Reduction in SOCE and Associated Aggregation in Platelets from Mice with Platelet-Specific Deletion of Orai1
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Reagents
2.3. Isolation of Washed Murine Platelets and Platelet Aggregation
2.4. Ca2+ Imaging of Mouse Platelets
2.5. Statistical Analysis
3. Results
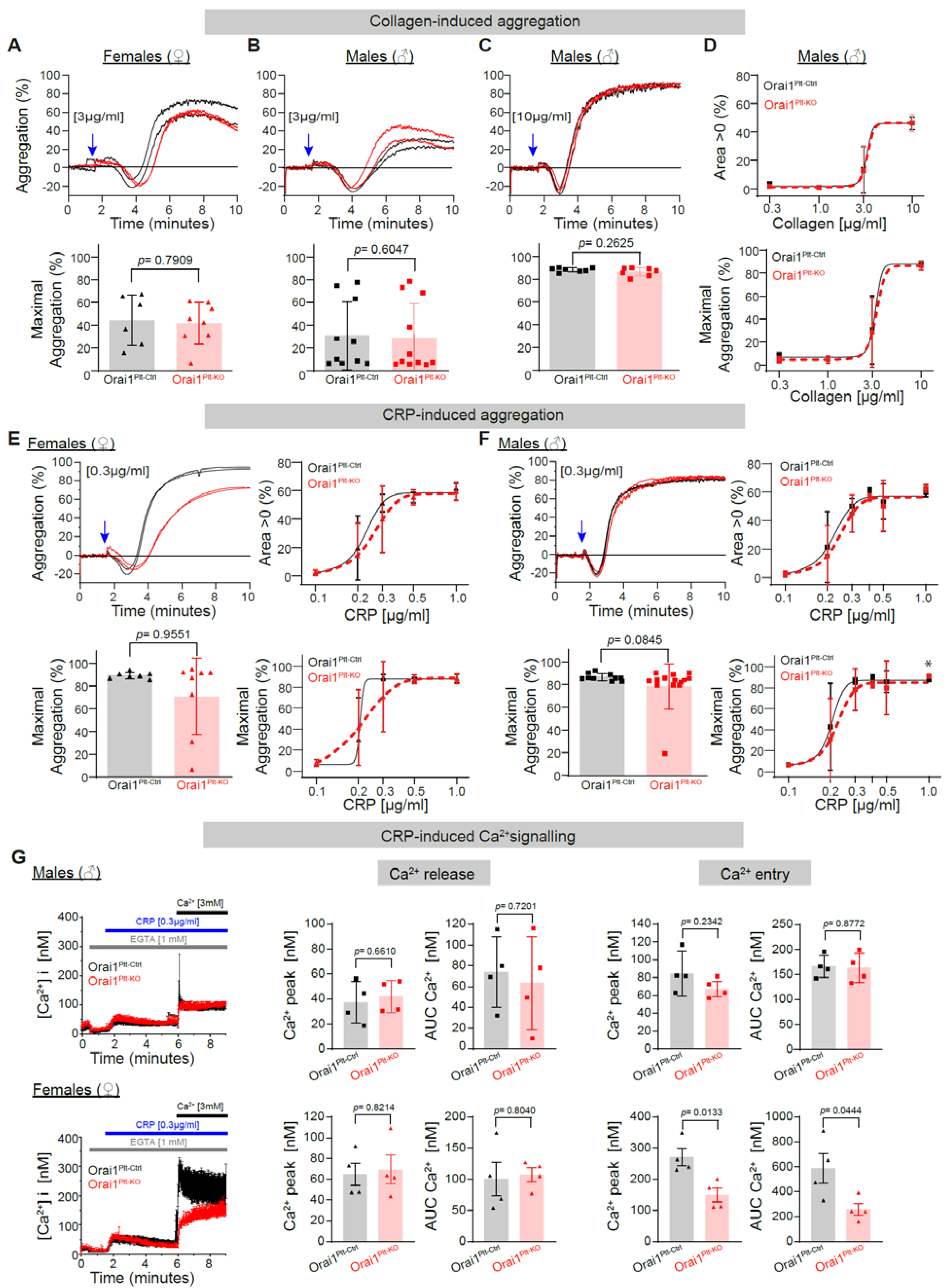
3.1. Analysis of the Role of Orai1 Proteins in Platelet Function Downstream of the Glycoprotein VI (GPVI) Receptor and the PLCγ Axis
3.2. Analysis of ORAI1’s Role in Platelet Function Downstream of G-Protein-Coupled Receptors and PLCβ Axis
3.3. Reduction in Store Operated Calcium Entry (SOCE) and Associated Platelet Aggregation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilius, B. Store-Operated Ca2+ Entry Channels: Still Elusive! Sci. STKE 2004, 2004, pe36. [Google Scholar] [CrossRef]
- Parekh, A.B.; Putney, J.W., Jr. Store-Operated Calcium Channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.S. The molecular choreography of a store-operated calcium channel. Nature 2007, 446, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Gudermann, T.; Hofmann, T.; Mederos y Schnitzler, M.; Dietrich, A. Activation, subunit composition and physiological relevance of DAG-sensitive TRPC proteins. Novartis Found. Symp. 2004, 58, 103–118. [Google Scholar]
- Trebak, M.; Lemonnier, L.; Smyth, J.T.; Vazquez, G.; Putney, J.W. Phospholipase C-Coupled Receptors and Activation of TRPC Channels. Handb. Exp. Pharmacol. 2007, 593–614. [Google Scholar] [CrossRef]
- Gudermann, T.; Schnitzler, M.M.Y.; Dietrich, A. Receptor-Operated Cation Entry—More than Esoteric Terminology? Sci. STKE 2004, 2004, pe35. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, T.J. Receptor-Activated Calcium Entry Channels—Who Does What, and When? Sci. STKE 2004, 2004, pe40. [Google Scholar] [CrossRef] [PubMed]
- Rosado, J. Acidic Ca(2+) stores in platelets. Cell Calcium 2011, 50, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Fernández, D.I.; Kuijpers, M.J.E.; Heemskerk, J.W.M. Platelet calcium signaling by G-protein coupled and ITAM-linked receptors regulating anoctamin-6 and procoagulant activity. Platelets 2020, 32, 863–871. [Google Scholar] [CrossRef]
- Munzer, P.; Borst, O. CRACking the Molecular Regulatory Mechanism of SOCE during Platelet Activation in Thrombo-Occlusive Diseases. Cells 2022, 11, 619. [Google Scholar] [CrossRef]
- Clemetson, J.M.; Clemetson, K.J. Platelet Collagen Receptors. Thromb. Haemost. 2001, 86, 189–197. [Google Scholar] [CrossRef]
- Heemskerk, J.W.; Kuijpers, M.J.; Munnix, I.C.; Siljander, P.R. Platelet Collagen Receptors and Coagulation. A Characteristic Platelet Response as Possible Target for Antithrombotic Treatment. Trends Cardiovasc. Med. 2005, 15, 86–92. [Google Scholar] [CrossRef]
- Jung, S.M.; Moroi, M. Platelet Glycoprotein VI. Adv. Exp. Med. Biol. 2008, 640, 53–63. [Google Scholar] [CrossRef]
