Magnetoreceptory Function of European Robin Retina: Electrophysiological and Morphological Non-Homogeneity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
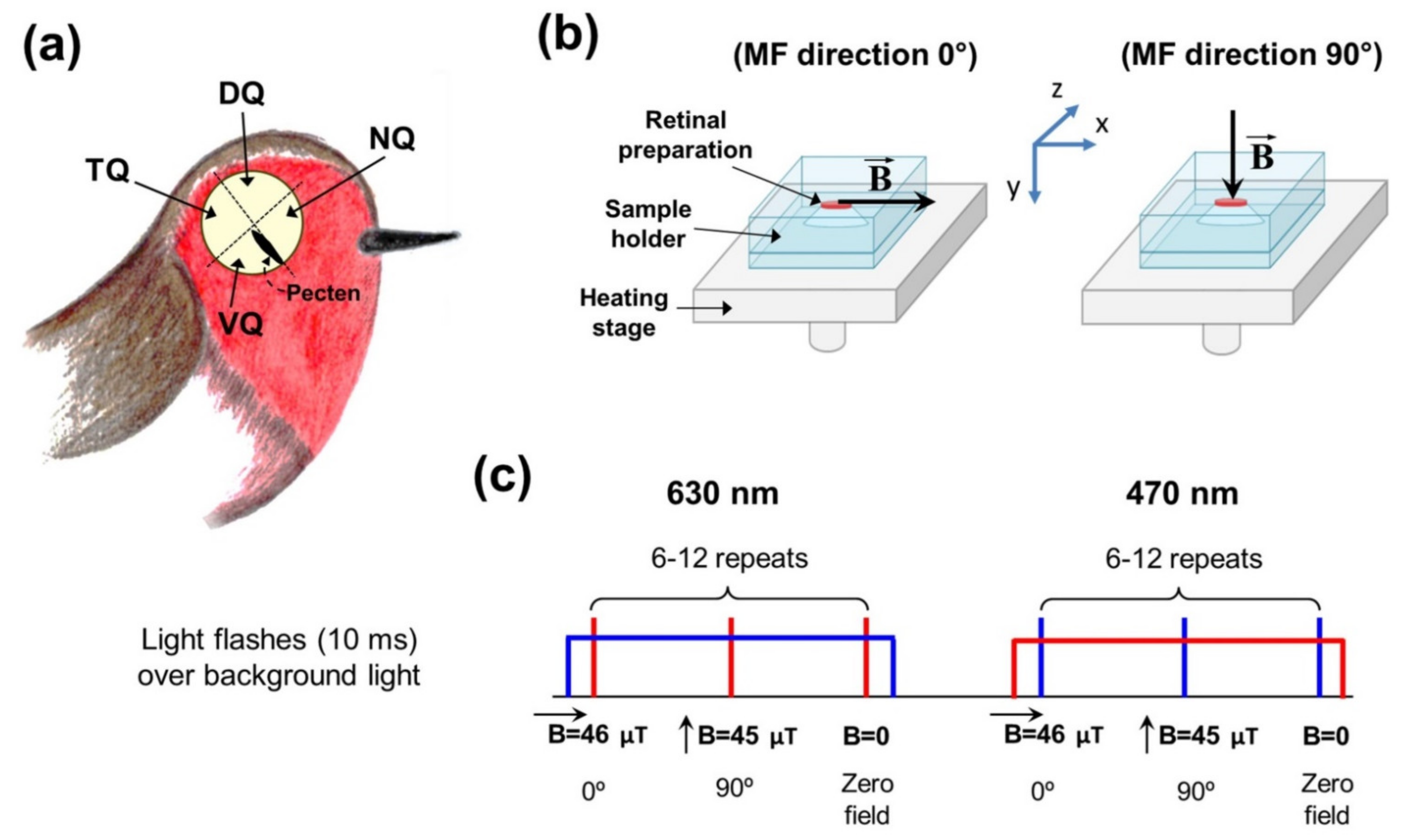
2.2. Ex Vivo Electroretinography with Magnetic Field Modulation
2.3. Light Microscopy
2.4. Microspectrophotometry of Oil Droplets
2.5. Data Analysis
3. Results
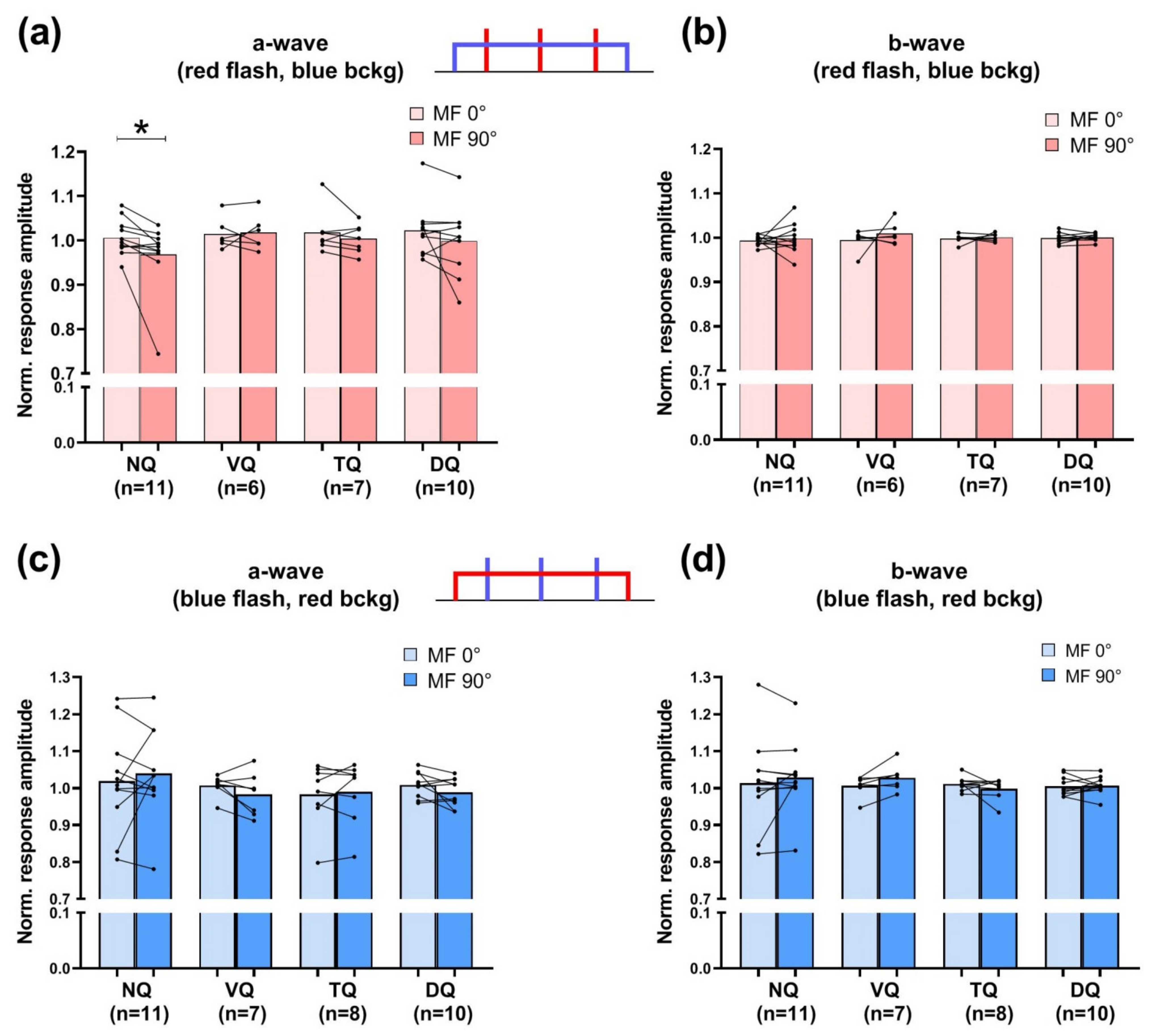
3.1. Electroretinography under Background Light and Magnetic Field Modulation
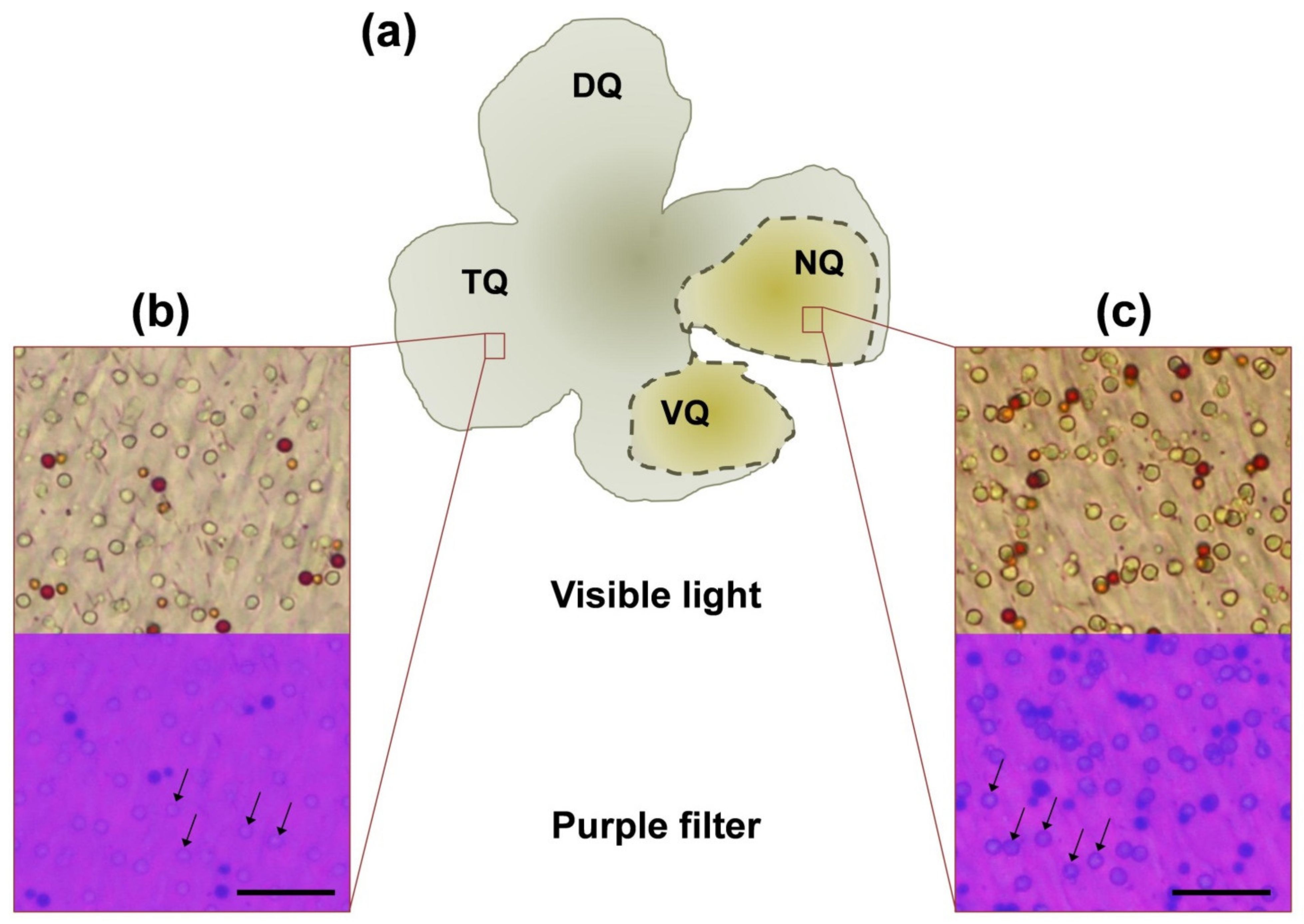
3.2. Morphological Features of Four Quadrants of European Robin’s Retina
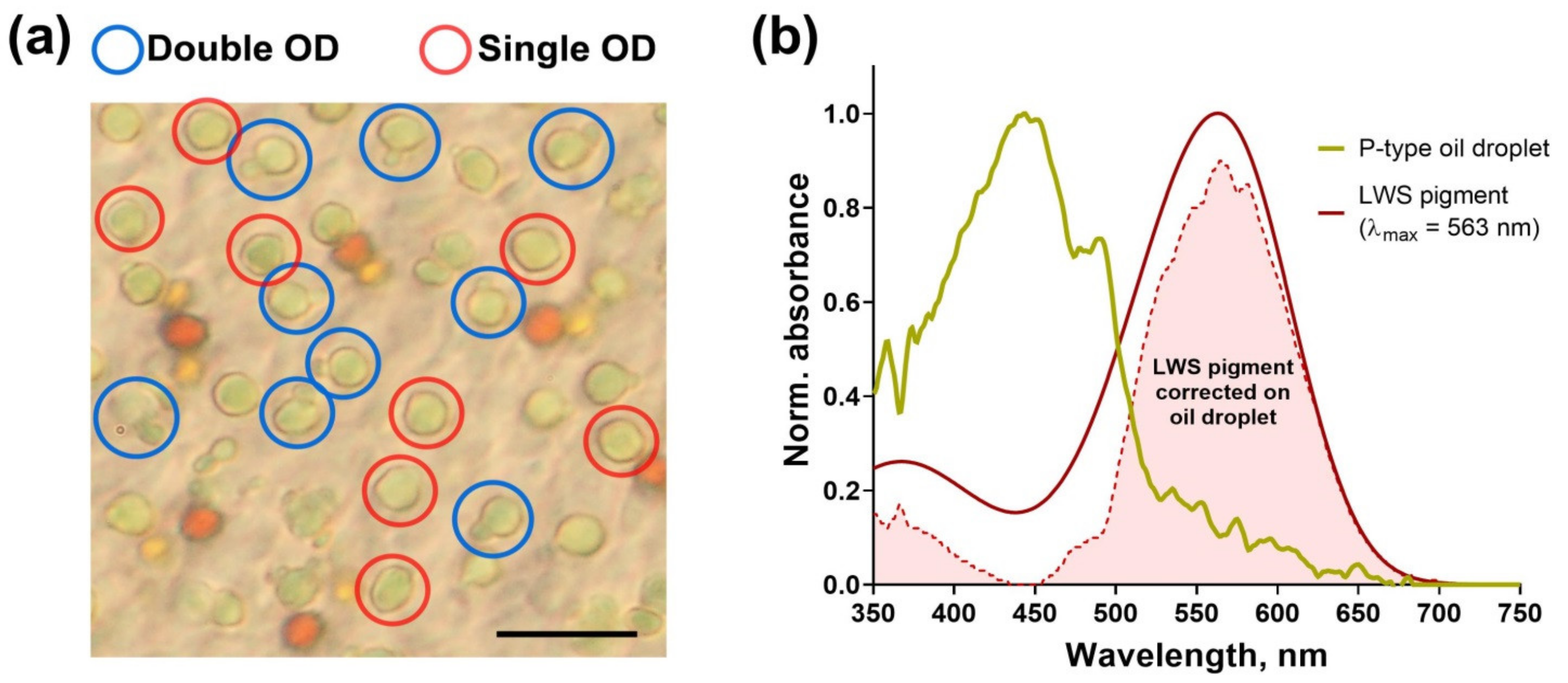
3.3. Spectral Characteristics of Oil Droplets from Different Quadrants of European Robin’s Retina
4. Discussion
4.1. Insight into Basic Properties of Magnetoreception from Electrophysiological Data
4.2. Morphological Features of European Robin’s Nasal Retina: Possible Connection of Oil Droplets with Magnetoreceptive Function
4.3. Nasal Retinal Double Cones as Potential Magnetoreceptors
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiltschko, W.; Wiltschko, R. Magnetic compass of European robins. Science 1972, 176, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Kishkinev, D.; Chernetsov, N.; Pakhomov, A.; Heyers, D.; Mouritsen, H. Eurasian reed warblers compensate for virtual magnetic displacement. Curr. Biol. 2015, 5, R822–R824. [Google Scholar] [CrossRef] [PubMed]
- Munro, U.H.; Munro, J.A.; Phillips, J.B.; Wiltschko, W. Effect of wavelength of light and pulse magnetization on different magnetoreception systems in a migratory bird. Aust. J. Zool. 1997, 45, 189–198. [Google Scholar] [CrossRef]
- Rappl, R.; Wiltschko, R.; Weindler, P.; Berthold, P.; Wiltschko, W. Orientation behavior of garden warblers (Sylvia borin) under monochromatic light of various wavelengths. Auk 2000, 117, 256–260. [Google Scholar] [CrossRef]
