Bisphenol Exposure Disrupts Cytoskeletal Organization and Development of Pre-Implantation Embryos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. In Vitro Fertilization (IVF), Embryo Culture and Bisphenol Exposure
2.3. Live Cell Imaging
2.4. Immunofluorescence
2.5. Statistical Analysis
3. Results
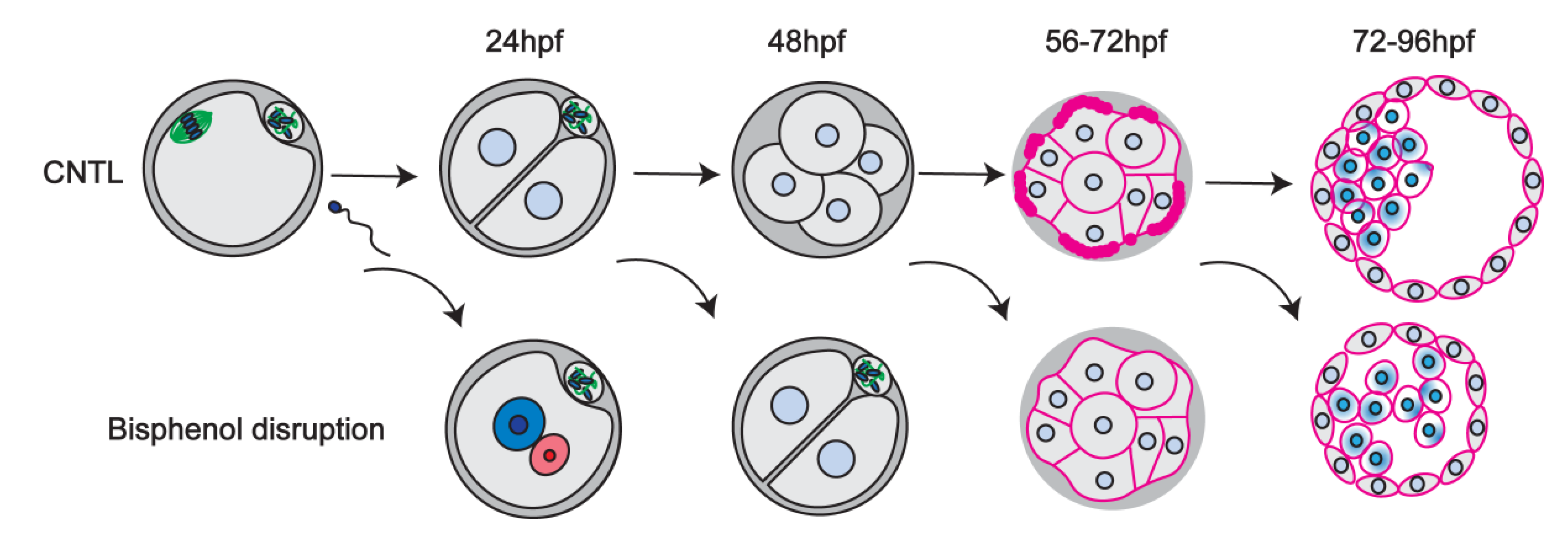
3.1. Bisphenol Exposure Shortly after Fertilization Disrupts the First Cleavage Division
3.2. Transient Bisphenol Exposure at the Zygote Stage Disrupts the Cortical Actomyosin Network and Limits Subsequent Embryo Development
3.3. Bisphenol Exposure during the 4–8 Cell Stage Disrupts Initial Cell Polarization and Division
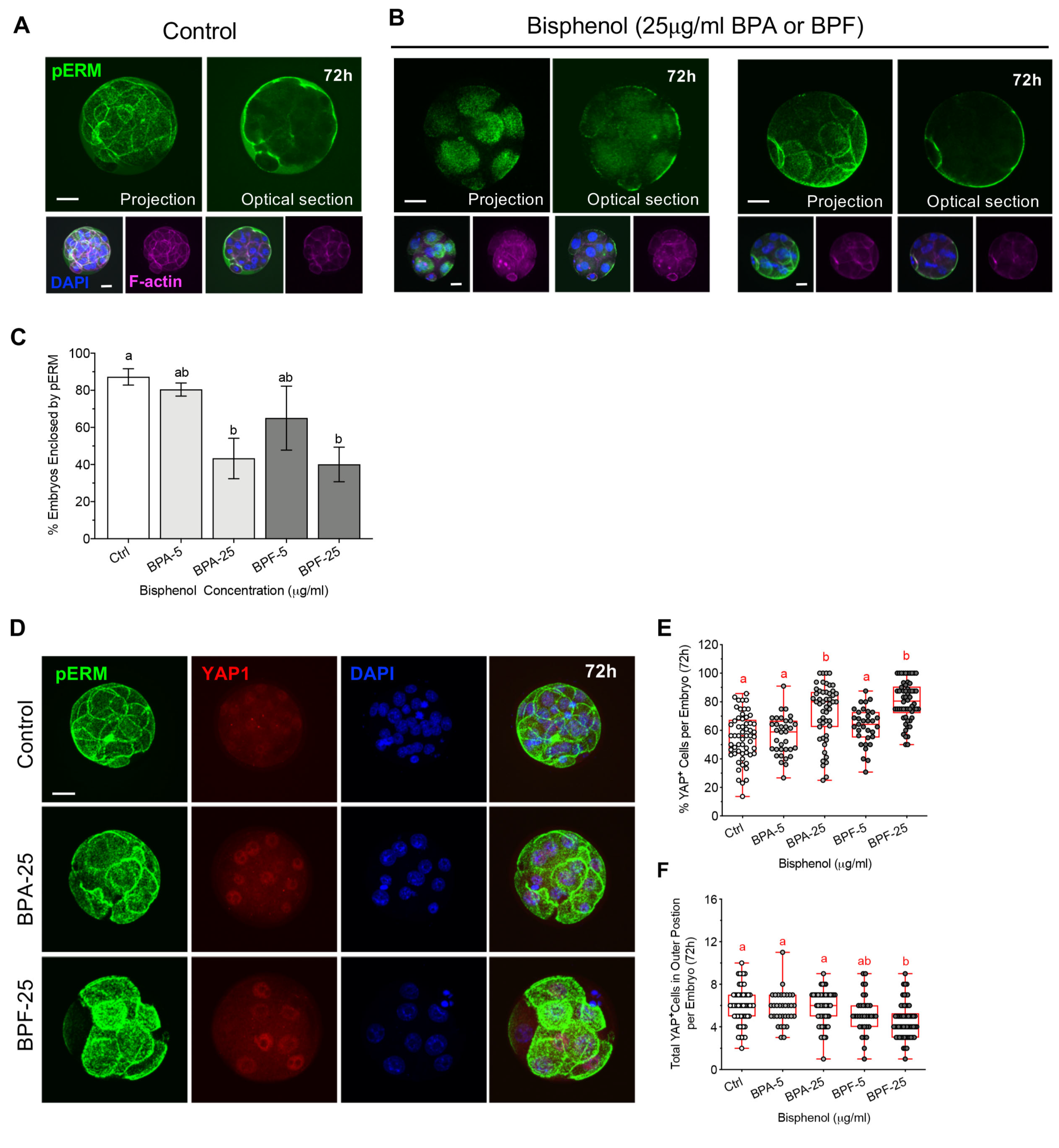
3.4. Bisphenols Disrupt the Actin Ring “Zippering” Process during Morula Formation
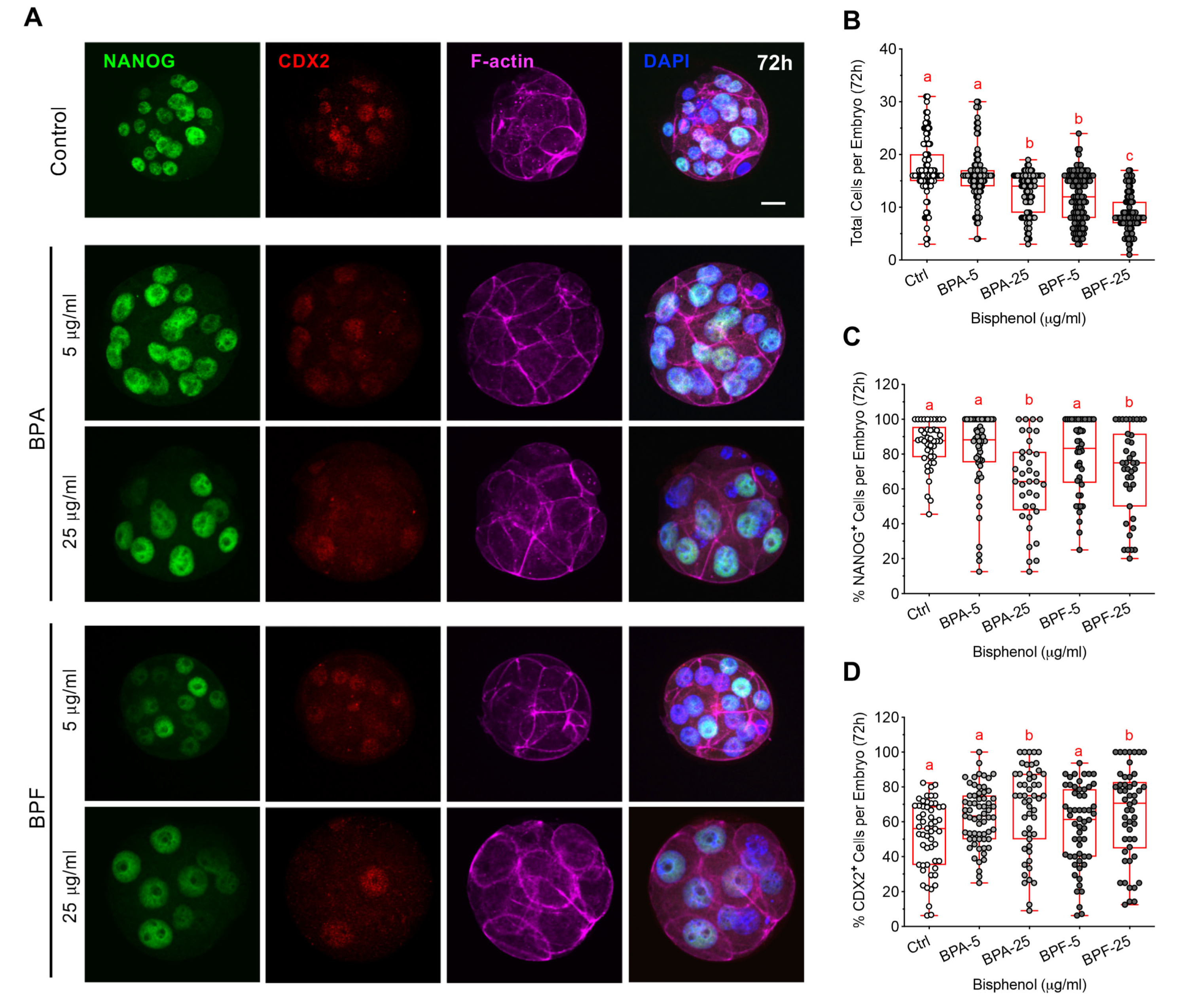
3.5. Bisphenol Exposure during Morula Formation Disrupts Cell Polarity, YAP1+pos Cell Distribution and Lineage Marker Profiles
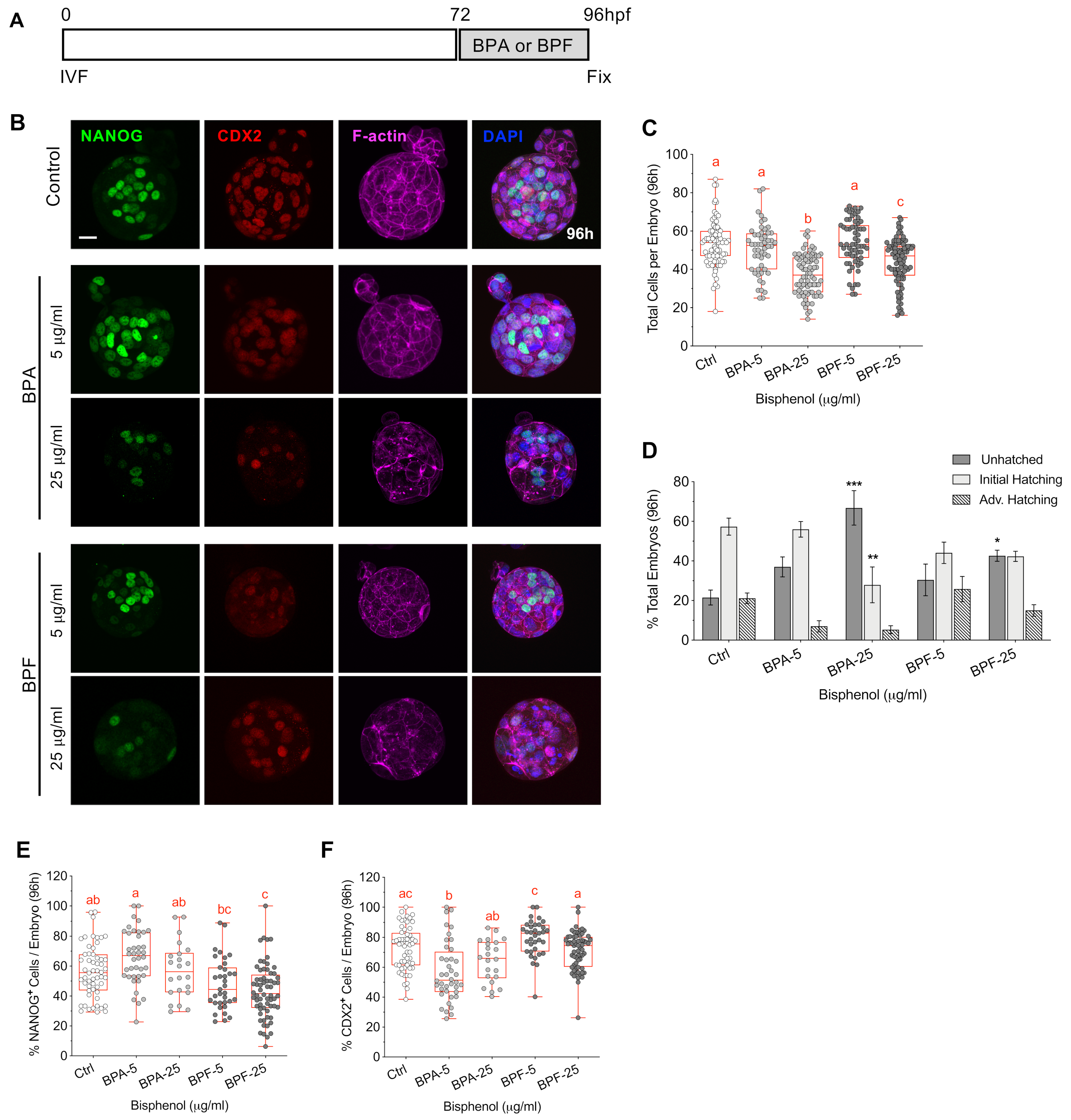
3.6. Bisphenol Exposure during Blastocyst Formation Disrupts Cell Division, Embryo Hatching and Lineage Marker Profiles
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Choi, K.; Park, J.; Moon, H.B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.J.; Eun, S.H.; Kim, G.H.; et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs. Sci. Total Environ. 2018, 626, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Ikezuki, Y.; Tsutsumi, O.; Takai, Y.; Kamei, Y.; Taketani, Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002, 17, 2839–2841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dualde, P.; Pardo, O.; Corpas-Burgos, F.; Kuligowski, J.; Gormaz, M.; Vento, M.; Pastor, A.; Yusà, V. Biomonitoring of bisphenols A, F, S in human milk and probabilistic risk assessment for breastfed infants. Sci. Total Environ. 