Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications
Abstract
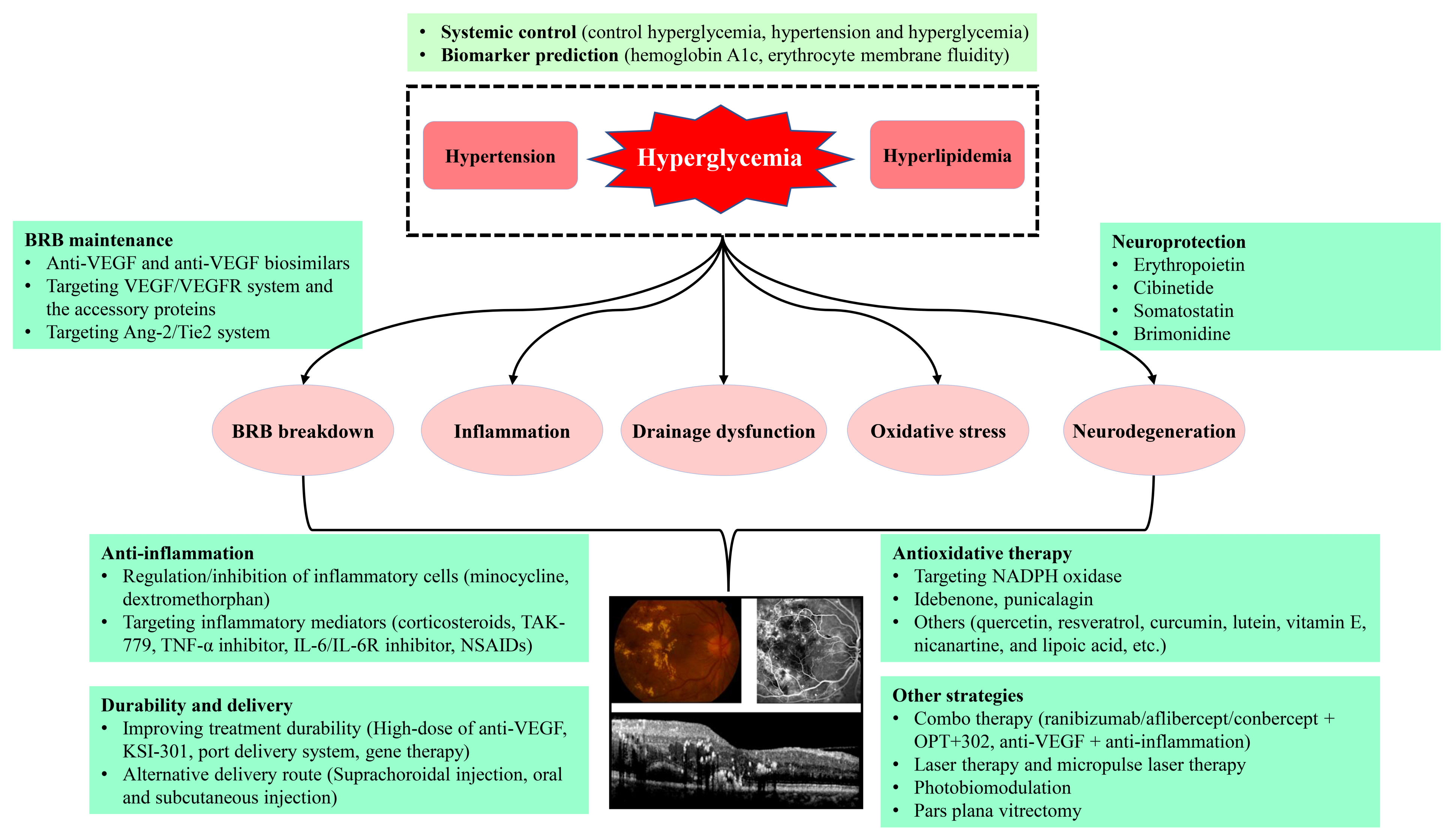
:1. Overview of Diabetic Retinopathy (DR) and Diabetic Macular Edema (DME)
2. OCT-Based DME Classification and Evaluation
3. The Pathogenesis of DME
3.1. Two Forms of Edema in DME: Intracellular Edema and Extracellular Edema
3.2. Drainage Dysfunction of Müller Cells Leading to Intracellular Edema
3.3. BRB Breakdown Leading to Increased Fluid Leakage into Retina
3.4. Inflammatory Effect Contributing to DME Formation
3.4.1. Inflammation-Related Factors Are Upregulated in DME
3.4.2. Inflammatory Cells Are Activated in DME
3.5. Diabetic Retinal Neurodegeneration (DRN) Aggregating the Functional Outcome in DME
3.6. Proteomics and Metabolomics Leading to Deep Understanding and Targeted Treatments for DME
4. Therapeutic Strategies for DME
4.1. Control of Systemic Risk Factors
4.2. Laser Therapy
4.3. Intravitreal Injection of Anti-VEGF Agents
4.4. Emerging Therapeutic Strategies Targeting VEGF/VEGFR System and the Accessory Proteins
4.4.1. Abicipar Pegol
4.4.2. OPT-302
4.4.3. Anti-VEGF Biosimilars
4.4.4. KSI-301
4.4.5. Port Delivery System (PDS) with Ranibizumab
4.4.6. High-Dose of Anti-VEGF Agents
4.4.7. Gene Therapy to Deliver Anti-VEGF Agents
4.4.8. Targeting VEGFRs
4.4.9. Targeting Neuropilin-1
4.5. Anti-Inflammatory Therapy
4.5.1. Minocycline and Dextromethorphan
4.5.2. Difluprednate and Dexamethasone-Cyclodextrin
4.5.3. TAK-779
4.5.4. Targeting Integrin
4.5.5. Targeting TNF-α
4.5.6. Targeting IL-6/IL-6R
4.5.7. Vascular Adhesion Protein-1 (VAP-1) Inhibitor
4.5.8. Non-Steroid Anti-Inflammatory Drugs (NSAIDs)
4.5.9. Suprachoroidal Injection of Steroid
4.6. Targeting Ang-2/Tyrosine Kinase with Immunoglobulin-like and Epidermal Growth Factor-like Domains 2 (Tie2) System
4.6.1. Targeting Ang-2
4.6.2. Bispecific Drug
4.6.3. Targeting VE-PTP
4.7. Neuroprotection in DME Management
4.8. Antioxidative Therapy
4.9. Combo Therapy and Other Strategies
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashemi, H.; Rezvan, F.; Pakzad, R.; Ansaripour, A.; Heydarian, S.; Yekta, A.; Ostadimoghaddam, H.; Pakbin, M.; Khabazkhoob, M. Global and Regional Prevalence of Diabetic Retinopathy; A Comprehensive Systematic Review and Meta-Analysis. Semin. Ophthalmol. 2022, 37, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.-R.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of Macular Edema: Beyond the Surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.L.; Dunstan, F.D.; Luzio, S.D.; Chowdhury, S.R.; North, R.V.; Hale, S.L.; Gibbins, R.L.; Owens, D.R. Prevalence of Diabetic Retinopathy within a National Diabetic Retinopathy Screening Service. Br. J. Ophthalmol. 2015, 99, 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Im, J.H.B.; Jin, Y.-P.; Chow, R.; Yan, P. Prevalence of Diabetic Macular Edema Based on Optical Coherence Tomography in People with Diabetes: A Systematic Review and Meta-Analysis. Surv. Ophthalmol. 2022, 67, 1244–1251. [Google Scholar] [CrossRef]
- Otani, T.; Kishi, S.; Maruyama, Y. Patterns of Diabetic Macular Edema with Optical Coherence Tomography. Am. J. Ophthalmol. 1999, 127, 688–693. [Google Scholar] [CrossRef]
- Arf, S.; Sayman Muslubas, I.; Hocaoglu, M.; Ersoz, M.G.; Ozdemir, H.; Karacorlu, M. Spectral Domain Optical Coherence Tomography Classification of Diabetic Macular Edema: A New Proposal to Clinical Practice. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1165–1172. [Google Scholar] [CrossRef]
- Fujiwara, A.; Kanzaki, Y.; Kimura, S.; Hosokawa, M.; Shiode, Y.; Doi, S.; Takahashi, K.; Matoba, R.; Morizane, Y. En Face Image-Based Classification of Diabetic Macular Edema Using Swept Source Optical Coherence Tomography. Sci. Rep. 2021, 11, 7665. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, F.; Wong, R.; Lok, J.; Szeto, S.K.H.; Chan, J.C.K.; Chan, C.K.M.; Tham, C.C.; Ng, D.S.; Cheung, C.Y. OCT Angiography Metrics Predict Progression of Diabetic Retinopathy and Development of Diabetic Macular Edema: A Prospective Study. Ophthalmology 2019, 126, 1675–1684. [Google Scholar] [CrossRef]
- Toto, L.; D’Aloisio, R.; Chiarelli, A.M.; Di Antonio, L.; Evangelista, F.; D’Onofrio, G.; Merla, A.; Parravano, M.; Di Marzio, G.; Mastropasqua, R. A Custom-Made Semiautomatic Analysis of Retinal Nonperfusion Areas After Dexamethasone for Diabetic Macular Edema. Transl. Vis. Sci. Technol. 2020, 9, 13. [Google Scholar] [CrossRef]
- Bringmann, A.; Reichenbach, A.; Wiedemann, P. Pathomechanisms of Cystoid Macular Edema. Ophthalmic Res. 2004, 36, 241–249. [Google Scholar] [CrossRef]
- Reichenbach, A.; Wurm, A.; Pannicke, T.; Iandiev, I.; Wiedemann, P.; Bringmann, A. Müller Cells as Players in Retinal Degeneration and Edema. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Villarroel, M.; Corraliza, L.; Hernández, C.; Garcia-Ramírez, M. The Retinal Pigment Epithelium: Something More than a Constituent of the Blood-Retinal Barrier—Implications for the Pathogenesis of Diabetic Retinopathy. J. Biomed. Biotechnol. 2010, 2010, 190724. [Google Scholar] [CrossRef] [Green Version]
- Reichenbach, A.; Bringmann, A. New Functions of Müller Cells. Glia 2013, 61, 651–678. [Google Scholar] [CrossRef] [PubMed]
- Caplan, M.J. Membrane Polarity in Epithelial Cells: Protein Sorting and Establishment of Polarized Domains. Am. J. Physiol. 1997, 272, F425–F429. [Google Scholar] [CrossRef]
