The Cytotoxicity and Genotoxicity of Bioactive Dental Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pulp Capping Materials
2.2. Cell Line and Eluate Preparation
2.3. Cytotoxicity Analysis
2.4. Genotoxicity Assessment
2.5. Apoptosis Detection
2.6. Cell Cycle Analysis
2.7. Statistical Analysis
3. Results
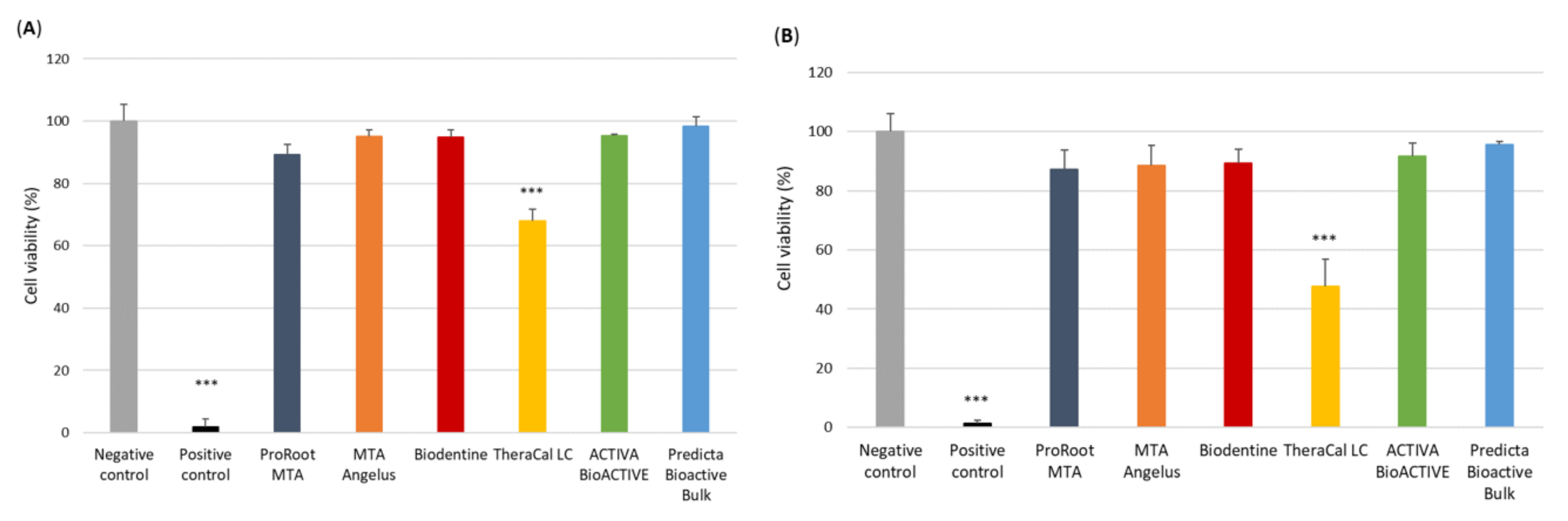
3.1. Analysis of the Cytotoxicity of the Pulp Capping Agents
3.2. Analysis of the Genotoxicity of the Pulp Capping Agents
3.3. Apoptosis Detection by FITC Annexin V/PI Double Staining of the Pulp Capping Agents
3.4. Analysis of the Cell Cycle Progression by PI Staining of the Pulp Capping Agents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.; Lee, D.; Song, D.; Kim, H.M.; Kim, S.Y. Biocompatibility and bioactivity of set direct pulp capping materials on human dental pulp stem cells. Materials 2020, 13, 3925. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça Petta, T.; Pedroni, A.C.F.; Saavedra, D.F.; Faial, K.D.C.F.; Marques, M.M.; D’Almeida Couto, R.S. The effect of three different pulp capping cements on mineralization of dental pulp stem cells. Dent. Mater. J. 2020, 39, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghilotti, J.; Sanz, J.L.; López-García, S.; Guerrero-Gironés, J.; Pecci-Lloret, M.P.; Lozano, A.; Llena, C.; Rodríguez-Lozano, F.J.; Forner, L.; Spagnuolo, G. Comparative surface morphology, chemical composition, and cytocompatibility of Bio-C repair, biodentine, and proroot MTA on hDPCs. Materials 2020, 13, 2189. [Google Scholar] [CrossRef] [PubMed]
- Akbiyik, S.Y.; Bakir, E.P.; Bakir, S. Evaluation of the Bond Strength of Different Pulp Capping Materials to Dental Adhesive Systems: An In Vitro Study. J. Adv. Oral Res. 2021, 12, 286–295. [Google Scholar] [CrossRef]
- Hardan, L.; Mancino, D.; Bourgi, R.; Alvarado-Orozco, A.; Rodríguez-Vilchis, L.E.; Flores-Ledesma, A.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M.; Eid, A.; Danhache, M.-L.; et al. Bond Strength of Adhesive Systems to Calcium Silicate-Based Materials: A Systematic Review and Meta-Analysis of In Vitro Studies. Gels 2022, 8, 311. [Google Scholar] [CrossRef]
- Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; Markvart, M.; et al. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [CrossRef] [Green Version]
- Schwendicke, F.; Frencken, J.E.; Bjørndal, L.; Maltz, M.; Manton, D.J.; Ricketts, D.; Van Landuyt, K.; Banerjee, A.; Campus, G.; Doméjean, S.; et al. Managing Carious Lesions: Consensus Recommendations on Carious Tissue Removal. Adv. Dent. Res. 2016, 28, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Maciel Pires, P.; Ionescu, A.C.; Pérez-Gracia, M.T.; Vezzoli, E.; Soares, I.P.M.; Brambilla, E.; de Almeida Neves, A.; Sauro, S. Assessment of the remineralisation induced by contemporary ion-releasing materials in mineral-depleted dentine. Clin. Oral Investig. 2022, 26, 6195–6207. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Siboni, F.; Botero, T.; Bossù, M.; Riccitiello, F.; Prati, C. Calcium silicate and calcium hydroxide materials for pulp capping: Biointeractivity, porosity, solubility and bioactivity of current formulations. J. Appl. Biomater. Funct. Mater. 2015, 13, 41–60. [Google Scholar] [CrossRef]
- Mickenautsch, S.; Yengopal, V.; Banerjee, A. Pulp response to resin-modified glass ionomer and calcium hydroxide cements in deep cavities: A quantitative systematic review. Dent. Mater. 2010, 26, 761–770. [Google Scholar] [CrossRef]
