The SOCE Machinery: An Unbalanced Knowledge between Left and Right Ventricular Pathophysiology
Abstract
1. Introduction
2. Ca2+ Signaling in the Left and Right Ventricles
3. Generality about the SOCE Machinery
4. The SOCE in the Ventricles
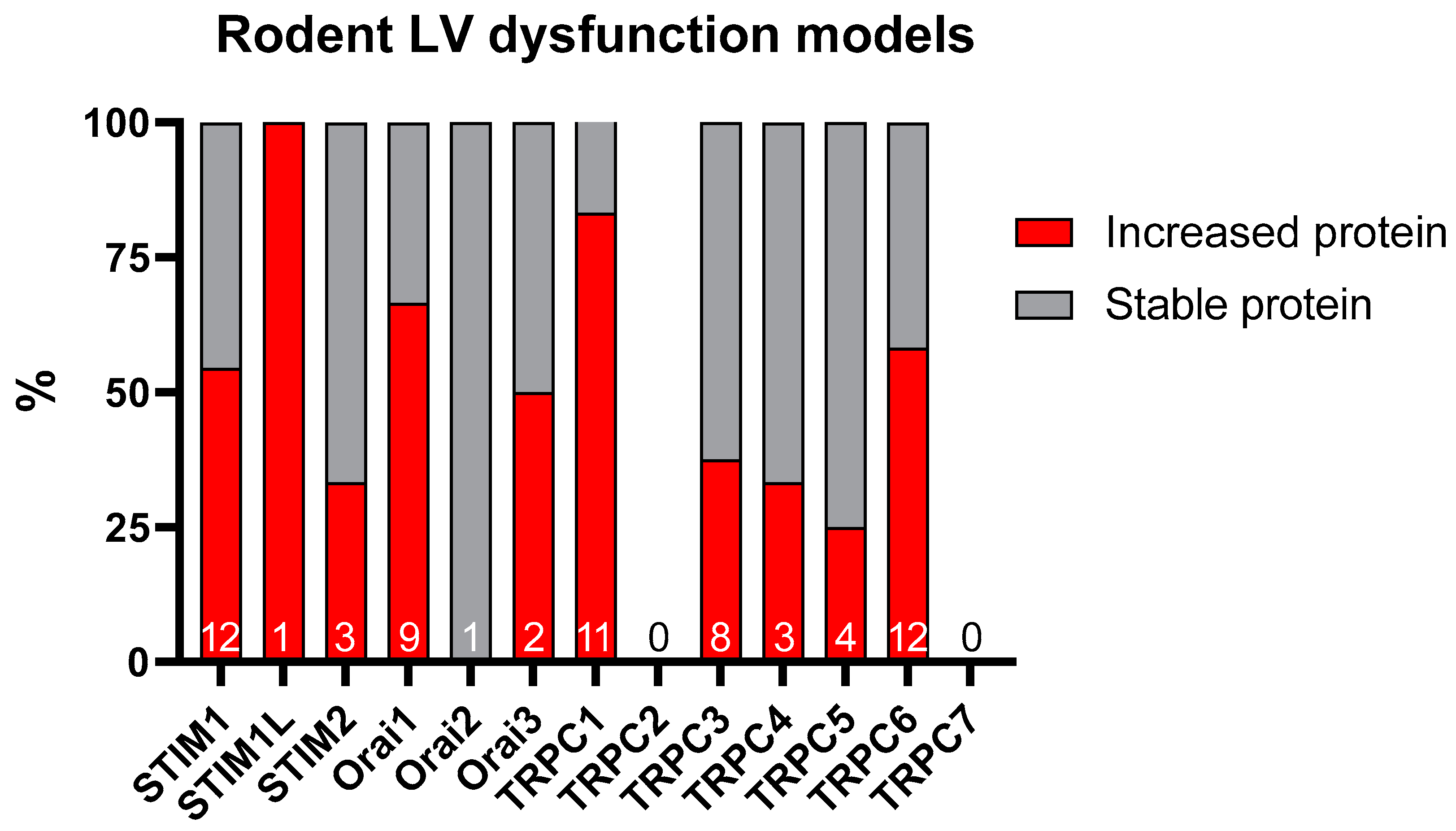
5. The SOCE in the Adverse LV Remodeling
5.1. Role of STIM1
5.2. Role of Orai Channels
5.3. Role of TRPC Channels
| SOCE Molecules | Expression Level | SOCE Function | Species | Induction of the LV Remodeling | References |
|---|---|---|---|---|---|
| STIM1L | ↑ (mRNA/Protein) | ↑ SOCE | Mouse | Thoracic aortic banding (3 weeks) | [46] |
| STIM1 | ↔ (Protein) | ||||
| STIM2 | ↔ (mRNA) | ||||
| TRPC6 | ↑ (mRNA) | ND | Mouse | Thoracic aortic banding (3 weeks)/Calcineurin Tg mouse | [100] |
| Human | Dilated cardiomyopathy | ||||
| TRPC1, TRPC3, TRPC4 | ↔ (mRNA) | Mouse | Calcineurin Tg mouse | ||
| TRPC3 | ↑ (Protein) | ND | Rat | Isoproterenol (4 mg/kg/day, 4 days) | [93] |
| Spontaneous hypertensive heart failure (SHHR, 19 months) | |||||
| Mouse | Thoracic aortic banding (7 days) | ||||
| Calcineurin Tg mouse (2 months) | |||||
| TRPC5 | ↑ (mRNA/Protein) | ND | Human | Idiopathic dilated cardiomyopathy | |
| TRPC1, TRPC4, TRPC6 | ↔ (mRNA/Protein) | ||||
| STIM1 | ↑ (mRNA) | ND | Human | Severe LV heart failure (NYHA III-IV class) | [60] |
| Orai1 | ↔ (mRNA in female) ↓ (mRNA in male) | ||||
| STIM2, Orai2, Orai3 | ↔ (mRNA) | ||||
| TRPC4 | ↓ (mRNA) | ND | Human | Ischemic cardiomyopathy | [107] |
| TRPC1 | ↑ (mRNA) | ND | Human | Hypertrophic cardiomyopathy, Heart failure | [106] |
| TRPC1, TRPC5 | ↑ (mRNA) | ND | Human | End-stage heart failure (NYHA III-IV class) | [105] |
| TRPC3, TRPC6 | ↔ (mRNA) | ||||
| TRPC4 | ↓ (mRNA) | ||||
| Orai1, TRPC5 | ↑ (mRNA/Protein) | ↑ SOCE | Rat | Ischemia (45 min)/Reperfusion (1 week) injury | [83] |
| STIM1, Orai1, TRPC1 | ↑ (mRNA/Protein) | ND | Mouse | Ischemia (30 min)/Reperfusion (24 h) injury | [82] |
| STIM1, Orai1 | ↑ (mRNA/Protein) | ND | Mouse | Abdominal aortic banding (4 weeks) | [79] |
| TRPC1 | ↑ (mRNA/Protein) | ND | Rat | Abdominal aortic banding (4 weeks) | [57] |
| STIM1 | ↔ (mRNA/Protein) | ||||
| TRPC5, TRPC6 | ↔ (Protein) | ||||
| TRPC1 | ↑ (mRNA/Protein) | ND | Rat | Abdominal aortic banding (4 weeks) | [92] |
| TRPC3, TRPC5, TRPC6 | ↔ (mRNA/Protein) | ||||
| STIM1 | ↑ (Protein) | ↑ ISOC and ICRAC | Rat | Abdominal aortic banding (14 days and 28 days) | [69] |
| STIM1 | ↑ (mRNA/Protein) | ND | Cat | Ascending aorta banding (4 months) | [74] |
| Orai3 | ↑ (mRNA) | ||||
| Orai1, STIM2 | ↔ (mRNA/Protein) | ||||
| TRPC6 | ↑ (Protein) | ND | Mouse | Ascending aortic banding (6 weeks) | [103] |
| TRPC1 | ↔ (Protein) | ||||
| Orai1 | ↑ (mRNA/Protein) | ND | Mouse | Transverse aortic banding (4 days) Myocardial infarction (4 days) | [64] |
| STIM1 | ↑ (Protein) | ND | Mouse | Transverse aortic banding (2 weeks) | [61] |
| STIM1, Orai1, Orai3 | ↑ interaction between Orai3 and STIM1/Orai1 (Protein) | ↑ Orai3-mediated Ca2+ entry and ↑ IARC | Rat | Transverse aortic banding (4 weeks) | [63] |
| STIM1, Orai1, Orai3 | ↔ (Protein) | ||||
| TRPC3, TRPC6 | ↑ (mRNA) | ND | Mouse | Transverse aortic banding (7 days) | [94] |
| TRPC6 | ↑ (Protein) | ||||
| TRPC1 | ↔ (mRNA) | ||||
| TRPC6 | ↑ (mRNA) | ND | Mouse | Transverse aortic banding (8 weeks) | [104] |
| TRPC1 | ↑ (Protein) | ND | Mouse | Transverse aortic banding (4 weeks) | [65] |
| TRPC3, TRPC4, TRPC6, STIM2 | ↔ (Protein) | ||||
| Orai1, Orai3, TRPC6, STIM2 | ↑ (mRNA/Protein) | ↑ SOCE | Mouse | Transverse aortic banding (5 weeks) | [72] |
| TRPC1, TRPC3, TRPC4, TRPC5, STIM1, Orai2 | ↔ (mRNA/Protein) | ||||
| TRPC1 | ↑ (Protein) | ↑ ISOC | Mouse | Transverse aortic banding (4, 8 weeks) | [54] |
| TRPC3, TRPC6 | ↔ (Protein) | ||||
| STIM1 | ↑ (Protein) | ND | Mouse | Transverse aortic banding (28 days) | [71] |
| TRPC4α, TRPC4β | ↑ (Protein) | ND | Mouse | Transverse aortic banding (8 weeks) | [97] |
| Orai1, Orai2, Orai3, STIM1, TRPC4, TRPC6 | ↑ (mRNA) | ND | Mouse | Angiontensin II infusion (3 mg/kg/day, 2 weeks) | [81] |
| TRPC1, TRPC3, STIM2 | ↔ (mRNA) | ||||
| STIM1 | ↑ (mRNA/Protein) | ND | Mouse | Angiontensin II infusion (400 ng/kg/min, 4 weeks) | [75] |
| TRPC1, TRPC3, TRPC4, TRPC6 | ↑ (mRNA) | ↑ SOCE | Mouse | Myocardial infarction (1, 2, 6 weeks) | [59] |
| TRPC2, TRPC5 | ↔ (mRNA) | ||||
| TRPC6 | ↑ (mRNA/Protein) | ND | Rat | Myocardial infarction (1 month) | [95] |
| TRPC3, TRPC6 | ↑ (Protein) | ND | Rat | Myocardial infarction (1, 6, 24 h) | [96] |
| TRPC1 | ↑ (Protein) | ↑ Strech-activated Ca2+ entry | Rat | Isoproterenol (5 mg/kg/5 days, 5 weeks) | [88] |
| TRPC3, TRPC6 | ↔ (Protein) | ||||
| TRPC6 | ↑ (mRNA) | ↑ ISOC | Mouse | Isoproterenol (2 mg/kg/day, 10 days) | [101] |
| TRPC1 | ↑ (mRNA/Protein) | ND | Rat | Spontaneously hypertensive rat (SHR) | [89] |
| TRPC6 | ↔ (mRNA) ↑ (Protein) | ND | Rat | Spontaneously hypertensive rat (SHR) | [102] |
| TRPC1 | ↑ (Protein) | ↑ Strech-activated Ca2+ entry | Mouse | mdx with dilated cardiomyopathy (9–12 months) | [91] |
| TRPC1, TRPC6 | ↑ (mRNA/Protein) | ND | Mouse | Tg dn-NRSF (neuron-restrictive silencer factor, 12–16 weeks) | [90] |
| TRPC2 | ↑ (mRNA) | ||||
| TRPC3 | ↓ (mRNA), ↑ (Protein) |
5.4. The SOCE in the Adverse RV Remodeling
5.5. Available Pharmacological Tools to Target SOCE in Heart Failure
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vonk Noordegraaf, A.; Chin, K.M.; Haddad, F.; Hassoun, P.M.; Hemnes, A.R.; Hopkins, S.R.; Kawut, S.M.; Langleben, D.; Lumens, J.; Naeije, R. Pathophysiology of the Right Ventricle and of the Pulmonary Circulation in Pulmonary Hypertension: An Update. Eur. Respir. J. 2019, 53, 1801900. [Google Scholar] [CrossRef]
