Circulating miR-499a and miR-125b as Potential Predictors of Left Ventricular Ejection Fraction Improvement after Cardiac Resynchronization Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Echocardiographic Evaluation
2.3. Device Implantation
2.4. Blood Collection
2.5. RNA Extraction and miRNA Quantification
2.6. MicroRNA Pathway Analysis and Target Prediction
2.7. Statistical Analysis
3. Results
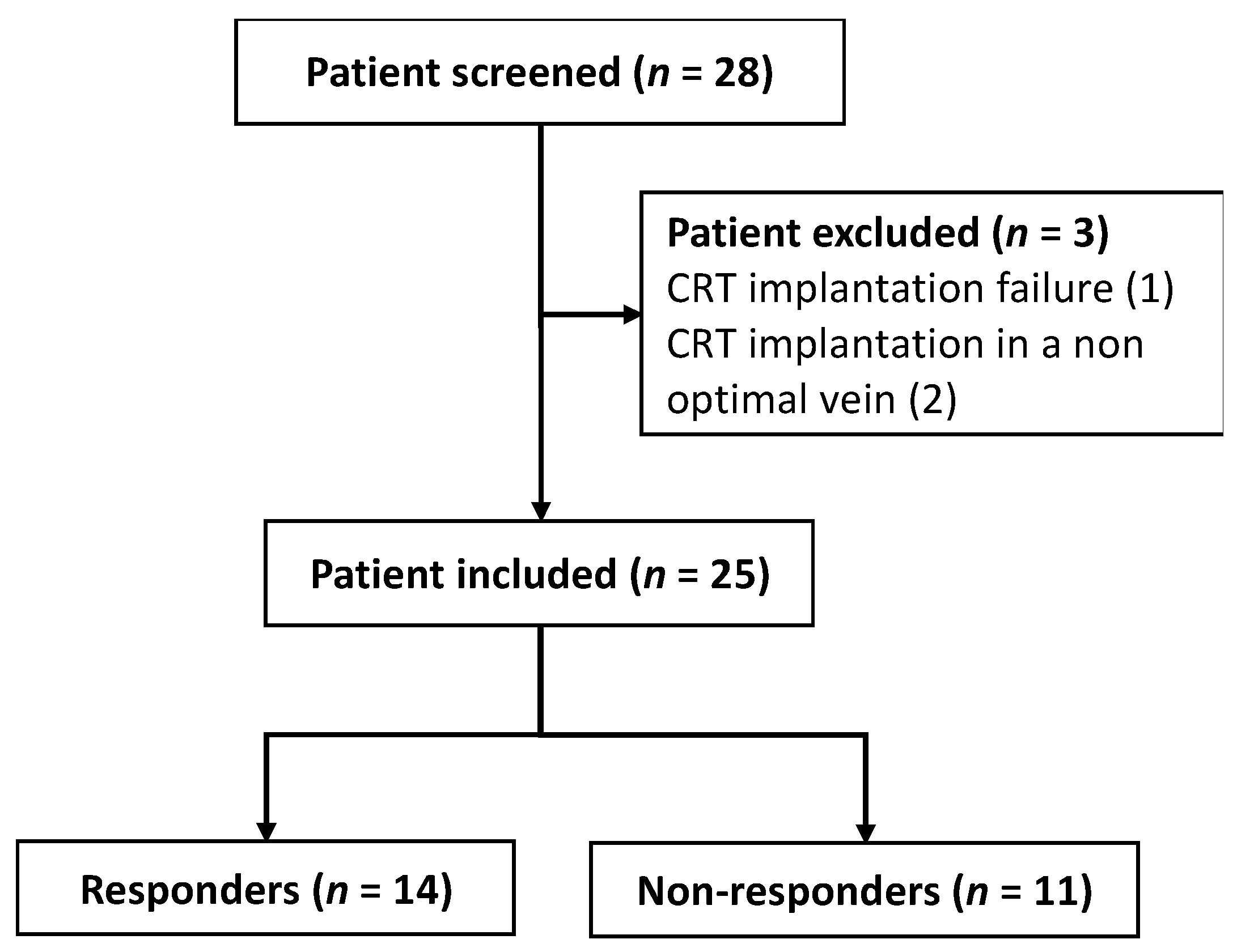
3.1. Study Population
3.2. Baseline Differences and Longitudinal Changes in miRNA Profiles after CRT
3.3. Follow-Up Changes in miRNA Profiles after CRT
3.4. MicroRNAs Predictive Value of CRT Response
3.5. MicroRNA Correlation between Echocardiographic and Clinical Parameters
3.6. Predictive Analysis of miR-499-5p and miR-125b-5p in CRT Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohn, J.N. Structural basis for heart failure: Ventricular remodeling and its pharmacological inhibition. Circulation 1995, 91, 2504–2507. [Google Scholar] [CrossRef]
- Verbeek, X.A.; Vernooy, K.; Peschar, M.; Cornelussen, R.N.; Prinzen, F.W. Intra-ventricular resynchronization for optimal left ventricular function during pacing in experimental left bundle branch block. J. Am. Coll. Cardiol. 2003, 42, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.-M.; Chau, E.; Sanderson, J.E.; Fan, K.; Tang, M.-O.; Fung, W.-H.; Lin, H.; Kong, S.-L.; Lam, Y.-M.; Hill, M.R.; et al. Tissue Doppler Echocardiographic Evidence of Reverse Remodeling and Improved Synchronicity by Simultaneously Delaying Regional Contraction After Biventricular Pacing Therapy in Heart Failure. Circulation 2002, 105, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Daubert, C.; Behar, N.; Martins, R.P.; Mabo, P.; Leclercq, C. Avoiding non-responders to cardiac resynchronization therapy: A practical guide. Eur. Heart J. 2016, 38, 1463–1472. [Google Scholar] [CrossRef]
- Lopez-Andrès, N.; Rossignol, P.; Iraqi, W.; Fay, R.; Nuée, J.; Ghio, S.; Cleland, J.G.; Zannad, F.; Lacolley, P. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: Insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur. J. Heart Fail. 2012, 14, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell. Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Köppel, T.; Jahantigh, M.N.; Lutgens, E.; et al. Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12-Dependent Vascular Protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laterza, O.F.; Lim, L.; Garrett-Engele, P.W.; Vlasakova, K.; Muniappa, N.; Tanaka, W.K.; Johnson, J.M.; Sina, J.F.; Fare, T.L.; Sistare, F.D.; et al. Plasma MicroRNAs as Sensitive and Specific Biomarkers of Tissue Injury. Clin. Chem. 2009, 55, 1977–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating MicroRNA-208b and MicroRNA-499 Reflect Myocardial Damage in Cardiovascular Disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef]
