Direct Differentiation of Human Embryonic Stem Cells to 3D Functional Hepatocyte-like Cells in Alginate Microencapsulation Sphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Two-Dimensional Cell Culture and Cell Differentiation
2.2. Alginate Encapsulation
2.3. HE and PAS Staining
2.4. Immunofluorescence Staining
2.5. RNA Isolation and qRT-PCR
2.6. Permeability of Microspheres
2.7. Assessment of Intracapsular Cell Viability
2.8. MTT Assay
2.9. Western Blotting
2.10. Albumin Secretion
2.11. Ammonia Clearance Assay
2.12. Indocyanine Green Assay
2.13. Statistical Analysis
3. Results
3.1. Enzymatic Detachment Affects Hepatic Characteristics of 2D hESC-Heps in The Hepatocyte Stage
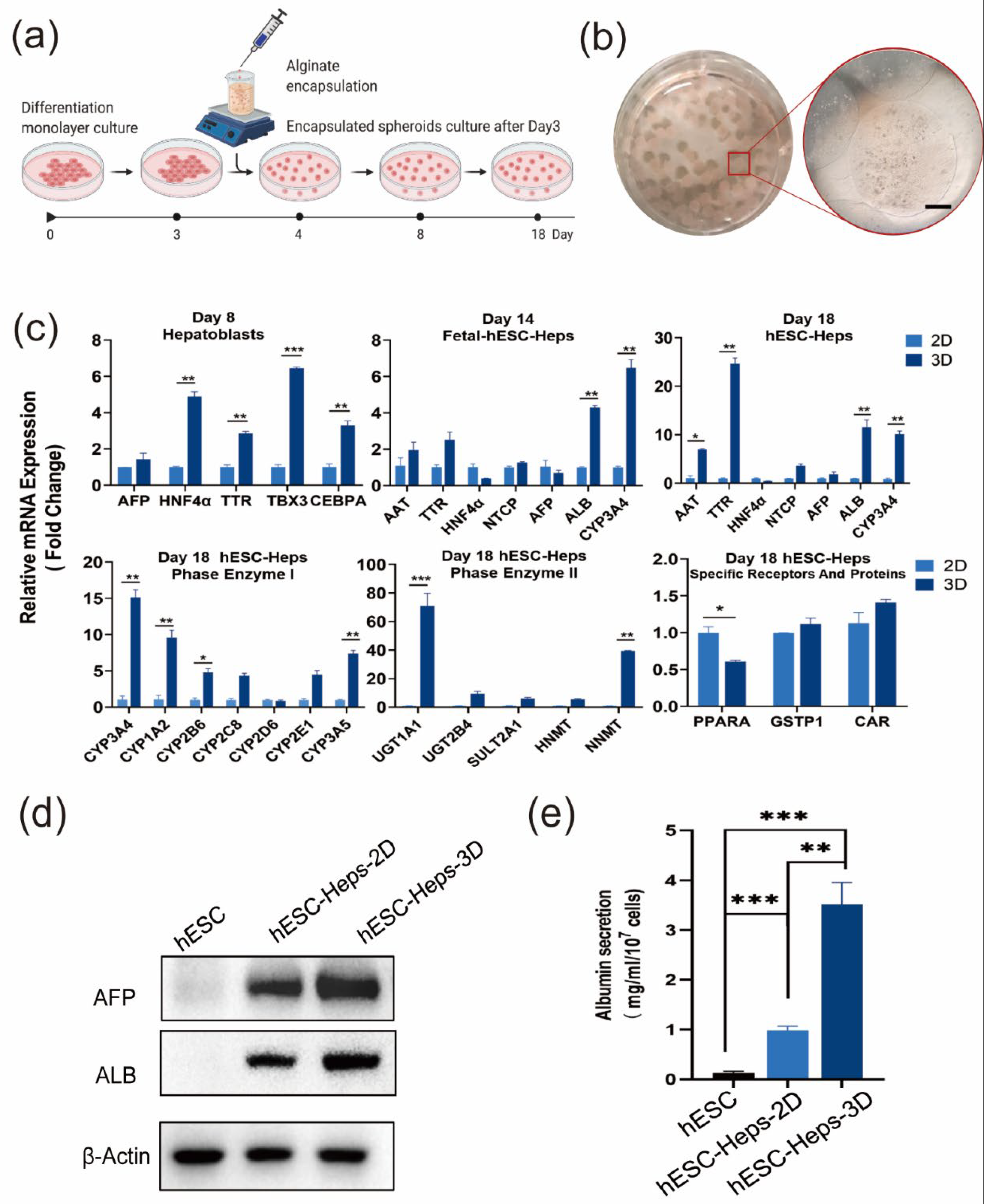
3.2. Establishment of hESC-Hep Encapsulation Conditions
3.3. hESC-Heps in 3D Microspheres Display More Mature Hepatocyte Characteristics Than hESC-Heps in 2D Culture
3.4. Induction of Hepatocyte Functions in hESC-Heps and Antiviral Properties by 3D Microspheres
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forbes, S.J.; Gupta, S.; Dhawan, A. Cell therapy for liver disease: From liver transplantation to cell factory. J. Hepatol. 2015, 62, S157–S169. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.T.; Wang, Y.; Nyberg, S.L. Cell therapy in chronic liver disease. Curr. Opin. Gastroenterol. 2016, 32, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Behbahan, I.S.; Duan, Y.; Lam, A.; Khoobyari, S.; Ma, X.; Ahuja, T.P.; Zern, M.A. New approaches in the differentiation of human embryonic stem cells and induced pluripotent stem cells toward hepatocytes. Stem. Cell Rev. Rep. 2011, 7, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Siller, R.; Greenhough, S.; Naumovska, E.; Sullivan, G.J. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem. Cell Rep. 2015, 4, 939–952. [Google Scholar] [CrossRef]
- Asumda, F.Z.; Hatzistergos, K.E.; Dykxhoorn, D.M.; Jakubski, S.; Edwards, J.; Thomas, E.; Schiff, E.R. Differentiation of hepatocyte-like cells from human pluripotent stem cells using small molecules. Differentiation 2018, 101, 16–24. [Google Scholar] [CrossRef]
- Feng, S.; Wu, J.; Qiu, W.L.; Yang, L.; Deng, X.; Zhou, Y.; Chen, Y.; Li, X.; Yu, L.; Li, H.; et al. Large-scale Generation of Functional and Transplantable Hepatocytes and Cholangiocytes from Human Endoderm Stem Cells. Cell Rep. 2020, 33, 108455. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Chen, L.; Liu, T.; Li, Y.; Wang, Y.; Geng, Y. Efficient large-scale generation of functional hepatocytes from mouse embryonic stem cells grown in a rotating bioreactor with exogenous growth factors and hormones. Stem. Cell Res. Ther. 2013, 4, 145. [Google Scholar] [CrossRef]
- Gieseck, R.L., 3rd; Hannan, N.R.; Bort, R.; Hanley, N.A.; Drake, R.A.; Cameron, G.W.; Wynn, T.A.; Vallier, L. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS ONE 2014, 9, e86372. [Google Scholar] [CrossRef]
