth via the PD-L1-Dependent Signaling Pathway. Cells. 2022; 11(19):3050. https://doi.org/10.3390/cells11193050
Chicago/Turabian StyleSu, Kuan-Wei, Hung-Yun Lin, Hsien-Chung Chiu, Shin-Yu Shen, Chun A. ChangOu, Dana R. Crawford, Yu-Chen S. H. Yang, Ya-Jung Shih, Zi-Lin Li, Haw-Ming Huang, and et al. 2022. "Thyroid Hormone Induces Oral Cancer Growth via the PD-L1-Dependent Signaling Pathway" Cells 11, no. 19: 3050. https://doi.org/10.3390/cells11193050
APA StyleSu, K.-W., Lin, H.-Y., Chiu, H.-C., Shen, S.-Y., ChangOu, C. A., Crawford, D. R., Yang, Y.-C. S. H., Shih, Y.-J., Li, Z.-L., Huang, H.-M., Whang-Peng, J., Ho, Y., & Wang, K. (2022). Thyroid Hormone Induces Oral Cancer Growth via the PD-L1-Dependent Signaling Pathway. Cells, 11(19), 3050. https://doi.org/10.3390/cells11193050










