Abstract
Non-syndromic cleft lip with or without cleft palate (NSCL/P) is a complex disease with a strong genetic component. More than 40 loci have been identified to be associated with the risk of NSCL/P by genome-wide association studies (GWASs), but the majority of these variants are mapped to non-coding regions of the genome. Expression quantitative trait locus (eQTL) studies have increasingly been integrated with GWASs to identify target genes for these non-coding variants. In this study, we generated a unique, lip-specific eQTL dataset from 40 NSCL/P patients. A total of 5158 eQTL SNPs (eSNPs) -689 eQTL genes were identified after multiple corrections. Then, we integrated nominal eQTL SNPs with NSCL/P risk SNPs and identified 243 variants associated with the expression of 18 genes in lip tissues. Functional annotation analysis indicated that these risk eSNPs were significantly enriched in transcription regulation and chromatin open regions on the genome. These susceptible genes were enriched in cell fate determination, the pluripotency of stem cells, and Wnt signaling pathways. Finally, 8 of the 18 susceptible genes were differentially expressed in NSCL/P case-control studies. In summary, we have generated a unique lip-specific eQTL resource and identified multiple associations for NSCL/P risk loci, which should inform functional studies of NSCL/P biology.
1. Introduction
Non-syndromic cleft lip with or without cleft palate (NSCL/P) is one of the most common birth defects characterized by a cleft in the lip or palate, with an incidence of 1:1000 worldwide [1]. Environmental exposures, such as malnutrition and smoking during pregnancy, have been shown to play a role in the diseases [2]. Apart from these effects, it has been proven that NSCL/P has a compelling genetic predisposition through twin and familial clustering studies [3]. More than 25% to 35% of cleft lip and palate cases had a family history.
Genome-wide association studies (GWASs) have been widely conducted in the analysis and prediction of disease pathogenesis. So far, more than 40 risk loci have been identified through GWASs [4,5,6,7]. However, GWASs do not necessarily determine causal variants and genes. The majority of the association signals are localized in non-coding regions of the genome, which is a great challenge for biological interpretation [8,9]. In addition, it has been determined that casual genetic variants have usually been mapped to regulatory regions in disease-associated cell types or tissues, altering the expression of downstream target genes. Expression quantitative trait locus (eQTL) analyses, which associate genetic variants with gene expression, have increasingly been integrated with GWASs to identify disease-susceptibility genes [10,11,12,13]. However, many studies have used eQTL data non-specified for NSCL/P, with the exception of a study using the orbicularis oris muscle of NSCL/P-affected individuals [14], there is a lack of eQTL data that is specific for NSCL/P.
To identify susceptibility genes of NSCL/P, we generated an eQTL dataset using 40 lip tissues that were obtained from NSCL/P patients. We combined this information with published GWAS data to identify genetic regulatory patterns of NSCL/P and to gain a better understanding of its biological mechanisms.
2. Materials and Methods
2.1. Subjects
We obtained redundant lip tissues and venous blood samples from 40 NSCL/P patients, which was approved by the Institutional Review Board of Nanjing Medical University (NJMUERC [2008] No. 20). All patients signed informed consents. All patients were clinically assessed by an experienced oral surgeon to ensure that individuals with other congenital deformities or syndromic orofacial clefts were excluded. The basic information of the participants is shown in Table S1.
2.2. RNA Sequencing and Genotype Data
The total RNA from these lip tissues was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The quality control of RNA was determined using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and 1% agarose electrophoresis. Sequencing libraries were generated using Nova6000 (Illumina, San Diego, CA, USA) by Genergy Bio (Shanghai, China), following the manufacturer’s recommendations. The transcript per million (TPM) values were used to normalize the expression levels of the genes.
Genotyping of the 40 patients was conducted via the Affymetrix Axiom Genome-Wide CHB1 and CHB2 Array by the CapitalBio Corporation. The imputation of ungenotyped SNPs was calculated using IMPUTE2 [15] from the 1000 Genomes Project. Basic quality control was completed by removing the SNPs that did not meet the locating conditions in autosomal chromosomes, having a call rate <95%, or having a genotype distribution in the controls that deviated from Hardy–Weinberg equilibrium (HWE; p < 1.0 × 10−5).
2.3. eQTL Analysis
eQTL identification was conducted using gene expression as the outcome, SNP dosage as the independent variable, and gender/age as the covariates. The MatrixEQTL [16] package is a toolset implemented in R to identify eQTLs, and detects the association between genotypes and gene expression with a linear regression model. We used this algorithm to test, on a large-scale basis, the associations in our data. Correction for multiple testing was calculated for each of the association tests between SNPs and the transcription start site (TSS), within 1 megabase (MB).
2.4. Integrative Analysis of eQTL and GWAS Data
NSCL/P risk SNPs were retrieved from the GWAS catalog database [17], which included 70 independent tag SNPs. In addition, the linkage disequilibrium analysis, based on the 1000 Genomes Project, was conducted to identify all susceptibility SNPs. A direct overlap was performed between NSCL/P risk SNPs and eQTL SNPs. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses of the eGene regulated by risk SNPs were determined using KOBAS-i [18]. We selected the top 6 pathways or biological processes with the lowest FDR.
2.5. Histone Mark and Transcription Factor Binding Site Enrichment Analysis
Histone and transcription factor modifications suggest the potential regulatory regions of the genome. We used this information to ascertain which regions the eSNPs were enriched in and to interpret the latent functions of these eSNPs.