- Yao, R.; Wang, J.; Li, X.; Jung Jung, D.; Qi, H.; Kee, K.K.; Du, Y. Hepatic differentiation of human embryonic stem cells as microscaled multilayered colonies leading to enhanced homogeneity and maturation. Small 2014, 10, 4311–4323. [Google Scholar] [CrossRef]
- Zimmermann, H.; Shirley, S.G.; Zimmermann, U. Alginate-based encapsulation of cells: Past, present, and future. Curr. Diab. Rep. 2007, 7, 314–320. [Google Scholar] [CrossRef]
- Goren, A.; Dahan, N.; Goren, E.; Baruch, L.; Machluf, M. Encapsulated human mesenchymal stem cells: A unique hypoimmunogenic platform for long-term cellular therapy. FASEB J. 2010, 24, 22–31. [Google Scholar] [CrossRef]
- Paul, A.; Ge, Y.; Prakash, S.; Shum-Tim, D. Microencapsulated stem cells for tissue repairing: Implications in cell-based myocardial therapy. Regen. Med. 2009, 4, 733–745. [Google Scholar] [CrossRef]
- Dawson, E.; Mapili, G.; Erickson, K.; Taqvi, S.; Roy, K. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 2008, 60, 215–228. [Google Scholar] [CrossRef]
- Serra, M.; Correia, C.; Malpique, R.; Brito, C.; Jensen, J.; Bjorquist, P.; Carrondo, M.J.; Alves, P.M. Microencapsulation technology: A powerful tool for integrating expansion and cryopreservation of human embryonic stem cells. PLoS ONE 2011, 6, e23212. [Google Scholar] [CrossRef]
- Chayosumrit, M.; Tuch, B.; Sidhu, K. Alginate microcapsule for propagation and directed differentiation of hESCs to definitive endoderm. Biomaterials 2010, 31, 505–514. [Google Scholar] [CrossRef]
- Dean, S.K.; Yulyana, Y.; Williams, G.; Sidhu, K.S.; Tuch, B.E. Differentiation of encapsulated embryonic stem cells after transplantation. Transplantation 2006, 82, 1175–1184. [Google Scholar] [CrossRef]
- Addae, C.; Yi, X.; Gernapudi, R.; Cheng, H.; Musto, A.; Martinez-Ceballos, E. All-trans-retinoid acid induces the differentiation of encapsulated mouse embryonic stem cells into GABAergic neurons. Differentiation 2012, 83, 233–241. [Google Scholar] [CrossRef]
- Kim, J.; Sachdev, P.; Sidhu, K. Alginate microcapsule as a 3D platform for the efficient differentiation of human embryonic stem cells to dopamine neurons. Stem. Cell Res. 2013, 11, 978–989. [Google Scholar] [CrossRef]
- Bidarra, S.l.J.; Barrias, C.C.; Barbosa, M.r.A.; Soares, R.; Granja, P.L. Immobilization of human mesenchymal stem cells within RGD-grafted alginate microspheres and assessment of their angiogenic potential. Biomacromolecules 2010, 11, 1956–1964. [Google Scholar] [CrossRef]
- Raof, N.A.; Raja, W.K.; Castracane, J.; Xie, Y. Bioengineering embryonic stem cell microenvironments for exploring inhibitory effects on metastatic breast cancer cells. Biomaterials 2011, 32, 4130–4139. [Google Scholar] [CrossRef]
