Molecular Mechanisms Underlying Twin-to-Twin Transfusion Syndrome
Abstract
1. Introduction
2. Placental Expression of Vasoactive Proteins
3. Renin–Angiotensin and Endothelin Systems
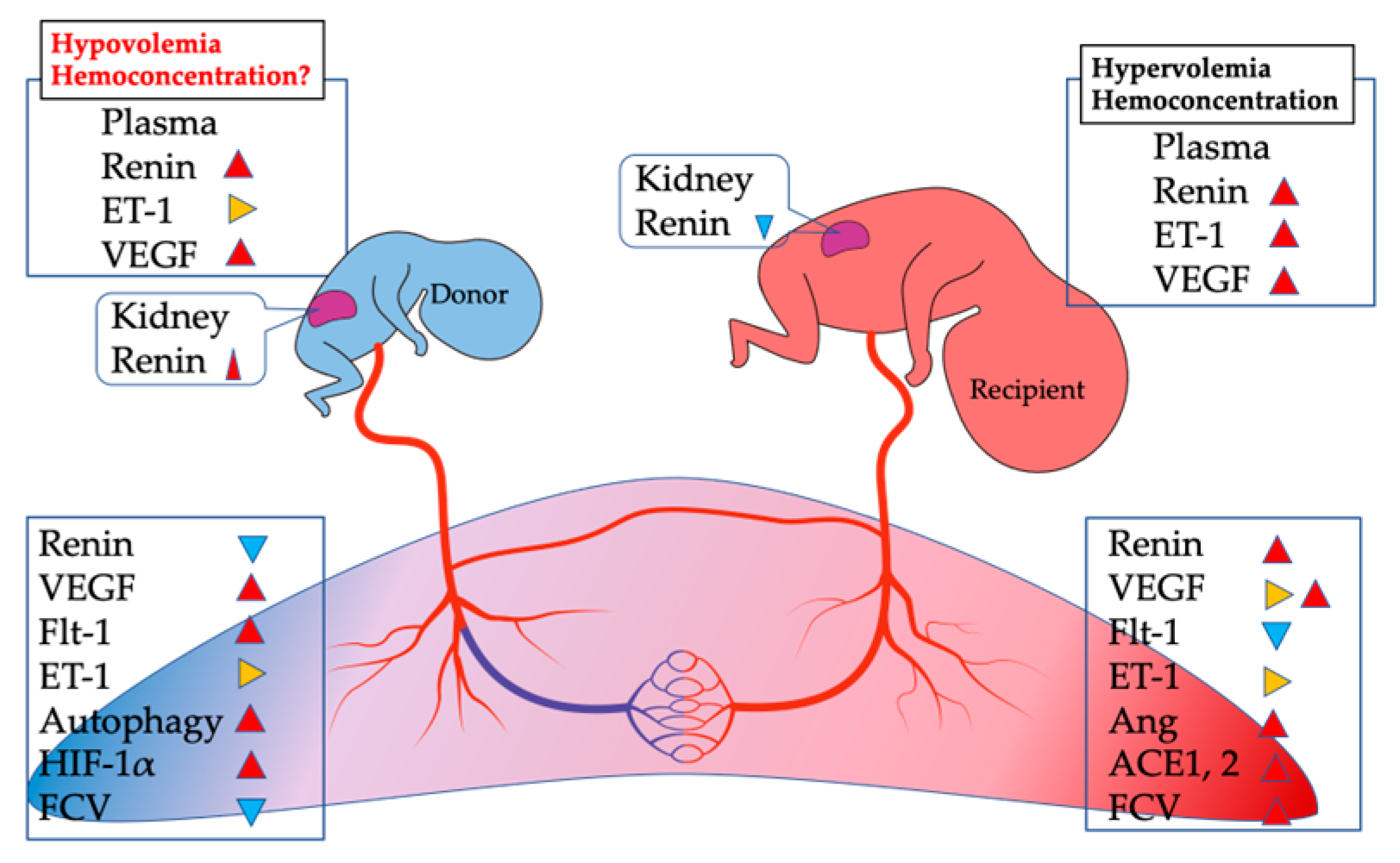
4. Differences in RAS between PE and TTTS
5. Expression of ACE2 in TTTS and TAPS Placentas
6. Is There an Anemic Condition in the Donor Placenta from TTTS?
7. Pathological Changes
8. Hypoxia-Related Factors
9. OS
10. Autophagic Activity
11. IRI
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | angiotensin-converting enzyme |
| AGA | appropriate for gestational age |
| Ang | angiotensin |
| AT-1 | angiotensin II type 1 |
| AT-2 | angiotensin II type 2 |
| AVA | arterio–venous anastomosis |
| CA | carbonic anhydrous |
| DVM | delayed villous maturation |
| ET-1 | endothelin-1 |
| FD | fetal demise |
| FC | fold changes |
| FGR | fetal growth restriction |
| FLP | fetoscopic laser photocoagulation |
| FTV | fetal thrombotic vasculopathy |
| HIF-1α | hypoxia inducible factor 1α |
| HMOX1 | heme oxygenase 1 |
| IGF | insulin-like growth factor |
| IRI | ischemia-reperfusion injury |
| LC3 | light chain 3 |
| MC | monochorionic |
| MCA-PSV | middle cerebral artery peak systolic value |
| MDA | malondialdehyde |
| mtDNA | mitochondrial DNA |
| NFE2L2 | nuclear factor erythroid 2 like 2 |
| OS | oxidative stress |
| PE | preeclampsia |
| PlGF | placental growth factor |
| RAS | renin–angiotensin system |
| RI | resistance index |
| ROS | reactive oxygen species |
| sENG | soluble endoglin |
| sFGR | selective fetal growth restriction |
| sFlt1 | soluble fms-like tyrosine kinase 1 |
| StO2% | tissue venous oxygenation |
| TAPS | twin anemia-polycythemia sequence |
| TTTS | twin-to-twin transfusion syndrome |
| VEGF | vascular endothelial growth factor |
| Δ | delta |
References
- Denbow, M.L.; Cox, P.; Taylor, M.; Hammal, D.M.; Fisk, N.M. Placental angioarchitecture in monochorionic twin pregnancies: Relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am. J. Obstet. Gynecol. 2000, 182, 417–426. [Google Scholar] [CrossRef]
- Quintero, R.A. Twin-twin transfusion syndrome. Clin. Perinatol. 2003, 30, 591–600. [Google Scholar] [CrossRef]
- Sago, H.; Ishii, K.; Sugibayashi, R.; Ozawa, K.; Sumie, M.; Wada, S. Fetoscopic laser photocoagulation for twin-twin transfusion syndrome. J. Obstet. Gynaecol. Res. 2018, 44, 831–839. [Google Scholar] [CrossRef]
- Senat, M.-V.; Deprest, J.; Boulvain, M.; Paupe, A.; Winer, N.; Ville, Y. Endoscopic Laser Surgery versus Serial Amnioreduction for Severe Twin-to-Twin Transfusion Syndrome. N. Engl. J. Med. 2004, 351, 136–144. [Google Scholar] [CrossRef]
- Baschat, A.A.; Barber, J.; Pedersen, N.; Turan, O.M.; Harman, C.R. Outcome after fetoscopic selective laser ablation of placental anastomoses vs equatorial laser dichorionization for the treatment of twin-to-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2013, 209, 234.e1–234.e8. [Google Scholar] [CrossRef]
- Ruano, R.; Rodo, C.; Peiro, J.L.; Shamshirsaz, A.A.; Haeri, S.; Nomura, M.L.; Salustiano, E.M.A.; de Andrade, K.K.; Sangi-Haghpeykar, H.; Carreras, E.; et al. Fetoscopic laser ablation of placental anastomoses in twin-twin transfusion syndrome using ‘Solomon technique’. Ultrasound Obstet. Gynecol. 2013, 42, 434–439. [Google Scholar] [CrossRef]
- Slaghekke, F.; Lewi, L.; Middeldorp, J.M.; Weingertner, A.S.; Klumper, F.J.; Dekoninck, P.; Devlieger, R.; Lanna, M.M.; Deprest, J.; Favre, R.; et al. Residual anastomoses in twin-twin transfusion syndrome after laser: The Solomon randomized trial. Am. J. Obstet. Gynecol. 2014, 211, 285.e1–285.e7. [Google Scholar] [CrossRef]
- Diehl, W.; Diemert, A.; Grasso, D.; Sehner, S.; Wegscheider, K.; Hecher, K. Fetoscopic laser coagulation in 1020 pregnancies with twin-twin transfusion syndrome demonstrates improvement in double-twin survival rate. Ultrasound Obstet. Gynecol. 2017, 50, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Egawa, M.; Hayashi, S.; Yang, L.; Sakamoto, N.; Sago, H. Chorioamniotic membrane separation after fetoscopic laser surgery for twin-twin transfusion syndrome. Prenat. Diagn. 2012, 33, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.C.; Vanderbilt, D.; Chmait, R.H. Neurodevelopmental outcomes after laser therapy for twin-twin transfusion syndrome: A systematic review and meta-analysis. Obstet. Gynecol. 2011, 118, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- van Klink, J.M.; Koopman, H.M.; Oepkes, D.; Walther, F.J.; Lopriore, E. Long-term neurodevelopmental outcome in monochorionic twins after fetal therapy. Early Hum. Dev. 2011, 87, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Snowise, S.; Moise, K.J.; Johnson, A.; Bebbington, M.W.; Papanna, R. Donor Death After Selective Fetoscopic Laser Surgery for Twin–Twin Transfusion Syndrome. Obstet. Gynecol. 