- Scharf, R.E. Platelet Signaling in Primary Haemostasis and Arterial Thrombus Formation: Part 1. Hamostaseologie 2018, 38, 203–210. [Google Scholar] [CrossRef]
- Borst, O.; Gawaz, M. Glycoprotein VI-novel target in antiplatelet medication. Pharmacol. Ther. 2020, 217, 107630. [Google Scholar] [CrossRef]
- Vilahur, G.; Gutiérrez, M.; Arzanauskaite, M.; Mendieta, G.; Ben-Aicha, S.; Badimon, L. Intracellular platelet signalling as a target for drug development. Vasc. Pharmacol. 2018, 111, 22–25. [Google Scholar] [CrossRef]
- Offermanns, S. Activation of Platelet Function Through G Protein–Coupled Receptors. Circ. Res. 2006, 99, 1293–1304. [Google Scholar] [CrossRef]
- Abrams, C.S. Intracellular signaling in platelets. Curr. Opin. Hematol. 2005, 12, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, W.; Stefanini, L. Novel molecules in calcium signaling in platelets. J. Thromb. Haemost. 2009, 7, 187–190. [Google Scholar] [CrossRef]
- Rivera, J.; Lozano, M.L.; Navarro-Nuñez, L.; Vicente, V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica 2009, 94, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Mammadova-Bach, E.; Nagy, M.; Heemskerk, J.W.; Nieswandt, B.; Braun, A. Store-operated calcium entry in thrombosis and thrombo-inflammation. Cell Calcium 2018, 77, 39–48. [Google Scholar] [CrossRef]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.-H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef]
- Prakriya, M.; Feske, S.; Gwack, Y.; Srikanth, S.; Rao, A.; Hogan, P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature 2006, 443, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Vig, M.; DeHaven, W.I.; Bird, G.S.; Billingsley, J.M.; Wang, H.; Rao, P.E.; Hutchings, A.B.; Jouvin, M.-H.; Putney, J.; Kinet, J.-P. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release–activated calcium channels. Nat. Immunol. 2007, 9, 89–96. [Google Scholar] [CrossRef]
- Zhang, S.L.; Yeromin, A.V.; Zhang, X.H.-F.; Yu, Y.; Safrina, O.; Penna, A.; Roos, J.; Stauderman, K.A.; Cahalan, M.D. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci. USA 2006, 103, 9357–9362. [Google Scholar] [CrossRef]
- Gross, S.A.; Wissenbach, U.; Philipp, S.E.; Freichel, M.; Cavalié, A.; Flockerzi, V. Murine ORAI2 Splice Variants Form Functional Ca2+ Release-activated Ca2+ (CRAC) Channels. J. Biol. Chem. 2007, 282, 19375–19384. [Google Scholar] [CrossRef]
- Hou, X.; Pedi, L.; Diver, M.M.; Long, S.B. Crystal Structure of the Calcium Release-Activated Calcium Channel Orai. Science 2012, 338, 1308–1313. [Google Scholar] [CrossRef]
- Vaeth, M.; Yang, J.; Yamashita, M.; Zee, I.; Eckstein, M.; Knosp, C.; Kaufmann, U.; Jani, P.K.; Lacruz, R.S.; Flockerzi, V.; et al. ORAI2 modulates store-operated calcium entry and T cell-mediated immunity. Nat. Commun. 2017, 8, 14714. [Google Scholar] [CrossRef]