- Fang, S.; Qiu, Y.D.; Mao, L.; Shi, X.L.; Yu, D.C.; Ding, Y.T. Differentiation of embryoid-body cells derived from embryonic stem cells into hepatocytes in alginate microbeads in vitro. Acta Pharmacol. Sin. 2007, 28, 1924–1930. [Google Scholar] [CrossRef]
- Pasqua, M.; Pereira, U.; Messina, A.; de Lartigue, C.; Vigneron, P.; Dubart-Kupperschmitt, A.; Legallais, C. HepaRG Self-Assembled Spheroids in Alginate Beads Meet the Clinical Needs for Bioartificial Liver. Tissue Eng. Part A 2020, 26, 613–622. [Google Scholar] [CrossRef]
- Zhang, F.T.; Wan, H.J.; Li, M.H.; Ye, J.; Yin, M.J.; Huang, C.Q.; Yu, J. Transplantation of microencapsulated umbilical-cord-blood-derived hepatic-like cells for treatment of hepatic failure. World J. Gastroenterol. 2011, 17, 938–945. [Google Scholar] [CrossRef]
- Chang, T.M.S. Artificial Cells Containing Hepatocytes and/or Stem Cells in Regenerative Medicine. In Book Artificial Cells: Biotechnology, Nanotechnology, Blood Substitutes, Regenerative Medicine, Bioencapsulation, Cell/Stem Cell Therapy; World Scientific Publisher/Imperial College Press: Singapore, 2007; pp. 225–251. [Google Scholar]
- Chan, H.F.; Zhang, Y.; Leong, K.W. Efficient One-Step Production of Microencapsulated Hepatocyte Spheroids with Enhanced Functions. Small 2016, 12, 2720–2730. [Google Scholar] [CrossRef]
- Ishikawa, T.; Aibe, Y.; Matsuda, T.; Iwamoto, T.; Takami, T.; Sakaida, I. Plasma Glucose Level Is Predictive of Serum Ammonia Level After Retrograde Occlusion of Portosystemic Shunts. AJR Am. J. Roentgenol. 2017, 209, W169–W176. [Google Scholar] [CrossRef]
- Walker, V. Ammonia metabolism and hyperammonemic disorders. Adv. Clin. Chem. 2014, 67, 73–150. [Google Scholar] [CrossRef]
- Griffin, J.W.D.; Bradshaw, P.C. Effects of a high protein diet and liver disease in an in silico model of human ammonia metabolism. Theor. Biol. Med. Model. 2019, 16, 11. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Tran, N.M.; Dufresne, M.; Helle, F.; Hoffmann, T.W.; Francois, C.; Brochot, E.; Paullier, P.; Legallais, C.; Duverlie, G.; Castelain, S. Alginate hydrogel protects encapsulated hepatic HuH-7 cells against hepatitis C virus and other viral infections. PLoS ONE 2014, 9, e109969. [Google Scholar] [CrossRef]
- Pavelkova, M.; Vyslouzil, J.; Kubova, K.; Pavlokova, S.; Molinkova, D.; Celer, V.; Pechova, A.; Masek, J.; Vetchy, D. Assessment of Antimicrobic, Antivirotic and Cytotoxic Potential of Alginate Beads Cross-Linked by Bivalent Ions for Vaginal Administration. Pharmaceutics 2021, 13, 165. [Google Scholar] [CrossRef]