Histone modification data of the embryonic craniofacial tissues were retrieved from GSE97752 [19]. It is ideal to use ChIP-seq data from lip tissues for this enrichment analysis. However, due to the limited size of the lip samples, we failed to create more specific ChIP-seq data from the lip tissues. Given that NSCL/P is a congenital craniofacial malformation and resulted from disturbed processes during embryonic development, the transcription factor ChIP-seq data from ESCs (embryonic stem cells) were retrieved from Cistromes (http://cistrome.org/db) and applied for the enrichment analysis. GREGOR [20] (Genomic Regulatory Elements and Gwas Overlap algorithm) v1.4.0, implemented in Perl, was used to assess eSNP enrichment scores in the above functional genomic regions.
2.6. Differentially Expression Analysis
To determine whether candidate genes were differentially expressed in NSCL/P pathogenesis, we retrieved and downloaded two datasets, GSE42589 [21] and GSE85748 [14], which preserved gene expression profiles from the GEO database. GSE42589 included 7 NSCL/P patients and 6 healthy individuals, and GSE85748 contained 42 NSCL/P-affected individuals and 4 healthy controls. The candidate gene expression levels between the cases and controls were assessed using Student’s t-test. A p-value less than 0.05 was considered to be statistically significant.
3. Results
3.1. eQTL Analysis of 40 Lip Tissues from NSCL/P Patients
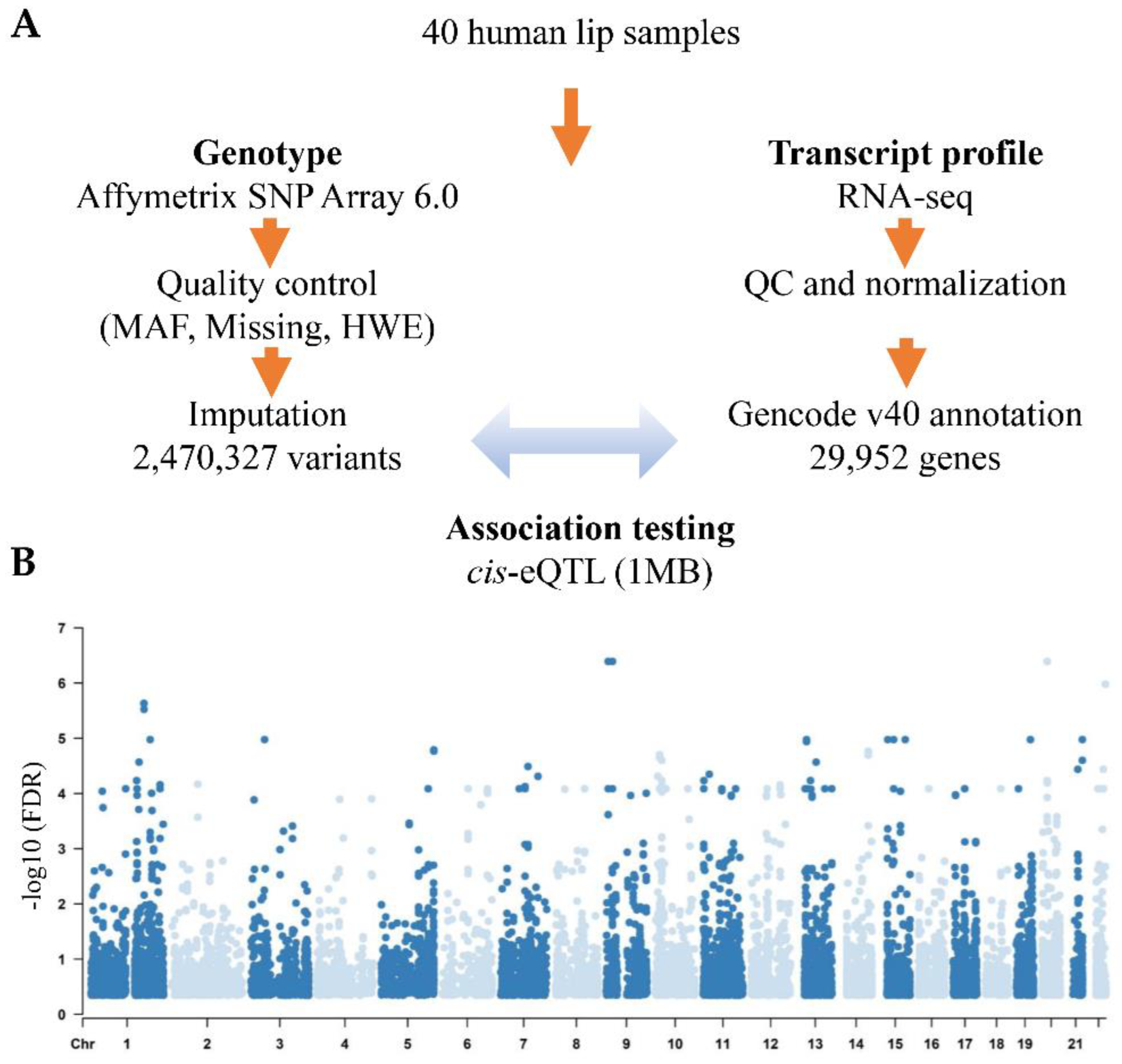
Forty lip samples were obtained from the Chinese Han population; sample characteristics, including demographics and subtype classifications, are listed in Table S1. We determined the association between 2.4 million genetic variants and the expression of 29,952 genes using MatrixeQTL (Figure 1A). The analysis was limited to cis-eQTLs in which SNPs were within 1 megabase (Mb) distance from the transcription start site of the genes, and we identified 689 eGenes and 5158 eQTL SNPs (eSNPs) (FDR < 0.05) (Figure 1B, Table S2). Then, we performed GO enrichment analyses on these eGenes and found that they were enriched in transcription regulation, G protein-coupled receptor signaling, keratinization, and olfactory perception pathways (Table S3), indicating that these eGenes play vital roles in cell energy synthesis, gene expression, and normal craniofacial function development.