- Muheim, R.; Bäckman, J.; Åkesson, S. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J. Exp. Biol. 2002, 205, 3845–3856. [Google Scholar] [CrossRef]
- Pinzon-Rodriguez, A.; Muheim, R. Zebra finches have a light-dependent magnetic compass similar to migratory birds. J. Exp. Biol. 2017, 220, 1202–1209. [Google Scholar] [CrossRef]
- Ritz, T.; Adem, S.; Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 2000, 78, 707–718. [Google Scholar] [CrossRef]
- Solov’yov, I.A.; Mouritsen, H.; Schulten, K. Acuity of a cryptochrome and vision-based magnetoreception system in birds. Biophys. J. 2010, 99, 40–49. [Google Scholar] [CrossRef]
- Maeda, K.; Robinson, A.J.; Henbest, K.B.; Hogben, H.J.; Biskup, T.; Ahmad, M.; Schleicher, E.; Weber, S.; Timmel, C.R.; Hore, P.J. Magnetically sensitive lightinduced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl Acad. Sci. USA 2012, 109, 4774–4779. [Google Scholar] [CrossRef]
- Nießner, C.; Denzau, S.; Gross, J.C.; Peichl, L.; Bischof, H.J.; Fleißner, G.; Wiltschko, W.; Wiltschko, R. Avian ultraviolet/violet cones identified as probable magnetoreceptors. PLoS ONE 2011, 6, e20091. [Google Scholar] [CrossRef] [Green Version]
- Bolte, P.; Bleibaum, F.; Einwich, A.; Günther, A.; Liedvogel, M.; Heyers, D.; Depping, A.; Wöhlbrand, L.; Rabus, R.; Janssen-Bienhold, U.; et al. Localisation of the putative magnetoreceptive protein cryptochrome 1b in the retinae of migratory birds and homing pigeons. PLoS ONE 2016, 11, e0147819. [Google Scholar] [CrossRef]
- Günther, A.; Einwich, A.; Sjulstok, E.; Feederle, R.; Bolte, P.; Koch, K.W.; Solov’yov, I.A.; Mouritsen, H. Double-cone localization and seasonal expression pattern suggest a role in magnetoreception for European robin cryptochrome 4. Curr. Biol. 2018, 28, 211–223. [Google Scholar] [CrossRef]