2019, 668, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the Great Divide: A Review of Controversies in the Field of Endocrine Disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef] [Green Version]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose Response 2015, 13, 1559325815610582. [Google Scholar] [CrossRef] [Green Version]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Siracusa, J.S.; Yin, L.; Measel, E.; Liang, S.; Yu, X. Effects of bisphenol A and its analogs on reproductive health: A mini review. Reprod. Toxicol. 2018, 79, 96–123. [Google Scholar] [CrossRef]
- Pivonello, C.; Muscogiuri, G.; Nardone, A.; Garifalos, F.; Provvisiero, D.P.; Verde, N.; De Angelis, C.; Conforti, A.; Piscopo, M.; Auriemma, R.S.; et al. Bisphenol A: An emerging threat to female fertility. Reprod. Biol. Endocrinol. 2020, 18, 22. [Google Scholar] [CrossRef] [Green Version]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Sartain, C.V.; Hunt, P.A. An old culprit but a new story: Bisphenol A and “NextGen” bisphenols. Fertil. Steril. 2016, 106, 820–826. [Google Scholar] [CrossRef]
- Pelch, K.; Wignall, J.A.; Goldstone, A.E.; Ross, P.K.; Blain, R.B.; Shapiro, A.; Holmgren, S.D.; Hsieh, J.-H.; Svoboda, D.; Auerbach, S.S.; et al. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology 2019, 424, 152235. [Google Scholar] [CrossRef]
- Xiao, S.; Diao, H.; Smith, M.A.; Song, X.; Ye, X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod. Toxicol. 2011, 32, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Wang, X.; Sun, Y.; Dou, Z.; Li, Z. Inhibitory effects of preimplantation exposure to bisphenol-A on blastocyst development and implantation. Int. J. Clin. Exp. Med. 2015, 8, 8720–8729. [Google Scholar]
- Yuan, L.; Qian, L.; Qian, Y.; Liu, J.; Yang, K.; Huang, Y.; Wang, C.; Li, Y.; Mu, X. Bisphenol F-Induced Neurotoxicity toward Zebrafish Embryos. Environ. Sci. Technol. 2019, 53, 14638–14648. [Google Scholar] [CrossRef]