- Paula, A.; Laranjo, M.; Marto, C.M.; Abrantes, A.M.; Casalta-Lopes, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Ferreira, M.M.; Botelho, M.F.; Carrilho, E. BiodentineTM boosts, WhiteProRoot®MTA increases and Life® suppresses odontoblast activity. Materials 2019, 12, 1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birant, S.; Gokalp, M.; Duran, Y.; Koruyucu, M.; Akkoc, T.; Seymen, F. Cytotoxicity of NeoMTA Plus, ProRoot MTA and Biodentine on human dental pulp stem cells. J. Dent. Sci. 2021, 16, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Che, J.-L.; Kim, J.-H.; Kim, S.-M.; Choi, N.; Moon, H.-J.; Hwang, M.-J.; Song, H.-J.; Park, Y.-J. Comparison of Setting Time, Compressive Strength, Solubility, and pH of Four Kinds of MTA. Korean J. Dent. Mater. 2016, 43, 61–72. [Google Scholar] [CrossRef]
- Vivan, R.R.; Zapata, R.O.; Zeferino, M.A.; Bramante, C.M.; Bernardineli, N.; Garcia, R.B.; Hungaro Duarte, M.A.; Tanomaru Filho, M.; Gomes De Moraes, I. Evaluation of the physical and chemical properties of two commercial and three experimental root-end filling materials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 110, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Pornamazeh, T.; Yadegari, Z.; Ghasemi, A.; Sheykh-al-Eslamian, S.M.; Shojaeian, S.H. In vitro cytotoxicity and setting time assessment of calcium-enriched mixture cement, retro mineral trioxide aggregate and mineral trioxide aggregate. Iran. Endod. J. 2017, 12, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Kaup, M.; Schäfer, E.; Dammaschke, T. An in vitro study of different material properties of Biodentine compared to ProRoot MTA. Head Face Med. 2015, 11, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.; Park, S.J.; Lee, S.H.; Hwang, Y.C.; Yu, M.K.; Min, K.S. Biological effects and washout resistance of a newly developed fast-setting pozzolan cement. J. Endod. 2013, 39, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Marques, J.A.; Falacho, R.I.; Vinagre, A.; Santos, J.M.; Ramos, J.C. Does delayed restoration improve shear bond strength of different restorative protocols to calcium silicate-based cements? Materials 2018, 11, 2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grech, L.; Mallia, B.; Camilleri, J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent. Mater. 2013, 29, e20–e28. [Google Scholar] [CrossRef]
- Kunert, M.; Lukomska-Szymanska, M. Bio-Inductive Materials in Direct and Indirect Pulp Capping—A Review Article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Deepa, V.; Dhamaraju, B.; Bollu, I.; Balaji, T. Shear bond strength evaluation of resin composite bonded to three different liners: TheraCal LC, Biodentine, and resin-modified glass ionomer cement using universal adhesive: An in vitro study. J. Conserv. Dent. 2016, 19, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandolfi, M.G.; Siboni, F.; Prati, C. Chemical-physical properties of TheraCal, a novel light-curable MTA-like material for pulp capping. Int. Endod. J. 2012, 45, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Kamal, E.; Nabih, S.; Obeid, R.; Abdelhameed, M. The reparative capacity of different bioactive dental materials for direct pulp capping. Dent. Med. Probl. 2018, 55, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Suh, B.I. Cytotoxicity and biocompatibility of resin-free and resin-modified direct pulp capping materials: A state-of-the-art review. Dent. Mater. J. 2017, 36, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savas, S.; Botsali, M.S.; Kucukyilmaz, E.; Sari, T. Evaluation of temperature changes in the pulp chamber during polymerization of light-cured pulp-capping materials by using a VALO LED light curing unit at different curing distances. Dent. Mater. J. 2014, 33, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Jeanneau, C.; Laurent, P.; Rombouts, C.; Giraud, T.; About, I. Light-cured Tricalcium Silicate Toxicity to the Dental Pulp. J. Endod. 2017, 43, 2074–2080. [Google Scholar] [CrossRef]