2015, 126, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.W.; Cachianes, G.; Kuang, W.-J.; Goeddel, D.V.; Ferrara, N. Vascular Endothelial Growth Factor Is a Secreted Angiogenic Mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Morine, M.; Nobunaga, T.; Mizutani, T.; Yamanaka, K.; Wasada, K.; Maeda, K.; Suehara, N.; Yasui, T.; Irahara, M. Vascular endothelial growth factor in monochorionic twins with twin-twin transfusion syndrome. J. Endocrinol. Investig. 2008, 31, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Poole, T.J.; Coffin, J.D. Vasculogenesis and angiogenesis: Two distinct morphogenetic mechanisms establish embryonic vascular pattern. J. Exp. Zool. 1989, 251, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Houck, K.; Jakeman, L.; Leung, D.W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr. Rev. 1992, 13, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Jackson, M.; Carney, E.; Lye, S.; Ritchie, J.K. Localization of two angiogenic growth factors (PDECGF and VEGF) in human placentae throughout gestation. Placenta 1994, 15, 341–353. [Google Scholar] [CrossRef]
- Clark, D.E.; Smith, S.K.; Sharkey, A.M.; Charnock-Jones, D.S. Localization of VEGF and expression of its receptors flt and KDR in human placenta throughout pregnancy. Hum. Reprod. 1996, 11, 1090–1098. [Google Scholar] [CrossRef]
- Sharkey, A.M.; Charnock-Jones, D.S.; Boocock, C.A.; Brown, K.D.; Smith, S.K. Expression of mRNA for vascular endothelial growth factor in human placenta. Reproduction 1993, 99, 609–615. [Google Scholar] [CrossRef]
- Anthony, F.W.; Wheeler, T.; Elcock, C.L.; Pickett, M.; Thomas, E.J. Short report: Identification of a specific pattern of vascular endothelial growth factor mRNA expression in human placenta and cultured placental fibroblasts. Placenta 1994, 15, 557–561. [Google Scholar] [CrossRef]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of preeclampsia. Annu. Rev. Pathol. 2010, 5, 173–192. [Google Scholar] [CrossRef]
- Roberts, J.M.; Rajakumar, A. Preeclampsia and Soluble fms-Like Tyrosine Kinase 1. J. Clin. Endocrinol. Metab. 2009, 94, 2252–2254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; McMaster, M.; Woo, K.; Janatpour, M.; Perry, J.; Karpanen, T.; Alitalo, K.; Damsky, C.; Fisher, S.J. Vascular Endothelial Growth Factor Ligands and Receptors That Regulate Human Cytotrophoblast Survival Are Dysregulated in Severe Preeclampsia and Hemolysis, Elevated Liver Enzymes, and Low Platelets Syndrome. Am. J. Pathol. 2002, 160, 1405–1423. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L.; Staff, A.C. IFPA Senior Award Lecture: Making sense of pre-eclampsia-two placental causes of preeclampsia? Placenta 2014, 35, S20–S25. [Google Scholar] [CrossRef]
- Staff, A.C. The two-stage placental model of preeclampsia: An update. J. Reprod. Immunol. 2019, 134–135, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gu, B.; Zhang, Y.; Lewis, D.; Wang, Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta 2005, 26, 210–217. [Google Scholar] [CrossRef]
- Nevo, O.; Soleymanlou, N.; Wu, Y.; Xu, J.; Kingdom, J.; Many, A.; Zamudio, S.; Caniggia, I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am. J. Physiol. Integr. Comp. Physiol. 2006, 291, R1085–R1093. [Google Scholar] [CrossRef]
- Nagamatsu, T.; Fujii, T.; Kusumi, M.; Zou, L.; Yamashita, T.; Osuga, Y.; Momoeda, M.; Kozuma, S.; Taketani, Y. Cytotrophoblasts Up-Regulate Soluble Fms-Like Tyrosine Kinase-1 Expression under Reduced Oxygen: An Implication for the Placental Vascular Development and the Pathophysiology of Preeclampsia. Endocrinology 2004, 145, 4838–4845. [Google Scholar] [CrossRef]
- Kumazaki, K.; Nakayama, M.; Suehara, N.; Wada, Y. Expression of vascular endothelial growth factor, placental growth factor, and their receptors Flt-1 and KDR in human placenta under pathologic conditions. Hum. Pathol. 2002, 33, 1069–1077. [Google Scholar] [CrossRef]
- Gathiram, P.; Moodley, J. Pre-eclampsia: Its pathogenesis and pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.E.; Lash, G.E.; Pretlove, S.J.; Chan, B.C.; Holder, R.; Kilby, M.D. Maternal plasma and amniotic fluid angiogenic factors and their receptors in monochorionic twin pregnancies complicated by twin-to-twin transfusion syndrome. Ultrasound Obstet. Gynecol. 2009, 35, 695–701. [Google Scholar] [CrossRef]
- Chon, A.H.; Chavira, E.R.; Wilson, M.; Ingles, S.A.; Llanes, A.; Chmait, R.H. The impact of laser surgery on angiogenic and anti-angiogenic factors in twin–twin transfusion syndrome: A prospective study. J. Matern. Neonatal Med. 2017, 31, 1085–1091. [Google Scholar] [CrossRef]
- Matijevic, R.; Ward, S.; Bajoria, R. Non-invasive Method of Evaluation of Trophoblast Invasion of Spiral Arteries in Monochorionic Twins with Discordant Birthweight. Placenta 2002, 23, 93–99. [Google Scholar] [CrossRef]