- Hore, P.J.; Mouritsen, H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Kavokin, K. The puzzle of magnetic resonance effect on the magnetic compass of migratory birds. Bioelectromagnetics 2009, 30, 402–410. [Google Scholar] [CrossRef]
- Hiscock, H.G.; Mouritsen, H.; Manolopoulos, D.E.; Hore, P.J. Disruption of magnetic compass orientation in migratory birds by radiofrequency electromagnetic fields. Biophys. J. 2017, 113, 1475–1484. [Google Scholar] [CrossRef]
- Xu, J.; Jarocha, L.E.; Zollitsch, T.; Konowalczyk, M.; Henbest, K.B.; Richert, S.; Golesworthy, M.J.; Schmidt, J.; Déjean, V.; Sowood, D.J.C.; et al. Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature 2021, 594, 535–540. [Google Scholar] [CrossRef]
- Wu, H.; Scholten, A.; Einwich, A.; Mouritsen, H.; Koch, K.W. Protein-protein interaction of the putative magnetoreceptor cryptochrome 4 expressed in the avian retina. Sci. Rep. 2020, 10, 7364. [Google Scholar] [CrossRef]
- Görtemaker, K.; Yee, C.; Bartölke, R.; Behrmann, H.; Voß, J.O.; Schmidt, J.; Xu, J.; Solovyeva, V.; Leberecht, B.; Behrmann, E.; et al. Direct interaction of avian cryptochrome 4 with a cone specific G-protein. Cells 2022, 11, 2043. [Google Scholar] [CrossRef]
- Worster, S.; Mouritsen, H.; Hore, P.J. A light-dependent magnetoreception mechanism insensitive to light intensity and polarization. J. R. Soc. Interface 2017, 14, 20170405. [Google Scholar] [CrossRef]
- Rotov, A.; Cherbunin, R.V.; Kavokin, K.V.; Firsov, M.L.; Chernetsov, N.; Astakhova, L.A. Magnetoreception in the retina of the domestic pigeon Columbia livia: A retinographic search. J. Evol. Biochem. Physiol. 2018, 54, 498–501. [Google Scholar] [CrossRef]
- Rotov, A.; Cherbunin, R.V.; Anashina, A.; Kavokin, K.V.; Chernetsov, N.; Firsov, M.L.; Astakhova, L.A. Searching for magnetic compass mechanism in pigeon retinal photoreceptors. PLoS ONE 2020, 15, e0229142. [Google Scholar] [CrossRef]