Figure 1.
cis-eQTL analysis of lip tissues from NSCL/P patients. (A) Flowchart of 40 samples used for eQTL analysis, depicting the steps used to generate the genotype and gene expression data used in the eQTL analysis. (B) eQTL SNP-gene pairs across the genome. The x-axis shows the chromosome number, and the y-axis shows the -log10 (FDR) value.
3.2. Integrative Analysis of Lip eQTL and NSCL/P Risk SNPs
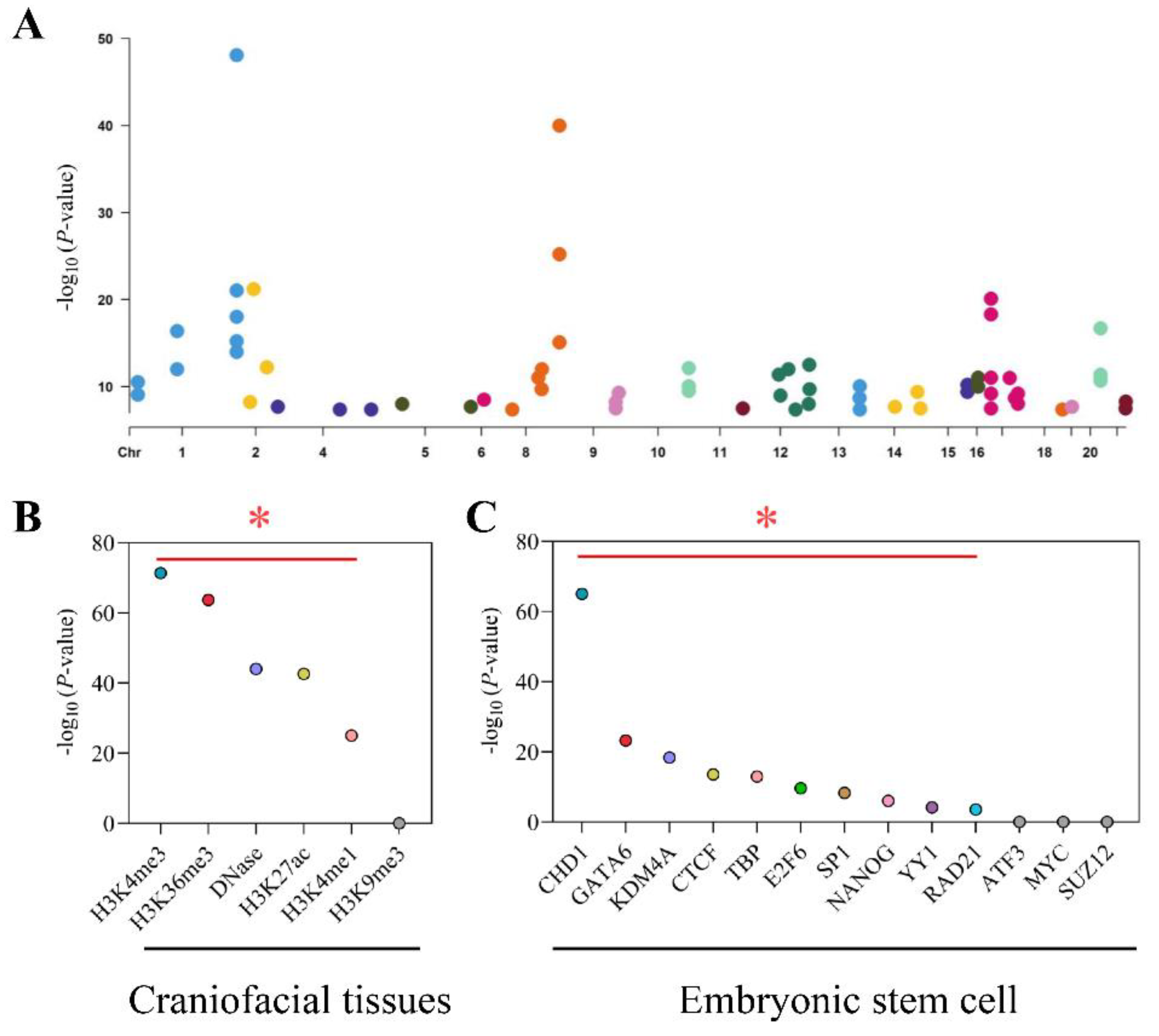
From the GWAS catalog database, we screened out 70 NSCL/P risk SNPs, which met the condition that p < 5 × 10−8 (Figure 2A). Then, we performed linkage disequilibrium analyses and identified 2832 tagging SNPs. Together, a total of 2902 risk SNPs were collected for NSCL/P. After overlapping these SNPs and nominal eSNPs (p < 0.05), 243 risk-eSNPs with 303 associations were obtained. These SNPs, which were all located within 250 kb of the eGene TSS, regulated 18 genes, such as TAF1B, WNT9B, and RAD54B (Table S4).

Figure 2.
Identification and functional annotation of risk-eSNPs. (A) Significant NSCL/P risk SNPs across the genome. The x-axis shows the chromosome number, and the y-axis shows the -log10 (p) value. (B,C) The -log10 (p) value of eSNPs enrichment in histone marks and transcription factors, respectively. Marks with a significance level of p-value < 0.01 are highlighted above the red dotted line. * p < 0.01.