- Galea, P.; Barigye, O.; Wee, L.; Jain, V.; Sullivan, M.; Fisk, N. The Placenta Contributes to Activation of the Renin Angiotensin System in Twin–Twin Transfusion Syndrome. Placenta 2008, 29, 734–742. [Google Scholar] [CrossRef]
- Miller, J.W.; Adamis, A.P.; Shima, D.T.; D’Amore, P.A.; Moulton, R.S.; O’Reilly, M.S.; Folkman, J.; Dvorak, H.F.; Brown, L.F.; Berse, B. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am. J. Pathol. 1994, 145, 574–584. [Google Scholar] [CrossRef]
- Pe’Er, J.; Shweiki, D.; Itin, A.; Hemo, I.; Gnessin, H.; Keshet, E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab. Investig. 1995, 72, 638–645. [Google Scholar]
- Diguisto, C.; Piver, E.; Le Gouge, A.; Eboue, F.; Le Vaillant, C.; Maréchaud, M.; Goua, V.; Giraudeau, B.; Perrotin, F. First trimester uterine artery Doppler, sFlt-1 and PlGF to predict preeclampsia in a high-risk population. J. Matern. Neonatal Med. 2017, 30, 1514–1519. [Google Scholar] [CrossRef]
- Crovetto, F.; Figueras, F.; Triunfo, S.; Crispi, F.; Rodriguez-Sureda, V.; Peguero, A.; Dominguez, C.; Gratacos, E. Added Value of Angiogenic Factors for the Prediction of Early and Late Preeclampsia in the First Trimester of Pregnancy. Fetal Diagn. Ther. 2014, 35, 258–266. [Google Scholar] [CrossRef]
- Dröge, L.; Herraìz, I.; Zeisler, H.; Schlembach, D.; Stepan, H.; Küssel, L.; Henrich, W.; Galindo, A.; Verlohren, S. Maternal serum sFlt-1/PlGF ratio in twin pregnancies with and without pre-eclampsia in comparison with singleton pregnancies. Ultrasound Obstet. Gynecol. 2015, 45, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Bdolah, Y.; Lam, C.; Rajakumar, A.; Shivalingappa, V.; Mutter, W.; Sachs, B.P.; Lim, K.H.; Bdolah-Abram, T.; Epstein, F.H.; Karumanchi, S.A. Twin pregnancy and the risk of preeclampsia: Bigger placenta or relative ischemia? Am. J. Obstet. Gynecol. 2008, 198, 428.e1–428.e6. [Google Scholar] [CrossRef] [PubMed]
- Mackie, F.L.; Whittle, R.; Morris, R.K.; Hyett, J.; Riley, R.D.; Kilby, M.D. First-trimester ultrasound measurements and maternal serum biomarkers as prognostic factors in monochorionic twins: A cohort study. Diagn. Progn. Res. 2019, 3, 9. [Google Scholar] [CrossRef][Green Version]
- Kusanovic, J.P.; Romero, R.; Espinoza, J.; Nien, J.K.; Kim, C.J.; Mittal, P.; Edwin, S.; Erez, O.; Gotsch, F.; Mazaki-Tovi, S.; et al. Twin-to-twin transfusion syndrome: An antiangiogenic state? Am J. Obstet. Gynecol. 2008, 198, 382.e1–382.e8. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Mao, Q.; Shapiro, S.; De Paepe, M. Placental endoglin levels in diamniotic-monochorionic twin gestations: Correlation with clinical and placental characteristics. Placenta 2013, 34, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2020, 226, S1019–S1034. [Google Scholar] [CrossRef] [PubMed]
- Mahieu-Caputo, D.; Muller, F.; Joly, D.; Gubler, M.-C.; Lebidois, J.; Fermont, L.; Dumez, Y.; Dommergues, M. Pathogenesis of Twin-Twin Transfusion Syndrome: The Renin-Angiotensin System Hypothesis. Fetal Diagn. Ther. 2001, 16, 241–244. [Google Scholar] [CrossRef]
- Kilby, M.D.; Platt, C.; Whittle, M.J.; Oxley, J.; Lindop, G.B. Renin Gene Expression in Fetal Kidneys of Pregnancies Complicated by Twin-Twin Transfusion Syndrome. Pediatr. Dev. Pathol. 2001, 4, 175–179. [Google Scholar] [CrossRef]
- Wee, L.; Sullivan, M.; Humphries, K.; Fisk, N. Longitudinal Blood Flow in Shared (Arteriovenous Anastomoses) and Non-Shared Cotyledons in Monochorionic Placentae. Placenta 2007, 28, 516–522. [Google Scholar] [CrossRef]
- Gussi, I.; Nizard, J.; Yamamoto, M.; Robyr, R.; Ville, Y. Maternal pseudo primary hyperaldosteronism in twin-to-twin transfusion syndrome. BJOG: Int. J. Obstet. Gynaecol. 2006, 114, 65–69. [Google Scholar] [CrossRef]
- Luhtala, S.; Vaajanen, A.; Oksala, O.; Valjakka, J.; Vapaatalo, H. Activities of Angiotensin-Converting Enzymes ACE1 and ACE2 and Inhibition by Bioactive Peptides in Porcine Ocular Tissues. J. Ocul. Pharmacol. Ther. 2009, 25, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A Novel Angiotensin-Converting Enzyme–Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9. Circ. Res. 2000, 87, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Mehr, A.P.; Kreutz, R. Physiology of Local Renin-Angiotensin Systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Hagemann, A.; Nielsen, A.H.; Poulsen, K. The uteroplacental renin-angiotensin system: A review. Exp. Clin. Endocrinol. Diabetes 1994, 102, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Knock, G.; Sullivan, M.H.; McCarthy, A.; Elder, M.G.; Polak, J.M.; Wharton, J. Angiotensin II (AT1) vascular binding sites in human placentae from normal-term, preeclamptic and growth retarded pregnancies. J. Pharmacol. Exp. Ther. 1994, 271, 1007–1015. [Google Scholar] [PubMed]