- Rangasamy, S.; McGuire, P.G.; Franco Nitta, C.; Monickaraj, F.; Oruganti, S.R.; Das, A. Chemokine Mediated Monocyte Trafficking into the Retina: Role of Inflammation in Alteration of the Blood-Retinal Barrier in Diabetic Retinopathy. PLoS ONE 2014, 9, e108508. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Pareja-Rios, A.; Lopez-Galvez, M.; Navarro-Gil, R.; Verges, R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J. Diabetes Res. 2016, 2016, 2156273. [Google Scholar] [CrossRef] [Green Version]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressler, N.M.; Beaulieu, W.T.; Glassman, A.R.; Blinder, K.J.; Bressler, S.B.; Jampol, L.M.; Melia, M.; Wells, J.A. Diabetic Retinopathy Clinical Research Network Persistent Macular Thickening Following Intravitreous Aflibercept, Bevacizumab, or Ranibizumab for Central-Involved Diabetic Macular Edema With Vision Impairment: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Kohno, T.; Ishibashi, T.; Inomata, H.; Ikui, H.; Taniguchi, Y. Experimental Macular Edema of Commotio Retinae: Preliminary Report. Jpn. J. Ophthalmol. 1983, 27, 149–156. [Google Scholar]
- Yanoff, M.; Fine, B.S.; Brucker, A.J.; Eagle, R.C. Pathology of Human Cystoid Macular Edema. Surv. Ophthalmol. 1984, 28 (Suppl. 2), 505–511. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller Cells in the Healthy and Diseased Retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Retinal Vascular Cystoid Macular Edema: Review and New Theory. Retina 2016, 36, 1823–1842. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, P.; Biedermann, B.; Siddharthan, V.; Raap, M.; Iandiev, I.; Milenkovic, I.; Thomzig, A.; Veh, R.W.; Bringmann, A.; Reichenbach, A. Kir Potassium Channel Subunit Expression in Retinal Glial Cells: Implications for Spatial Potassium Buffering. Glia 2002, 39, 292–303. [Google Scholar] [CrossRef]
- Fort, P.E.; Sene, A.; Pannicke, T.; Roux, M.J.; Forster, V.; Mornet, D.; Nudel, U.; Yaffe, D.; Reichenbach, A.; Sahel, J.A.; et al. Kir4.1 and AQP4 Associate with Dp71- and Utrophin-DAPs Complexes in Specific and Defined Microdomains of Müller Retinal Glial Cell Membrane. Glia 2008, 56, 597–610. [Google Scholar] [CrossRef]
- Sene, A.; Tadayoni, R.; Pannicke, T.; Wurm, A.; El Mathari, B.; Benard, R.; Roux, M.J.; Yaffe, D.; Mornet, D.; Reichenbach, A.; et al. Functional Implication of Dp71 in Osmoregulation and Vascular Permeability of the Retina. PLoS ONE 2009, 4, e7329. [Google Scholar] [CrossRef]
- Pannicke, T.; Iandiev, I.; Uckermann, O.; Biedermann, B.; Kutzera, F.; Wiedemann, P.; Wolburg, H.; Reichenbach, A.; Bringmann, A. A Potassium Channel-Linked Mechanism of Glial Cell Swelling in the Postischemic Retina. Mol. Cell. Neurosci. 2004, 26, 493–502. [Google Scholar] [CrossRef]
- Rehak, M.; Hollborn, M.; Iandiev, I.; Pannicke, T.; Karl, A.; Wurm, A.; Kohen, L.; Reichenbach, A.; Wiedemann, P.; Bringmann, A. Retinal Gene Expression and Müller Cell Responses after Branch Retinal Vein Occlusion in the Rat. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2359–2367. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, C.; Xie, H.; Jiang, M.; Tian, H.; Lu, L.; Xu, G.-T.; Liu, L.; Zhang, J. Anti-VEGF Therapy Prevents Müller Intracellular Edema by Decreasing VEGF-A in Diabetic Retinopathy. Eye Vis. Lond. Engl. 2021, 8, 13. [Google Scholar] [CrossRef]
- McDowell, R.E.; Barabas, P.; Augustine, J.; Chevallier, O.; McCarron, P.; Chen, M.; McGeown, J.G.; Curtis, T.M. Müller Glial Dysfunction during Diabetic Retinopathy in Rats Is Reduced by the Acrolein-Scavenging Drug, 2-Hydrazino-4,6-Dimethylpyrimidine. Diabetologia 2018, 61, 2654–2667. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Valamanesh, F.; Celerier, I.; Savoldelli, M.; Jonet, L.; Jeanny, J.-C.; Jaisser, F.; Farman, N.; Behar-Cohen, F. The Neuroretina Is a Novel Mineralocorticoid Target: Aldosterone up-Regulates Ion and Water Channels in Müller Glial Cells. FASEB J. 2010, 24, 3405–3415. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Hadziahmetovic, M.; Zhang, J.; Li, W. Region-Specific Ischemia, Neovascularization and Macular Oedema in Treatment-Naïve Proliferative Diabetic Retinopathy. Clin. Experiment. Ophthalmol. 2018, 46, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zhang, C.; Qin, H.; Xie, H.; Luo, D.; Qiu, Q.; Liu, K.; Zhang, J.; Xu, G.; Zhang, J. Hyperreflective Foci and Subretinal Fluid Are Potential Imaging Biomarkers to Evaluate Anti-VEGF Effect in Diabetic Macular Edema. Front. Physiol. 2021, 12, 791442. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, G.; Clementi, M.E.; Sampaolese, B.; Serantoni, C.; Abeltino, A.; De Spirito, M.; Sasson, S.; Maulucci, G. Investigation of DHA-Induced Regulation of Redox Homeostasis in Retinal Pigment Epithelium Cells through the Combination of Metabolic Imaging and Molecular Biology. Antioxid. Basel Switz. 2022, 11, 1072. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. Ilastik: Interactive Machine Learning for (Bio)Image Analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef]
- Bianchetti, G.; Ciccarone, F.; Ciriolo, M.R.; De Spirito, M.; Pani, G.; Maulucci, G. Label-Free Metabolic Clustering through Unsupervised Pixel Classification of Multiparametric Fluorescent Images. Anal. Chim. Acta 2021, 1148, 238173. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-Retinal Barrier. Eur. J. Ophthalmol. 2011, 21, 3–9. [Google Scholar] [CrossRef]
- Cunha-Vaz, J. Diabetic Macular Edema. Eur. J. Ophthalmol. 1998, 8, 127–130. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, H.; Yang, Q.; Yang, Y.; Li, W.; Tian, H.; Lu, L.; Wang, F.; Xu, J.-Y.; Gao, F.; et al. Erythropoietin Protects Outer Blood-Retinal Barrier in Experimental Diabetic Retinopathy by up-Regulating ZO-1 and Occludin. Clin. Experiment. Ophthalmol. 2019, 47, 1182–1197. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Jin, Y.; Ji, F.; Sinclair, S.H.; Luo, Y.; Xu, G.; Lu, L.; Dai, W.; Yanoff, M.; et al. Intravitreal Injection of Erythropoietin Protects Both Retinal Vascular and Neuronal Cells in Early Diabetes. Investig. Ophthalmol. Vis. Sci. 2008, 49, 732–742. [Google Scholar] [CrossRef]