- Tolhurst, G.; Carter, R.N.; Amisten, S.; Holdich, J.P.; Erlinge, D.; Mahaut-Smith, M.P. Expression profiling and electrophysiological studies suggest a major role for Orai1 in the store-operated Ca2+ influx pathway of platelets and megakaryocytes. Platelets 2008, 19, 308–313. [Google Scholar] [CrossRef][Green Version]
- van Kruchten, R.; Braun, A.; Feijge, M.A.H.; Kuijpers, M.J.E.; Rivera-Galdos, R.; Kraft, P.; Stoll, G.; Kleinschnitz, C.; Bevers, E.M.; Nieswandt, B.; et al. Antithrombotic Potential of Blockers of Store-Operated Calcium Channels in Platelets. Arter. Thromb. Vasc. Biol. 2012, 32, 1717–1723. [Google Scholar] [CrossRef]
- Berna-Erro, A.; Jardín, I.; Smani, T.; Rosado, J.A. Regulation of Platelet Function by Orai, STIM and TRP. In Calcium Entry Pathways in Non-excitable Cells; Springer: Cham, Switzerland, 2016; Volume 898, pp. 157–181. [Google Scholar] [CrossRef]
- Jardin, I.; Lopez, J.J.; Salido, G.M.; Rosado, J. Orai1 Mediates the Interaction between STIM1 and hTRPC1 and Regulates the Mode of Activation of hTRPC1-forming Ca2+ Channels. J. Biol. Chem. 2008, 283, 25296–25304. [Google Scholar] [CrossRef]
- Galán, C.; Zbidi, H.; Bartegi, A.; Salido, G.; Rosado, J. STIM1, Orai1 and hTRPC1 are important for thrombin- and ADP-induced aggregation in human platelets. Arch. Biochem. Biophys. 2009, 490, 137–144. [Google Scholar] [CrossRef]
- Zbidi, H.; Jardin, I.; Woodard, G.E.; Lopez, J.J.; Berna-Erro, A.; Salido, G.M.; Rosado, J.A. STIM1 and STIM2 Are Located in the Acidic Ca2+ Stores and Associates with Orai1 upon Depletion of the Acidic Stores in Human Platelets. J. Biol. Chem. 2011, 286, 12257–12270. [Google Scholar] [CrossRef]
- Lopez, J.J.; Albarrán, L.; Jardín, I.; Sanchez-Collado, J.; Redondo, P.C.; Bermejo, N.; Bobe, R.; Smani, T.; Rosado, J.A. Filamin A Modulates Store-Operated Ca2+ Entry by Regulating STIM1 (Stromal Interaction Molecule 1)–Orai1 Association in Human Platelets. Arter. Thromb. Vasc. Biol. 2018, 38, 386–397. [Google Scholar] [CrossRef]
- Bergmeier, W.; Oh-Hora, M.; McCarl, C.-A.; Roden, R.C.; Bray, P.F.; Feske, S. R93W mutation in Orai1 causes impaired calcium influx in platelets. Blood 2009, 113, 675–678. [Google Scholar] [CrossRef]
- Braun, A.; Varga-Szabo, D.; Kleinschnitz, C.; Pleines, I.; Bender, M.; Austinat, M.; Bösl, M.; Stoll, G.; Nieswandt, B. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood 2009, 113, 2056–2063. [Google Scholar] [CrossRef]
- Gilio, K.; van Kruchten, R.; Braun, A.; Berna-Erro, A.; Feijge, M.A.H.; Stegner, D.; van der Meijden, P.E.J.; Kuijpers, M.J.E.; Varga-Szabo, D.; Heemskerk, J.W.M.; et al. Roles of Platelet STIM1 and Orai1 in Glycoprotein VI- and Thrombin-dependent Procoagulant Activity and Thrombus Formation. J. Biol. Chem. 2010, 285, 23629–23638. [Google Scholar] [CrossRef]