- Masaki, T. Possible role of endothelin in endothelial regulation of vascular tone. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 235–255. [Google Scholar] [CrossRef] [PubMed]
- Nussdorfer, G.G.; Rossi, G.P.; Belloni, A.S. The Role of Endothelins in the Paracrine Control of the Secretion and Growth of the Adrenal Cortex. Int. Rev. Cytol. 1997, 171, 267–308. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Sacchetto, A.; Cesari, M.; Pessina, A.C. Interactions between endothelin-1 and the renin–angiotensin–aldosterone system. Cardiovasc. Res. 1999, 43, 300–307. [Google Scholar] [CrossRef]
- Bajoria, R.; Ward, S.; Chatterjee, R. Brain natriuretic peptide and endothelin-1 in the pathogenesis of polyhydramnios-oligohydramnios in monochorionic twins. Am. J. Obstet. Gynecol. 2003, 189, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Bajoria, R.; Sullivan, M.; Fisk, N. Endothelin concentrations in monochorionic twins with severe twin–twin transfusion syndrome. Hum. Reprod. 1999, 14, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Bajoria, R.; Ward, S.; Chatterjee, R. Natriuretic peptides in the pathogenesis of cardiac dysfunction in the recipient fetus of twin-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2002, 186, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Wharton, J.; Rutherford, R.A.D.; Gordon, L.; Moscoso, G.; Schiemberg, I.; Gaer, J.A.R.; Taylor, K.M.; Polak, J.M. Localization of Endothelin Binding Sites and Endothelin-Like Immunoreactivity in Human Fetal Heart. J. Cardiovasc. Pharmacol. 1991, 17, S378–S384. [Google Scholar] [CrossRef]
- Lopriore, E.; Bökenkamp, R.; Rijlaarsdam, M.; Sueters, M.; Vandenbussche, F.P.; Walther, F.J. Congenital Heart Disease in Twin-to-twin Transfusion Syndrome Treated with Fetoscopic Laser Surgery. Congenit. Hear. Dis. 2007, 2, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Discher, D.J.; Bishopric, N.H.; Webster, K.A. Hypoxia Regulates Expression of the Endothelin-1 Gene through a Proximal Hypoxia-Inducible Factor-1 Binding Site on the Antisense Strand. Biochem. Biophys. Res. Commun. 1998, 245, 894–899. [Google Scholar] [CrossRef]
- Ito, H.; Adachi, S.; Tamamori, M.; Fujisaki, H.; Tanaka, M.; Lin, M.; Akimoto, H.; Marumo, F.; Hiroe, M. Mild Hypoxia Induces Hypertrophy of Cultured Neonatal Rat Cardiomyocytes: A Possible Endogenous Endothelin-1-mediated Mechanism. J. Mol. Cell. Cardiol. 1996, 28, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, E.; Ishida, J.; Sugiyama, F.; Horiguchi, H.; Murakami, K.; Fukamizu, A. Hypertension Induced in Pregnant Mice by Placental Renin and Maternal Angiotensinogen. Science 1996, 274, 995–998. [Google Scholar] [CrossRef]
- Herse, F.; Dechend, R.; Harsem, N.K.; Wallukat, G.; Janke, J.; Qadri, F.; Hering, L.; Muller, D.N.; Luft, F.; Staff, A.C. Dysregulation of the Circulating and Tissue-Based Renin-Angiotensin System in Preeclampsia. Hypertension 2007, 49, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Gathiram, P.; Moodley, J. The Role of the Renin-Angiotensin-Aldosterone System in Preeclampsia: A Review. Curr. Hypertens. Rep. 2020, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- Sugulle, M.; Heidecke, H.; Maschke, U.; Herse, F.; Danser, A.J.; Mueller, D.N.; Staff, A.C.; Dechend, R. Soluble (pro)renin receptor in preeclampsia and diabetic pregnancies. J. Am. Soc. Hypertens. 2017, 11, 644–652. [Google Scholar] [CrossRef]
- Lumbers, E.R.; Gunn, A.J.; Zhang, D.Y.; Wu, J.J.; Maxwell, L.; Bennet, L. Nonimmune hydrops fetalis and activation of the renin-angiotensin system after asphyxia in preterm fetal sheep. Am. J. Physiol. Integr. Comp. Physiol. 2001, 280, R1045–R1051. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Chhabra, K.H.; Chodavarapu, H.; Lazartigues, E. Angiotensin converting enzyme 2: A new important player in the regulation of glycemia. IUBMB Life 2013, 65, 731–738. [Google Scholar] [CrossRef]
- Pringle, K.G.; Tadros, M.A.; Callister, R.J.; Lumbers, E.R. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: Roles in trophoblast invasion and angiogenesis? Placenta 2011, 32, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Imperio, G.E.; Lye, P.; Mughis, H.; Hamada, H.; Bloise, E.; Lye, S.J.; Matthews, S.G. Hypoxia alters the expression of ACE2 and TMPRSS2 SARS-CoV-2 cell entry mediators in hCMEC/D3 brain endothelial cells. Microvasc. Res. 2021, 138, 104232. [Google Scholar] [CrossRef] [PubMed]
- Cuffe, J.; Walton, S.; Steane, S.; Singh, R.; Simmons, D.; Moritz, K. The effects of gestational age and maternal hypoxia on the placental renin angiotensin system in the mouse. Placenta 2014, 35, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Su, H.; Ma, X.; Xu, X.; Liang, L.; Ma, G.; Shi, L. MiRNA let-7b promotes the development of hypoxic pulmonary hypertension by targeting ACE2. Am. J. Physiol. Cell. Mol. Physiol. 2019, 316, L547–L557. [Google Scholar] [CrossRef] [PubMed]