- Urias, E.A.; Urias, G.A.; Monickaraj, F.; McGuire, P.; Das, A. Novel Therapeutic Targets in Diabetic Macular Edema: Beyond VEGF. Vision Res. 2017, 139, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic Retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, R.N. Diabetic Retinopathy. N. Engl. J. Med. 2004, 350, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, G.; Viti, L.; Scupola, A.; Di Leo, M.; Tartaglione, L.; Flex, A.; De Spirito, M.; Pitocco, D.; Maulucci, G. Erythrocyte Membrane Fluidity as a Marker of Diabetic Retinopathy in Type 1 Diabetes Mellitus. Eur. J. Clin. Investig. 2021, 51, e13455. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Chan, P.-S. Oxidative Stress and Diabetic Retinopathy. Exp. Diabetes Res. 2007, 2007, 43603. [Google Scholar] [CrossRef] [Green Version]
- Rudraraju, M.; Narayanan, S.P.; Somanath, P.R. Regulation of Blood-Retinal Barrier Cell-Junctions in Diabetic Retinopathy. Pharmacol. Res. 2020, 161, 105115. [Google Scholar] [CrossRef]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Gandhi, J.K.; Zhong, X.; Wei, Y.; Gong, J.; Duh, E.J.; Vinores, S.A. TNFalpha Is Required for Late BRB Breakdown in Diabetic Retinopathy, and Its Inhibition Prevents Leukostasis and Protects Vessels and Neurons from Apoptosis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1336–1344. [Google Scholar] [CrossRef] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Biarnés Costa, M.; Gerhardinger, C. IL-1β Is Upregulated in the Diabetic Retina and Retinal Vessels: Cell-Specific Effect of High Glucose and IL-1β Autostimulation. PLoS ONE 2012, 7, e36949. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Feener, E.P. Plasma Kallikrein-Kinin System and Diabetic Retinopathy. Biol. Chem. 2013, 394, 319–328. [Google Scholar] [CrossRef]
- Villarroel, M.; García-Ramírez, M.; Corraliza, L.; Hernández, C.; Simó, R. Effects of High Glucose Concentration on the Barrier Function and the Expression of Tight Junction Proteins in Human Retinal Pigment Epithelial Cells. Exp. Eye Res. 2009, 89, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Crider, J.Y.; Yorio, T.; Sharif, N.A.; Griffin, B.W. The Effects of Elevated Glucose on Na+/K(+)-ATPase of Cultured Bovine Retinal Pigment Epithelial Cells Measured by a New Nonradioactive Rubidium Uptake Assay. J. Ocul. Pharmacol. Ther. 1997, 13, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Rizzolo, L.J. Effects of Diabetic Retinopathy on the Barrier Functions of the Retinal Pigment Epithelium. Vis. Res. 2017, 139, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Ruia, S.; Prasad, S.; Jain, A.; Mishra, N.; Natu, S.M.; Meyer, C.H.; Gilhotra, J.S.; Kruzliak, P.; Akduman, L. Increased Serum Levels of Urea and Creatinine Are Surrogate Markers for Disruption of Retinal Photoreceptor External Limiting Membrane and Inner Segment Ellipsoid Zone in Type 2 Diabetes Mellitus. Retina 2017, 37, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Ţălu, Ş.; Nicoara, S.D. Malfunction of Outer Retinal Barrier and Choroid in the Occurrence and Progression of Diabetic Macular Edema. World J. Diabetes 2021, 12, 437–452. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in Diabetic Retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [Green Version]
- Semeraro, F.; Morescalchi, F.; Cancarini, A.; Russo, A.; Rezzola, S.; Costagliola, C. Diabetic Retinopathy, a Vascular and Inflammatory Disease: Therapeutic Implications. Diabetes Metab. 2019, 45, 517–527. [Google Scholar] [CrossRef]
- Ambrosini, E.; Aloisi, F. Chemokines and Glial Cells: A Complex Network in the Central Nervous System. Neurochem. Res. 2004, 29, 1017–1038. [Google Scholar] [CrossRef]
- Adamis, A.P.; Berman, A.J. Immunological Mechanisms in the Pathogenesis of Diabetic Retinopathy. Semin. Immunopathol. 2008, 30, 65–84. [Google Scholar] [CrossRef]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A Central Role for Inflammation in the Pathogenesis of Diabetic Retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jang, H.; Choi, Y.A.; Kim, H.C.; Chung, H. Association Between Soluble CD14 in the Aqueous Humor and Hyperreflective Foci on Optical Coherence Tomography in Patients With Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2018, 59, 715–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esser, P.; Heimann, K.; Wiedemann, P. Macrophages in Proliferative Vitreoretinopathy and Proliferative Diabetic Retinopathy: Differentiation of Subpopulations. Br. J. Ophthalmol. 1993, 77, 731–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Yokoyama, T.; Ebihara, N.; Murakami, A. Histopathologic Analysis of the Internal Limiting Membrane Surgically Peeled from Eyes with Diffuse Diabetic Macular Edema. Jpn. J. Ophthalmol. 2012, 56, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal Microglia: Just Bystander or Target for Therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Krady, J.K.; Basu, A.; Allen, C.M.; Xu, Y.; LaNoue, K.F.; Gardner, T.W.; Levison, S.W. Minocycline Reduces Proinflammatory Cytokine Expression, Microglial Activation, and Caspase-3 Activation in a Rodent Model of Diabetic Retinopathy. Diabetes 2005, 54, 1559–1565. [Google Scholar] [CrossRef] [Green Version]
- Saijo, K.; Glass, C.K. Microglial Cell Origin and Phenotypes in Health and Disease. Nat. Rev. Immunol. 2011, 11, 775–787. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, C.; Liu, D.; Yang, Q.; Tang, L.; Wang, T.; Tian, H.; Lu, L.; Xu, J.-Y.; Gao, F.; et al. Erythropoietin Protects the Inner Blood-Retinal Barrier by Inhibiting Microglia Phagocytosis via Src/Akt/Cofilin Signalling in Experimental Diabetic Retinopathy. Diabetologia 2021, 64, 211–225. [Google Scholar] [CrossRef]