- Haddad, F.; Hunt, S.A.; Rosenthal, D.N.; Murphy, D.J. Right Ventricular Function in Cardiovascular Disease, Part I: Anatomy, Physiology, Aging, and Functional Assessment of the Right Ventricle. Circulation 2008, 117, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Bogaard, H.J.; Abe, K.; Vonk Noordegraaf, A.; Voelkel, N.F. The Right Ventricle under Pressure: Cellular and Molecular Mechanisms of Right-Heart Failure in Pulmonary Hypertension. Chest 2009, 135, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Harjola, V.-P.; Mebazaa, A.; Čelutkienė, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary Management of Acute Right Ventricular Failure: A Statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Acute Pulmonary Embolism Developed in Collaboration with the European Respiratory Society (ERS): The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur. Respir. J. 2019, 54, 1901647. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Raina, A.; Humbert, M. Risk Assessment in Pulmonary Arterial Hypertension. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2016, 25, 390–398. [Google Scholar] [CrossRef]
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef]
- Dell’Italia, L.J. The Right Ventricle: Anatomy, Physiology, and Clinical Importance. Curr. Probl. Cardiol. 1991, 16, 653–720. [Google Scholar] [CrossRef]
- Ashley, L.M. A Determination of the Diameters of Ventricular Myocardial Fibers in Man and Other Mammals. Am. J. Anat. 1945, 77, 325–363. [Google Scholar] [CrossRef]
- Kelly, R.G.; Buckingham, M.E.; Moorman, A.F. Heart Fields and Cardiac Morphogenesis. Cold Spring Harb. Perspect. Med. 2014, 4, a015750. [Google Scholar] [CrossRef] [PubMed]
- Kiserud, T.; Acharya, G. The Fetal Circulation. Prenat. Diagn. 2004, 24, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Waskova-Arnostova, P.; Elsnicova, B.; Kasparova, D.; Sebesta, O.; Novotny, J.; Neckar, J.; Kolar, F.; Zurmanova, J. Right-to-Left Ventricular Differences in the Expression of Mitochondrial Hexokinase and Phosphorylation of Akt. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2013, 31, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Antigny, F.; Mercier, O.; Humbert, M.; Sabourin, J. Excitation-Contraction Coupling and Relaxation Alteration in Right Ventricular Remodelling Caused by Pulmonary Arterial Hypertension. Arch. Cardiovasc. Dis. 2020, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Benitah, J.P.; Gomez, A.M.; Virsolvy, A.; Richard, S. New Perspectives on the Key Role of Calcium in the Progression of Heart Disease. J. Muscle Res. Cell Motil. 2003, 24, 275–283. [Google Scholar] [CrossRef]
- Putney, J.W. A Model for Receptor-Regulated Calcium Entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Wen, H.; Gwathmey, J.K.; Xie, L.-H. Role of Transient Receptor Potential Canonical Channels in Heart Physiology and Pathophysiology. Front. Cardiovasc. Med. 2020, 7, 24. [Google Scholar] [CrossRef]
- Antigny, F.; Koenig, S.; Bernheim, L.; Frieden, M. During Post-Natal Human Myogenesis, Normal Myotube Size Requires TRPC1- and TRPC4-Mediated Ca2+ Entry. J. Cell Sci. 2013, 126, 2525–2533. [Google Scholar] [CrossRef]
- Antigny, F.; Sabourin, J.; Saüc, S.; Bernheim, L.; Koenig, S.; Frieden, M. TRPC1 and TRPC4 Channels Functionally Interact with STIM1L to Promote Myogenesis and Maintain Fast Repetitive Ca(2+) Release in Human Myotubes. Biochim. Biophys. Acta 2017, 1864, 806–813. [Google Scholar] [CrossRef]
- Antigny, F.; Girardin, N.; Frieden, M. Transient Receptor Potential Canonical Channels Are Required for in Vitro Endothelial Tube Formation. J. Biol. Chem. 2012, 287, 5917–5927. [Google Scholar] [CrossRef]
- Sabourin, J.; Le Gal, L.; Saurwein, L.; Haefliger, J.-A.; Raddatz, E.; Allagnat, F. Store-Operated Ca2+ Entry Mediated by Orai1 and TRPC1 Participates to Insulin Secretion in Rat β-Cells. J. Biol. Chem. 2015, 290, 30530–30539. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, J.; Lamiche, C.; Vandebrouck, A.; Magaud, C.; Rivet, J.; Cognard, C.; Bourmeyster, N.; Constantin, B. Regulation of TRPC1 and TRPC4 Cation Channels Requires an Alpha1-Syntrophin-Dependent Complex in Skeletal Mouse Myotubes. J. Biol. Chem. 2009, 284, 36248–36261. [Google Scholar] [CrossRef] [PubMed]
- Masson, B.; Montani, D.; Humbert, M.; Capuano, V.; Antigny, F. Role of Store-Operated Ca2+ Entry in the Pulmonary Vascular Remodeling Occurring in Pulmonary Arterial Hypertension. Biomolecules 2021, 11, 1781. [Google Scholar] [CrossRef] [PubMed]
- Hoth, M. Calcium and Barium Permeation through Calcium Release-Activated Calcium (CRAC) Channels. Pflug. Arch. 1995, 430, 315–322. [Google Scholar] [CrossRef]
- Hoth, M.; Penner, R. Depletion of Intracellular Calcium Stores Activates a Calcium Current in Mast Cells. Nature 1992, 355, 353–356. [Google Scholar] [CrossRef]
- Zweifach, A.; Lewis, R.S. Rapid Inactivation of Depletion-Activated Calcium Current (ICRAC) Due to Local Calcium Feedback. J. Gen. Physiol. 1995, 105, 209–226. [Google Scholar] [CrossRef]
- Darbellay, B.; Arnaudeau, S.; Bader, C.R.; Konig, S.; Bernheim, L. STIM1L Is a New Actin-Binding Splice Variant Involved in Fast Repetitive Ca2+ Release. J. Cell Biol. 2011, 194, 335–346. [Google Scholar] [CrossRef]
- Ramesh, G.; Jarzembowski, L.; Schwarz, Y.; Poth, V.; Konrad, M.; Knapp, M.L.; Schwär, G.; Lauer, A.A.; Grimm, M.O.W.; Alansary, D.; et al. A Short Isoform of STIM1 Confers Frequency-Dependent Synaptic Enhancement. Cell Rep. 2021, 34, 108844. [Google Scholar] [CrossRef]
- Miederer, A.-M.; Alansary, D.; Schwär, G.; Lee, P.-H.; Jung, M.; Helms, V.; Niemeyer, B.A. A STIM2 Splice Variant Negatively Regulates Store-Operated Calcium Entry. Nat. Commun. 2015, 6, 6899. [Google Scholar] [CrossRef]
- Liou, J.; Kim, M.L.; Heo, W.D.; Jones, J.T.; Myers, J.W.; Ferrell, J.E.; Meyer, T. STIM Is a Ca2+ Sensor Essential for Ca2+-Store-Depletion-Triggered Ca2+ Influx. Curr. Biol. CB 2005, 15, 1235–1241. [Google Scholar] [CrossRef]