- Harnett, K.G.; Moore, L.G.; Chin, A.; Cohen, I.C.; Lautrup, R.R.; Schuh, S.M. Teratogenicity and toxicity of the new BPA alternative TMBPF, and BPA, BPS, and BPAF in chick embryonic development. Curr. Res. Toxicol. 2021, 2, 399–410. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, M.-H.; Shin, K.-T.; Niu, Y.-J.; Ahn, Y.-D.; Kim, N.-H.; Cui, X.-S. The possible molecular mechanisms of bisphenol A action on porcine early embryonic development. Sci. Rep. 2017, 7, 8632. [Google Scholar] [CrossRef]
- Lim, H.Y.G.; Plachta, N. Cytoskeletal control of early mammalian development. Nat. Rev. Mol. Cell Biol. 2021, 22, 548–562. [Google Scholar] [CrossRef]
- Nikas, G.; Ao, A.; Winston, R.M.; Handyside, A.H. Compaction and Surface Polarity in the Human Embryo in Vitro. Biol. Reprod. 1996, 55, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Eckert, J.J.; Velazquez, M.A.; Fleming, T.P. Cell Signalling During Blastocyst Morphogenesis. Adv. Exp. Med. Biol. 2015, 843, 1–21. [Google Scholar] [CrossRef]
- Zenker, J.; White, M.D.; Gasnier, M.; Alvarez, Y.D.; Lim, H.Y.G.; Bissiere, S.; Biro, M.; Plachta, N. Expanding Actin Rings Zipper the Mouse Embryo for Blastocyst Formation. Cell 2018, 173, 776–791.e17. [Google Scholar] [CrossRef] [Green Version]
- Tsukita, S.; Yonemura, S. Cortical Actin Organization: Lessons from ERM (Ezrin/Radixin/Moesin) Proteins. J. Biol. Chem. 1999, 274, 34507–34510. [Google Scholar] [CrossRef]
- Louvet, S.; Aghion, J.; Santa-Maria, A.; Mangeat, P.; Maro, B. Ezrin Becomes Restricted to Outer Cells Following Asymmetrical Division in the Preimplantation Mouse Embryo. Dev. Biol. 1996, 177, 568–579. [Google Scholar] [CrossRef] [Green Version]
- Nishioka, N.; Inoue, K.; Adachi, K.; Kiyonari, H.; Ota, M.; Ralston, A.; Yabuta, N.; Hirahara, S.; Stephenson, R.O.; Ogonuki, N.; et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 2009, 16, 398–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handyside, A.H. Distribution of antibody- and lectin-binding sites on dissociated blastomeres from mouse morulae: Evidence for polarization at compaction. J. Embryol. Exp. Morphol. 1980, 60, 99–116. [Google Scholar] [CrossRef]