3.3. Risk-eSNPs were Enriched at Regulatory Regions
Given that risk-eSNPs influenced gene expression, we analyzed whether risk-eSNPs were enriched on regulatory or chromatin open regions. We overlapped risk-eSNPs with each histone or DNase mark of the craniofacial tissues and compared them with randomly generated SNPs that were matched by number, frequency, and TSS distance. We found that risk-eSNPs were statistically significantly enriched (~25–70 fold) in regions with active epigenetic modifications, including trimethylation of histone H3 at lysine 4 (H3K4me3) and trimethylation of histone H3 at lysine 36 (H3K36me3), but not in regions with the repressive modification trimethylation of histone H3 at lysine 9 (H3K9me3) (Figure 2B). These data indicated that risk-eSNPs were enriched in promoter or chromatin open regions of the craniofacial tissues.
Another feature of regulatory regions is that they are enriched in transcription factor binding sites. We next extended the analysis on binding transcription factors and found that risk-eSNPs were statistically significantly enriched in transcription factor binding sites, such as CHD1, GATA6, KDM4A, CTCF, TBP, E2F6, SP1, NANOG, YY1, and RAD21 (Figure 2C). Many of these transcription factors have been shown to be important in lip development or NSCL/P [22,23,24,25,26]. In addition, the eSNP-TF-eGene regulatory relationships for the top five TFs are listed in Table S5. In summary, risk-eSNPs were enriched in regulatory regions, thus indicating the robustness of the approach.
3.4. Pathway Enrichment Analysis
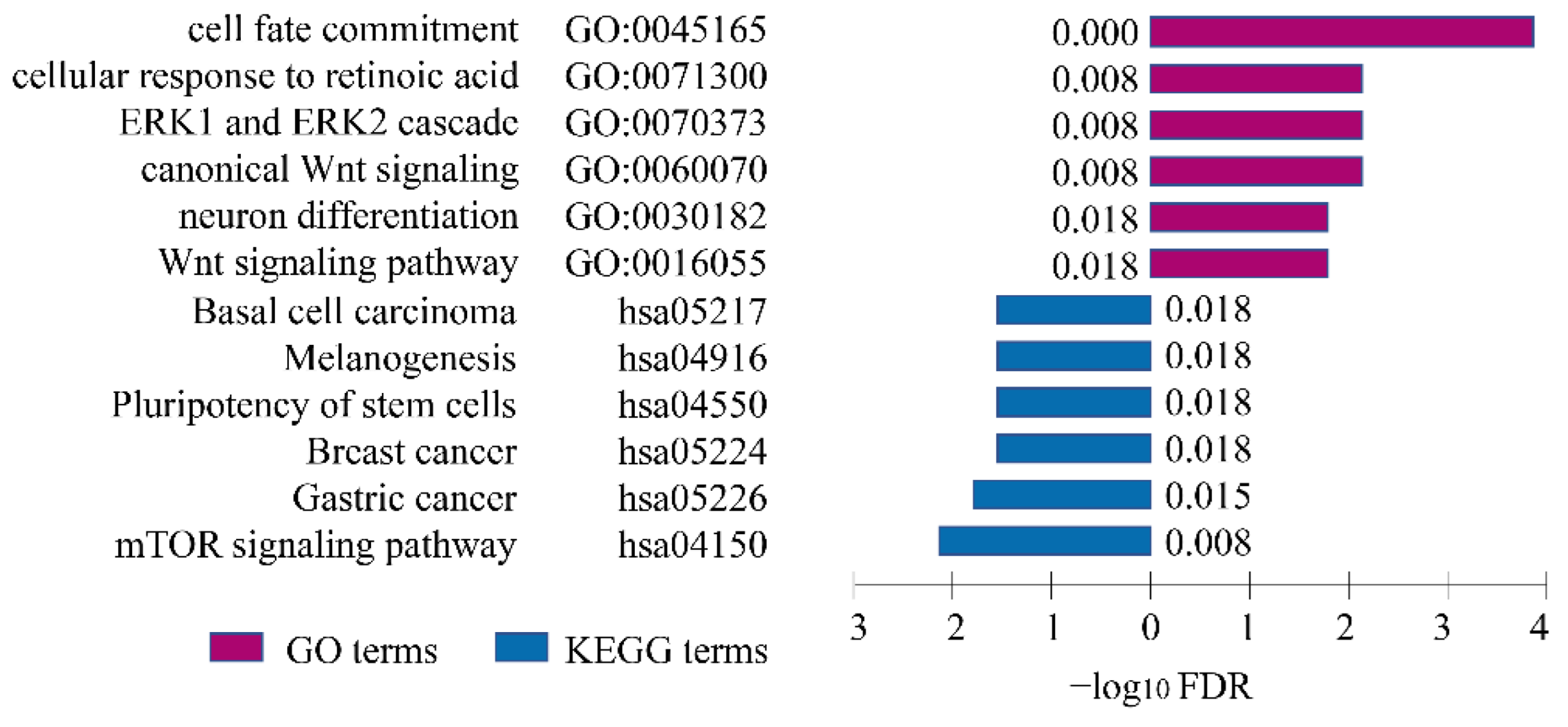
GO and KEGG enrichment analyses were performed to explore the underlying risky biological processes and pathways. The GO enrichment analyses demonstrated that eGenes regulated by risk-eSNPs were mainly enriched in cell fate commitment, cellular response to retinoic acid, ERK1 and ERK2 cascade, neuron differentiation, and Wnt signaling pathways; they were also enriched in carcinoma, cancers, the pluripotency of stem cells, and mTOR signaling pathways in KEGG analyses (Figure 3).

Figure 3.
Pathway enrichment analyses. GO and KEGG pathway enrichment analyses for genes regulated by NSCL/P risk SNPs. Purple columns represent GO terms and blue columns represent KEGG terms.