- Jiang, M.; Xie, H.; Zhang, C.; Wang, T.; Tian, H.; Lu, L.; Xu, J.-Y.; Xu, G.-T.; Liu, L.; Zhang, J. Enhancing Fractalkine/CX3CR1 Signalling Pathway Can Reduce Neuroinflammation by Attenuating Microglia Activation in Experimental Diabetic Retinopathy. J. Cell. Mol. Med. 2022, 26, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Shinozaki, Y.; Kashiwagi, K.; Ohno, N.; Eto, K.; Wake, H.; Nabekura, J.; Koizumi, S. Microglia Mediate Non-Cell-Autonomous Cell Death of Retinal Ganglion Cells. Glia 2018, 66, 2366–2384. [Google Scholar] [CrossRef]
- Graeber, M.B.; Li, W.; Rodriguez, M.L. Role of Microglia in CNS Inflammation. FEBS Lett. 2011, 585, 3798–3805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taghavi, Y.; Hassanshahi, G.; Kounis, N.G.; Koniari, I.; Khorramdelazad, H. Monocyte Chemoattractant Protein-1 (MCP-1/CCL2) in Diabetic Retinopathy: Latest Evidence and Clinical Considerations. J. Cell Commun. Signal. 2019, 13, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Green, W.R.; Tso, M.O.M. Microglial Activation in Human Diabetic Retinopathy. Arch. Ophthalmol. Chic. Ill 1960 2008, 126, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaštelan, S.; Orešković, I.; Bišćan, F.; Kaštelan, H.; Gverović Antunica, A. Inflammatory and Angiogenic Biomarkers in Diabetic Retinopathy. Biochem. Med. 2020, 30, 030502. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S. Contributions of Inflammatory Processes to the Development of the Early Stages of Diabetic Retinopathy. Exp. Diabetes Res. 2007, 2007, 95103. [Google Scholar] [CrossRef] [Green Version]
- Spencer, B.G.; Estevez, J.J.; Liu, E.; Craig, J.E.; Finnie, J.W. Pericytes, Inflammation, and Diabetic Retinopathy. Inflammopharmacology 2020, 28, 697–709. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. [Google Scholar] [CrossRef] [Green Version]
- Abcouwer, S.F.; Gardner, T.W. Diabetic Retinopathy: Loss of Neuroretinal Adaptation to the Diabetic Metabolic Environment. Ann. N. Y. Acad. Sci. 2014, 1311, 174–190. [Google Scholar] [CrossRef] [Green Version]
- Simó, R.; Hernández, C.; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration in the Diabetic Eye: New Insights and Therapeutic Perspectives. Trends Endocrinol. Metab. TEM 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C. Novel Approaches for Treating Diabetic Retinopathy Based on Recent Pathogenic Evidence. Prog. Retin. Eye Res. 2015, 48, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.-P.; Simó, R.; et al. The Progress in Understanding and Treatment of Diabetic Retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural Apoptosis in the Retina during Experimental and Human Diabetes. Early Onset and Effect of Insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Kern, T.S.; Barber, A.J. Retinal Ganglion Cells in Diabetes. J. Physiol. 2008, 586, 4401–4408. [Google Scholar] [CrossRef]
- Abu-El-Asrar, A.M.; Dralands, L.; Missotten, L.; Al-Jadaan, I.A.; Geboes, K. Expression of Apoptosis Markers in the Retinas of Human Subjects with Diabetes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2760–2766. [Google Scholar] [CrossRef]
- Park, S.-H.; Park, J.-W.; Park, S.-J.; Kim, K.-Y.; Chung, J.-W.; Chun, M.-H.; Oh, S.-J. Apoptotic Death of Photoreceptors in the Streptozotocin-Induced Diabetic Rat Retina. Diabetologia 2003, 46, 1260–1268. [Google Scholar] [CrossRef]
- Énzsöly, A.; Szabó, A.; Kántor, O.; Dávid, C.; Szalay, P.; Szabó, K.; Szél, Á.; Németh, J.; Lukáts, Á. Pathologic Alterations of the Outer Retina in Streptozotocin-Induced Diabetes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3686–3699. [Google Scholar] [CrossRef] [Green Version]
- Oshitari, T.; Yamamoto, S.; Hata, N.; Roy, S. Mitochondria- and Caspase-Dependent Cell Death Pathway Involved in Neuronal Degeneration in Diabetic Retinopathy. Br. J. Ophthalmol. 2008, 92, 552–556. [Google Scholar] [CrossRef]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.B.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.J.; et al. Retinal Neurodegeneration May Precede Microvascular Changes Characteristic of Diabetic Retinopathy in Diabetes Mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef] [Green Version]
- Srividya, G.; Jain, M.; Mahalakshmi, K.; Gayathri, S.; Raman, R.; Angayarkanni, N. A Novel and Less Invasive Technique to Assess Cytokine Profile of Vitreous in Patients of Diabetic Macular Oedema. Eye Lond. Engl. 2018, 32, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, Q.; Lu, P. Quantitative Proteomics Analysis of Vitreous Body from Type 2 Diabetic Patients with Proliferative Diabetic Retinopathy. BMC Ophthalmol. 2018, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Purohit, S.; Sharma, A.; Hopkins, D.; Steed, L.; Bode, B.; Anderson, S.W.; Caldwell, R.; She, J.-X. Elevated Serum Levels of Soluble TNF Receptors and Adhesion Molecules Are Associated with Diabetic Retinopathy in Patients with Type-1 Diabetes. Mediat. Inflamm. 2015, 2015, 279393. [Google Scholar] [CrossRef] [Green Version]
- Youngblood, H.; Robinson, R.; Sharma, A.; Sharma, S. Proteomic Biomarkers of Retinal Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2019, 20, 4755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, M.; West, K.; Crabb, J.W.; Kinoshita, S.; Kamei, M. Proteomic Analysis of Vitreous from Diabetic Macular Edema. Exp. Eye Res. 2005, 81, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Gong, R.; Liu, W.; Xu, G. Proteome Changes Associated with the VEGFR Pathway and Immune System in Diabetic Macular Edema Patients at Different Diabetic Retinopathy Stages. Curr. Eye Res. 2022, 47, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Laíns, I.; Gantner, M.; Murinello, S.; Lasky-Su, J.A.; Miller, J.W.; Friedlander, M.; Husain, D. Metabolomics in the Study of Retinal Health and Disease. Prog. Retin. Eye Res. 2019, 69, 57–79. [Google Scholar] [CrossRef]