- Long, R.; Liu, Y.; Wang, S.; Ye, L.; He, P. Co-microencapsulation of BMSCs and mouse pancreatic beta cells for improving the efficacy of type I diabetes therapy. Int. J. Artif. Organs 2017, 40, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Bussche, L.; Harman, R.M.; Syracuse, B.A.; Plante, E.L.; Lu, Y.C.; Curtis, T.M.; Ma, M.; Van de Walle, G.R. Microencapsulated equine mesenchymal stromal cells promote cutaneous wound healing in vitro. Stem. Cell Res. Ther. 2015, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; McClarren, B.; Aijaz, A.; Chalaby, R.; Cook-Chennault, K.; Olabisi, R.M. The effect of low-magnitude, high-frequency vibration on poly(ethylene glycol)-microencapsulated mesenchymal stem cells. J. Tissue Eng. 2018, 9, 2041731418800101. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.P.; Mahou, R.; Morel, P.; Meyer, J.; Montanari, E.; Muller, Y.D.; Christofilopoulos, P.; Wandrey, C.; Gonelle-Gispert, C.; Buhler, L.H. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J. Hepatol. 2015, 62, 634–641. [Google Scholar] [CrossRef]
- Montanari, E.; Meier, R.P.H.; Mahou, R.; Seebach, J.D.; Wandrey, C.; Gerber-Lemaire, S.; Buhler, L.H.; Gonelle-Gispert, C. Multipotent mesenchymal stromal cells enhance insulin secretion from human islets via N-cadherin interaction and prolong function of transplanted encapsulated islets in mice. Stem. Cell Res. Ther. 2017, 8, 199. [Google Scholar] [CrossRef]
- Montanari, E.; Pimenta, J.; Szabo, L.; Noverraz, F.; Passemard, S.; Meier, R.P.H.; Meyer, J.; Sidibe, J.; Thomas, A.; Schuurman, H.J.; et al. Beneficial Effects of Human Mesenchymal Stromal Cells on Porcine Hepatocyte Viability and Albumin Secretion. J. Immunol. Res. 2018, 2018, 1078547. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, J.; Wen, X.; Wang, H.; Wang, Y.; Lin, Q.; Du, Z.; Duan, C.; Wang, C.; Wang, C. Transplantation of co-microencapsulated hepatocytes and HUVECs for treatment of fulminant hepatic failure. Int. J. Artif. Organs 2012, 35, 458–465. [Google Scholar] [CrossRef]
- Serrano-Aroca, A.; Ferrandis-Montesinos, M.; Wang, R. Antiviral Properties of Alginate-Based Biomaterials: Promising Antiviral Agents against SARS-CoV-2. ACS Appl. Bio Mater. 2021, 4, 5897–5907. [Google Scholar] [CrossRef]
- Jitraruch, S.; Dhawan, A.; Hughes, R.D.; Filippi, C.; Soong, D.; Philippeos, C.; Lehec, S.C.; Heaton, N.D.; Longhi, M.S.; Mitry, R.R. Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure. PLoS ONE 2014, 9, e113609. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J.H.; Ha, J.; Jeong, B.I.; Jung, Y.C.; Lee, G.S.; Woo, H.M.; Kang, B.J. Intra-Articular Injection of Alginate-Microencapsulated Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Osteoarthritis in Rabbits. Stem. Cells Int. 2018, 2018, 2791632. [Google Scholar] [CrossRef]
- Karpov, A.A.; Puzanov, M.V.; Ivkin, D.Y.; Krasnova, M.V.; Anikin, N.A.; Docshin, P.M.; Moiseeva, O.M.; Galagudza, M.M. Non-inferiority of microencapsulated mesenchymal stem cells to free cells in cardiac repair after myocardial infarction: A rationale for using paracrine factor(s) instead of cells. Int. J. Exp. Pathol. 2019, 100, 102–113. [Google Scholar] [CrossRef]