- Astakhova, L.A.; Rotov, A.Y.; Cherbunin, R.V.; Goriachenkov, A.A.; Kavokin, K.V.; Firsov, M.L.; Chernetsov, N. Electroretinographic study of the magnetic compass in European robins. Proc. R. Soc. B 2020, 287, 20202507. [Google Scholar] [CrossRef]
- Hart, N.S. The visual ecology of avian photoreceptors. Prog. Retin. Eye Res. 2001, 20, 675–703. [Google Scholar] [CrossRef]
- Kelber, A. Bird colour vision–from cones to perception. Curr. Opin. Behav. Sci. 2019, 30, 34–40. [Google Scholar] [CrossRef]
- Walls, G.L. The Vertebrate Eye and Its Adaptive Radiation; Hafner: New York, NY, USA, 1942. [Google Scholar]
- Meyer, D.B.; Cooper, T.G.; Gernez, C. Retinal oil droplets. In II Symposium the Structure of the Eye, Proceedings of the 8th International Congress of Anatomists, Wiesbaden, Germany, 8–13 August 1965; Schattauer: Stuttgart, Germany, 1965. [Google Scholar]
- Toomey, M.B.; Collins, A.M.; Frederiksen, R.; Cornwall, M.C.; Timlin, J.A.; Corbo, J.C. A complex carotenoid palette tunes avian colour vision. J. R. Soc. Interface 2015, 12, 20150563. [Google Scholar] [CrossRef]
- Victory, N.; Segovia, Y.; García, M. Cone distribution and visual resolution of the yellow-legged gull, Larus michahellis (Naumann, 1840). Anat. Histol. Embryol. 2022, 51, 197–214. [Google Scholar] [CrossRef]
- Hart, N.S.; Partridge, J.C.; Cuthill, I.C.; Bennett, A.T.D. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: The blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J. Comp. Physiol. A 2000, 186, 375–387. [Google Scholar] [CrossRef]
- Zueva, L.V.; Golubeva, T.B.; Kerov, V.S.; Zuev, A.V. Heterogeneous maturation of retina of the pied flycatcher Ficedula hypoleuca. J. Evol. Biochem. Physiol. 2003, 39, 724–731. [Google Scholar] [CrossRef]