3.5. Differential Expression Analysis
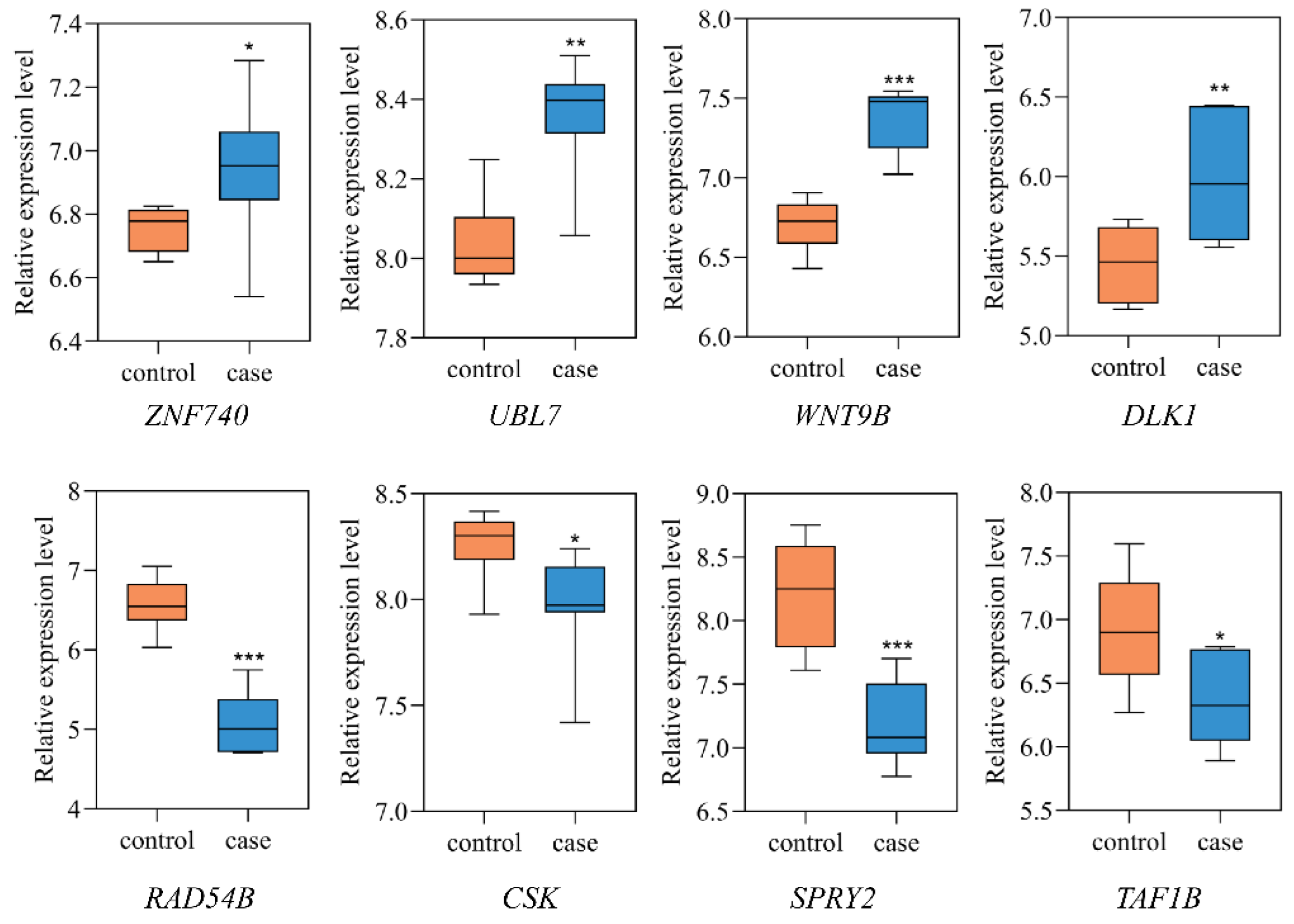
For the above 18 NSCL/P functional genes displaying eQTL signals, we analyzed mRNA expression levels in two gene expression microarray datasets (GSE42589 and GSE85748). In these two expression data sets, we found that 8 of the 18 effect genes were differentially expressed in at least one study (p < 0.05). For example, ZNF740 (p = 2.75 × 10−2) were upregulated in GSE85748. Meanwhile, UBL7 (p = 3.97 × 10−3), WNT9B (p = 3.66 × 10−5), and DLK1 (p = 7.14 × 10−3) were upregulated, while RAD54B (p = 1.69 × 10−6), CSK (p = 4.69 × 10−2), SPRY2 (p = 1.32 × 10−4), and TAF1B (p = 2.33 × 10−2) were downregulated in GSE42589 (Figure 4).

Figure 4.
Eight differentially expressed genes between NSCL/P and healthy controls. The expression levels of differentially expressed genes in dental pulp stem cells (GSE42589) or orbicularis oris muscles (GSE85748) were compared between NSCL/P patients and healthy controls. * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Discussion
In this study, we identified a number of SNP gene association pairs in lip tissues from patients with non-syndromic cleft lips with or without cleft palate (NSCL/P). By integrating nominal eSNPs with NSCL/P risk SNPs, we identified 243 risk-eSNPs and 18 susceptibility genes, which broadened our understanding of the genetic factors of NSCL/P.
Integrating functional genomics data to annotate specific sets of SNPs provided valuable information for understanding the underlying mechanisms of these SNPs [27]. In this study, we found that risk-eSNPs were enriched in open chromatin regions and transcription factors, which played critical roles in the development of NSCL/P. For example, abnormal neural crest migration was observed in Xenopus laevis embryos deficient in Chd1 [22]. In addition, missense and copy number variants of CHD1 contributed to craniofacial malformations, including cleft palate in human patients [23]. GATA6, which played an important role in the regulation of cellular differentiation and organogenesis during vertebrate development, was involved in the dexamethasone-inducing cleft palate [24]. Our study highlighted the importance of these regulatory elements and transcription factors in the development of NSCL/P.