- Condorelli, G.; Latronico, M.V.G.; Cavarretta, E. MicroRNAs in cardiovascular diseases: Current knowledge and the road ahead. J. Am. Coll. Cardiol. 2014, 63, 2177–2187. [Google Scholar] [CrossRef] [Green Version]
- Castaldi, A.; Zaglia, T.; Di Mauro, V.; Carullo, P.; Viggiani, G.; Borile, G.; di Stefano, A.B.; Schiattarella, G.G.; Gualazzi, M.G.; Elia, L.; et al. MicroRNA-133 Modulates the β1-Adrenergic Receptor Transduction Cascade. Circ. Res. 2014, 115, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ransom, J.F.; Li, A.; Vedantham, V.; von Drehle, M.; Muth, A.N.; Tsuchihashi, T.; McManus, M.; Schwartz, R.J.; Srivastava, D. Dysregulation of Cardiogenesis, Cardiac Conduction, and Cell Cycle in Mice Lacking miRNA-1-2. Cell 2007, 129, 303–317. [Google Scholar] [CrossRef] [Green Version]
- Matkovich, S.J.; Wang, W.; Tu, Y.; Eschenbacher, W.H.; Dorn, L.E.; Condorelli, G.; Diwan, A.; Nerbonne, J.M.; Dorn, G.W. MicroRNA-133a Protects Against Myocardial Fibrosis and Modulates Electrical Repolarization Without Affecting Hypertrophy in Pressure-Overloaded Adult Hearts. Circ. Res. 2010, 106, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duisters, R.F.; Tijsen, A.J.; Schroen, B.; Leenders, J.J.; Lentink, V.; van der Made, I.; Herias, V.; van Leeuwen, R.E.; Schellings, M.W.; Barenbrug, P.; et al. miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 2009, 104, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.-L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef]
- Vogel, B.; Keller, A.; Frese, K.S.; Leidinger, P.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Kloos, W.; Backe, C.; Thanaraj, A.; Brefort, T.; et al. Multivariate miRNA signatures as biomarkers for non-ischaemic systolic heart failure. Eur. Heart J. 2013, 34, 2812–2823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viereck, J.; Thum, T. Circulating Noncoding RNAs as Biomarkers of Cardiovascular Disease and Injury. Circ. Res. 2017, 120, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Topkara, V.K.; Mann, D.L. Role of MicroRNAs in cardiac remodeling and heart failure. Cardiovasc. Drugs Ther. 2011, 25, 171–182. [Google Scholar] [CrossRef]
- Marques, F.Z.; Vizi, D.; Khammy, O.; Mariani, J.A.; Kaye, D.M. The transcardiac gradient of cardio-microRNAs in the failing heart. Eur. J. Heart Fail. 2016, 18, 1000–1008. [Google Scholar] [CrossRef] [Green Version]