- Roos, J.; DiGregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an Essential and Conserved Component of Store-Operated Ca2+ Channel Function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Luik, R.M.; Wu, M.M.; Buchanan, J.; Lewis, R.S. The Elementary Unit of Store-Operated Ca2+ Entry: Local Activation of CRAC Channels by STIM1 at ER-Plasma Membrane Junctions. J. Cell Biol. 2006, 174, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Hoover, P.J.; Mullins, F.M.; Bachhawat, P.; Covington, E.D.; Raunser, S.; Walz, T.; Garcia, K.C.; Dolmetsch, R.E.; Lewis, R.S. STIM1 Clusters and Activates CRAC Channels via Direct Binding of a Cytosolic Domain to Orai1. Cell 2009, 136, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wei, M.; He, L.; Liu, C.; Wu, B.; Zhang, S.L.; Jing, J.; Liang, X.; Senes, A.; Tan, P.; et al. Inside-out Ca(2+) Signalling Prompted by STIM1 Conformational Switch. Nat. Commun. 2015, 6, 7826. [Google Scholar] [CrossRef]
- Choi, S.; Maleth, J.; Jha, A.; Lee, K.P.; Kim, M.S.; So, I.; Ahuja, M.; Muallem, S. The TRPCs-STIM1-Orai Interaction. Handb. Exp. Pharmacol. 2014, 223, 1035–1054. [Google Scholar] [CrossRef]
- Zeng, W.; Yuan, J.P.; Kim, M.S.; Choi, Y.J.; Huang, G.N.; Worley, P.F.; Muallem, S. STIM1 Gates TRPC Channels, but Not Orai1, by Electrostatic Interaction. Mol. Cell 2008, 32, 439–448. [Google Scholar] [CrossRef]
- Takemura, T.; Matsui, Y.; Saiki, S.; Mikami, R. Pulmonary Vascular Involvement in Sarcoidosis: A Report of 40 Autopsy Cases. Hum. Pathol. 1992, 23, 1216–1223. [Google Scholar] [CrossRef]
- Hunton, D.L.; Lucchesi, P.A.; Pang, Y.; Cheng, X.; Dell’Italia, L.J.; Marchase, R.B. Capacitative Calcium Entry Contributes to Nuclear Factor of Activated T-Cells Nuclear Translocation and Hypertrophy in Cardiomyocytes. J. Biol. Chem. 2002, 277, 14266–14273. [Google Scholar] [CrossRef]
- Uehara, A.; Yasukochi, M.; Imanaga, I.; Nishi, M.; Takeshima, H. Store-Operated Ca2+ Entry Uncoupled with Ryanodine Receptor and Junctional Membrane Complex in Heart Muscle Cells. Cell Calcium 2002, 31, 89–96. [Google Scholar] [CrossRef]
- Huang, J.; van Breemen, C.; Kuo, K.-H.; Hove-Madsen, L.; Tibbits, G.F. Store-Operated Ca2+ Entry Modulates Sarcoplasmic Reticulum Ca2+ Loading in Neonatal Rabbit Cardiac Ventricular Myocytes. Am. J. Physiol. Cell Physiol. 2006, 290, C1572–C1582. [Google Scholar] [CrossRef]
- Kojima, A.; Kitagawa, H.; Omatsu-Kanbe, M.; Matsuura, H.; Nosaka, S. Presence of Store-Operated Ca2+ Entry in C57BL/6J Mouse Ventricular Myocytes and Its Suppression by Sevoflurane. Br. J. Anaesth. 2012, 109, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhao, Z.; Fefelova, N.; Xie, L.-H. Potential Arrhythmogenic Role of TRPC Channels and Store-Operated Calcium Entry Mechanism in Mouse Ventricular Myocytes. Front. Physiol. 2018, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Eder, P.; Chang, B.; Molkentin, J.D. TRPC Channels Are Necessary Mediators of Pathologic Cardiac Hypertrophy. Proc. Natl. Acad. Sci. USA 2010, 107, 7000–7005. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, F.; Wang, W.; Makarewich, C.A.; Zhang, H.; Kubo, H.; Berretta, R.M.; Barr, L.A.; Molkentin, J.D.; Houser, S.R. Ca(2+) Influx through L-Type Ca(2+) Channels and Transient Receptor Potential Channels Activates Pathological Hypertrophy Signaling. J. Mol. Cell. Cardiol. 2012, 53, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Touchberry, C.D.; Elmore, C.J.; Nguyen, T.M.; Andresen, J.J.; Zhao, X.; Orange, M.; Weisleder, N.; Brotto, M.; Claycomb, W.C.; Wacker, M.J. Store-Operated Calcium Entry Is Present in HL-1 Cardiomyocytes and Contributes to Resting Calcium. Biochem. Biophys. Res. Commun. 2011, 416, 45–50. [Google Scholar] [CrossRef]
- Luo, X.; Hojayev, B.; Jiang, N.; Wang, Z.V.; Tandan, S.; Rakalin, A.; Rothermel, B.A.; Gillette, T.G.; Hill, J.A. STIM1-Dependent Store-Operated Ca2+ Entry Is Required for Pathological Cardiac Hypertrophy. J. Mol. Cell. Cardiol. 2012, 52, 136–147. [Google Scholar] [CrossRef]
- Sabourin, J.; Bartoli, F.; Antigny, F.; Gomez, A.M.; Benitah, J.-P. Transient Receptor Potential Canonical (TRPC)/Orai1-Dependent Store-Operated Ca2+ Channels: NEW TARGETS OF ALDOSTERONE IN CARDIOMYOCYTES. J. Biol. Chem. 2016, 291, 13394–13409. [Google Scholar] [CrossRef]
- Bartoli, F.; Sabourin, J. Cardiac Remodeling and Disease: Current Understanding of STIM1/Orai1-Mediated Store-Operated Ca2+ Entry in Cardiac Function and Pathology. Adv. Exp. Med. Biol. 2017, 993, 523–534. [Google Scholar] [CrossRef]
- Bonilla, I.M.; Belevych, A.E.; Baine, S.; Stepanov, A.; Mezache, L.; Bodnar, T.; Liu, B.; Volpe, P.; Priori, S.; Weisleder, N.; et al. Enhancement of Cardiac Store Operated Calcium Entry (SOCE) within Novel Intercalated Disk Microdomains in Arrhythmic Disease. Sci. Rep. 2019, 9, 10179. [Google Scholar] [CrossRef]
- Pan, Z.; Brotto, M.; Ma, J. Store-Operated Ca2+ Entry in Muscle Physiology and Diseases. BMB Rep. 2014, 47, 69–79. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, A.; Ruiz-Hurtado, G.; Sabourin, J.; Gómez, A.M.; Alvarez, J.L.; Benitah, J.-P. Proarrhythmic Effect of Sustained EPAC Activation on TRPC3/4 in Rat Ventricular Cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 87, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Rietdorf, K. Tissue Specificity: Store-Operated Ca2+ Entry in Cardiac Myocytes. Adv. Exp. Med. Biol. 2017, 993, 363–387. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, J.; Robin, E.; Raddatz, E. A Key Role of TRPC Channels in the Regulation of Electromechanical Activity of the Developing Heart. Cardiovasc. Res. 2011, 92, 226–236. [Google Scholar] [CrossRef]