- Johnson, M.; A Ziomek, C. Induction of polarity in mouse 8-cell blastomeres: Specificity, geometry, and stability. J. Cell Biol. 1981, 91, 303–308. [Google Scholar] [CrossRef]
- Strumpf, D.; Mao, C.-A.; Yamanaka, Y.; Ralston, A.; Chawengsaksophak, K.; Beck, F.; Rossant, J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 2005, 132, 2093–2102. [Google Scholar] [CrossRef] [Green Version]
- Chazaud, C.; Yamanaka, Y. Lineage specification in the mouse preimplantation embryo. Development 2016, 143, 1063–1074. [Google Scholar] [CrossRef] [Green Version]
- George, O.; Bryant, B.K.; Chinnasamy, R.; Corona, C.; Arterburn, J.B.; Shuster, C.B. Bisphenol A Directly Targets Tubulin to Disrupt Spindle Organization in Embryonic and Somatic Cells. ACS Chem. Biol. 2008, 3, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Baumann, C.; De La Fuente, R.; Viveiros, M.M. Mechanisms underlying disruption of oocyte spindle stability by bisphenol compounds. Reproduction 2020, 159, 383–396. [Google Scholar] [CrossRef]
- Ma, W.; Baumann, C.; Viveiros, M.M. Lack of protein kinase C-delta (PKCdelta) disrupts fertilization and embryonic development. Mol. Reprod. Dev. 2015, 82, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Combelles, C.M.; Missmer, S.A.; Correia, K.F.; Williams, P.; Hauser, R.; Racowsky, C. Bisphenol-A and human oocyte maturation in vitro. Hum. Reprod. 2013, 28, 2735–2745. [Google Scholar] [CrossRef]
- Nakano, K.; Nishio, M.; Kobayashi, N.; Hiradate, Y.; Hoshino, Y.; Sato, E.; Tanemura, K. Comparison of the effects of BPA and BPAF on oocyte spindle assembly and polar body release in mice. Zygote 2015, 24, 172–180. [Google Scholar] [CrossRef]