- Park, K.S.; Xu, C.L.; Cui, X.; Tsang, S.H. Reprogramming the Metabolome Rescues Retinal Degeneration. Cell. Mol. Life Sci. CMLS 2018, 75, 1559–1566. [Google Scholar] [CrossRef]
- Hou, X.-W.; Wang, Y.; Pan, C.-W. Metabolomics in Diabetic Retinopathy: A Systematic Review. Investig. Ophthalmol. Vis. Sci. 2021, 62, 4. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, J.; Jin, E.; Zhong, Y.; Zhang, L.; Han, X.; Liu, J.; Cheng, Y.; Hou, J.; Shi, X.; et al. Serum Untargeted Metabolomics Reveal Potential Biomarkers of Progression of Diabetic Retinopathy in Asians. Front. Mol. Biosci. 2022, 9, 871291. [Google Scholar] [CrossRef]
- The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33). Lancet Lond. Engl. 1998, 352, 837–853. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study Group. Tight Blood Pressure Control and Risk of Macrovascular and Microvascular Complications in Type 2 Diabetes: UKPDS 38. BMJ 1998, 317, 703–713. [Google Scholar] [CrossRef] [Green Version]
- The ACCORD Study Group and ACCORD Eye Study Group. Effects of Medical Therapies on Retinopathy Progression in Type 2 Diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for Diabetic Macular Edema. Early Treatment Diabetic Retinopathy Study Report Number 1. Arch. Ophthalmol. Chic. Ill 1960 1985, 103, 1796–1806. [Google Scholar]
- Passos, R.M.; Malerbi, F.K.; Rocha, M.; Maia, M.; Farah, M.E. Real-Life Outcomes of Subthreshold Laser Therapy for Diabetic Macular Edema. Int. J. Retina Vitr. 2021, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Scholz, P.; Altay, L.; Fauser, S. A Review of Subthreshold Micropulse Laser for Treatment of Macular Disorders. Adv. Ther. 2017, 34, 1528–1555. [Google Scholar] [CrossRef] [Green Version]
- Lavinsky, D.; Wang, J.; Huie, P.; Dalal, R.; Lee, S.J.; Lee, D.Y.; Palanker, D. Nondamaging Retinal Laser Therapy: Rationale and Applications to the Macula. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2488–2500. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Sramek, C.; Palanker, D.; Spink, C.J.; Musch, D.C. Long-Term Safety, High-Resolution Imaging, and Tissue Temperature Modeling of Subvisible Diode Micropulse Photocoagulation for Retinovascular Macular Edema. Retina 2012, 32, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Lavinsky, D.; Sramek, C.; Wang, J.; Huie, P.; Dalal, R.; Mandel, Y.; Palanker, D. Subvisible Retinal Laser Therapy: Titration Algorithm and Tissue Response. Retina 2014, 34, 87–97. [Google Scholar] [CrossRef]
- Mainster, M.A. Wavelength Selection in Macular Photocoagulation. Tissue Optics, Thermal Effects, and Laser Systems. Ophthalmology 1986, 93, 952–958. [Google Scholar] [CrossRef]
- Frizziero, L.; Calciati, A.; Midena, G.; Torresin, T.; Parrozzani, R.; Pilotto, E.; Midena, E. Subthreshold Micropulse Laser Modulates Retinal Neuroinflammatory Biomarkers in Diabetic Macular Edema. J. Clin. Med. 2021, 10, 3134. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasad, S.; Sandhu, R.; Tandon, A.; Sayed-Ahmed, K.; McHugh, D.A. Subthreshold Micropulse Diode Laser Photocoagulation for Clinically Significant Diabetic Macular Oedema: A Three-Year Follow Up. Clin. Experiment. Ophthalmol. 2007, 35, 640–644. [Google Scholar] [CrossRef]
- Chhablani, J.; Alshareef, R.; Kim, D.T.; Narayanan, R.; Goud, A.; Mathai, A. Comparison of Different Settings for Yellow Subthreshold Laser Treatment in Diabetic Macular Edema. BMC Ophthalmol. 2018, 18, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vujosevic, S.; Martini, F.; Longhin, E.; Convento, E.; Cavarzeran, F.; Midena, E. Subthreshold Micropulse Yellow Laser Versus Subthreshold Micropulse Infrared Laser in Center-Involving Diabetic Macular Edema: Morphologic and Functional Safety. Retina 2015, 35, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lin, W.V.; Rodriguez, S.M.; Chen, A.; Loya, A.; Weng, C.Y. Treatment of Diabetic Macular Edema. Curr. Diab. Rep. 2019, 19, 68. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Funatsu, H.; Yamashita, H.; Sakata, K.; Noma, H.; Mimura, T.; Suzuki, M.; Eguchi, S.; Hori, S. Vitreous Levels of Vascular Endothelial Growth Factor and Intercellular Adhesion Molecule 1 Are Related to Diabetic Macular Edema. Ophthalmology 2005, 112, 806–816. [Google Scholar] [CrossRef]
- Glassman, A.R.; Wells, J.A.; Josic, K.; Maguire, M.G.; Antoszyk, A.N.; Baker, C.; Beaulieu, W.T.; Elman, M.J.; Jampol, L.M.; Sun, J.K. Five-Year Outcomes after Initial Aflibercept, Bevacizumab, or Ranibizumab Treatment for Diabetic Macular Edema (Protocol T Extension Study). Ophthalmology 2020, 127, 1201–1210. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Harris, A.; McIntyre, N.; Jonescu-Cuypers, C. Treatment of Diabetic Macular Edema with Sustained-Release Glucocorticoids: Intravitreal Triamcinolone Acetonide, Dexamethasone Implant, and Fluocinolone Acetonide Implant. Expert Opin. Pharmacother. 2014, 15, 953–959. [Google Scholar] [CrossRef]