- Tabari, K.; Rahbar, M.; Safyari, L.; Safarvand, H. Comparison of compressive strength and setting time of four experimental nanohybrid mineral trioxide aggregates and angelus mineral trioxide aggregate. World J. Dent. 2017, 8, 386–392. [Google Scholar] [CrossRef]
- Abou ElReash, A.; Hamama, H.; Grawish, M.; Saeed, M.; Zaen El-Din, A.M.; Shahin, M.A.; Zhenhuan, W.; Xiaoli, X. A laboratory study to test the responses of human dental pulp stem cells to extracts from three dental pulp capping biomaterials. Int. Endod. J. 2021, 54, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Bakir, E.P.; Yildirim, Z.S.; Bakir, Ş.; Ketani, A. Are resin-containing pulp capping materials as reliable as traditional ones in terms of local and systemic biological effects? Dent. Mater. J. 2021, 41, 78–86. [Google Scholar] [CrossRef]
- Pedano, M.S.; Li, X.; Yoshihara, K.; Van Landuyt, K.; Van Meerbeek, B. Cytotoxicity and bioactivity of dental pulp-capping agents towards human tooth-pulp cells: A systematic review of in-vitro studies and meta-analysis of randomized and controlled clinical trials. Materials 2020, 13, 2670. [Google Scholar] [CrossRef] [PubMed]
- Giraud, T.; Jeanneau, C.; Rombouts, C.; Bakhtiar, H.; Laurent, P.; About, I. Pulp capping materials modulate the balance between inflammation and regeneration. Dent. Mater. 2019, 35, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safety Data Sheet, Predicta TM Flow Dual Cure Bulk-Fill Composite; Parkell Inc.: Brentwood, NY, USA, 2018; Volume 77, pp. 1–25.
- Wawrzynkiewicz, A.; Rozpedek-Kaminska, W.; Galita, G.; Lukomska-Szymanska, M.; Lapinska, B.; Sokolowski, J.; Majsterek, I. The toxicity of universal dental adhesives: An in vitro study. Polymers 2021, 13, 2653. [Google Scholar] [CrossRef] [PubMed]
- Pieklarz, K.; Galita, G.; Tylman, M.; Maniukiewicz, W.; Kucharska, E.; Majsterek, I.; Modrzejewska, Z. Physico-chemical properties and biocompatibility of thermosensitive chitosan lactate and chitosan chloride hydrogels developed for tissue engineering application. J. Funct. Biomater. 2021, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Wawrzynkiewicz, A.; Rozpedek-Kaminska, W.; Galita, G.; Lukomska-Szymanska, M.; Lapinska, B.; Sokolowski, J.; Majsterek, I. The cytotoxicity and genotoxicity of three dental universal adhesives—An in vitro study. Int. J. Mol. Sci. 2020, 21, 3950. [Google Scholar] [CrossRef] [PubMed]
- Manaspon, C.; Jongwannasiri, C.; Chumprasert, S.; Sa-Ard-Iam, N.; Mahanonda, R.; Pavasant, P.; Porntaveetus, T.; Osathanon, T. Human dental pulp stem cell responses to different dental pulp capping materials. BMC Oral Health 2021, 21, 209. [Google Scholar] [CrossRef]
- Sanz, J.L.; Soler-Doria, A.; López-García, S.; García-Bernal, D.; Rodríguez-Lozano, F.J.; Lozano, A.; Llena, C.; Forner, L.; Guerrero-Gironés, J.; Melo, M. Comparative Biological Properties and Mineralization Potential of 3 Endodontic Materials for Vital Pulp Therapy: Theracal PT, Theracal LC, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2021, 47, 1896–1906. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, F.J.; López-García, S.; García-Bernal, D.; Sanz, J.L.; Lozano, A.; Pecci-Lloret, M.P.; Melo, M.; López-Ginés, C.; Forner, L. Cytocompatibility and bioactive properties of the new dual-curing resin-modified calcium silicate-based material for vital pulp therapy. Clin. Oral Investig. 2021, 25, 5009–5024. [Google Scholar] [CrossRef]
- Safety Data Sheet, TheraCalTM LC. 2018. Available online: https://www.bisco.com/assets/1/22/TheraCal_LC_SDS_US_English1.pdf (accessed on 12 September 2022).