- Fenton, G.E.; Nath, K.; Malkemper, E.P. Electrophysiology and the magnetic sense: A guide to best practice. J. Comp. Physiol. A 2021, 208, 185–195. [Google Scholar] [CrossRef]
- Wiltschko, R.; Thalau, P.; Gehring, D.; Nießner, C.; Ritz, T.; Wiltschko, W. Magnetoreception in birds: The effect of radio-frequency fields. J. R. Soc. Interface 2015, 12, 20141103. [Google Scholar] [CrossRef]
- Kram, Y.A.; Mantey, S.; Corbo, J.C. Avian cone photoreceptors tile the retina as five independent, self-organizing mosaics. PLoS ONE 2010, 5, e8992. [Google Scholar] [CrossRef] [PubMed]
- Govardovskii, V.I.; Fyhrquist, N.; Reuter, T.; Kuzmin, D.G.; Donner, K. In search of the visual pigment template. Vis. Neurosci. 2000, 17, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Lipetz, L.E. A new method for determining peak absorbance of dense pigment samples and its application to the cone oil droplets of Emydoidea blandingii. Vis. Res. 1984, 24, 597–604. [Google Scholar] [CrossRef]
- Tyrrell, L.P.; Teixeira, L.B.; Dubielzig, R.R.; Pita, D.; Baumhardt, P.; Moore, B.A.; Fernández-Juricic, E. A novel cellular structure in the retina of insectivorous birds. Sci. Rep. 2019, 9, 15230. [Google Scholar] [CrossRef]
- Rahman, M.L.; Aoyama, M.; Sugita, S. Regional specialization of the tree sparrow Passer montanus retina: Ganglion cell density and oil droplet distribution. Ornithol. Sci. 2007, 6, 95–105. [Google Scholar] [CrossRef]
- Bowmaker, J.K. Color vision in birds and the role of oil droplets. Trends Neurosci. 1980, 3, 196–199. [Google Scholar] [CrossRef]
- Govardovskii, V.I. On the role of oil drops in color vision. Vis. Res. 1983, 23, 1739–1740. [Google Scholar] [CrossRef]
- Vorobyev, M. Coloured oil droplets enhance colour discrimination. Proc. R. Soc. B 2003, 270, 1255–1261. [Google Scholar] [CrossRef]
- Bowmaker, J.K.; Heath, L.A.; Wilkie, S.E.; Hunt, D.M. Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vis. Res. 1997, 37, 2183–2194. [Google Scholar] [CrossRef]