- Chen, W.; Thielmann, I.; Gupta, S.; Subramanian, H.; Stegner, D.; van Kruchten, R.; Dietrich, A.; Gambaryan, S.; Heemskerk, J.W.M.; Hermanns, H.M.; et al. Orai1-induced store-operated Ca2+ entry enhances phospholipase activity and modulates canonical transient receptor potential channel 6 function in murine platelets. J. Thromb. Haemost. 2014, 12, 528–539. [Google Scholar] [CrossRef]
- Golde, W.T.; Gollobin, P.; Rodriguez, L.L. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim. 2005, 34, 39–43. [Google Scholar] [CrossRef]
- Ahuja, M.; Schwartz, D.; Tandon, M.; Son, A.; Zeng, M.; Swaim, W.; Eckhaus, M.; Hoffman, V.; Cui, Y.; Xiao, B.; et al. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab. 2017, 25, 635–646. [Google Scholar] [CrossRef]
- Harper, M.T.; Londoño, J.E.C.; Quick, K.; Londoño, J.C.; Flockerzi, V.; Philipp, S.E.; Birnbaumer, L.; Freichel, M.; Poole, A.W. Transient Receptor Potential Channels Function as a Coincidence Signal Detector Mediating Phosphatidylserine Exposure. Sci. Signal. 2013, 6, ra50. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Vorndran, C.; Minta, A.; Poenie, M. New fluorescent calcium indicators designed for cytosolic retention or measuring calcium near membranes. Biophys. J. 1995, 69, 2112–2124. [Google Scholar] [CrossRef][Green Version]
- Schoenmakers, T.J.; Visser, G.J.; Flik, G.; Theuvenet, A.P. Theuvenet, CHELATOR: An improved method for computing metal ion concentrations in physiological solutions. Biotechniques 1992, 12, 870–876. [Google Scholar]
- Gwack, Y.; Srikanth, S.; Oh-Hora, M.; Hogan, P.G.; Lamperti, E.D.; Yamashita, M.; Gelinas, C.; Neems, D.S.; Sasaki, Y.; Feske, S.; et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell. Biol. 2008, 28, 5209–5222. [Google Scholar] [CrossRef]
- Tsvilovskyy, V.; Solís-López, A.; Schumacher, D.; Medert, R.; Roers, A.; Kriebs, U.; Freichel, M. Deletion of Orai2 augments endogenous CRAC currents and degranulation in mast cells leading to enhanced anaphylaxis. Cell Calcium 2018, 71, 24–33. [Google Scholar] [CrossRef]
- Wettschureck, N.; Offermanns, S. Mammalian G Proteins and Their Cell Type Specific Functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef]
- Lanza, F.; Beretz, A.; Stierle, A.; Hanau, D.; Kubina, M.; Cazenave, J.P. Epinephrine potentiates human platelet activation but is not an aggregating agent. Am. J. Physiol. Circ. Physiol. 1988, 255, H1276–H1288. [Google Scholar] [CrossRef]
- Yun-Choi, H.S.; Park, K.M.; Pyo, M.K. Epinephrine induced platelet aggregation in rat platelet-rich plasma. Thromb. Res. 2000, 100, 511–518. [Google Scholar] [CrossRef]
- Pozgajova, M.; Sachs, U.J.H.; Hein, L.; Nieswandt, B. Reduced thrombus stability in mice lacking the α2A-adrenergic receptor. Blood 2006, 108, 510–514. [Google Scholar] [CrossRef]
- Varga-Szabo, D.; Braun, A.; Nieswandt, B. STIM and Orai in platelet function. Cell Calcium 2011, 50, 270–278. [Google Scholar] [CrossRef]