- Seth, M.; Zhang, Z.-S.; Mao, L.; Graham, V.; Burch, J.; Stiber, J.; Tsiokas, L.; Winn, M.; Abramowitz, J.; Rockman, H.A.; et al. TRPC1 Channels Are Critical for Hypertrophic Signaling in the Heart. Circ. Res. 2009, 105, 1023–1030. [Google Scholar] [CrossRef]
- Watanabe, H.; Murakami, M.; Ohba, T.; Takahashi, Y.; Ito, H. TRP Channel and Cardiovascular Disease. Pharmacol. Ther. 2008, 118, 337–351. [Google Scholar] [CrossRef]
- Watanabe, H.; Murakami, M.; Ohba, T.; Ono, K.; Ito, H. The Pathological Role of Transient Receptor Potential Channels in Heart Disease. Circ. J. Off. J. Jpn. Circ. Soc. 2009, 73, 419–427. [Google Scholar] [CrossRef]
- Ohba, T.; Watanabe, H.; Murakami, M.; Sato, T.; Ono, K.; Ito, H. Essential Role of STIM1 in the Development of Cardiomyocyte Hypertrophy. Biochem. Biophys. Res. Commun. 2009, 389, 172–176. [Google Scholar] [CrossRef]
- Sabourin, J.; Antigny, F.; Robin, E.; Frieden, M.; Raddatz, E. Activation of Transient Receptor Potential Canonical 3 (TRPC3)-Mediated Ca2+ Entry by A1 Adenosine Receptor in Cardiomyocytes Disturbs Atrioventricular Conduction. J. Biol. Chem. 2012, 287, 26688–26701. [Google Scholar] [CrossRef]
- Makarewich, C.A.; Zhang, H.; Davis, J.; Correll, R.N.; Trappanese, D.M.; Hoffman, N.E.; Troupes, C.D.; Berretta, R.M.; Kubo, H.; Madesh, M.; et al. Transient Receptor Potential Channels Contribute to Pathological Structural and Functional Remodeling after Myocardial Infarction. Circ. Res. 2014, 115, 567–580. [Google Scholar] [CrossRef]
- Čendula, R.; Dragún, M.; Gažová, A.; Kyselovič, J.; Hulman, M.; Máťuš, M. Changes in STIM Isoforms Expression and Gender-Specific Alterations in Orai Expression in Human Heart Failure. Physiol. Res. 2019, 68, S165–S172. [Google Scholar] [CrossRef]
- Correll, R.N.; Goonasekera, S.A.; van Berlo, J.H.; Burr, A.R.; Accornero, F.; Zhang, H.; Makarewich, C.A.; York, A.J.; Sargent, M.A.; Chen, X.; et al. STIM1 Elevation in the Heart Results in Aberrant Ca2+ Handling and Cardiomyopathy. J. Mol. Cell. Cardiol. 2015, 87, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Malette, J.; Degrandmaison, J.; Giguère, H.; Berthiaume, J.; Frappier, M.; Parent, J.-L.; Auger-Messier, M.; Boulay, G. MURC/CAVIN-4 Facilitates Store-Operated Calcium Entry in Neonatal Cardiomyocytes. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Saliba, Y.; Keck, M.; Marchand, A.; Atassi, F.; Ouillé, A.; Cazorla, O.; Trebak, M.; Pavoine, C.; Lacampagne, A.; Hulot, J.-S.; et al. Emergence of Orai3 Activity during Cardiac Hypertrophy. Cardiovasc. Res. 2014, 105, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Völkers, M.; Dolatabadi, N.; Gude, N.; Most, P.; Sussman, M.A.; Hassel, D. Orai1 Deficiency Leads to Heart Failure and Skeletal Myopathy in Zebrafish. J. Cell Sci. 2012, 125, 287–294. [Google Scholar] [CrossRef]
- Ohba, T.; Watanabe, H.; Murakami, M.; Iino, K.; Adachi, T.; Baba, Y.; Kurosaki, T.; Ono, K.; Ito, H. Stromal Interaction Molecule 1 Haploinsufficiency Causes Maladaptive Response to Pressure Overload. PLoS ONE 2017, 12, e0187950. [Google Scholar] [CrossRef]
- Wolkowicz, P.E.; Huang, J.; Umeda, P.K.; Sharifov, O.F.; Tabengwa, E.; Halloran, B.A.; Urthaler, F.; Grenett, H.E. Pharmacological Evidence for Orai Channel Activation as a Source of Cardiac Abnormal Automaticity. Eur. J. Pharmacol. 2011, 668, 208–216. [Google Scholar] [CrossRef]
- Vig, M.; Peinelt, C.; Beck, A.; Koomoa, D.L.; Rabah, D.; Koblan-Huberson, M.; Kraft, S.; Turner, H.; Fleig, A.; Penner, R.; et al. CRACM1 Is a Plasma Membrane Protein Essential for Store-Operated Ca2+ Entry. Science 2006, 312, 1220–1223. [Google Scholar] [CrossRef]
- Collins, H.E.; He, L.; Zou, L.; Qu, J.; Zhou, L.; Litovsky, S.H.; Yang, Q.; Young, M.E.; Marchase, R.B.; Chatham, J.C. Stromal Interaction Molecule 1 Is Essential for Normal Cardiac Homeostasis through Modulation of ER and Mitochondrial Function. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1231–H1239. [Google Scholar] [CrossRef] [PubMed]
- Hulot, J.-S.; Fauconnier, J.; Ramanujam, D.; Chaanine, A.; Aubart, F.; Sassi, Y.; Merkle, S.; Cazorla, O.; Ouillé, A.; Dupuis, M.; et al. Critical Role for Stromal Interaction Molecule 1 in Cardiac Hypertrophy. Circulation 2011, 124, 796–805. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, A.Y.; Kim, J.J.; Graham, V.; Finch, E.A.; Nepliouev, I.; Zhao, G.; Li, T.; Lederer, W.J.; Stiber, J.A.; et al. STIM1-Ca2+ Signaling Modulates Automaticity of the Mouse Sinoatrial Node. Proc. Natl. Acad. Sci. USA 2015, 112, E5618–E5627. [Google Scholar] [CrossRef]
- Baine, S.; Bonilla, I.; Belevych, A.; Stepanov, A.; Dorn, L.E.; Terentyeva, R.; Terentyev, D.; Accornero, F.; Carnes, C.A.; Gyorke, S. Pyridostigmine Improves Cardiac Function and Rhythmicity through RyR2 Stabilization and Inhibition of STIM1-mediated Calcium Entry in Heart Failure. J. Cell. Mol. Med. 2021, 25, 4637–4648. [Google Scholar] [CrossRef]
- Bartoli, F.; Bailey, M.A.; Rode, B.; Mateo, P.; Antigny, F.; Bedouet, K.; Gerbaud, P.; Gosain, R.; Plante, J.; Norman, K.; et al. Orai1 Channel Inhibition Preserves Left Ventricular Systolic Function and Normal Ca2+ Handling After Pressure Overload. Circulation 2020, 141, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.S.; Buckley, C.L.; Alvarez, E.M.; Schorlemmer, A.; Stokes, A.J. The Calcium Release-Activated Calcium Channel Orai1 Represents a Crucial Component in Hypertrophic Compensation and the Development of Dilated Cardiomyopathy. Channels 2014, 8, 35–48. [Google Scholar] [CrossRef]
- Troupes, C.D.; Wallner, M.; Borghetti, G.; Zhang, C.; Mohsin, S.; von Lewinski, D.; Berretta, R.M.; Kubo, H.; Chen, X.; Soboloff, J.; et al. Role of STIM1 in Hypertrophy-Related Contractile Dysfunction. Circ. Res. 2017, 121, 125–136. [Google Scholar] [CrossRef]