- Gidlof, O.; Smith, J.G.; Miyazu, K.; Gilje, P.; Spencer, A.; Blomquist, S.; Erlinge, D. Circulating cardio-enriched microRNAs are associated with long-term prognosis following myocardial infarction. BMC Cardiovasc. Disord. 2013, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Ben-Zvi, I.; Volinsky, N.; Grosman-Rimon, L.; Haviv, I.; Rozen, G.; Andria, N.; Asulin, N.; Margalit, N.; Marai, I.; Amir, O. Cardiac-peripheral transvenous gradients of microRNA expression in systolic heart failure patients. ESC Heart Fail. 2020, 7, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Akat, K.M.; Moore-McGriff, D.; Morozov, P.; Brown, M.; Gogakos, T.; da Rosa, J.C.; Mihailovic, A.; Sauer, M.; Ji, R.; Ramarathnam, A.; et al. Comparative RNA-sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. Proc. Natl. Acad. Sci. USA 2014, 111, 11151–11156. [Google Scholar] [CrossRef] [Green Version]
- Marfella, R.; Di Filippo, C.; Potenza, N.; Sardu, C.; Rizzo, M.R.; Siniscalchi, M.; Musacchio, E.; Barbieri, M.; Mauro, C.; Mosca, N.; et al. Circulating microRNA changes in heart failure patients treated with cardiac resynchronization therapy: Responders vs. non-responders. Eur. J. Heart Fail. 2013, 15, 1277–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramani, R.; Vela, D.; Segura, A.; McNamara, D.; Lemster, B.; Samarendra, V.; Kormos, R.; Toyoda, Y.; Bermudez, C.; Frazier, O.; et al. A Micro-Ribonucleic Acid Signature Associated with Recovery From Assist Device Support in 2 Groups of Patients with Severe Heart Failure. J. Am. Coll. Cardiol. 2011, 58, 2270–2278. [Google Scholar] [CrossRef] [Green Version]
- Strauss, D.G.; Selvester, R.H.; Wagner, G.S. Defining Left Bundle Branch Block in the Era of Cardiac Resynchronization Therapy. Am. J. Cardiol. 2011, 107, 927–934. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Schulze, P.C. MicroRNAs in heart failure: Non-coding regulators of metabolic function. Biochim. Biophys. Acta 2016, 1862, 2276–2287. [Google Scholar] [CrossRef]
- Bie, Z.-D.; Sun, L.-Y.; Geng, C.-L.; Meng, Q.-G.; Lin, X.-J.; Wang, Y.-F.; Wang, X.-B.; Yang, J. MiR-125b regulates SFRP5 expression to promote growth and activation of cardiac fibroblasts. Cell Biol. Int. 2016, 40, 1224–1234. [Google Scholar] [CrossRef]
- Melman, Y.F.; Shah, R.; Danielson, K.; Xiao, J.; Simonson, B.; Barth, A.; Chakir, K.; Lewis, G.D.; Lavender, Z.; Truong, Q.A.; et al. Circulating MicroRNA-30d Is Associated with Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis. Circulation 2015, 131, 2202–2216. [Google Scholar] [CrossRef]
- van Rooij, E.; Quiat, D.; Johnson, B.A.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Kelm, R.J.; Olson, E.N. A Family of microRNAs Encoded by Myosin Genes Governs Myosin Expression and Muscle Performance. Dev. Cell 2009, 17, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Yue, B.; Lan, X.; Wang, Y.; Fang, X.; Ma, Y.; Bai, Y.; Qi, X.; Zhang, C.; Chen, H. MiR-499 regulates myoblast proliferation and differentiation by targeting transforming growth factor β receptor 1. J. Cell. Physiol. 2019, 234, 2523–2536. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Su, T.; Li, H.; Huang, Q.; Wu, D.; Yang, C.; Han, Z. Circulating miR-499 are novel and sensitive biomarker of acute myocardial infarction. J. Thorac. Dis. 2015, 7, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhu, J.-Q.; Zhang, J.-T.; Li, Q.; Li, Y.; He, J.; Qin, Y.-W.; Jing, Q. Circulating microRNA: A novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Soler-Botija, C.; Gálvez-Montón, C.; Bayes-Genis, A. Epigenetic Biomarkers in Cardiovascular Diseases. Front. Genet. 2019, 10, 950. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Rumsey, J.; Hazen, B.C.; Lai, L.; Leone, T.C.; Vega, R.B.; Xie, H.; Conley, K.E.; Auwerx, J.; Smith, S.R.; et al. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J. Clin. Investig. 2013, 123, 2564–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooren, F.C.; Viereck, J.; Krüger, K.; Thum, T. Circulating micrornas as potential biomarkers of aerobic exercise capacity. Am. J. Physiol. Circ. Physiol. 2014, 306, H557–H563. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandra, Y.; Chiesa, M.; Carena, M.C.; Beltrami, A.P.; Rizzo, P.; Buzzetti, M.; Ricci, V.; Ferrari, R.; Fucili, A.; Livi, U.; et al. Differential Role of Circulating microRNAs to Track Progression and Pre-Symptomatic Stage of Chronic Heart Failure: A Pilot Study. Biomedicines 2020, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.T.C.; Huang, Y.; Gilmore, J.; Srivastava, D. Elevated miR-499 Levels Blunt the Cardiac Stress Response. PLoS ONE 2011, 6, e19481. [Google Scholar] [CrossRef] [Green Version]
- Hromádka, M.; Černá, V.; Pešta, M.; Kučerová, A.; Jarkovský, J.; Rajdl, D.; Rokyta, R.; Moťovská, Z. Prognostic Value of MicroRNAs in Patients after Myocardial Infarction: A Substudy of PRAGUE-18. Dis. Markers 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.V.N.; Gupta, M.K.; Duan, Z.-H.; Surampudi, V.S.K.; Liu, C.-G.; Kotwal, A.; Moravec, C.S.; Starling, R.C.; Perez, D.M.; Sen, S.; et al. A unique microRNA profile in end-stage heart failure indicates alterations in specific cardiovascular signaling networks. PLoS ONE 2017, 12, e0170456. [Google Scholar] [CrossRef] [Green Version]
- Linna-Kuosmanen, S.; Hartikainen, J.; Hippeläinen, M.; Kokki, H.; Levonen, A.-L.; Tavi, P. MicroRNA Profiling of Pericardial Fluid Samples from Patients with Heart Failure. PLoS ONE 2015, 10, e0119646. [Google Scholar] [CrossRef] [Green Version]
- Busk, P.K.; Cirera, S. MicroRNA profiling in early hypertrophic growth of the left ventricle in rats. Biochem. Biophys. Res. Commun. 2010, 396, 989–993. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.; Lu, S.; Yan, L.; Hu, F.; Wang, Z.; Cheng, B. Androgen receptor regulates cardiac fibrosis in mice with experimental autoimmune myocarditis by increasing microRNA-125b expression. Biochem. Biophys. Res. Commun. 