- Baumann, C.; Viveiros, M.M. Meiotic Spindle Assessment in Mouse Oocytes by siRNA-mediated Silencing. J. Vis. Exp. 2015, 104, e53586. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.; Peters, A.H.; Otte, A.P.; Reik, W.; Dean, W. Dynamic chromatin modifications characterise the first cell cycle in mouse embryos. Dev. Biol. 2005, 280, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Gentile, L.; Fuchikami, T.; Sutter, J.; Psathaki, K.; Esteves, T.C.; Araúzo-Bravo, M.J.; Ortmeier, C.; Verberk, G.; Abe, K.; et al. Initiation of trophectoderm lineage specification in mouse embryos is independent of Cdx2. Development 2010, 137, 4159–4169. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, K.; Fujimori, T. Multiple phases in regulation of Nanog expression during pre-implantation development. Dev. Growth Differ. 2015, 57, 648–656. [Google Scholar] [CrossRef] [Green Version]
- Niwa, H.; Toyooka, Y.; Shimosato, D.; Strumpf, D.; Takahashi, K.; Yagi, R.; Rossant, J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 2005, 123, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Maître, J.-L.; Niwayama, R.; Turlier, H.; Nédélec, F.; Hiiragi, T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat. Cell Biol. 2015, 17, 849–855. [Google Scholar] [CrossRef]
- Zhu, M.; Zernicka-Goetz, M. Building an apical domain in the early mouse embryo: Lessons, challenges and perspectives. Curr. Opin. Cell Biol. 2019, 62, 144–149. [Google Scholar] [CrossRef]
- Saini, D.; Yamanaka, Y. Cell Polarity-Dependent Regulation of Cell Allocation and the First Lineage Specification in the Preimplantation Mouse Embryo. Curr. Top. Dev. Biol. 2018, 128, 11–35. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Z.; Shi, X.; Li, W.; Liu, C.; Wang, D.; Ye, X.; Liu, L.; Na, J.; Cheng, H.; et al. Atypical PKC, regulated by Rho GTPases and Mek/Erk, phosphorylates Ezrin during eight-cell embryocompaction. Dev. Biol. 2013, 375, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Marikawa, Y.; Alarcón, V.B. Establishment of trophectoderm and inner cell mass