- Kassan, M.; Ait-Aissa, K.; Radwan, E.; Mali, V.; Haddox, S.; Gabani, M.; Zhang, W.; Belmadani, S.; Irani, K.; Trebak, M.; et al. Essential Role of Smooth Muscle STIM1 in Hypertension and Cardiovascular Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1900–1909. [Google Scholar] [CrossRef]
- Bénard, L.; Oh, J.G.; Cacheux, M.; Lee, A.; Nonnenmacher, M.; Matasic, D.S.; Kohlbrenner, E.; Kho, C.; Pavoine, C.; Hajjar, R.J.; et al. Cardiac Stim1 Silencing Impairs Adaptive Hypertrophy and Promotes Heart Failure Through Inactivation of MTORC2/Akt Signaling. Circulation 2016, 133, 1458–1471, discussion 1471. [Google Scholar] [CrossRef]
- Parks, C.; Alam, M.A.; Sullivan, R.; Mancarella, S. STIM1-Dependent Ca(2+) Microdomains Are Required for Myofilament Remodeling and Signaling in the Heart. Sci. Rep. 2016, 6, 25372. [Google Scholar] [CrossRef]
- Cacheux, M.; Strauss, B.; Raad, N.; Ilkan, Z.; Hu, J.; Benard, L.; Feske, S.; Hulot, J.-S.; Akar, F.G. Cardiomyocyte-Specific STIM1 (Stromal Interaction Molecule 1) Depletion in the Adult Heart Promotes the Development of Arrhythmogenic Discordant Alternans. Circ. Arrhythm. Electrophysiol. 2019, 12, e007382. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, Y.; Wang, Q.; Li, D.; Yang, Y.; Ma, S.; Yang, D. Overexpression of SARAF Ameliorates Pressure Overload-Induced Cardiac Hypertrophy Through Suppressing STIM1-Orai1 in Mice. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 47, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, M.; Liu, J.-J.; He, X.; Yu, X.-J.; Liu, L.-Z.; Sun, L.; Chen, L.-N.; Zang, W.-J. Long-Term Administration of Pyridostigmine Attenuates Pressure Overload-Induced Cardiac Hypertrophy by Inhibiting Calcineurin Signalling. J. Cell. Mol. Med. 2017, 21, 2106–2116. [Google Scholar] [CrossRef]
- Segin, S.; Berlin, M.; Richter, C.; Flockerzi, R.M.V.; Worley, P.; Freichel, M.; Londoño, J.E.C. Cardiomyocyte-Specific Deletion of Orai1 Reveals Its Protective Role in Angiotensin-II-Induced Pathological Cardiac Remodeling. Cells 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wu, Q.; Xu, B.; Wang, X.; Wu, J.; Huang, L.; Cheng, J. Suppression of Stim1 Reduced Intracellular Calcium Concentration and Attenuated Hypoxia/Reoxygenation Induced Apoptosis in H9C2 Cells. Biosci. Rep. 2017, 37, BSR20171249. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, A.; Mayoral-Gonzalez, I.; Avila-Medina, J.; de Rojas-de Pedro, E.S.; Calderón-Sánchez, E.; Díaz, I.; Hmadcha, A.; Castellano, A.; Rosado, J.A.; Benitah, J.-P.; et al. Urocortin-2 Prevents Dysregulation of Ca2+ Homeostasis and Improves Early Cardiac Remodeling After Ischemia and Reperfusion. Front. Physiol. 2018, 9, 813. [Google Scholar] [CrossRef]
- Ross, G.R.; Bajwa, T.; Edwards, S.; Emelyanova, L.; Rizvi, F.; Holmuhamedov, E.L.; Werner, P.; Downey, F.X.; Tajik, A.J.; Jahangir, A. Enhanced Store-Operated Ca2+ Influx and ORAI1 Expression in Ventricular Fibroblasts from Human Failing Heart. Biol. Open 2017, 6, 326–332. [Google Scholar] [CrossRef]
- Petersen, C.E.; Tripoli, B.A.; Schoborg, T.A.; Smyth, J.T. Analysis of Drosophila Cardiac Hypertrophy by Microcomputerized Tomography for Genetic Dissection of Heart Growth Mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H296–H309. [Google Scholar] [CrossRef]
- Gammons, J.; Trebak, M.; Mancarella, S. Cardiac-Specific Deletion of Orai3 Leads to Severe Dilated Cardiomyopathy and Heart Failure in Mice. J. Am. Heart Assoc. 2021, 10, e019486. [Google Scholar] [CrossRef]
- Keck, M.; Flamant, M.; Mougenot, N.; Favier, S.; Atassi, F.; Barbier, C.; Nadaud, S.; Lompré, A.-M.; Hulot, J.-S.; Pavoine, C. Cardiac Inflammatory CD11b/c Cells Exert a Protective Role in Hypertrophied Cardiomyocyte by Promoting TNFR2- and Orai3- Dependent Signaling. Sci. Rep. 2019, 9, 6047. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-S.; Xiao, J.-H.; Wang, Y.; Xu, B.-M.; Gao, L.; Wang, J.-L. Upregulation of TRPC1 Contributes to Contractile Function in Isoproterenol-Induced Hypertrophic Myocardium of Rat. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2013, 32, 951–959. [Google Scholar] [CrossRef]
- Zou, G.; Hong, H.; Lin, X.; Shi, X.; Wu, Y.; Chen, L. TRPC1, CaN and NFATC3 Signaling Pathway in the Pathogenesis and Progression of Left Ventricular Hypertrophy in Spontaneously Hypertensive Rats. Clin. Exp. Hypertens. 2015, 37, 223–234. [Google Scholar] [CrossRef]
- Ohba, T.; Watanabe, H.; Takahashi, Y.; Suzuki, T.; Miyoshi, I.; Nakayama, S.; Satoh, E.; Iino, K.; Sasano, H.; Mori, Y.; et al. Regulatory Role of Neuron-Restrictive Silencing Factor in Expression of TRPC1. Biochem. Biophys. Res. Commun. 2006, 351, 764–770. [Google Scholar] [CrossRef]
- Ward, M.-L.; Williams, I.A.; Chu, Y.; Cooper, P.J.; Ju, Y.-K.; Allen, D.G. Stretch-Activated Channels in the Heart: Contributions to Length-Dependence and to Cardiomyopathy. Prog. Biophys. Mol. Biol. 2008, 97, 232–249. [Google Scholar] [CrossRef]
- Ohba, T.; Watanabe, H.; Murakami, M.; Takahashi, Y.; Iino, K.; Kuromitsu, S.; Mori, Y.; Ono, K.; Iijima, T.; Ito, H. Upregulation of TRPC1 in the Development of Cardiac Hypertrophy. J. Mol. Cell. Cardiol. 2007, 42, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Bush, E.W.; Hood, D.B.; Papst, P.J.; Chapo, J.A.; Minobe, W.; Bristow, M.R.; Olson, E.N.; McKinsey, T.A. Canonical Transient Receptor Potential Channels Promote Cardiomyocyte Hypertrophy through Activation of Calcineurin Signaling. J. Biol. Chem. 2006, 281, 33487–33496. [Google Scholar] [CrossRef]
- Koitabashi, N.; Aiba, T.; Hesketh, G.G.; Rowell, J.; Zhang, M.; Takimoto, E.; Tomaselli, G.F.; Kass, D.A. Cyclic GMP/PKG-Dependent Inhibition of TRPC6 Channel Activity and Expression Negatively Regulates Cardiomyocyte NFAT Activation Novel Mechanism of Cardiac Stress Modulation by PDE5 Inhibition. J. Mol. Cell. Cardiol. 2010, 48, 713–724. [Google Scholar] [CrossRef]