- Wiltschko, R.; Wiltschko, W. Magnetoreception in birds. J. R. Soc. Interface 2019, 16, 20190295. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, S.; Mattern, E.; Ritz, T. Light-dependent magnetoreception: Quantum catches and opponency mechanisms of possible photosensitive molecules. J. Exp. Biol. 2007, 210, 3171–3178. [Google Scholar] [CrossRef]
- Wiltschko, R.; Stapput, K.; Thalau, P.; Wiltschko, W. Directional orientation of birds by the magnetic field under different light conditions. J. R. Soc. Interface 2010, 7, S163–S177. [Google Scholar] [CrossRef]
- Zapka, M.; Heyers, D.; Liedvogel, M.; Jarvis, E.D.; Mouritsen, H. Night-time neuronal activation of Cluster N in a day-and night-migrating songbird. Eur. J. Neurosci. 2010, 32, 619–624. [Google Scholar] [CrossRef]
- Arshavsky, V.Y.; Burns, M.E. Photoreceptor signaling: Supporting vision across a wide range of light intensities. J. Biol. Chem. 2012, 2873, 1620–1626. [Google Scholar] [CrossRef]
- Hart, N.S.; Partridge, J.C.; Cuthill, I.C. Visual pigments, oil droplets and cone photoreceptor distribution in the European starling (Sturnus vulgaris). J. Exp. Biol. 1998, 201, 1433–1446. [Google Scholar] [CrossRef]
- Goldsmith, T.H.; Collins, J.S.; Licht, S. The cone oil droplets of avian retinas. Vis. Res. 1984, 24, 1661–1671. [Google Scholar] [CrossRef]
- Das, D.; Wilkie, S.E.; Hunt, D.M.; Bowmaker, J.K. Visual pigments and oil droplets in the retina of a passerine bird, the canary Serinus canaria: Microspectrophotometry and opsin sequences. Vis. Res. 1999, 39, 2801–2815. [Google Scholar] [CrossRef]
- Hart, N.S.; Partridge, J.C.; Bennett, A.T.D.; Cuthill, I.C. Visual pigments, cone oil droplets and ocular media in four species of estrildid finch. J. Comp. Physiol. 2000, 186, 681–694. [Google Scholar] [CrossRef]