- Barszczewska-Rybarek, I.M.; Chrószcz, M.W.; Chladek, G. Novel Urethane-Dimethacrylate Monomers and Compositions for Use as Matrices in Dental Restorative Materials. Int. J. Mol. Sci. 2020, 21, 2644. [Google Scholar] [CrossRef] [Green Version]
- De Nys, S.; Duca, R.C.; Vervliet, P.; Covaci, A.; Boonen, I.; Elskens, M.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Bisphenol A as degradation product of monomers used in resin-based dental materials. Dent. Mater. 2021, 37, 1020–1029. [Google Scholar] [CrossRef]
- Li, L.; Wang, Q.; Zhang, Y.; Niu, Y.; Yao, X.; Liu, H. The molecular mechanism of bisphenol A (BPA) as an endocrine disruptor by interacting with nuclear receptors: Insights from molecular dynamics (MD) simulations. PLoS ONE 2015, 10, e0120330. [Google Scholar] [CrossRef] [PubMed]
- Hampe, T.; Wiessner, A.; Frauendorf, H.; Alhussein, M.; Karlovsky, P.; Bürgers, R.; Krohn, S. Monomer Release from Dental Resins: The Current Status on Study Setup, Detection and Quantification for In Vitro Testing. Polymers 2022, 14, 1790. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.D.; Achilias, D.S. Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.M.; Chang, Y.C. Cytotoxicity of dentine-bonding agents on human pulp cells in vitro. Int. Endod. J. 2002, 35, 905–909. [Google Scholar] [CrossRef]
- Polydorou, O.; Trittler, R.; Hellwig, E.; Kümmerer, K. Elution of monomers from two conventional dental composite materials. Dent. Mater. 2007, 23, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.M.; Tai, K.W.; Chou, M.Y.; Chang, Y.C. Cytotoxicity of resin-, zinc oxide–eugenol-, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int. Endod. J. 2002, 35, 153–158. [Google Scholar] [CrossRef]
- ISO 10993-11:2017; Biological Evaluation of Medical Devices—Part 11: Tests for Systemic Toxicity. ISO: Geneva, Switzerland, 1 September 2017.
- Lipski, M.; Nowicka, A.; Kot, K.; Postek-Stefańska, L.; Wysoczańska-Jankowicz, I.; Borkowski, L.; Andersz, P.; Jarząbek, A.; Grocholewicz, K.; Sobolewska, E.; et al. Factors affecting the outcomes of direct pulp capping using Biodentine. Clin. Oral Investig. 2018, 22, 2021–2029. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunert, M.; Rozpedek-Kaminska, W.; Galita, G.; Sauro, S.; Bourgi, R.; Hardan, L.; Majsterek, I.; Lukomska-Szymanska, M. The Cytotoxicity and Genotoxicity of Bioactive Dental Materials. Cells 2022, 11, 3238. https://doi.org/10.3390/cells11203238
Kunert M, Rozpedek-Kaminska W, Galita G, Sauro S, Bourgi R, Hardan L, Majsterek I, Lukomska-Szymanska M. The Cytotoxicity and Genotoxicity of Bioactive Dental Materials. Cells. 2022; 11(20):3238. https://doi.org/10.3390/cells11203238
Chicago/Turabian StyleKunert, Marta, Wioletta Rozpedek-Kaminska, Grzegorz Galita, Salvatore Sauro, Rim Bourgi, Louis Hardan, Ireneusz Majsterek, and Monika Lukomska-Szymanska. 2022. "The Cytotoxicity and Genotoxicity of Bioactive Dental Materials" Cells 11, no. 20: 3238. https://doi.org/10.3390/cells11203238
APA StyleKunert, M., Rozpedek-Kaminska, W., Galita, G., Sauro, S., Bourgi, R., Hardan, L., Majsterek, I., & Lukomska-Szymanska, M. (2022). The Cytotoxicity and Genotoxicity of Bioactive Dental Materials. Cells, 11(20), 3238. https://doi.org/10.3390/cells11203238