A pathway enrichment analysis showed that the genes we identified were significantly enriched in classical developmental pathways, such as the Wnt signal pathway, which was a critical regulator of lip and palate formation [28,29]. Moreover, a KEGG analysis also found that many cancer-related pathways were enriched, such as breast cancer, gastric cancer, and basal cell cancer. Interestingly, several studies have reported the co-occurrence of NSCL/P and a variety of cancer types [30,31]. Our results further suggested that NSCL/P shared some genetic factors with cancers.
Among the eight differentially expressed genes, some are famous and known for their association with NSCL/P. Take WNT9B for instance, it belongs to the Wnt signaling pathway, which is a classic pathway in the development of the branchial arches, lip fusion, and normal morphogenesis of the face [32,33,34,35]. A Pbx-dependent Wnt-p63-Irf6 regulatory module in the midfacial ectoderm was established in a mouse line lacking Pbx genes in the cephalic ectoderm, and it was conserved within mammals. The abnormal expression of Pbx in the cephalic ectoderm led to a fully penetrant cleft lip with or without cleft palate (CL/P) and perturbed Wnt signaling, while ectopic Wnt ectodermal expression in Pbx mutants rescues the cleft. These findings indicated the significant role of adequate WNT9B expression in midface development and its association with NSCL/P [36,37].
Among some unfamiliar genes in NSCL/P, TAF1B, and ZNF740 are transcription factors, which belong to the TATA-box binding protein-associated factors family and the zinc finger protein family, respectively. They involve in the universal transcription regulation process in cells [38,39,40] and play important roles in growth, aging, and responses to abiotic and biotic stresses. Despite their similar functions within their families, their independent roles and interactions with other family members or genes are still largely unveiled. In addition, their roles in NSCL/P still need to be validated in populations and verified by in vivo as well as in vitro experiments.
DLK1, delta-like non-canonical Notch ligand 1, is a member of the Notch signaling pathway. It has been found to decrease expression and increase differentiation as gestation proceeds, and its expression is restricted to a few tissues and progenitor cells in adults, but it would be re-expressed during disease and regeneration [41]. Here, we found that it was significantly upregulated in NSCL/P cases, which is in accordance with the previous findings and suggests its involvement in the occurrence of NSCL/P.
There were still some limitations in this study. Firstly, due to the limited sample size, some eGenes and eSNPs may be missed. Secondly, the technology principle of eQTL analysis, which mainly detected the genes with relatively high expression in the tissues, may lead to some undetected signals. Thirdly, the SNP array used for lip sample genotyping did not include all NSCL/P risk SNPs, which may also contribute to some variants missing in the integrative analysis. Finally, these lip tissues were sourced several weeks after the likely molecular events that led to the clefts happened. That means our findings cannot fully reflect the mechanism of the clefts. These problems will be overcome with the emergence of higher-quality data.
In conclusion, we generated a unique, lip-specific eQTL resource from 40 NSCL/P patients and identified multiple associations for NSCL/P risk loci. These findings provided new evidence for susceptibility genes of NSCL/P and new research directions for the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11203281/s1, Table S1: Demographics of the Study Cohort; Table S2: All significant SNP-eGene pairs; Table S3: Gene Ontology Pathway Enrichment Analysis on eGenes at Biological Process Level; Table S4: All nominal risk-eSNP-eGene pairs. Table S5: eSNP-TF-eGene regulatory relationships.
Author Contributions
Conceptualization, Y.P. and L.M.; project administration, X.L. and Y.T.; validation, L.Q., G.Z. and S.L.; data curation, Y.G.; writing—original draft preparation, X.L. and Y.T.; writing—review and editing, Y.P. and L.M.; visualization, X.L.; funding acquisition, Y.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81970969, 81830031, 82001088, 82101054), the State Key Lab of Reproductive Medicine of Nanjing Medical University (JX116GSP 20171416), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD-2018-87), the Natural Science Foundation of Jiangsu Province (15KJA320002, BK20180667), the Qing Lan Project (to Y.P.), Six Distinguished Talent (2016-WSW-008), Jiangsu Provincial Medical Talent (ZDRCC2016023 to YP), the Jiangsu Provincial Key Medical Discipline (zdxka2016026), and the Graduate Student Scientific Research Innovation Projects in Jiangsu Province (KYCX19_1148).
Institutional Review Board Statement
The studies involving human participants were approved by the Institutional Review Board of Nanjing Medical University (NJMUERC [2008] No. 20).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in insert article or Supplementary Material here.