- Zhou, R.; Hang, P.; Zhu, W.; Su, Z.; Liang, H.; Du, Z. Whole Genome Network Analysis of Ion Channels and Connexins in Myocardial Infarction. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2011, 27, 299–304. [Google Scholar] [CrossRef]
- Hang, P.; Zhao, J.; Cai, B.; Tian, S.; Huang, W.; Guo, J.; Sun, C.; Li, Y.; Du, Z. Brain-Derived Neurotrophic Factor Regulates TRPC3/6 Channels and Protects against Myocardial Infarction in Rodents. Int. J. Biol. Sci. 2015, 11, 536–545. [Google Scholar] [CrossRef]
- Kirschmer, N.; Bandleon, S.; von Ehrlich-Treuenstätt, V.; Hartmann, S.; Schaaf, A.; Lamprecht, A.-K.; Miranda-Laferte, E.; Langsenlehner, T.; Ritter, O.; Eder, P. TRPC4α and TRPC4β Similarly Affect Neonatal Cardiomyocyte Survival during Chronic GPCR Stimulation. PLoS ONE 2016, 11, e0168446. [Google Scholar] [CrossRef]
- Onohara, N.; Nishida, M.; Inoue, R.; Kobayashi, H.; Sumimoto, H.; Sato, Y.; Mori, Y.; Nagao, T.; Kurose, H. TRPC3 and TRPC6 Are Essential for Angiotensin II-Induced Cardiac Hypertrophy. EMBO J. 2006, 25, 5305–5316. [Google Scholar] [CrossRef] [PubMed]
- Brenner, J.S.; Dolmetsch, R.E. TrpC3 Regulates Hypertrophy-Associated Gene Expression without Affecting Myocyte Beating or Cell Size. PLoS ONE 2007, 2, e802. [Google Scholar] [CrossRef]
- Kuwahara, K.; Wang, Y.; McAnally, J.; Richardson, J.A.; Bassel-Duby, R.; Hill, J.A.; Olson, E.N. TRPC6 Fulfills a Calcineurin Signaling Circuit during Pathologic Cardiac Remodeling. J. Clin. Investig. 2006, 116, 3114–3126. [Google Scholar] [CrossRef]
- Xie, J.; Cha, S.-K.; An, S.-W.; Kuro-o, M.; Birnbaumer, L.; Huang, C.-L. Cardioprotection by Klotho through Downregulation of TRPC6 Channels in the Mouse Heart. Nat. Commun. 2012, 3, 1238. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, E.; Beresneva, O.; Galkina, O.; Zubina, I.; Ivanova, G.; Parastaeva, M.; Semenova, N.; Dobronravov, V. Myocardial Hypertrophy and Fibrosis Are Associated with Cardiomyocyte Beta-Catenin and TRPC6/Calcineurin/NFAT Signaling in Spontaneously Hypertensive Rats with 5/6 Nephrectomy. Int. J. Mol. Sci. 2021, 22, 4645. [Google Scholar] [CrossRef] [PubMed]
- Vindis, C.; D’Angelo, R.; Mucher, E.; Nègre-Salvayre, A.; Parini, A.; Mialet-Perez, J. Essential Role of TRPC1 Channels in Cardiomyoblasts Hypertrophy Mediated by 5-HT2A Serotonin Receptors. Biochem. Biophys. Res. Commun. 2010, 391, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.L.; Matera, D.; Doerner, J.F.; Zheng, N.; del Camino, D.; Mishra, S.; Bian, H.; Zeveleva, S.; Zhen, X.; Blair, N.T.; et al. In Vivo Selective Inhibition of TRPC6 by Antagonist BI 749327 Ameliorates Fibrosis and Dysfunction in Cardiac and Renal Disease. Proc. Natl. Acad. Sci. USA 2019, 116, 10156–10161. [Google Scholar] [CrossRef] [PubMed]
- Dragún, M.; Gažová, A.; Kyselovič, J.; Hulman, M.; Máťuš, M. TRP Channels Expression Profile in Human End-Stage Heart Failure. Med. Kaunas Lith. 2019, 55, 380. [Google Scholar] [CrossRef]
- Tang, L.; Yao, F.; Wang, H.; Wang, X.; Shen, J.; Dai, B.; Wu, H.; Zhou, D.; Guo, F.; Wang, J.; et al. Inhibition of TRPC1 Prevents Cardiac Hypertrophy via NF-ΚB Signaling Pathway in Human Pluripotent Stem Cell-Derived Cardiomyocytes. J. Mol. Cell. Cardiol. 2019, 126, 143–154. [Google Scholar] [CrossRef]
- Gronich, N.; Kumar, A.; Zhang, Y.; Efimov, I.R.; Soldatov, N.M. Molecular Remodeling of Ion Channels, Exchangers and Pumps in Atrial and Ventricular Myocytes in Ischemic Cardiomyopathy. Channels 2010, 4, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Eder, P. Cardiac Remodeling and Disease: SOCE and TRPC Signaling in Cardiac Pathology. Adv. Exp. Med. Biol. 2017, 993, 505–521. [Google Scholar] [CrossRef]
- Kinoshita, H.; Kuwahara, K.; Nishida, M.; Jian, Z.; Rong, X.; Kiyonaka, S.; Kuwabara, Y.; Kurose, H.; Inoue, R.; Mori, Y.; et al. Inhibition of TRPC6 Channel Activity Contributes to the Antihypertrophic Effects of Natriuretic Peptides-Guanylyl Cyclase-A Signaling in the Heart. Circ. Res. 2010, 106, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Cooley, N.; Grubb, D.R.; Luo, J.; Woodcock, E.A. The Phosphatidylinositol(4,5)Bisphosphate-Binding Sequence of Transient Receptor Potential Channel Canonical 4α Is Critical for Its Contribution to Cardiomyocyte Hypertrophy. Mol. Pharmacol. 2014, 86, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Camacho Londoño, J.E.; Tian, Q.; Hammer, K.; Schröder, L.; Camacho Londoño, J.; Reil, J.C.; He, T.; Oberhofer, M.; Mannebach, S.; Mathar, I.; et al. A Background Ca2+ Entry Pathway Mediated by TRPC1/TRPC4 Is Critical for Development of Pathological Cardiac Remodelling. Eur. Heart J. 2015, 36, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Camacho Londoño, J.E.; Kuryshev, V.; Zorn, M.; Saar, K.; Tian, Q.; Hübner, N.; Nawroth, P.; Dietrich, A.; Birnbaumer, L.; Lipp, P.; et al. Transcriptional Signatures Regulated by TRPC1/C4-Mediated Background Ca2+ Entry after Pressure-Overload Induced Cardiac Remodelling. Prog. Biophys. Mol. Biol. 2021, 159, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Domes, K.; Patrucco, E.; Loga, F.; Dietrich, A.; Birnbaumer, L.; Wegener, J.W.; Hofmann, F. Murine Cardiac Growth, TRPC Channels, and CGMP Kinase I. Pflug. Arch. 2015, 467, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Lin, S.; Wang, B.; Wang, Q.; Xia, W.; Zhang, H.; Cui, Y.; He, C.; Wu, H.; Sun, F.; et al. TRPC3 Deficiency Attenuates High Salt-Induced Cardiac Hypertrophy by Alleviating Cardiac Mitochondrial Dysfunction. Biochem. Biophys. Res. Commun. 2019, 519, 674–681. [Google Scholar] [CrossRef]
- Han, J.W.; Lee, Y.H.; Yoen, S.-I.; Abramowitz, J.; Birnbaumer, L.; Lee, M.G.; Kim, J.Y. Resistance to Pathologic Cardiac Hypertrophy and Reduced Expression of CaV1.2 in Trpc3-Depleted Mice. Mol. Cell. Biochem. 2016, 421, 55–65. [Google Scholar] [CrossRef]