- Serebrovska, Z.O.; Chong, E.Y.; Serebrovska, T.V.; Tumanovska, L.V.; Xi, L. Hypoxia, HIF-1α, and COVID-19: From pathogenic factors to potential therapeutic targets. Acta Pharmacol. Sin. 2020, 41, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Niecknig, H.; Tug, S.; Reyes, B.D.; Kirsch, M.; Fandrey, J.; Berchner-Pfannschmidt, U. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic. Res. 2012, 46, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Valdés, G.; Corthorn, J.; Bharadwaj, M.S.; Joyner, J.; Schneider, D.; Brosnihan, K.B. Utero-placental expression of angiotensin-(1–7) and ACE2 in the pregnant guinea-pig. Reprod. Biol. Endocrinol. 2013, 11, 5. [Google Scholar] [CrossRef]
- Mao, Q.; Chu, S.; Shapiro, S.; Bliss, J.M.; De Paepe, M.E. Increased placental expression of angiotensin-converting enzyme 2, the receptor of SARS-CoV-2, associated with hypoxia in twin anemia-polycythemia sequence (TAPS). Placenta 2021, 105, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Chu, S.; Shapiro, S.; Yao, H.; De Paepe, M.E. Discordant placental oxygenation and autophagy in twin anemia-polycythemia sequence (TAPS). Placenta 2019, 90, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Tongprasert, F.; Srisupundit, K.; Luewan, S.; Tongsong, T. Comparison of maternal serum PlGF and sFlt-1 between pregnancies with and without fetal hemoglobin Bart’s disease. Prenat. Diagn. 2013, 33, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.; Sass, N.; Boute, T.; Moron, A.F. sFlt-1 and PlGF levels in a patient with mirror syndrome related to cytomegalovirus infection. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 366–367. [Google Scholar] [CrossRef]
- Espinoza, J.; Romero, R.; Nien, J.K.; Kusanovic, J.P.; Richani, K.; Gomez, R.; Kim, C.J.; Mittal, P.; Gotsh, F.; Erez, O. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne’s syndrome). J. Matern Fetal Neonatal Med. 2006, 19, 607–613. [Google Scholar] [CrossRef]
- Kaiser, I. Ballantyne and triple edema. Am. J. Obstet. Gynecol. 1971, 110, 115–120. [Google Scholar] [CrossRef]
- Stepan, H.; Faber, R. Elevated sFlt1 Level and Preeclampsia with Parvovirus-Induced Hydrops. N. Engl. J. Med. 2006, 354, 1857–1858. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Seyama, T.; Mimura, N.; Furuya, H.; Nakayama, T.; Iriyama, T.; Nagamatsu, T.; Osuga, Y.; Fujii, T. Elevation of maternal serum sFlt-1 in pregnancy with mirror syndrome caused by fetal cardiac failure. Oxf. Med. Case Rep. 2018, 2018, omx112. [Google Scholar] [CrossRef] [PubMed]
- Barker, G.; Boyd, R.; D’Souza, S.; Donnai, P.; Fox, H.; Sibley, C. Placental water content and distribution. Placenta 1994, 15, 47–56. [Google Scholar] [CrossRef]
- Alvarez, H.; Sala, M.A.; Benedetti, W.L. Intervillous space reduction in the edematous placenta. Am. J. Obstet. Gynecol. 1972, 112, 819–820. [Google Scholar] [CrossRef]
- Naeye, R.L.; Maisels, M.J.; Lorenz, R.P.; Botti, J.J. The clinical significance of placental villous edema. Pediatrics 1983, 71, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Kovalovszki, L.; Villányi, E.; Benkó, G. Placental villous edema: A possible cause of antenatal hypoxia. Acta Paediatr Hung 1990, 30, 209–215. [Google Scholar]
- Sala, A.M.; Matheus, M. Placental characteristics in twin transfusion syndrome. Arch. Gynecol. Obstet. 1989, 246, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kontopoulos, E.V.; Quintero, R.A. Assessment of the peak systolic velocity of the middle cerebral artery in twin-twin transfusion syndrome. Part I: Preoperative assessment. Am. J. Obstet. Gynecol. 2009, 200, 61.e1–61.e5. [Google Scholar] [CrossRef] [PubMed]
- Denbow, M.; Fogliani, R.; Kyle, P.; Letsky, E.; Nicolini, U.; Fisk, N. Haematological indices at fetal blood sampling in monochorionic pregnancies complicated by feto-fetal transfusion syndrome. Prenat. Diagn. 1998, 18, 941–946. [Google Scholar] [CrossRef]
- Mahieu-Caputo, D.; Meulemans, A.; Martinovic, J.; Gubler, M.-C.; Delezoide, A.-L.; Muller, F.; Madelenat, P.; Fisk, N.; Dommergues, M. Paradoxic Activation of the Renin-Angiotensin System in Twin-Twin Transfusion Syndrome: An Explanation for Cardiovascular Disturbances in the Recipient. Pediatr. Res. 2005, 58, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Lopriore, E.; Slaghekke, F.; Oepkes, D.; Middeldorp, J.M.; Vandenbussche, F.P.H.A.; Walther, F. Hematological characteristics in neonates with twin anemia-polycythemia sequence (TAPS). Prenat. Diagn. 2010, 30, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, S.; Katsumura, K.; Kobayashi, T.; Puro, D.G. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am. J. Physiol. Circ. Physiol. 2006, 290, H925–H934. [Google Scholar] [CrossRef]