Acknowledgments
We thank all the study participants, research staff, and students who contributed to this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Gen. 2011, 12, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Fell, M.; Dack, K.; Chummun, S.; Sandy, J.; Wren, Y.; Lewis, S. Maternal Cigarette Smoking and Cleft Lip and Palate: A Systematic Review and Meta-Analysis. Cleft Palate-Craniofacial J. 2022, 59, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Marazita, M.L.; Field, L.L.; Cooper, M.E.; Tobias, R.; Maher, B.S.; Peanchitlertkajorn, S.; Liu, Y.E. Nonsyndromic cleft lip with or without cleft palate in China: Assessment of candidate regions. Cleft Palate-Craniofacial J. 2002, 39, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.U.; Böhmer, A.C.; Bowes, J.; Nikolic, M.; Ishorst, N.; Wyatt, N.; Hammond, N.L.; Gölz, L.; Thieme, F.; Barth, S.; et al. Imputation of orofacial clefting data identifies novel risk loci and sheds light on the genetic background of cleft lip ± cleft palate and cleft palate only. Hum. Mol. Genet. 2017, 26, 829–842. [Google Scholar] [CrossRef]
- Beaty, T.H.; Marazita, M.L.; Leslie, E.J. Genetic factors influencing risk to orofacial clefts: Today’s challenges and tomorrow’s opportunities. F1000Research 2016, 5, 2800. [Google Scholar] [CrossRef]
- Mostowska, A.; Gaczkowska, A.; Żukowski, K.; Ludwig, K.U.; Hozyasz, K.K.; Wójcicki, P.; Mangold, E.; Böhmer, A.C.; Heilmann-Heimbach, S.; Knapp, M.; et al. Common variants in DLG1 locus are associated with non-syndromic cleft lip with or without cleft palate. Clin. Genet. 2018, 93, 784–793. [Google Scholar] [CrossRef]
- Yu, Y.; Zuo, X.; He, M.; Gao, J.; Fu, Y.; Qin, C.; Meng, L.; Wang, W.; Song, Y.; Cheng, Y.; et al. Genome-wide analyses of non-syndromic cleft lip with palate identify 14 novel loci and genetic heterogeneity. Nat. Commun. 2017, 8, 14364. [Google Scholar] [CrossRef]
- Edwards, S.L.; Beesley, J.; French, J.D.; Dunning, A.M. Beyond GWASs: Illuminating the dark road from association to function. Am. J. Hum. Genet. 2013, 93, 779–797. [Google Scholar] [CrossRef]
- Lee, I.; Blom, U.M.; Wang, P.I.; Shim, J.E.; Marcotte, E.M. Prioritizing candidate disease genes by network-based boosting of genome-wide association data. Genome Res. 2011, 21, 1109–1121. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.; Hu, H.; Bakshi, A.; Robinson, M.R.; Powell, J.E.; Montgomery, G.W.; Goddard, M.E.; Wray, N.R.; Visscher, P.M.; et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 2016, 48, 481–487. [Google Scholar] [CrossRef]
- Ratnapriya, R.; Sosina, O.A.; Starostik, M.R.; Kwicklis, M.; Kapphahn, R.J.; Fritsche, L.G.; Walton, A.; Arvanitis, M.; Gieser, L.; Pietraszkiewicz, A.; et al. Retinal transcriptome and eQTL analyses identify genes associated with age-related macular degeneration. Nat. Genet. 2019, 51, 606–610. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Zhu, G.; Wang, R.; Lou, S.; Zhu, W.; Fu, C.; Liu, J.; Fan, L.; Li, D.; et al. Integrating GWAS and eQTL to predict genes and pathways for non-syndromic cleft lip with or without palate. Oral Dis. 2021, 27, 1747–1754. [Google Scholar] [CrossRef]
- Mullin, B.H.; Zhu, K.; Xu, J.; Brown, S.J.; Mullin, S.; Tickner, J.; Pavlos, N.J.; Dudbridge, F.; Walsh, J.P.; Wilson, S.G. Expression Quantitative Trait Locus Study of Bone Mineral Density GWAS Variants in Human Osteoclasts. J. Bone Miner. Res. 2018, 33, 1044–1051. [Google Scholar] [CrossRef]
- Masotti, C.; Brito, L.A.; Nica, A.C.; Ludwig, K.U.; Nunes, K.; Savastano, C.P.; Malcher, C.; Ferreira, S.G.; Kobayashi, G.S.; Bueno, D.F.; et al. MRPL53, a New Candidate Gene for Orofacial Clefting, Identified Using an eQTL Approach. J. Dent. Res. 2018, 97, 33–40. [Google Scholar] [CrossRef]
- Howie, B.N.; Donnelly, P.; Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009, 5, e1000529. [Google Scholar] [CrossRef]
- Shabalin, A.A. Matrix eQTL: Ultra fast eQTL analysis via large matrix operations. Bioinformatics 2012, 28, 1353–1358. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Wilderman, A.; VanOudenhove, J.; Kron, J.; Noonan, J.P.; Cotney, J. High-Resolution Epigenomic Atlas of Human Embryonic Craniofacial Development. Cell Rep. 2018, 23, 1581–1597. [Google Scholar] [CrossRef]
- Schmidt, E.M.; Zhang, J.; Zhou, W.; Chen, J.; Mohlke, K.L.; Chen, Y.E.; Willer, C.J. GREGOR: Evaluating global enrichment of trait-associated variants in epigenomic features using a systematic, data-driven approach. Bioinformatics 2015, 31, 2601–2606. [Google Scholar] [CrossRef]
- Kobayashi, G.S.; Alvizi, L.; Sunaga, D.Y.; Francis-West, P.; Kuta, A.; Almada, B.V.; Ferreira, S.G.; de Andrade-Lima, L.C.; Bueno, D.F.; Raposo-Amaral, C.E.; et al. Susceptibility to DNA damage as a molecular mechanism for non-syndromic cleft lip and palate. PLoS ONE 2013, 8, e65677. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Mendoza, C.; Goodenberger, M.L.; Kuhl, A.; Rice, G.M.; Hoppman, N. Familial segregation of a 5q15-q21.2 deletion associated with facial dysmorphism and speech delay. Clin. Case Rep. 2019, 7, 1154–1160. [Google Scholar] [CrossRef]