- Xie, Y.-P.; Chen, B.; Sanders, P.; Guo, A.; Li, Y.; Zimmerman, K.; Wang, L.-C.; Weiss, R.M.; Grumbach, I.M.; Anderson, M.E.; et al. Sildenafil Prevents and Reverses Transverse-Tubule Remodeling and Ca(2+) Handling Dysfunction in Right Ventricle Failure Induced by Pulmonary Artery Hypertension. Hypertension 2012, 59, 355–362. [Google Scholar] [CrossRef]
- Seo, K.; Rainer, P.P.; Shalkey Hahn, V.; Lee, D.; Jo, S.-H.; Andersen, A.; Liu, T.; Xu, X.; Willette, R.N.; Lepore, J.J.; et al. Combined TRPC3 and TRPC6 Blockade by Selective Small-Molecule or Genetic Deletion Inhibits Pathological Cardiac Hypertrophy. Proc. Natl. Acad. Sci. USA 2014, 111, 1551–1556. [Google Scholar] [CrossRef]
- Eder, P.; Molkentin, J.D. TRPC Channels as Effectors of Cardiac Hypertrophy. Circ. Res. 2011, 108, 265–272. [Google Scholar] [CrossRef]
- Kiyonaka, S.; Kato, K.; Nishida, M.; Mio, K.; Numaga, T.; Sawaguchi, Y.; Yoshida, T.; Wakamori, M.; Mori, E.; Numata, T.; et al. Selective and Direct Inhibition of TRPC3 Channels Underlies Biological Activities of a Pyrazole Compound. Proc. Natl. Acad. Sci. USA 2009, 106, 5400–5405. [Google Scholar] [CrossRef]
- Benoist, D.; Stones, R.; Benson, A.P.; Fowler, E.D.; Drinkhill, M.J.; Hardy, M.E.L.; Saint, D.A.; Cazorla, O.; Bernus, O.; White, E. Systems Approach to the Study of Stretch and Arrhythmias in Right Ventricular Failure Induced in Rats by Monocrotaline. Prog. Biophys. Mol. Biol. 2014, 115, 162–172. [Google Scholar] [CrossRef]
- Sabourin, J.; Boet, A.; Rucker-Martin, C.; Lambert, M.; Gomez, A.-M.; Benitah, J.-P.; Perros, F.; Humbert, M.; Antigny, F. Ca2+ Handling Remodeling and STIM1L/Orai1/TRPC1/TRPC4 Upregulation in Monocrotaline-Induced Right Ventricular Hypertrophy. J. Mol. Cell. Cardiol. 2018, 118, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Ohga, K.; Takezawa, R.; Arakida, Y.; Shimizu, Y.; Ishikawa, J. Characterization of YM-58483/BTP2, a Novel Store-Operated Ca2+ Entry Blocker, on T Cell-Mediated Immune Responses in Vivo. Int. Immunopharmacol. 2008, 8, 1787–1792. [Google Scholar] [CrossRef]
- Zitt, C.; Strauss, B.; Schwarz, E.C.; Spaeth, N.; Rast, G.; Hatzelmann, A.; Hoth, M. Potent Inhibition of Ca2+ Release-Activated Ca2+ Channels and T-Lymphocyte Activation by the Pyrazole Derivative BTP2. J. Biol. Chem. 2004, 279, 12427–12437. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, H.; Doleschal, B.; Lichtenegger, M.; Oppenrieder, R.; Derler, I.; Frischauf, I.; Glasnov, T.N.; Kappe, C.O.; Romanin, C.; Groschner, K. Novel Pyrazole Compounds for Pharmacological Discrimination between Receptor-Operated and Store-Operated Ca(2+) Entry Pathways. Br. J. Pharmacol. 2012, 167, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Derler, I.; Schindl, R.; Fritsch, R.; Heftberger, P.; Riedl, M.C.; Begg, M.; House, D.; Romanin, C. The Action of Selective CRAC Channel Blockers Is Affected by the Orai Pore Geometry. Cell Calcium 2013, 53, 139–151. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Rovedatti, L.; Kaur, R.; Spencer, J.P.; Brown, J.T.; Morisset, V.D.; Biancheri, P.; Leakey, N.A.B.; Wilde, J.I.; Scott, L.; et al. Targeting Gut T Cell Ca2+ Release-Activated Ca2+ Channels Inhibits T Cell Cytokine Production and T-Box Transcription Factor T-Bet in Inflammatory Bowel Disease. J. Immunol. 2009, 183, 3454–3462. [Google Scholar] [CrossRef] [PubMed]
- Waldherr, L.; Tiffner, A.; Mishra, D.; Sallinger, M.; Schober, R.; Frischauf, I.; Schmidt, T.; Handl, V.; Sagmeister, P.; Köckinger, M.; et al. Blockage of Store-Operated Ca2+ Influx by Synta66 Is Mediated by Direct Inhibition of the Ca2+ Selective Orai1 Pore. Cancers 2020, 12, 2876. [Google Scholar] [CrossRef]
- Ng, S.W.; di Capite, J.; Singaravelu, K.; Parekh, A.B. Sustained Activation of the Tyrosine Kinase Syk by Antigen in Mast Cells Requires Local Ca2+ Influx through Ca2+ Release-Activated Ca2+ Channels. J. Biol. Chem. 2008, 283, 31348–31355. [Google Scholar] [CrossRef] [PubMed]
- Sadaghiani, A.M.; Lee, S.M.; Odegaard, J.I.; Leveson-Gower, D.B.; McPherson, O.M.; Novick, P.; Kim, M.R.; Koehler, A.N.; Negrin, R.; Dolmetsch, R.E.; et al. Identification of Orai1 Channel Inhibitors by Using Minimal Functional Domains to Screen Small Molecule Microarrays. Chem. Biol. 2014, 21, 1278–1292. [Google Scholar] [CrossRef]
- Masson, B.; Le Ribeuz, H.; Sabourin, J.; Laubry, L.; Woodhouse, E.; Foster, R.; Ruchon, Y.; Dutheil, M.; Boët, A.; Ghigna, M.-R.; et al. Orai1 Inhibitors as Potential Treatments for Pulmonary Arterial Hypertension. Circ. Res. 2022, 131, e109–e119. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-D.; Srikanth, S.; Tan, Y.-V.; Yee, M.-K.; Jew, M.; Damoiseaux, R.; Jung, M.E.; Shimizu, S.; An, D.S.; Ribalet, B.; et al. Calcium Signaling via Orai1 Is Essential for Induction of the Nuclear Orphan Receptor Pathway To Drive Th17 Differentiation. J. Immunol. 2014, 192, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Bruen, C.; Schnaus, M.; Zhang, J.; Ali, S.; Lind, A.; Stoecker, Z.; Stauderman, K.; Hebbar, S. Auxora versus Standard of Care for the Treatment of Severe or Critical COVID-19 Pneumonia: Results from a Randomized Controlled Trial. Crit. Care 2020, 24, 502. [Google Scholar] [CrossRef] [PubMed]
- CalciMedica, Inc. An Open-Label, Dose-Response Study of CM4620 Injectable Emulsion (CM4620-IE) in Patients with Acute Pancreatitis and Accompanying Systemic Inflammatory Response Syndrome (SIRS); CalciMedica, Inc.: La Jolla, CA, USA, 2019. [Google Scholar]
- Rhizen Pharmaceuticals SA. A Phase I/IIa, Randomized, Double-Blind, Placebo Controlled Study to Evaluate the Safety, and Pharmacokinetics of Single and Multiple Ascending Dose of RP3128 in HV and Effect on LAR to Allergen Challenge in Mild Asthmatics. Rhizen Pharmaceuticals SA: Basel, Switzerland, 2019. [Google Scholar]