- Wee, L.; Sebire, N.; Bhundia, J.; Sullivan, M.; Fisk, N. Histomorphometric Characterisation of Shared and Non-shared Cotyledonary Villus Territories of Monochorionic Placentae in Relation to Pregnancy Complications. Placenta 2006, 27, 475–482. [Google Scholar] [CrossRef]
- Redline, R.W.; Patterson, P. Patterns of placental injury. Correlations with gestational age, placental weight, and clinical diagnoses. Arch. Pathol. Lab Med. 1994, 118, 698–701. [Google Scholar]
- Redline, R.W.; Pappin, A. Fetal thrombotic vasculopathy: The clinical significance of extensive avascular villi. Hum. Pathol. 1995, 26, 80–85. [Google Scholar] [CrossRef]
- Redline, R.W.; O’Riordan, M.A. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch. Pathol. Lab. Med. 2000, 124, 1785–1791. [Google Scholar] [CrossRef]
- Vern, T.Z.; Alles, A.J.; Kowal-Vern, A.; Longtine, J.; Roberts, D.J. Frequency of factor V(Leiden) and prothrombin G20210A in placentas and their relationship with placental lesions. Hum. Pathol. 2000, 31, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.P.; Hecht, J.L.; Kane, S.E. Incidence and clinicopathologic correlation of fetal vessel thrombosis in mono- and dichorionic twin placentas. J. Perinatol. 2010, 30, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Quintero, R.A.; Morales, W.J.; Allen, M.H.; Bornick, P.W.; Johnson, P.K.; Kruger, M. Staging of Twin-Twin Transfusion Syndrome. J. Perinatol. 1999, 19, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Redline, R.W.; Shah, D.; Sakar, H.; Schluchter, M.; Salvator, A. Placental Lesions Associated with Abnormal Growth in Twins. Pediatr. Dev. Pathol. 2001, 4, 473–481. [Google Scholar] [CrossRef]
- Emmrich, P. Pathology of the placenta. XI. Feto-fetal transfusion syndrome. Zent. Pathol. 1992, 138, 255–259. [Google Scholar]
- Steffensen, T.S.; Gilbert-Barness, E.; Spellacy, W.; Quintero, R.A. Placental pathology in trap sequence: Clinical and pathogenetic implications. Fetal Pediatr. Pathol. 2008, 27, 13–29. [Google Scholar] [CrossRef]
- Jaiman, S.; Romero, R.; Pacora, P.; Jung, E.J.; Kacerovsky, M.; Bhatti, G.; Yeo, L.; Hsu, C.-D. Placental delayed villous maturation is associated with evidence of chronic fetal hypoxia. J. Périnat. Med. 2020, 48, 516–518. [Google Scholar] [CrossRef]
- Quintero, R.A.; Chmait, R.H.; Carver, J.; Bornick, P.W.; Allen, M.H.; Kontopoulos, E.V. In utero fetal oximetry via visible light spectroscopy in twin-twin transfusion syndrome. Am. J. Obstet. Gynecol. 2008, 199, 636.e1–636.e4. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K.; Kharazinejad, E.; Majidpoor, J.; Ahadi, R. Hypoxia in solid tumors: A key promoter of cancer stem cell (CSC) resistance. J. Cancer Res. Clin. Oncol. 2019, 146, 19–31. [Google Scholar] [CrossRef]
- Wu, Q.; You, L.; Nepovimova, E.; Heger, Z.; Wu, W.; Kuca, K.; Adam, V. Hypoxia-inducible factors: Master regulators of hypoxic tumor immune escape. J. Hematol. Oncol. 2022, 15, 77. [Google Scholar] [CrossRef]
- Zhang, G.-L.; He, Z.-M.; Shi, X.-M.; Gou, C.-Y.; Gao, Y.; Fang, Q. Discordant HIF1A mRNA levels and oxidative stress in placental shares of monochorionic twins with selective intra-uterine growth restriction. Placenta 2015, 36, 297–303. [Google Scholar] [CrossRef]
- Wu, J.; He, Z.; Gao, Y.; Zhang, G.; Huang, X.; Fang, Q. Placental NFE2L2 is discordantly activated in monochorionic twins with selective intrauterine growth restriction and possibly regulated by hypoxia. Free Radic. Res. 2017, 51, 351–359. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Chao, A.-S.; Chang, S.-D.; Cheng, P.-J. Placental glucose transporter 1 and 3 gene expression in Monochorionic twin pregnancies with selective fetal growth restriction. BMC Pregnancy Childbirth 2021, 21, 260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kastrenopoulou, A.; Larrouture, Q.; Athanasou, N.A.; Knowles, H.J. Angiopoietin-like 4 promotes osteosarcoma cell proliferation and migration and stimulates osteoclastogenesis. BMC Cancer 2018, 18, 536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, D.; Fang, Q.; Zhong, M. Aberrant hydroxymethylation of ANGPTL4 is associated with selective intrauterine growth restriction in monochorionic twin pregnancies. Epigenetics 2020, 15, 887–899. [Google Scholar] [CrossRef]
- Yang, L.L.; Shao, H.; Yuan, P.-B.; Guo, X.-Y.; Zhang, X.-W.; Zhao, Y.-Y. Expressions of HIF-α and its target gene in monochorionic twin placentas with twin-twin transfusion syndrome. Beijing Da Xue Xue Bao Yi Xue Ban 2011, 43, 792–797. [Google Scholar]
- Talbert, D.G.; Bajoria, R.; Sepulveda, W.; Bower, S.; Fisk, N.M. Hydrostatic and osmotic pressure gradients produce manifestations of fetofetal transfusion syndrome in a computerized model of monochorial twin pregnancy. Am. J. Obstet. Gynecol. 1996, 174, 598–608. [Google Scholar] [CrossRef]