- Wyatt, B.H.; Raymond, T.O.; Lansdon, L.A.; Darbro, B.W.; Murray, J.C.; Manak, J.R.; Dickinson, A.J.G. Using an aquatic model, Xenopus laevis, to uncover the role of chromodomain 1 in craniofacial disorders. Genesis 2021, 59, e23394. [Google Scholar] [CrossRef]
- Lan, S.J.; Yang, X.G.; Chen, Z.; Yang, T.Y.; Xiang, C.H.; Zhang, D.; Li, Y.X.; Rong, L. Role of GATA-6 and Bone Morphogenetic Protein-2 in Dexamethasone-Induced Cleft Palate Formation in Institute of Cancer Research Mice. J. Craniofacial Surg. 2016, 27, 1600–1605. [Google Scholar] [CrossRef]
- Lan, S.; Yang, X.; Li, T.; Yang, T.; Rong, L. Dexamethasone Induces Apoptosis of Embryonic Palatal Mesenchymal Cells through the GATA-6/Bone Morphogenetic Protein-2/p38 MAPK Pathway. J. Craniofacial Surg. 2022, 33, 1335–1340. [Google Scholar] [CrossRef]
- Chen, S.; Jia, Z.; Cai, M.; Ye, M.; Wu, D.; Wan, T.; Zhang, B.; Wu, P.; Xu, Y.; Guo, Y.; et al. SP1-Mediated Upregulation of Long Noncoding RNA ZFAS1 Involved in Non-syndromic Cleft Lip and Palate via Inactivating WNT/β-Catenin Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 662780. [Google Scholar] [CrossRef]
- Chen, R.; Yang, Z.; Liu, J.; Cai, X.; Huo, Y.; Zhang, Z.; Li, M.; Chang, H.; Luo, X.J. Functional genomic analysis delineates regulatory mechanisms of GWAS-identified bipolar disorder risk variants. Genome Med. 2022, 14, 53. [Google Scholar] [CrossRef]
- Maili, L.; Letra, A.; Silva, R.; Buchanan, E.P.; Mulliken, J.B.; Greives, M.R.; Teichgraeber, J.F.; Blackwell, S.J.; Ummer, R.; Weber, R.; et al. PBX-WNT-P63-IRF6 pathway in nonsyndromic cleft lip and palate. Birth Defects Res. 2020, 112, 234–244. [Google Scholar] [CrossRef]
- Rochard, L.; Monica, S.D.; Ling, I.T.; Kong, Y.; Roberson, S.; Harland, R.; Halpern, M.; Liao, E.C. Roles of Wnt pathway genes wls, wnt9a, wnt5b, frzb and gpc4 in regulating convergent-extension during zebrafish palate morphogenesis. Development 2016, 143, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Bille, C.; Winther, J.F.; Bautz, A.; Murray, J.C.; Olsen, J.; Christensen, K. Cancer risk in persons with oral cleft—A population-based study of 8093 cases. Am. J. Epidemiol. 2005, 161, 1047–1055. [Google Scholar] [CrossRef]
- Bui, A.H.; Ayub, A.; Ahmed, M.K.; Taioli, E.; Taub, P.J. Association Between Cleft Lip and/or Cleft Palate and Family History of Cancer: A Case-Control Study. Ann. Plast. Surg. 2018, 80, S178–S181. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.R.; Han, X.H.; Nishimori, K.; Ben-Avraham, D.; Oh, Y.J.; Shim, J.W.; Yoon, J.K. Canonical WNT/β-Catenin Signaling Activated by WNT9b and RSPO2 Cooperation Regulates Facial Morphogenesis in Mice. Front. Cell Dev. Biol. 2020, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Jezewski, P.A.; Fang, P.K.; Payne-Ferreira, T.L.; Yelick, P.C. Zebrafish Wnt9b synteny and expression during first and second arch, heart, and pectoral fin bud morphogenesis. Zebrafish 2008, 5, 169–177. [Google Scholar] [CrossRef]
- Lan, Y.; Ryan, R.C.; Zhang, Z.; Bullard, S.A.; Bush, J.O.; Maltby, K.M.; Lidral, A.C.; Jiang, R. Expression of Wnt9b and activation of canonical Wnt signaling during midfacial morphogenesis in mice. Dev. Dyn. 2006, 235, 1448–1454. [Google Scholar] [CrossRef]
- Jin, Y.R.; Han, X.H.; Taketo, M.M.; Yoon, J.K. Wnt9b-dependent FGF signaling is crucial for outgrowth of the nasal and maxillary processes during upper jaw and lip development. Development 2012, 139, 1821–1830. [Google Scholar] [CrossRef]
- Ferretti, E.; Li, B.; Zewdu, R.; Wells, V.; Hebert, J.M.; Karner, C.; Anderson, M.J.; Williams, T.; Dixon, J.; Dixon, M.J.; et al. A conserved Pbx-Wnt-p63-Irf6 regulatory module controls face morphogenesis by promoting epithelial apoptosis. Dev. Cell 2011, 21, 627–641. [Google Scholar] [CrossRef]
- Fontoura, C.; Silva, R.M.; Granjeiro, J.M.; Letra, A. Association of WNT9B Gene Polymorphisms with Nonsyndromic Cleft Lip with or without Cleft Palate in Brazilian Nuclear Families. Cleft Palate-Craniofacial J. 2015, 52, 44–48. [Google Scholar] [CrossRef]
- Knutson, B.A.; Hahn, S. Yeast Rrn7 and human TAF1B are TFIIB-related RNA polymerase I general transcription factors. Science 2011, 333, 1637–1640. [Google Scholar] [CrossRef]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Li, H.T.; Liu, Y.; Liu, H.; Sun, X. Effect for Human Genomic Variation during the BMP4-Induced Conversion from Pluripotent Stem Cells to Trophoblast. Front. Genet. 2020, 11, 230. [Google Scholar] [CrossRef]
- Traustadóttir, G.; Lagoni, L.V.; Ankerstjerne, L.B.S.; Bisgaard, H.C.; Jensen, C.H.; Andersen, D.C. The imprinted gene Delta like non-canonical Notch ligand 1 (Dlk1) is conserved in mammals, and serves a growth modulatory role during tissue development and regeneration through Notch dependent and independent mechanisms. Cytokine Growth Factor Rev. 2019, 46, 17–27. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).