lineages in the mouse embryo. Mol. Reprod. Dev. 2009, 76, 1019–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenker, J.; White, M.D.; Templin, R.M.; Parton, R.G.; Thorn-Seshold, O.; Bissiere, S.; Plachta, N. A microtubule-organizing center directing intracellular transport in the early mouse embryo. Science 2017, 357, 925–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamakis, I.-D.S.; Panteris, E.; Eleftheriou, E.P. Tubulin Acetylation Mediates Bisphenol A Effects on the Microtubule Arrays of Allium cepa and Triticum turgidum. Biomolecules 2019, 9, 185. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, E.; Rosenberg, B.; Deuschel, S.; Metzler, M. Interference with microtubules and induction of micronuclei in vitro by various bisphenols. Mutat. Res. 1997, 390, 21–31. [Google Scholar] [CrossRef]
- Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C.R. Acute Toxicity, Teratogenic, and Estrogenic Effects of Bisphenol A and Its Alternative Replacements Bisphenol S, Bisphenol F, and Bisphenol AF in Zebrafish Embryo-Larvae. Environ. Sci. Technol. 2017, 51, 12796–12805. [Google Scholar] [CrossRef]
- Sun, S.C.; Wang, Q.L.; Gao, W.W.; Xu, Y.N.; Liu, H.L.; Cui, X.S.; Kim, N.H. Actin nucleator Arp2/3 complex is essential for mouse preimplantation embryo development. Reprod. Fertil. Dev. 2013, 25, 617–623. [Google Scholar] [CrossRef]
- Zhu, M.; Leung, C.Y.; Shahbazi, M.N.; Zernicka-Goetz, M. Actomyosin polarisation through PLC-PKC triggers symmetry breaking of the mouse embryo. Nat. Commun. 2017, 8, 921. [Google Scholar] [CrossRef] [Green Version]
- Maître, J.-L.; Turlier, H.; Illukkumbura, R.; Eismann, B.; Niwayama, R.; Nédélec, F.; Hiiragi, T. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature 2016, 536, 344–348. [Google Scholar] [CrossRef] [Green Version]