- PRCL Research Inc. Randomized, Double Blind, Placebo Controlled, Incomplete Crossover Single Oral Dose Escalation of PRCL-02 in Normal Healthy Volunteers (Part A) and Multiple Oral Dose Escalation in Normal Healthy Volunteers (Part B) and in Chronic Plaque Psoriasis Patients (Part C); PRCL Research Inc.: Montreal, QC, Canada, 2018. [Google Scholar]
- Stauderman, K.A. CRAC Channels as Targets for Drug Discovery and Development. Cell Calcium 2018, 74, 147–159. [Google Scholar] [CrossRef]
- Rubaiy, H.N.; Ludlow, M.J.; Henrot, M.; Gaunt, H.J.; Miteva, K.; Cheung, S.Y.; Tanahashi, Y.; Hamzah, N.; Musialowski, K.E.; Blythe, N.M.; et al. Picomolar, Selective, and Subtype-Specific Small-Molecule Inhibition of TRPC1/4/5 Channels. J. Biol. Chem. 2017, 292, 8158–8173. [Google Scholar] [CrossRef] [PubMed]
- Minard, A.; Bauer, C.C.; Wright, D.J.; Rubaiy, H.N.; Muraki, K.; Beech, D.J.; Bon, R.S. Remarkable Progress with Small-Molecule Modulation of TRPC1/4/5 Channels: Implications for Understanding the Channels in Health and Disease. Cells 2018, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Just, S.; Chenard, B.L.; Ceci, A.; Strassmaier, T.; Chong, J.A.; Blair, N.T.; Gallaschun, R.J.; del Camino, D.; Cantin, S.; D’Amours, M.; et al. Treatment with HC-070, a Potent Inhibitor of TRPC4 and TRPC5, Leads to Anxiolytic and Antidepressant Effects in Mice. PLoS ONE 2018, 13, e0191225. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Shi, J.; Zhu, Y.; Kustov, M.; Tian, J.; Stevens, A.; Wu, M.; Xu, J.; Long, S.; Yang, P.; et al. Identification of ML204, a Novel Potent Antagonist That Selectively Modulates Native TRPC4/C5 Ion Channels. J. Biol. Chem. 2011, 286, 33436–33446. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, D.; Quentin, D.; Yu-Strzelczyk, J.; Sitsel, O.; Merino, F.; Stabrin, M.; Hofnagel, O.; Yu, M.; Ledeboer, M.W.; Nagel, G.; et al. Structural Basis of TRPC4 Regulation by Calmodulin and Pharmacological Agents. eLife 2020, 9, e60603. [Google Scholar] [CrossRef]
- Yu, M.; Ledeboer, M.W.; Daniels, M.; Malojcic, G.; Tibbitts, T.T.; Coeffet-Le Gal, M.; Pan-Zhou, X.-R.; Westerling-Bui, A.; Beconi, M.; Reilly, J.F.; et al. Discovery of a Potent and Selective TRPC5 Inhibitor, Efficacious in a Focal Segmental Glomerulosclerosis Model. ACS Med. Chem. Lett. 2019, 10, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Washburn, D.G.; Holt, D.A.; Dodson, J.; McAtee, J.J.; Terrell, L.R.; Barton, L.; Manns, S.; Waszkiewicz, A.; Pritchard, C.; Gillie, D.J.; et al. The Discovery of Potent Blockers of the Canonical Transient Receptor Channels, TRPC3 and TRPC6, Based on an Anilino-Thiazole Pharmacophore. Bioorg. Med. Chem. Lett. 2013, 23, 4979–4984. [Google Scholar] [CrossRef]
- Häfner, S.; Burg, F.; Kannler, M.; Urban, N.; Mayer, P.; Dietrich, A.; Trauner, D.; Broichhagen, J.; Schaefer, M. A (+)-Larixol Congener with High Affinity and Subtype Selectivity toward TRPC6. ChemMedChem 2018, 13, 1028–1035. [Google Scholar] [CrossRef]
- Maier, T.; Follmann, M.; Hessler, G.; Kleemann, H.-W.; Hachtel, S.; Fuchs, B.; Weissmann, N.; Linz, W.; Schmidt, T.; Löhn, M.; et al. Discovery and Pharmacological Characterization of a Novel Potent Inhibitor of Diacylglycerol-Sensitive TRPC Cation Channels. Br. J. Pharmacol. 2015, 172, 3650–3660. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yu, X.; Chen, H.; Horne, D.; White, R.; Wu, X.; Lee, P.; Gu, Y.; Ghimire-Rijal, S.; Lin, D.C.-H.; et al. Structural Basis for Pharmacological Modulation of the TRPC6 Channel. eLife 2020, 9, e53311. [Google Scholar] [CrossRef]
- Walsh, L.; Reilly, J.F.; Cornwall, C.; Gaich, G.A.; Gipson, D.S.; Heerspink, H.J.L.; Johnson, L.; Trachtman, H.; Tuttle, K.R.; Farag, Y.M.K.; et al. Safety and Efficacy of GFB-887, a TRPC5 Channel Inhibitor, in Patients With Focal Segmental Glomerulosclerosis, Treatment-Resistant Minimal Change Disease, or Diabetic Nephropathy: TRACTION-2 Trial Design. Kidney Int. Rep. 2021, 6, 2575–2584. [Google Scholar] [CrossRef]
- Zhao, Y.; McVeigh, B.M.; Moiseenkova-Bell, V.Y. Structural Pharmacology of TRP Channels. J. Mol. Biol. 2021, 433, 166914. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Song, X.; Liu, R.; Zhang, J.; Li, Z. The Structure of TRPC Ion Channels. Cell Calcium 2019, 80, 25–28. [Google Scholar] [CrossRef] [PubMed]
| SOCE Molecules | Expression Level | SOCE Function | Species | Induction of the RV Remodeling | References |
|---|---|---|---|---|---|
| TRPC1 | ↓ (mRNA) | ND | Rat | Monocrotaline (60–80 mg/kg, 3–5 weeks) | [120] |
| TRPC6 | ↑ (mRNA) | ||||
| TRPC1, TRPC4, glycosylated Orai1, STIM1L | ↑ (Protein) | ↑ ISOC | Rat | Monocrotaline (60 mg/kg, 3 weeks) | [121] |
| STIM2, Orai3, TRPC3, TRPC6 | ↔ (Protein) | ||||
| TRPC5, STIM1 | ↓ (Protein) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabourin, J.; Beauvais, A.; Luo, R.; Montani, D.; Benitah, J.-P.; Masson, B.; Antigny, F. The SOCE Machinery: An Unbalanced Knowledge between Left and Right Ventricular Pathophysiology. Cells 2022, 11, 3282. https://doi.org/10.3390/cells11203282
Sabourin J, Beauvais A, Luo R, Montani D, Benitah J-P, Masson B, Antigny F. The SOCE Machinery: An Unbalanced Knowledge between Left and Right Ventricular Pathophysiology. Cells. 2022; 11(20):3282. https://doi.org/10.3390/cells11203282
Chicago/Turabian StyleSabourin, Jessica, Antoine Beauvais, Rui Luo, David Montani, Jean-Pierre Benitah, Bastien Masson, and Fabrice Antigny. 2022. "The SOCE Machinery: An Unbalanced Knowledge between Left and Right Ventricular Pathophysiology" Cells 11, no. 20: 3282. https://doi.org/10.3390/cells11203282
APA StyleSabourin, J., Beauvais, A., Luo, R., Montani, D., Benitah, J.-P., Masson, B., & Antigny, F. (2022). The SOCE Machinery: An Unbalanced Knowledge between Left and Right Ventricular Pathophysiology. Cells, 11(20), 3282. https://doi.org/10.3390/cells11203282