- Ambily, A.; Kaiser, W.; Pierro, C.; Chamberlain, E.; Li, Z.; Jones, C.; Kassouf, N.; Gibbins, J.; Authi, K. The role of plasma membrane STIM1 and Ca2+ entry in platelet aggregation. STIM1 binds to novel proteins in human platelets. Cell. Signal. 2013, 26, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Derler, I.; Schindl, R.; Fritsch, R.; Heftberger, P.; Riedl, M.C.; Begg, M.; House, D.; Romanin, C. The action of selective CRAC channel blockers is affected by the Orai pore geometry. Cell Calcium 2013, 53, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Boulaftali, Y.; Greene, T.K.; Ouellette, T.D.; Poncz, M.; Feske, S.; Bergmeier, W. Relative contributions of stromal interaction molecule 1 and CalDAG-GEFI to calcium-dependent platelet activation and thrombosis. J. Thromb. Haemost. 2011, 9, 2077–2086. [Google Scholar] [CrossRef]
- Peters, L.L.; Cheever, E.M.; Ellis, H.R.; Magnani, P.A.; Svenson, K.L.; Von Smith, R.; Bogue, M.A. Large-scale, high-throughput screening for coagulation and hematologic phenotypes in mice*. Physiol. Genom. 2002, 11, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.H.; Hong, S.Y.; Larrucea, S.; Zhang, W.; Li, T.T.; López, J.A.; Bray, P.F. Platelets of Female Mice Are Intrinsically More Sensitive to Agonists than Are Platelets of Males. Arter. Thromb. Vasc. Biol. 2004, 24, 376–381. [Google Scholar] [CrossRef]
- Berna-Erro, A.; Galan, C.; Dionisio, N.; Gomez, L.J.; Salido, G.M.; Rosado, J.A. Capacitative and non-capacitative signaling complexes in human platelets. Biochim. Biophys. Acta 2012, 1823, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W. Forms and functions of store-operated calcium entry mediators, STIM and Orai. Adv. Biol. Regul. 2018, 68, 88–96. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Ottenheijm, R.; Worley, P.; Freichel, M.; Camacho Londoño, J.E. Reduction in SOCE and Associated Aggregation in Platelets from Mice with Platelet-Specific Deletion of Orai1. Cells 2022, 11, 3225. https://doi.org/10.3390/cells11203225
Yang L, Ottenheijm R, Worley P, Freichel M, Camacho Londoño JE. Reduction in SOCE and Associated Aggregation in Platelets from Mice with Platelet-Specific Deletion of Orai1. Cells. 2022; 11(20):3225. https://doi.org/10.3390/cells11203225
Chicago/Turabian StyleYang, Linlin, Roger Ottenheijm, Paul Worley, Marc Freichel, and Juan E. Camacho Londoño. 2022. "Reduction in SOCE and Associated Aggregation in Platelets from Mice with Platelet-Specific Deletion of Orai1" Cells 11, no. 20: 3225. https://doi.org/10.3390/cells11203225
APA StyleYang, L., Ottenheijm, R., Worley, P., Freichel, M., & Camacho Londoño, J. E. (2022). Reduction in SOCE and Associated Aggregation in Platelets from Mice with Platelet-Specific Deletion of Orai1. Cells, 11(20), 3225. https://doi.org/10.3390/cells11203225