- Isozaki-Fukuda, Y.; Kojima, T.; Hirata, Y.; Ono, A.; Sawaragi, S.; Sawaragi, I.; Kobayashi, Y. Plasma Immunoreactive Endothelin-1 Concentration in Human Fetal Blood: Its Relation to Asphyxia. Pediatr. Res. 1991, 30, 244–247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.; Chen, S.J.; Chen, Y.F.; Meng, Q.C.; Durand, J.; Oparil, S.; Elton, T.S. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J. Appl. Physiol. 1994, 77, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Chang, S.-D.; Chao, A.-S.; Sieber, M.; Tsai, C.-L.; Cheng, P.-J. Effect of Hypoxia on Glucose Transporter 1 and 3 Gene Expression in Placental Mesenchymal Stem Cells Derived from Growth-Restricted Fetuses. Genes 2022, 13, 752. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1866, 165354. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Myatt, L. Placental adaptive responses and fetal programming. J. Physiol. 2006, 572, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Gür, S.; Türk, G.; Demirci, E.; Yüce, A.; Sönmez, M.; Özer, Ş.; Aksu, E. Effect of Pregnancy and Foetal Number on Diameter of Corpus Luteum, Maternal Progesterone Concentration and Oxidant/Antioxidant Balance in Ewes. Reprod. Domest. Anim. 2011, 46, 289–295. [Google Scholar] [CrossRef]
- Jantsch, L.B.; De Lucca, L.; Dorneles, B.N.; Konopka, C.K.; Gonçalves, T.D.L. Evaluation of oxidative stress and δ-aminolevulinate dehydratase activity in twin pregnancies. J. Matern. Neonatal Med. 2019, 33, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Cheng, Y.K.Y.; Wu, L.; Chaemsaithong, P.; Leung, M.B.W.; Chim, S.S.C.; Sahota, D.S.; Li, W.; Poon, L.C.Y.; Wang, C.C.; et al. Whole genome miRNA profiling revealed miR-199a as potential placental pathogenesis of selective fetal growth restriction in monochorionic twin pregnancies. Placenta 2020, 92, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yin, P.H.; Lu, C.Y.; Chi, C.W.; Wei, Y.H. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem. J. 2000, 348, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Wang, C.-N.; Wei, P.-C.; Peng, H.-H.; Chao, A.-S.; Chang, S.-D.; Cheng, P.-J.; Wang, T.-H. Mitochondrial activation in the growth-restricted fetus of monochorionic twins. Fertil. Steril. 2013, 100, 241–246.e2. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Chao, A.-S.; Peng, H.-H.; Chang, S.-D.; Su, S.-Y.; Chen, K.-J.; Wang, T.-H. Effects of inter-twin vascular anastomoses of monochorionic twins with selective intrauterine growth restriction on the contents of placental mitochondria DNA. BMC Pregnancy Childbirth 2018, 18, 74. [Google Scholar] [CrossRef]
- Levytska, K.; Kingdom, J.; Baczyk, D.; Drewlo, S. Heme oxygenase-1 in placental development and pathology. Placenta 2013, 34, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.M.; Torky, H.; Fahmed, M.A.; Gayed, S.; Salemouad, A.K.; GadAllah, S.H.; Waked, N.W.; Gayed, A.S.; Salem, A.K. Role of antioxidants in gestational diabetes mellitus and relation to fetal outcome: A randomized controlled trial. J. Matern. Fetal Neonatal Med. 2016, 29, 4049–4054. [Google Scholar] [CrossRef] [PubMed]
- Mert, I.; Oruc, A.S.; Yuksel, S.; Cakar, E.S.; Buyukkagnıcı, U.; Karaer, A.; Danısman, N. Role of oxidative stress in preeclampsia and intrauterine growth restriction. J. Obstet. Gynaecol. Res. 2012, 38, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Role of Redox in Fetal Development and Neonatal Diseases. Antioxidants Redox Signal. 2004, 6, 147–153. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Liu, D. Silibinin ameliorats H2O2-induced cell apoptosis and oxidative stress response by activating Nrf2 signaling in trophoblast cells. Acta Histochem. 2020, 122, 151620. [Google Scholar] [CrossRef]
- Mikhail, M.S.; Anyaegbunam, A.; Garfinkel, D.; Palan, P.R.; Basu, J.; Romney, S.L. Preeclampsia and antioxidant nutrients: Decreased plasma levels of reduced ascorbic acid, α-tocopherol, and beta-carotene in women with preeclampsia. Am. J. Obstet. Gynecol. 1994, 171, 150–157. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Shah, S.V. Autophagy in acute kidney injury. Kidney Int. 2016, 89, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-S.; Kim, G.J. The role of autophagy in the placenta as a regulator of cell death. Clin. Exp. Reprod. Med. 2014, 41, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Longtine, M.; Nelson, D.M. Hypoxia Induces Autophagy in Primary Human Trophoblasts. Endocrinology 2012, 153, 4946–4954. [Google Scholar] [CrossRef]