- Edmonds, D.T. A sensitive optically detected magnetic compass for animals. Proc. R. Soc. B 1996, 263, 295–298. [Google Scholar]
- Edelman, N.B.; Fritz, T.; Nimpf, S.; Pichler, P.; Lauwers, M.; Hickman, R.W.; Papadaki-Anastasopouloua, A.; Ushakova, L.; Heuserc, T.; Reschc, G.P.; et al. No evidence for intracellular magnetite in putative vertebrate magnetoreceptors identified by magnetic screening. Proc. Natl. Acad. Sci. USA 2015, 112, 262–267. [Google Scholar] [CrossRef]
- Knott, B.; Berg, M.L.; Morgan, E.R.; Buchanan, K.L.; Bowmaker, J.K.; Bennett, A.T. Avian retinal oil droplets: Dietary manipulation of colour vision? Proc. R. Soc. B 2010, 277, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Chetverikova, R.; Dautaj, G.; Schwigon, L.; Dedek, K.; Mouritsen, H. Double cones in the avian retina form an oriented mosaic which might facilitate magnetoreception and/or polarized light sensing. J. R. Soc. Interface 2022, 19, 20210877. [Google Scholar] [CrossRef]
- Wiltschko, R.; Nießner, C.; Wiltschko, W. The magnetic compass of birds: The role of cryptochrome. Front. Physiol. 2021, 12, 667000. [Google Scholar] [CrossRef]




| Quadrant and Region of the Retina | Type of OD, Density/mm2 | ||
|---|---|---|---|
| R-Type | Y-Type | P-Type | |
| Central region of NQ | 2495 ± 1095 | 2583 ± 1375 | 16,059 ± 9277 |
| Peripheral region of NQ | 1878 ± 982 | 2297 ± 1388 | 12,433 ± 7976 |
| Central region of VQ | 1861 ± 919 | 2295 ± 1083 | 12,412 ± 6192 |
| Peripheral region of VQ | 962 ± 291 | 1130 ± 361 | 5628 ± 2126 |
| Central region of TQ | 2458 ± 1102 | 3023 ± 1578 | 19,289 ± 11,102 |
| Peripheral region of TQ | 1178 ± 827 | 1364 ± 864 | 7634 ± 4461 |
| Central region of DQ | 2878 ± 1211 | 3630 ± 1560 | 23,250 ± 8788 |
| Peripheral region of DQ | 2283 ± 1277 | 2771 ± 1593 | 19,376 ± 12,940 |