- Rajendram, R.; Fraser-Bell, S.; Kaines, A.; Michaelides, M.; Hamilton, R.D.; Esposti, S.D.; Peto, T.; Egan, C.; Bunce, C.; Leslie, R.D.; et al. A 2-Year Prospective Randomized Controlled Trial of Intravitreal Bevacizumab or Laser Therapy (BOLT) in the Management of Diabetic Macular Edema: 24-Month Data: Report 3. Arch. Ophthalmol. Chic. Ill 1960 2012, 130, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Schmidt-Erfurth, U.; Do, D.V.; Holz, F.G.; Boyer, D.S.; Midena, E.; Heier, J.S.; Terasaki, H.; Kaiser, P.K.; Marcus, D.M.; et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology 2015, 122, 2044–2052. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Lang, G.E.; Holz, F.G.; Schlingemann, R.O.; Lanzetta, P.; Massin, P.; Gerstner, O.; Bouazza, A.S.; Shen, H.; Osborne, A.; et al. Three-Year Outcomes of Individualized Ranibizumab Treatment in Patients with Diabetic Macular Edema: The Restore Extension Study. Ophthalmology 2014, 121, 1045–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.M.; Emanuelli, A.; Bandello, F.; Barranco, J.J.E.; Figueira, J.; Souied, E.; Wolf, S.; Gupta, V.; Ngah, N.F.; Liew, G.; et al. KESTREL and KITE: 52-Week Results from Two Phase III Pivotal Trials of Brolucizumab for Diabetic Macular Edema. Am. J. Ophthalmol. 2022, 238, 157–172. [Google Scholar] [CrossRef]
- Thomas, C.N.; Sim, D.A.; Lee, W.H.; Alfahad, N.; Dick, A.D.; Denniston, A.K.; Hill, L.J. Emerging Therapies and Their Delivery for Treating Age-Related Macular Degeneration. Br. J. Pharmacol. 2022, 179, 1908–1937. [Google Scholar] [CrossRef]
- Souied, E.H.; Devin, F.; Mauget-Faÿsse, M.; Kolář, P.; Wolf-Schnurrbusch, U.; Framme, C.; Gaucher, D.; Querques, G.; Stumpp, M.T.; Wolf, S.; et al. Treatment of Exudative Age-Related Macular Degeneration with a Designed Ankyrin Repeat Protein That Binds Vascular Endothelial Growth Factor: A Phase I/II Study. Am. J. Ophthalmol. 2014, 158, 724–732.e2. [Google Scholar] [CrossRef] [Green Version]
- Campochiaro, P.A.; Channa, R.; Berger, B.B.; Heier, J.S.; Brown, D.M.; Fiedler, U.; Hepp, J.; Stumpp, M.T. Treatment of Diabetic Macular Edema with a Designed Ankyrin Repeat Protein That Binds Vascular Endothelial Growth Factor: A Phase I/II Study. Am. J. Ophthalmol. 2013, 155, 697–704.e2. [Google Scholar] [CrossRef]
- Krohne, T.U.; Liu, Z.; Holz, F.G.; Meyer, C.H. Intraocular Pharmacokinetics of Ranibizumab Following a Single Intravitreal Injection in Humans. Am. J. Ophthalmol. 2012, 154, 682–686.e2. [Google Scholar] [CrossRef]
- Kunimoto, D.; Yoon, Y.H.; Wykoff, C.C.; Chang, A.; Khurana, R.N.; Maturi, R.K.; Agostini, H.; Souied, E.; Chow, D.R.; Lotery, A.J.; et al. Efficacy and Safety of Abicipar in Neovascular Age-Related Macular Degeneration: 52-Week Results of Phase 3 Randomized Controlled Study. Ophthalmology 2020, 127, 1331–1344. [Google Scholar] [CrossRef]
- Callanan, D.; Kunimoto, D.; Maturi, R.K.; Patel, S.S.; Staurenghi, G.; Wolf, S.; Cheetham, J.K.; Hohman, T.C.; Kim, K.; López, F.J.; et al. Double-Masked, Randomized, Phase 2 Evaluation of Abicipar Pegol (an Anti-VEGF DARPin Therapeutic) in Neovascular Age-Related Macular Degeneration. J. Ocul. Pharmacol. Ther. 2018, 34, 700–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurana, R.N.; Kunimoto, D.; Yoon, Y.H.; Wykoff, C.C.; Chang, A.; Maturi, R.K.; Agostini, H.; Souied, E.; Chow, D.R.; Lotery, A.J.; et al. Two-Year Results of the Phase 3 Randomized Controlled Study of Abicipar in Neovascular Age-Related Macular Degeneration. Ophthalmology 2021, 128, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Striglia, E.; Caccioppo, A.; Castellino, N.; Reibaldi, M.; Porta, M. Emerging Drugs for the Treatment of Diabetic Retinopathy. Expert Opin. Emerg. Drugs 2020, 25, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Boyer, D.S.; Antoszyk, A.N.; Steinle, N.C.; Varenhorst, M.P.; Pearlman, J.A.; Gillies, M.C.; Finger, R.P.; Baldwin, M.E.; Leitch, I.M. Phase 1 Study of OPT-302 Inhibition of Vascular Endothelial Growth Factors C and D for Neovascular Age-Related Macular Degeneration. Ophthalmol. Retina 2020, 4, 250–263. [Google Scholar] [CrossRef]
- Boyer, D.S. Phase 1b/2a DME Study Results of OPT-302 to Block VEGF-C/-D in Combination with Aflibercept. In Proceedings of the AAO 2020, Virtual, 13 November 2020. [Google Scholar]
- Biosimilars for the Treatment of Wet AMD. Available online: Https://Www.Ophthalmologymanagement.Com/Newsletters/Amd-Update/July-2020 (accessed on 21 October 2022).
- Kapur, M.; Nirula, S.; Naik, M.P. Future of Anti-VEGF: Biosimilars and Biobetters. Int. J. Retina Vitr. 2022, 8, 2. [Google Scholar] [CrossRef]
- Sharma, A.; Reddy, P.; Kuppermann, B.D.; Bandello, F.; Lowenstein, A. Biosimilars in Ophthalmology: “Is There a Big Change on the Horizon?”. Clin. Ophthalmol. 2018, 12, 2137–2143. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kumar, N.; Kuppermann, B.D.; Francesco, B.; Lowenstein, A. Ophthalmic Biosimilars: Lessons from India. Indian J. Ophthalmol. 2019, 67, 1384–1385. [Google Scholar] [CrossRef]
- Kumar, A.; Agarwal, D.; Kumar, A. Commentary: Use of Biosimilars for Retinal Diseases in India: Challenges and Concerns. Indian J. Ophthalmol. 2021, 69, 357. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, N.; Parachuri, N.; Bandello, F.; Kuppermann, B.D.; Loewenstein, A. Biosimilars for Retinal Diseases: An Update. Am. J. Ophthalmol. 2021, 224, 36–42. [Google Scholar] [CrossRef]
- Kim, H.M.; Woo, S.J. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.R.; Madanagopalan, V.G. KSI-301: Antibody Biopolymer Conjugate in Retinal Disorders. Ther. Adv. Ophthalmol. 2021, 13, 25158414211027708. [Google Scholar] [CrossRef] [PubMed]
- Kodiak Sciences Announces Top-Line Results from Its Initial Phase 2b/3 Study of KSI-301 in Patients with Neovascular (Wet) Age-Related Macular Degeneration. Available online: Https://Ir.Kodiak.Com/News-Releases/News-Release-Details/Kodiak-Sciences-Announces-Top-Line-Results-Its-Initial-Phase-2b3 (accessed on 21 October 2022).