- Signorelli, P.; Avagliano, L.; Virgili, E.; Gagliostro, V.; Doi, P.; Braidotti, P.; Bulfamante, G.; Ghidoni, R.; Marconi, A. Autophagy in term normal human placentas. Placenta 2011, 32, 482–485. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Wang, T.-H.; Chang, S.-D.; Chao, A.-S.; Hsieh, P.C.C.; Wang, C.-N. Increased autophagy in the placental territory of selective intrauterine growth-restricted monochorionic twins. Prenat. Diagn. 2013, 33, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Wang, T.-H.; Chang, S.-D.; Chao, A.-S.; Hsieh, P.C. Fetoscopic laser coagulation of intertwin anastomoses reduces discordant placental autophagic activities in discordant twin growth. Taiwan. J. Obstet. Gynecol. 2015, 54, 580–582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.-Y.; Lee, S.-P.; Lee, J.-S.; Yoon, S.-J.; Jun, G.; Hwang, Y.-J. Telomerase and Apoptosis in the Placental Trophoblasts of Growth Discordant Twins. Yonsei Med, J. 2006, 47, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Almog, B.; Fainaru, O.; Gamzu, R.; Kupferminc, M.; Sasson, R.; Gold, R.; Lessing, J.; Amsterdam, A.; Many, A. Placental Apoptosis in Discordant Twins. Placenta 2002, 23, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, K.; Beharier, O.; Chng, C.-P.; Goff, J.P.; Ouyang, Y.; Croix, C.M.S.; Huang, C.; Kagan, V.E.; Hsia, K.J.; Sadovsky, Y. Ferroptosis induces membrane blebbing in placental trophoblasts. J. Cell Sci. 2021, 135, jcs255737. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Cortijo, D.; de la Calle, M.; Rodriguez-Rodriguez, P.; Phuthong, S.; de Pablo, L.L.; Martín-Cabrejas, M.A.; Arribas, S.M. First trimester elevations of hematocrit, lipid peroxidation and nitrates in women with twin pregnancies who develop preeclampsia. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2020, 22, 132–135. [Google Scholar] [CrossRef]
- Haram, K.; Mortensen, J.H.; Myking, O.; Magann, E.F.; Morrison, J.C. The Role of Oxidative Stress, Adhesion Molecules and Antioxidants in Preeclampsia. Curr. Hypertens. Rev. 2019, 15, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ortuño-Sahagún, D.; Pallàs, M.; Rojas-Mayorquín, A.E. Oxidative stress in aging: Advances in proteomic approaches. Oxid. Med. Cell Longev. 2014, 2014, 573208. [Google Scholar] [CrossRef] [PubMed]
- Many, A.; Hubel, C.A.; Fisher, S.J.; Roberts, J.M.; Zhou, Y. Invasive Cytotrophoblasts Manifest Evidence of Oxidative Stress in Preeclampsia. Am. J. Pathol. 2000, 156, 321–331. [Google Scholar] [CrossRef]
- Davidge, S.T.; Hubel, A.C.; Brayden, R.D.; Capeless, E.C.; McLaughlin, M.K. Sera antioxidant activity in uncomplicated and preeclamptic pregnancies. Obstet. Gynecol. 1992, 79, 897–901. [Google Scholar] [PubMed]
- Ries, M.; Beinder, E.; Gruner, C.; Zenker, M. Rapid development of hydrops fetalis in the donor twin following death of the recipient twin in twin-twin transfusion syndrome. J. Périnat. Med. 1999, 27, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Mahone, P.R.; Sherer, D.M.; Abramowicz, J.S.; Woods, J.R. Twin-twin transfusion syndrome: Rapid development of severe hydrops of the donor following selective feticide of the hydropic recipient. Am. J. Obstet. Gynecol. 1993, 169, 166–168. [Google Scholar] [CrossRef]
- Menger, M.D.; Rücker, M.; Vollmar, B. Capillary dysfunction in striated muscle ischemia/reperfusion: On the mechanisms of capillary “no-reflow”. Shock 1997, 8, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Verrier, E. The Microvascular Cell and Ischemia-Reperfusion Injury. J. Cardiovasc. Pharmacol. 1996, 27, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, A.; Giri, T.; Jiang, J.; Bice, A.; Quirk, J.D.; Conyers, S.B.; Maloney, S.E.; Raghuraman, N.; Bauer, A.Q.; Garbow, J.R.; et al. In utero exposure to transient ischemia-hypoxemia promotes long-term neurodevelopmental abnormalities in male rat offspring. JCI Insight 2020, 5, e133172. [Google Scholar] [CrossRef] [PubMed]
- Beharier, O.; Tyurin, V.A.; Goff, J.P.; Guerrero-Santoro, J.; Kajiwara, K.; Chu, T.; Tyurina, Y.Y.; Croix, C.M.S.; Wallace, C.T.; Parry, S.; et al. PLA2G6 guards placental trophoblasts against ferroptotic injury. Proc. Natl. Acad. Sci. USA 2020, 117, 27319–27328. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajiwara, K.; Ozawa, K.; Wada, S.; Samura, O. Molecular Mechanisms Underlying Twin-to-Twin Transfusion Syndrome. Cells 2022, 11, 3268. https://doi.org/10.3390/cells11203268
Kajiwara K, Ozawa K, Wada S, Samura O. Molecular Mechanisms Underlying Twin-to-Twin Transfusion Syndrome. Cells. 2022; 11(20):3268. https://doi.org/10.3390/cells11203268
Chicago/Turabian StyleKajiwara, Kazuhiro, Katsusuke Ozawa, Seiji Wada, and Osamu Samura. 2022. "Molecular Mechanisms Underlying Twin-to-Twin Transfusion Syndrome" Cells 11, no. 20: 3268. https://doi.org/10.3390/cells11203268
APA StyleKajiwara, K., Ozawa, K., Wada, S., & Samura, O. (2022). Molecular Mechanisms Underlying Twin-to-Twin Transfusion Syndrome. Cells, 11(20), 3268. https://doi.org/10.3390/cells11203268