2018, 506, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, V.; Rai, R.; Place, A.T.; Murphy, S.B.; Verma, S.K.; Ghosh, A.K.; Vaughan, D.E. MiR-125b Is Critical for Fibroblast-to-Myofibroblast Transition and Cardiac Fibrosis. Circulation 2016, 133, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Aoki, T.; Fukumoto, Y.; Sugimura, K.; Oikawa, M.; Satoh, K.; Nakano, M.; Nakayama, M.; Shimokawa, H. Prognostic Impact of Myocardial Interstitial Fibrosis in Non-Ischemic Heart Failure—Comparison Between Preserved and Reduced Ejection Fraction Heart Failure. Circ. J. 2011, 75, 2605–2613. [Google Scholar] [CrossRef] [Green Version]
- Massoullié, G.; Sapin, V.; Ploux, S.; Rossignol, P.; Mulliez, A.; Jean, F.; Marie, P.-Y.; Merlin, C.; Pereira, B.; Andronache, M.; et al. Low fibrosis biomarker levels predict cardiac resynchronization therapy response. Sci. Rep. 2019, 9, 6103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, H.; Yang, J.-J.; Shi, K.-H.; Li, J. Wnt signaling pathway in cardiac fibrosis: New insights and directions. Metabolism 2016, 65, 30–40. [Google Scholar] [CrossRef]
- Ma, Y.; Zou, H.; Zhu, X.-X.; Pang, J.; Xu, Q.; Jin, Q.-Y.; Ding, Y.-H.; Zhou, B.; Huang, D.-S. Transforming growth factor β: A potential biomarker and therapeutic target of ventricular remodeling. Oncotarget 2017, 8, 53780–53790. [Google Scholar] [CrossRef] [Green Version]
- Kishore, R.; Verma, S.K. Roles of STATs signaling in cardiovascular diseases. JAK-STAT 2012, 1, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Muslin, A.J. MAPK signalling in cardiovascular health and disease: Molecular mechanisms and therapeutic targets. Clin. Sci. 2008, 115, 203–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporali, A.; Emanueli, C. Cardiovascular Actions of Neurotrophins. Physiol. Rev. 2009, 89, 279–308. [Google Scholar] [CrossRef] [Green Version]
- Van Empel, V.P.; Bertrand, A.T.; Hofstra, L.; Crijns, H.J.; Doevendans, P.A.; De Windt, L.J. Myocyte apoptosis in heart failure. Cardiovasc. Res. 2005, 67, 21–29. [Google Scholar] [CrossRef]
- Sanchez-Soria, P.; Camenisch, T.D. ErbB signaling in cardiac development and disease. Semin. Cell Dev. Biol. 2010, 21, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Riehle, C.; Abel, E.D. Insulin Signaling and Heart Failure. Circ. Res. 2016, 118, 1151–1169. [Google Scholar] [CrossRef]
- Smekal, A.; Vaclavik, J. Adipokines and cardiovascular disease: A comprehensive review. Biomed. Pap. 2017, 161, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Total (n = 25) | Responders (n = 14) | Non-responders (n = 11) | |||
|---|---|---|---|---|---|
| Baseline | 6 m | Baseline | 6 m | ||
| Age, years | 77 (±8) | 75 (±5) | |||
| Sex, male/female | 15/10 | 9/5 | 6/5 | ||
| BMI, kg/m2 | 28.2 ± 0.83 | 28.3 ± 5.0 | 28.5 ± 5.6 | 28 ±3.18 | 28.8 ± 3.51 |
| Systolic blood pressure | 111 ± 15.62 | 115 ±19.42 | 111 ± 7.85 | ||