- Korotkevich, E.; Niwayama, R.; Courtois, A.; Friese, S.; Berger, N.; Buchholz, F.; Hiiragi, T. The Apical Domain Is Required and Sufficient for the First Lineage Segregation in the Mouse Embryo. Dev. Cell 2017, 40, 235–247.e7. [Google Scholar] [CrossRef] [Green Version]
- Prins, G.S.; Hu, W.-Y.; Shi, G.-B.; Hu, D.-P.; Majumdar, S.; Li, G.; Huang, K.; Nelles, J.L.; Ho, S.-M.; Walker, C.L.; et al. Bisphenol A Promotes Human Prostate Stem-Progenitor Cell Self-Renewal and Increases In Vivo Carcinogenesis in Human Prostate Epithelium. Endocrinology 2014, 155, 805–817. [Google Scholar] [CrossRef]
- Moreno-Gómez-Toledano, R.; Arenas, M.I.; González-Martínez, C.; Olea-Herrero, N.; Reventún, P.; Di Nunzio, M.; Sánchez-Esteban, S.; Arilla-Ferreiro, E.; Saura, M.; Bosch, R.J. Bisphenol A impaired cell adhesion by altering the expression of adhesion and cytoskeleton proteins on human podocytes. Sci. Rep. 2020, 10, 16638. [Google Scholar] [CrossRef]
- Anahara, R.; Yoshida, M.; Toyama, Y.; Maekawa, M.; Kai, M.; Ishino, F.; Toshimori, K.; Mori, C. Estrogen agonists, 17.BETA.-estradiol, bisphenol A, and diethylstilbestrol, decrease cortactin expression in the mouse testis. Arch. Histol. Cytol. 2006, 69, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.; Ding, Z.; Meng, F.; Zhang, X.; Wang, Y.; Chen, F.; Duan, Z.; Wu, D.; Zhang, S.; Miao, Y.; et al. The toxic effects of Fluorene-9-bisphenol on porcine oocyte in vitro maturation. Environ. Toxicol. 2019, 35, 152–158. [Google Scholar] [CrossRef]
- Pan, M.-H.; Wu, Y.-K.; Liao, B.-Y.; Zhang, H.; Li, C.; Wang, J.-L.; Hu, L.-L.; Ma, B. Bisphenol A Exposure Disrupts Organelle Distribution and Functions During Mouse Oocyte Maturation. Front. Cell Dev. Biol. 2021, 9, 661155. [Google Scholar] [CrossRef]
- Nguyen, M.; Sabry, R.; Davis, O.S.; Favetta, L.A. Effects of BPA, BPS, and BPF on Oxidative Stress and Antioxidant Enzyme Expression in Bovine Oocytes and Spermatozoa. Genes 2022, 13, 142. [Google Scholar] [CrossRef]