- Khanani, A.M.; Aziz, A.A.; Weng, C.Y.; Lin, W.V.; Vannavong, J.; Chhablani, J.; Danzig, C.J.; Kaiser, P.K. Port Delivery System: A Novel Drug Delivery Platform to Treat Retinal Diseases. Expert Opin. Drug Deliv. 2021, 18, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Marcus, D.M.; Awh, C.C.; Regillo, C.; Adamis, A.P.; Bantseev, V.; Chiang, Y.; Ehrlich, J.S.; Erickson, S.; Hanley, W.D.; et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial. Ophthalmology 2019, 126, 1141–1154. [Google Scholar] [CrossRef] [Green Version]
- Holekamp, N.M.; Campochiaro, P.A.; Chang, M.A.; Miller, D.; Pieramici, D.; Adamis, A.P.; Brittain, C.; Evans, E.; Kaufman, D.; Maass, K.F.; et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2022, 129, 295–307. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, Y.J.; Lee, S.; Son, J.Y.; Hong, H.K.; Ham, M.H.; Jin, X.; Chung, J.Y.; Park, K.H.; Park, K.D.; et al. Intraocular Pharmacokinetics of 10-Fold Intravitreal Ranibizumab Injection Dose in Rabbits. Transl. Vis. Sci. Technol. 2020, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- REGENXBIO Presents Positive Initial Data from Phase II ALTITUDE™ Trial of RGX-314 for the Treatment of Diabetic Retinopathy Using Suprachoroidal Delivery at American Society of Retina Specialists Annual Meeting. Available online: https://www.prnewswire.com/news-releases/regenxbio-presents-positive-initial-data-from-phase-ii-altitude-trial-of-rgx-314-for-the-treatment-of-diabetic-retinopathy-using-suprachoroidal-delivery-at-american-society-of-retina-specialists-annual-meeting-301396478.html (accessed on 21 October 2022).
- Grishanin, R.; Vuillemenot, B.; Sharma, P.; Keravala, A.; Greengard, J.; Gelfman, C.; Blumenkrantz, M.; Lawrence, M.; Hu, W.; Kiss, S.; et al. Preclinical Evaluation of ADVM-022, a Novel Gene Therapy Approach to Treating Wet Age-Related Macular Degeneration. Mol. Ther. 2019, 27, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-M.; Estcourt, M.J.; Himbeck, R.P.; Lee, S.-Y.; Yew-San Yeo, I.; Luu, C.; Loh, B.K.; Lee, M.W.; Barathi, A.; Villano, J.; et al. Preclinical Safety Evaluation of Subretinal AAV2.SFlt-1 in Non-Human Primates. Gene Ther. 2012, 19, 999–1009. [Google Scholar] [CrossRef] [Green Version]
- Rakoczy, E.P.; Lai, C.-M.; Magno, A.L.; Wikstrom, M.E.; French, M.A.; Pierce, C.M.; Schwartz, S.D.; Blumenkranz, M.S.; Chalberg, T.W.; Degli-Esposti, M.A.; et al. Gene Therapy with Recombinant Adeno-Associated Vectors for Neovascular Age-Related Macular Degeneration: 1 Year Follow-up of a Phase 1 Randomised Clinical Trial. Lancet Lond. Engl. 2015, 386, 2395–2403. [Google Scholar] [CrossRef] [Green Version]
- Constable, I.J.; Pierce, C.M.; Lai, C.-M.; Magno, A.L.; Degli-Esposti, M.A.; French, M.A.; McAllister, I.L.; Butler, S.; Barone, S.B.; Schwartz, S.D.; et al. Phase 2a Randomized Clinical Trial: Safety and Post Hoc Analysis of Subretinal RAAV.SFLT-1 for Wet Age-Related Macular Degeneration. EBioMedicine 2016, 14, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Heier, J.S.; Kherani, S.; Desai, S.; Dugel, P.; Kaushal, S.; Cheng, S.H.; Delacono, C.; Purvis, A.; Richards, S.; Le-Halpere, A.; et al. Intravitreous Injection of AAV2-SFLT01 in Patients with Advanced Neovascular Age-Related Macular Degeneration: A Phase 1, Open-Label Trial. Lancet Lond. Engl. 2017, 390, 50–61. [Google Scholar] [CrossRef]
- Atzori, M.G.; Tentori, L.; Ruffini, F.; Ceci, C.; Bonanno, E.; Scimeca, M.; Lacal, P.M.; Graziani, G. The Anti-Vascular Endothelial Growth Factor Receptor-1 Monoclonal Antibody D16F7 Inhibits Glioma Growth and Angiogenesis In Vivo. J. Pharmacol. Exp. Ther. 2018, 364, 77–86. [Google Scholar] [CrossRef]
- Lee, S.H. Tanibirumab (TTAC-0001): A Fully Human Monoclonal Antibody Targets Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2). Arch. Pharm. Res. 2011, 34, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.M.; Vaidya, A. Ramucirumab: First Global Approval. Drugs 2014, 74, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, R.; De Rosa, L.; Diana, D.; Fattorusso, R.; D’Andrea, L.D. Human Recombinant VEGFR2D4 Biochemical Characterization to Investigate Novel Anti-VEGFR2D4 Antibodies for Allosteric Targeting of VEGFR2. Mol. Biotechnol. 2019, 61, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Robinson, M.O. Development of Second-Generation VEGFR Tyrosine Kinase Inhibitors: Current Status. Curr. Oncol. Rep. 2011, 13, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Al-Khersan, H.; Hussain, R.M.; Ciulla, T.A.; Dugel, P.U. Innovative Therapies for Neovascular Age-Related Macular Degeneration. Expert Opin. Pharmacother. 2019, 20, 1879–1891. [Google Scholar] [CrossRef]
- Samanta, A.; Aziz, A.A.; Jhingan, M.; Singh, S.R.; Khanani, A.M.; Chhablani, J. Emerging Therapies in Neovascular Age-Related Macular Degeneration in 2020. Asia-Pac. J. Ophthalmol. 2020, 9, 250–259. [Google Scholar] [CrossRef]
- Hussain, R.M.; Shaukat, B.A.; Ciulla, L.M.; Berrocal, A.M.; Sridhar, J. Vascular Endothelial Growth Factor Antagonists: Promising Players in the Treatment of Neovascular Age-Related Macular Degeneration. Drug Des. Devel. Ther. 2021, 15, 2653–2665. [Google Scholar] [CrossRef]
- Graybug Vision Presents Top Line Results of Phase 1/2a ADAGIO Study at Hawaiian Eye & Retina 2019. Available online: https://www.businesswire.com/news/home/20190121005424/en/Graybug-Vision-Presents-Top-Line-Results-of-Phase-12a-ADAGIO-Study-at-Hawaiian-Eye-Retina-2019 (accessed on 21 October 2022).