| Quadrant and Region of the Retina | Type of OD, Diameter, µm | ||
|---|---|---|---|
| R-Type | Y-Type | P-Type | |
| Central region of NQ | 2.6 ± 0.3 | 1.8 ± 0.2 | 2.1 ± 0.4 |
| Peripheral region of NQ | 2.9 ± 0.3 | 2.2 ± 0.2 | 2.8 ± 0.3 |
| Central region of VQ | 2.7 ± 0.3 | 1.9 ± 0.3 | 2.3 ± 0.5 |
| Peripheral region of VQ | 3.2 ± 0.3 | 2.5 ± 0.3 | 3.3 ± 0.4 |
| Central region of TQ | 2.7 ± 0.4 | 2.0 ± 0.4 | 2.0 ± 0.3 |
| Peripheral region of TQ | 3.1 ± 0.3 | 2.2 ± 0.3 | 3.3 ± 0.6 |
| Central region of DQ | 2.5 ± 0.1 | 1.7 ± 0.2 | 1.9 ± 0.2 |
| Peripheral region of DQ | 2.7 ± 0.2 | 1.9 ± 0.2 | 2.5 ± 0.5 |
| Quadrant of the Retina | λcut of OD Type, nm | ||||
|---|---|---|---|---|---|
| R-Type | Y-Type | P-Type | C-Type * | T-Type * | |
| NQ | 566.4 ± 4.1 | 508.2 ± 3.7 | 457.6 ± 11.3 | 370.06 ± 4.04 | <330 nm |
| VQ | 564.0 ± 4.6 | 509.4 ± 4.2 | 431.2 ± 6.9 | ||
| TQ | 565.6 ± 2.4 | 505.1 ± 3.4 | 415.2 ± 2.8 | ||
| DQ | 565.9 ± 2.7 | 507.3 ± 3.3 | 404.0 ± 3.9 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotov, A.Y.; Goriachenkov, A.A.; Cherbunin, R.V.; Firsov, M.L.; Chernetsov, N.; Astakhova, L.A. Magnetoreceptory Function of European Robin Retina: Electrophysiological and Morphological Non-Homogeneity. Cells 2022, 11, 3056. https://doi.org/10.3390/cells11193056
Rotov AY, Goriachenkov AA, Cherbunin RV, Firsov ML, Chernetsov N, Astakhova LA. Magnetoreceptory Function of European Robin Retina: Electrophysiological and Morphological Non-Homogeneity. Cells. 2022; 11(19):3056. https://doi.org/10.3390/cells11193056
Chicago/Turabian StyleRotov, Alexander Yu., Arsenii A. Goriachenkov, Roman V. Cherbunin, Michael L. Firsov, Nikita Chernetsov, and Luba A. Astakhova. 2022. "Magnetoreceptory Function of European Robin Retina: Electrophysiological and Morphological Non-Homogeneity" Cells 11, no. 19: 3056. https://doi.org/10.3390/cells11193056