- Leslie, S.K.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Production of osteogenic and angiogenic factors by microencapsulated adipose stem cells varies with culture conditions. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1857–1867. [Google Scholar] [CrossRef]
- Montanucci, P.; Pescara, T.; Alunno, A.; Bistoni, O.; Basta, G.; Calafiore, R. Remission of hyperglycemia in spontaneously diabetic NOD mice upon transplant of microencapsulated human umbilical cord Wharton jelly-derived mesenchymal stem cells (hUCMS). Xenotransplantation 2019, 26, e12476. [Google Scholar] [CrossRef]
- Rebelo, S.P.; Costa, R.; Estrada, M.; Shevchenko, V.; Brito, C.; Alves, P.M. HepaRG microencapsulated spheroids in DMSO-free culture: Novel culturing approaches for enhanced xenobiotic and biosynthetic metabolism. Arch. Toxicol. 2015, 89, 1347–1358. [Google Scholar] [CrossRef]
- Liu, Z.C.; Chang, T.M. Transplantation of bioencapsulated bone marrow stem cells improves hepatic regeneration and survival of 90% hepatectomized rats: A preliminary report. Artif. Cells Blood Substit. Immobil. Biotechnol. 2005, 33, 405–410. [Google Scholar] [CrossRef]
- Liu, Z.C.; Chang, T.M. Transdifferentiation of bioencapsulated bone marrow cells into hepatocyte-like cells in the 90% hepatectomized rat model. Liver Transpl. 2006, 12, 566–572. [Google Scholar] [CrossRef]
- Baxter, M.; Withey, S.; Harrison, S.; Segeritz, C.P.; Zhang, F.; Atkinson-Dell, R.; Rowe, C.; Gerrard, D.T.; Sison-Young, R.; Jenkins, R.; et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J. Hepatol. 2015, 62, 581–589. [Google Scholar] [CrossRef]
- Takayama, K.; Kawabata, K.; Nagamoto, Y.; Kishimoto, K.; Tashiro, K.; Sakurai, F.; Tachibana, M.; Kanda, K.; Hayakawa, T.; Furue, M.K.; et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials 2013, 34, 1781–1789. [Google Scholar] [CrossRef]
- Twaroski, K.; Mallanna, S.K.; Jing, R.; DiFurio, F.; Urick, A.; Duncan, S.A. FGF2 mediates hepatic progenitor cell formation during human pluripotent stem cell differentiation by inducing the WNT antagonist NKD1. Genes Dev. 2015, 29, 2463–2474. [Google Scholar] [CrossRef]
- Pettinato, G.; Ramanathan, R.; Fisher, R.A.; Mangino, M.J.; Zhang, N.; Wen, X. Scalable Differentiation of Human iPSCs in a Multicellular Spheroid-based 3D Culture into Hepatocyte-like Cells through Direct Wnt/beta-catenin Pathway Inhibition. Sci. Rep. 2016, 6, 32888. [Google Scholar] [CrossRef]
- Mitani, S.; Takayama, K.; Nagamoto, Y.; Imagawa, K.; Sakurai, F.; Tachibana, M.; Sumazaki, R.; Mizuguchi, H. Human ESC/iPSC-Derived Hepatocyte-like Cells Achieve Zone-Specific Hepatic Properties by Modulation of WNT Signaling. Mol. Ther. 2017, 25, 1420–1433. [Google Scholar] [CrossRef]
- Mun, S.J.; Ryu, J.S.; Lee, M.O.; Son, Y.S.; Oh, S.J.; Cho, H.S.; Son, M.Y.; Kim, D.S.; Kim, S.J.; Yoo, H.J.; et al. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J. Hepatol. 2019, 71, 970–985. [Google Scholar] [CrossRef]