| Heart rate | 73 ± 19.63 | 77 ±22 | 68 ±17 | ||
| Dyslipidaemia % (n) | 44 (11) | 29 (4) | 64 (7) | ||
| Diabetes, % (n) | 36 (9) | 43 (6) | 27 (3) | ||
| Hypertension, % (n) | 68 (17) | 71 (10) | 64 (7) | ||
| COPD (n) | 25 (3) | 7 (1) | 18 (2) | ||
| GFR mL/min | 55 ± 22 | 56 ± 27 | 58 ± 21 | 53 ± 15 | 53 ± 24 |
| NT-proBNP, pg/mL | 4194 ± 4083 | 3361 ± 2697 | 1744 ± 1481 * | 5254 ± 5325 # | 12,914 ± 29,691 * |
| NYHA class, n | 2.64 ± 0.64 | 2.43 ± 0.51 | 1.64 ± 0.50 * | 2.91 ± 0.70 | 2.6 ± 0.97 |
| Aetiology, % (n) | |||||
| Ischaemic | 21.43 (3) | 18.18 (2) | |||
| Non-ischaemic | 78.57 (11) | 81.82 (9) | |||
| Medical treatment, % (n) | |||||
| ACEIs/ARBs | 56 (14) | 50 (7) | 36 (5) | 64 (7) | 45 (5) |
| Beta-blockers | 84 (21) | 86 (12) | 93 (13) | 82 (9) | 91 (10) |
| ARNI | 40 (10) | 50 (7) | 57 (8) | 27 (3) | 36 (4) |
| Spironolactone | 80 (20) | 79 (11) | 79 (11) | 82 (9) | 82 (9) |
| Diuretics | 88 (22) | 79 (11) | 64 (9) | 100 (11) | 64 (7) |
| Digoxin | 20 (5) | 21(3) | 18 (2) | ||
| Statin | 56 (14) | 50 (7) | 64 (7) | ||
| Echocardiographic data | |||||
| LVEDD (mm) | 64 ± 10 | 65 ± 9 | 57 ± 7 | 63 ± 12 | 60 ± 9 |
| LVESD (mm) | 57 ± 12 | 59 ± 10 | 48 ± 8 | 55 ± 13 | 52 ± 10 |
| LVEDVi, mL/m2 | 177 ± 87 | 187 ± 101 | 99 ± 44 * | 164 ± 68 | 171 ± 74 |
| LVESVi, mL/m2 | 129 ± 76 | 138 ± 87 | 59 ± 38 * | 116 ± 61 | 119 ± 70 |
| LVEF | 31 ± 9 | 30 ± 8 | 46 ± 12 * | 32 ± 10 | 35 ± 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moscoso, I.; Cebro-Márquez, M.; Martínez-Gómez, Á.; Abou-Jokh, C.; Martínez-Monzonís, M.A.; Martínez-Sande, J.L.; González-Melchor, L.; García-Seara, J.; Fernández-López, X.A.; Moraña-Fernández, S.; et al. Circulating miR-499a and miR-125b as Potential Predictors of Left Ventricular Ejection Fraction Improvement after Cardiac Resynchronization Therapy. Cells 2022, 11, 271. https://doi.org/10.3390/cells11020271
Moscoso I, Cebro-Márquez M, Martínez-Gómez Á, Abou-Jokh C, Martínez-Monzonís MA, Martínez-Sande JL, González-Melchor L, García-Seara J, Fernández-López XA, Moraña-Fernández S, et al. Circulating miR-499a and miR-125b as Potential Predictors of Left Ventricular Ejection Fraction Improvement after Cardiac Resynchronization Therapy. Cells. 2022; 11(2):271. https://doi.org/10.3390/cells11020271
Chicago/Turabian StyleMoscoso, Isabel, María Cebro-Márquez, Álvaro Martínez-Gómez, Charigan Abou-Jokh, María Amparo Martínez-Monzonís, José Luis Martínez-Sande, Laila González-Melchor, Javier García-Seara, Xesús Alberte Fernández-López, Sandra Moraña-Fernández, and et al. 2022. "Circulating miR-499a and miR-125b as Potential Predictors of Left Ventricular Ejection Fraction Improvement after Cardiac Resynchronization Therapy" Cells 11, no. 2: 271. https://doi.org/10.3390/cells11020271