- Balta, E.; Kramer, J.; Samstag, Y. Redox Regulation of the Actin Cytoskeleton in Cell Migration and Adhesion: On the Way to a Spatiotemporal View. Front. Cell Dev. Biol. 2021, 8, 618261. [Google Scholar] [CrossRef]
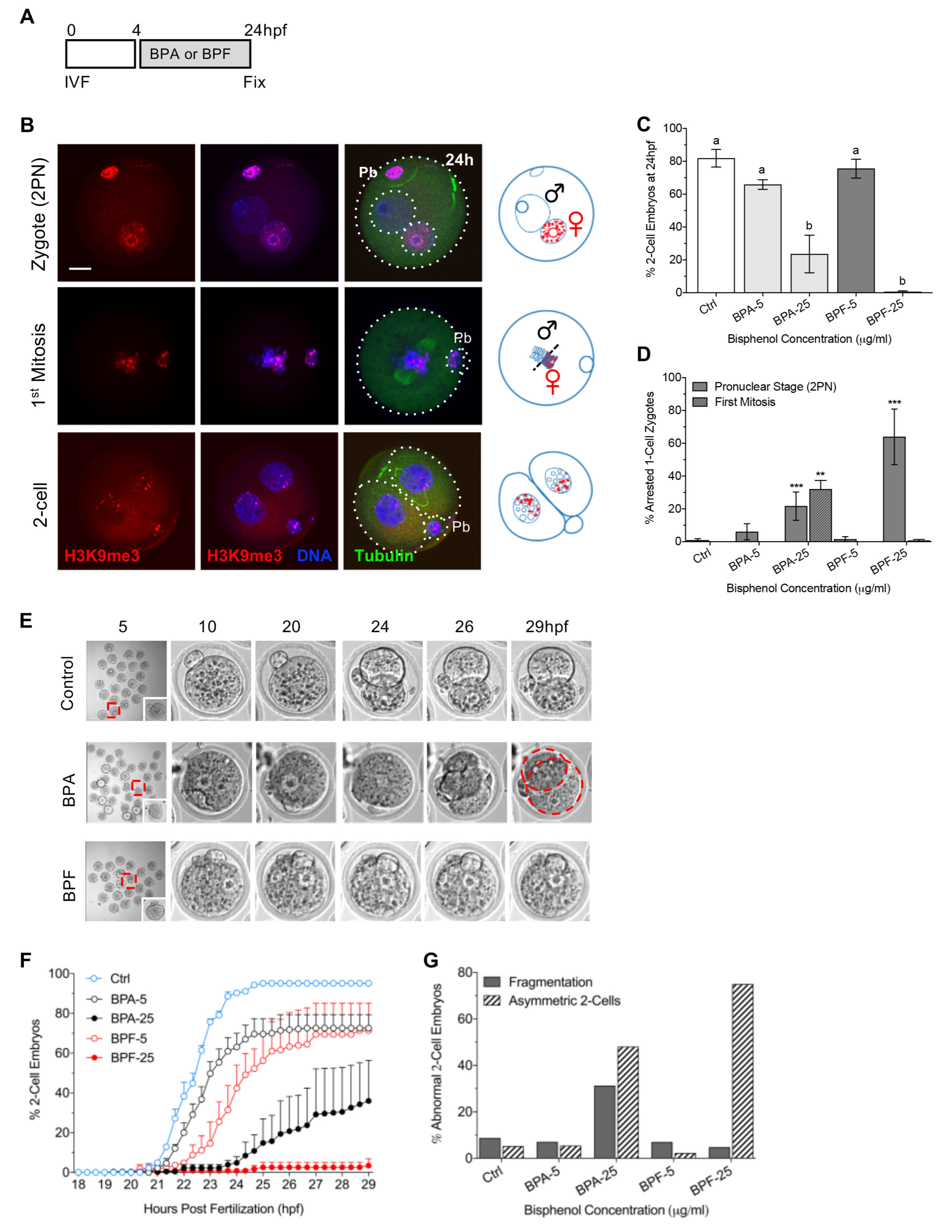
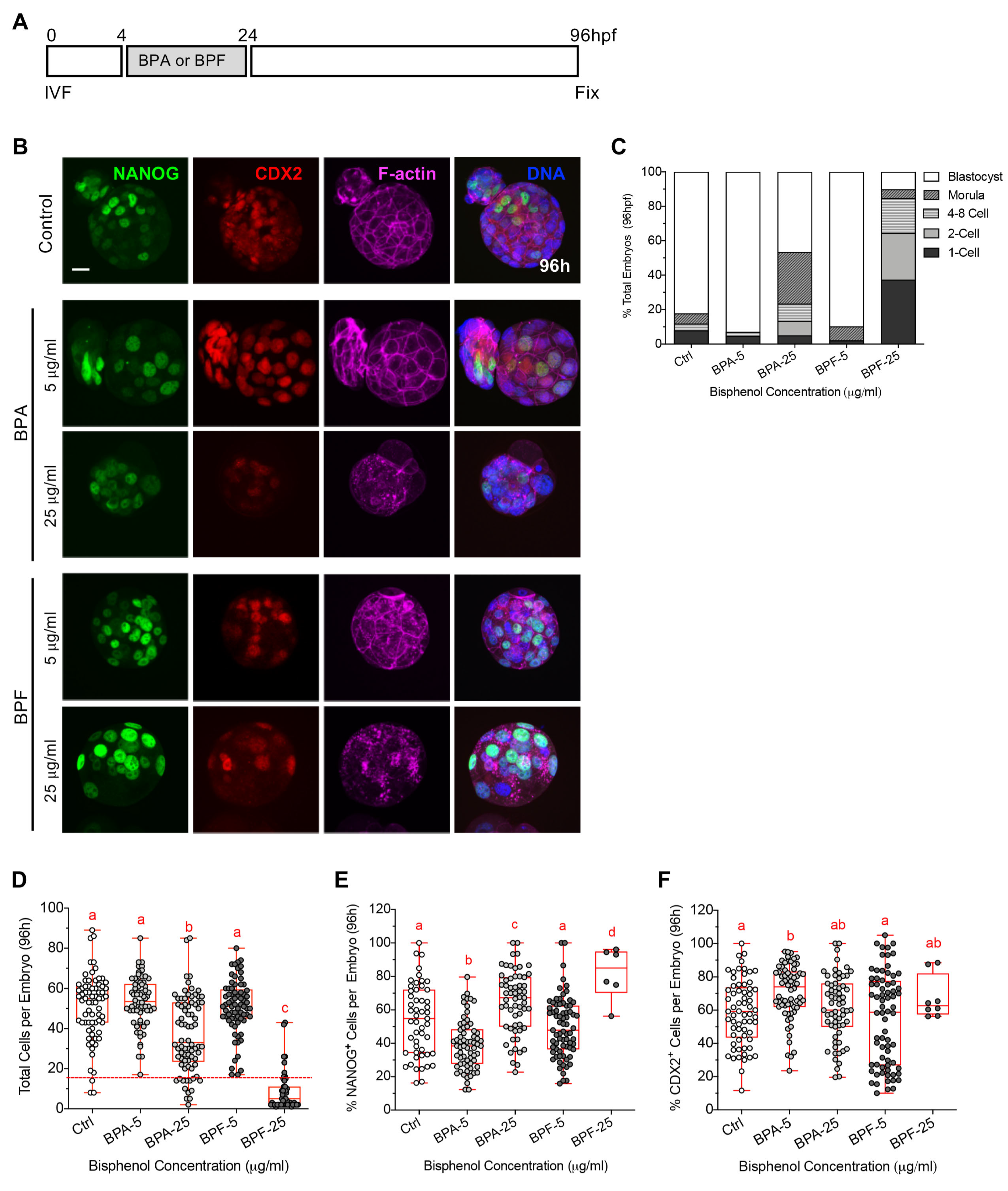
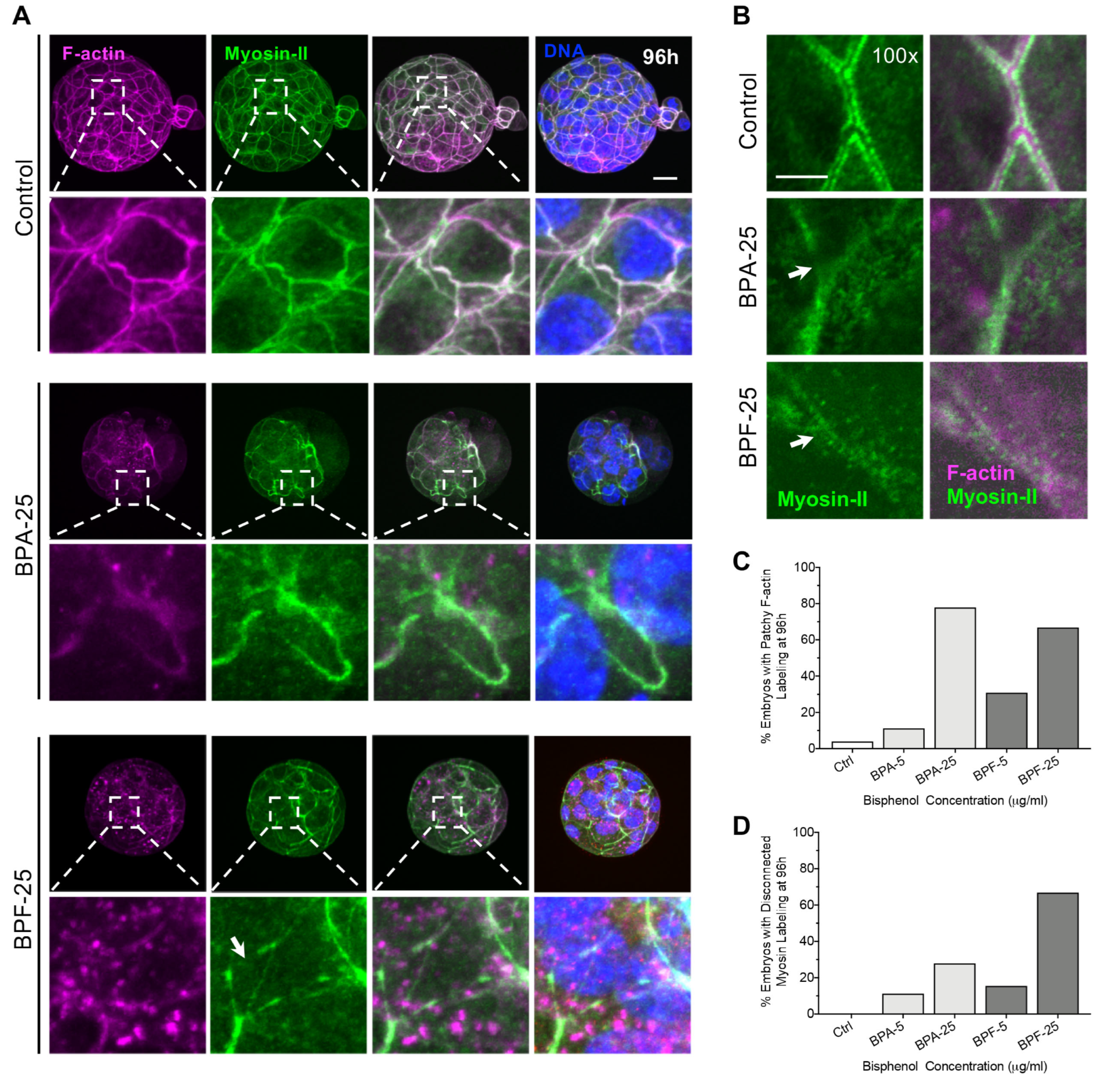

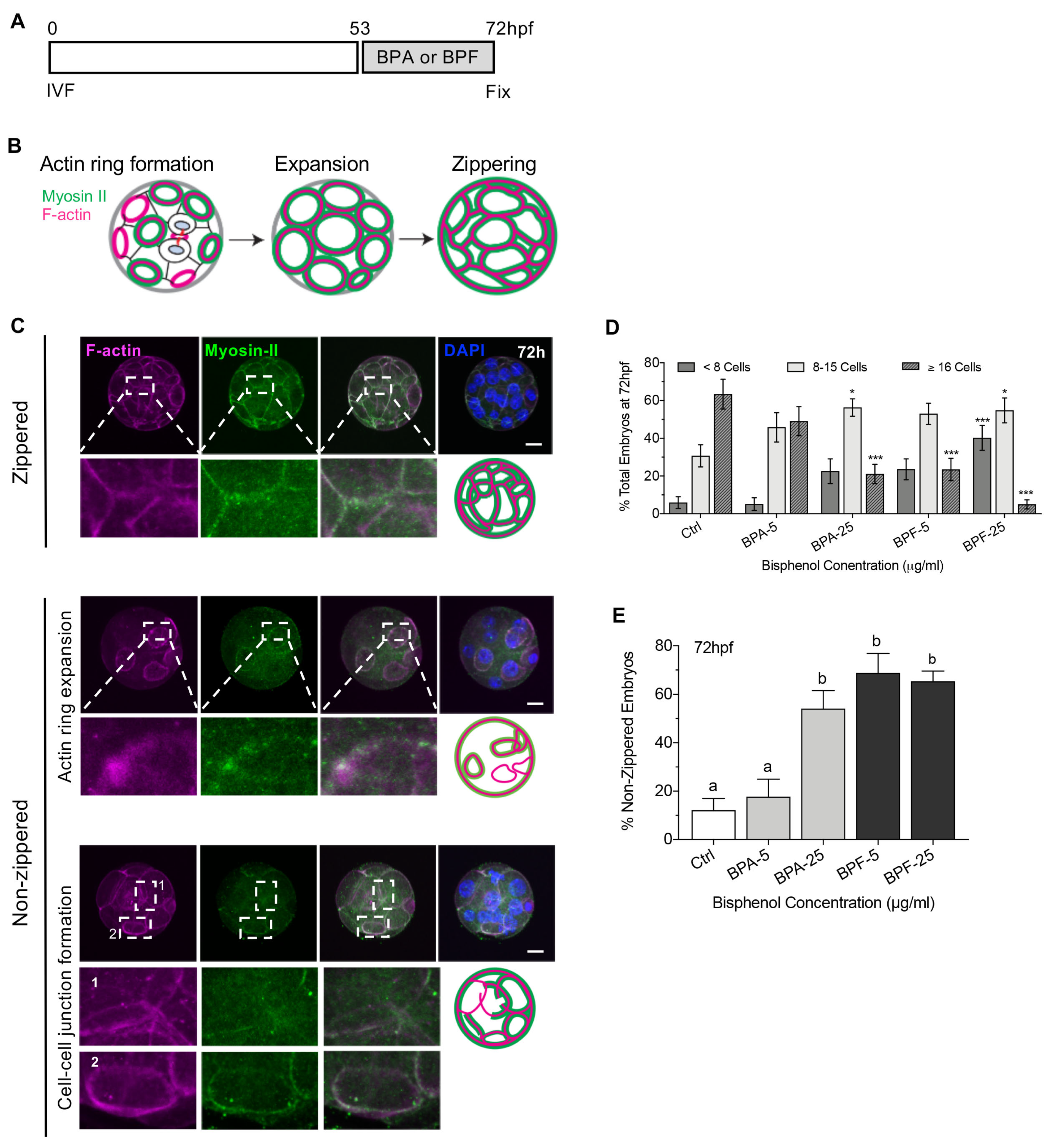
| Stage of Bisphenol Exposure and Embryo Fixation | Bisphenol-Mediate Disruptions |
|---|---|
 | Blocked or delayed onset of 2-cell cleavage BPF more detrimental |
 | Impaired embryo development Cell division defects Disrupted actomyosin network Altered lineage marker profiles (NANOG, CDX2) |
 | Cell division defects Disrupted cell polarization Fewer polar cells with apical domain |
 | Cell division defects Impaired embryo sealing Disrupted cell polarization & cell position Increased YAP1 positive cells Altered lineage marker profiles (NANOG, CDX2) |
 | Cell division defects Reduced blastocyst hatching rates Altered lineage marker profiles (NANOG, CDX2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Baumann, C.; De La Fuente, R.; Viveiros, M.M. Bisphenol Exposure Disrupts Cytoskeletal Organization and Development of Pre-Implantation Embryos. Cells 2022, 11, 3233. https://doi.org/10.3390/cells11203233
Yang L, Baumann C, De La Fuente R, Viveiros MM. Bisphenol Exposure Disrupts Cytoskeletal Organization and Development of Pre-Implantation Embryos. Cells. 2022; 11(20):3233. https://doi.org/10.3390/cells11203233
Chicago/Turabian StyleYang, Luhan, Claudia Baumann, Rabindranath De La Fuente, and Maria M. Viveiros. 2022. "Bisphenol Exposure Disrupts Cytoskeletal Organization and Development of Pre-Implantation Embryos" Cells 11, no. 20: 3233. https://doi.org/10.3390/cells11203233
APA StyleYang, L., Baumann, C., De La Fuente, R., & Viveiros, M. M. (2022). Bisphenol Exposure Disrupts Cytoskeletal Organization and Development of Pre-Implantation Embryos. Cells, 11(20), 3233. https://doi.org/10.3390/cells11203233