- Song, W.; Lu, Y.C.; Frankel, A.S.; An, D.; Schwartz, R.E.; Ma, M. Engraftment of human induced pluripotent stem cell-derived hepatocytes in immunocompetent mice via 3D co-aggregation and encapsulation. Sci. Rep. 2015, 5, 16884. [Google Scholar] [CrossRef]
- Rebelo, S.P.; Costa, R.; Silva, M.M.; Marcelino, P.; Brito, C.; Alves, P.M. Three-dimensional co-culture of human hepatocytes and mesenchymal stem cells: Improved functionality in long-term bioreactor cultures. J. Tissue Eng. Regen. Med. 2017, 11, 2034–2045. [Google Scholar] [CrossRef]
- Nagata, S.; Ozawa, F.; Nie, M.; Takeuchi, S. 3D culture of functional human iPSC-derived hepatocytes using a core-shell microfiber. PLoS ONE 2020, 15, e0234441. [Google Scholar] [CrossRef]
- Nebel, S.; Lux, M.; Kuth, S.; Bider, F.; Dietrich, W.; Egger, D.; Boccaccini, A.R.; Kasper, C. Alginate Core-Shell Capsules for 3D Cultivation of Adipose-Derived Mesenchymal Stem Cells. Bioengineering 2022, 9, 66. [Google Scholar] [CrossRef]
- Faulkner-Jones, A.; Fyfe, C.; Cornelissen, D.J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 044102. [Google Scholar] [CrossRef]
- Capone, S.H.; Dufresne, M.; Rechel, M.; Fleury, M.J.; Salsac, A.V.; Paullier, P.; Daujat-Chavanieu, M.; Legallais, C. Impact of alginate composition: From bead mechanical properties to encapsulated HepG2/C3A cell activities for in vivo implantation. PLoS ONE 2013, 8, e62032. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Zhou, X.; Liu, T.; Zhong, Z.; Zhou, Q.; Iqbal, W.; Xie, Q.; Wei, C.; Zhang, X.; Chang, T.M.S.; et al. Direct Differentiation of Human Embryonic Stem Cells to 3D Functional Hepatocyte-like Cells in Alginate Microencapsulation Sphere. Cells 2022, 11, 3134. https://doi.org/10.3390/cells11193134
Xie X, Zhou X, Liu T, Zhong Z, Zhou Q, Iqbal W, Xie Q, Wei C, Zhang X, Chang TMS, et al. Direct Differentiation of Human Embryonic Stem Cells to 3D Functional Hepatocyte-like Cells in Alginate Microencapsulation Sphere. Cells. 2022; 11(19):3134. https://doi.org/10.3390/cells11193134
Chicago/Turabian StyleXie, Xiaoling, Xiaoling Zhou, Tingdang Liu, Zhiqian Zhong, Qi Zhou, Waqas Iqbal, Qingdong Xie, Chiju Wei, Xin Zhang, Thomas Ming Swi Chang, and et al. 2022. "Direct Differentiation of Human Embryonic Stem Cells to 3D Functional Hepatocyte-like Cells in Alginate Microencapsulation Sphere" Cells 11, no. 19: 3134. https://doi.org/10.3390/cells11193134
APA StyleXie, X., Zhou, X., Liu, T., Zhong, Z., Zhou, Q., Iqbal, W., Xie, Q., Wei, C., Zhang, X., Chang, T. M. S., & Sun, P. (2022). Direct Differentiation of Human Embryonic Stem Cells to 3D Functional Hepatocyte-like Cells in Alginate Microencapsulation Sphere. Cells, 11(19), 3134. https://doi.org/10.3390/cells11193134