- Jackson, T.L.; Boyer, D.; Brown, D.M.; Chaudhry, N.; Elman, M.; Liang, C.; O’Shaughnessy, D.; Parsons, E.C.; Patel, S.; Slakter, J.S.; et al. Oral Tyrosine Kinase Inhibitor for Neovascular Age-Related Macular Degeneration: A Phase 1 Dose-Escalation Study. JAMA Ophthalmol. 2017, 135, 761–767. [Google Scholar] [CrossRef]
- Patnaik, A.; LoRusso, P.M.; Messersmith, W.A.; Papadopoulos, K.P.; Gore, L.; Beeram, M.; Ramakrishnan, V.; Kim, A.H.; Beyer, J.C.; Mason Shih, L.; et al. A Phase Ib Study Evaluating MNRP1685A, a Fully Human Anti-NRP1 Monoclonal Antibody, in Combination with Bevacizumab and Paclitaxel in Patients with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2014, 73, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Weekes, C.D.; Beeram, M.; Tolcher, A.W.; Papadopoulos, K.P.; Gore, L.; Hegde, P.; Xin, Y.; Yu, R.; Shih, L.M.; Xiang, H.; et al. A Phase I Study of the Human Monoclonal Anti-NRP1 Antibody MNRP1685A in Patients with Advanced Solid Tumors. Investig. New Drugs 2014, 32, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.S.; Sun, J.K.; Aiello, L.P. Role of Steroids in the Management of Diabetic Macular Edema and Proliferative Diabetic Retinopathy. Semin. Ophthalmol. 2009, 24, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.-Y.; Cui, H.; Hashad, Y.; Whitcup, S.M.; et al. Three-Year, Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmology 2014, 121, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Brown, D.M.; Pearson, A.; Chen, S.; Boyer, D.; Ruiz-Moreno, J.; Garretson, B.; Gupta, A.; Hariprasad, S.M.; Bailey, C.; et al. Sustained Delivery Fluocinolone Acetonide Vitreous Inserts Provide Benefit for at Least 3 Years in Patients with Diabetic Macular Edema. Ophthalmology 2012, 119, 2125–2132. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.; Ashton, P.; Iezzi, R.; Campochiaro, P.; Dugel, P.U.; Holz, F.G.; Weber, M.; Danis, R.P.; Kuppermann, B.D.; Bailey, C.; et al. Sustained Delivery Fluocinolone Acetonide Vitreous Implants: Long-Term Benefit in Patients with Chronic Diabetic Macular Edema. Ophthalmology 2014, 121, 1892–1903. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and Doxycycline: More Than Antibiotics. Curr. Mol. Pharmacol. 2021, 14, 1046–1065. [Google Scholar] [CrossRef]
- Cukras, C.A.; Petrou, P.; Chew, E.Y.; Meyerle, C.B.; Wong, W.T. Oral Minocycline for the Treatment of Diabetic Macular Edema (DME): Results of a Phase I/II Clinical Study. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3865–3874. [Google Scholar] [CrossRef]
- Valent, D.J.; Wong, W.T.; Chew, E.Y.; Cukras, C.A. Oral Dextromethorphan for the Treatment of Diabetic Macular Edema: Results From a Phase I/II Clinical Study. Transl. Vis. Sci. Technol. 2018, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Korenfeld, M.S.; Silverstein, S.M.; Cooke, D.L.; Vogel, R.; Crockett, R.S. Difluprednate Ophthalmic Emulsion 0.05% (Durezol) Study Group Difluprednate Ophthalmic Emulsion 0.05% for Postoperative Inflammation and Pain. J. Cataract. Refract. Surg. 2009, 35, 26–34. [Google Scholar] [CrossRef]
- Foster, C.S.; Davanzo, R.; Flynn, T.E.; McLeod, K.; Vogel, R.; Crockett, R.S. Durezol (Difluprednate Ophthalmic Emulsion 0.05%) Compared with Pred Forte 1% Ophthalmic Suspension in the Treatment of Endogenous Anterior Uveitis. J. Ocul. Pharmacol. Ther. 2010, 26, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Yamamoto, T.; Kirii, E.; Abe, S.; Yamashita, H. Steroid Eye Drop Treatment (Difluprednate Ophthalmic Emulsion) Is Effective in Reducing Refractory Diabetic Macular Edema. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 248, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Nakano Goto, S.; Yamamoto, T.; Kirii, E.; Abe, S.; Yamashita, H. Treatment of Diffuse Diabetic Macular Oedema Using Steroid Eye Drops. Acta Ophthalmol. 2012, 90, 628–632. [Google Scholar] [CrossRef]
- Tanito, M.; Hara, K.; Takai, Y.; Matsuoka, Y.; Nishimura, N.; Jansook, P.; Loftsson, T.; Stefánsson, E.; Ohira, A. Topical Dexamethasone-Cyclodextrin Microparticle Eye Drops for Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7944–7948. [Google Scholar] [CrossRef]
- Ohira, A.; Hara, K.; Jóhannesson, G.; Tanito, M.; Ásgrímsdóttir, G.M.; Lund, S.H.; Loftsson, T.; Stefánsson, E. Topical Dexamethasone γ-Cyclodextrin Nanoparticle Eye Drops Increase Visual Acuity and Decrease Macular Thickness in Diabetic Macular Oedema. Acta Ophthalmol. 2015, 93, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Monickaraj, F.; Oruganti, S.R.; McGuire, P.; Das, A. A Potential Novel Therapeutic Target in Diabetic Retinopathy: A Chemokine Receptor (CCR2/CCR5) Inhibitor Reduces Retinal Vascular Leakage in an Animal Model. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Bhatwadekar, A.D.; Kansara, V.; Luo, Q.; Ciulla, T. Anti-Integrin Therapy for Retinovascular Diseases. Expert Opin. Investig. Drugs 2020, 29, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Khosrof, S.; Bursell, S.E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of Leukostasis and Vascular Leakage in Streptozotocin-Induced Diabetic Retinopathy via Intercellular Adhesion Molecule-1 Inhibition. Proc. Natl. Acad. Sci. USA 1999, 96, 10836–10841. [Google Scholar] [CrossRef] [Green Version]
- Joussen, A.M.; Murata, T.; Tsujikawa, A.; Kirchhof, B.; Bursell, S.E.; Adamis, A.P. Leukocyte-Mediated Endothelial Cell Injury and Death in the Diabetic Retina. Am. J. Pathol. 2001, 158, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Barouch, F.C.; Miyamoto, K.; Allport, J.R.; Fujita, K.; Bursell, S.E.; Aiello, L.P.; Luscinskas, F.W.; Adamis, A.P. Integrin-Mediated Neutrophil Adhesion and Retinal Leukostasis in Diabetes. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1153–1158. [Google Scholar]
- Oliveira, L.B.; Meyer, C.H.; Kumar, J.; Tatebayashi, M.; Toth, C.A.; Wong, F.; Epstein, D.L.; McCuen, B.W. RGD Peptide-Assisted Vitrectomy to Facilitate Induction of a Posterior Vitreous Detachment: A New Principle in Pharmacological Vitreolysis. Curr. Eye Res. 2002, 25, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Hoffmann, S.; Eichler, W.; Friedrichs, U.; Wang, Y.-S.; Wiedemann, P. Inhibition of Experimental Choroidal Neovascularization in Rats by an Alpha(v)-Integrin Antagonist. Curr. Eye Res. 2004, 28, 359–366. [Google Scholar] [CrossRef]
- Shaw, L.T.; Mackin, A.; Shah, R.; Jain, S.; Jain, P.; Nayak, R.; Hariprasad, S.M. Risuteganib-a Novel Integrin Inhibitor for the Treatment of Non-Exudative (Dry) Age-Related Macular Degeneration and Diabetic Macular Edema. Expert Opin. Investig. Drugs 2020, 29, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-Berka, J.L.; Jones, D.; Taylor, G.; Jaworski, K.; Kelly, D.J.; Ludbrook, S.B.; Willette, R.N.; Kumar, S.; Gilbert, R.E. SB-267268, a Nonpeptidic Antagonist of Alpha(v)Beta3 and Alpha(v)Beta5 Integrins, Reduces Angiogenesis and VEGF Expression in a Mouse Model of Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1600–1605. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Yu, H.; Yu, Y.; Geng, Y.; Li, D.; Yang, C.; Lv, Q.; Lu, L.; Liu, T.; Li, G.; et al. Levels of Inflammatory Cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in Aqueous Humour of Patients with Diabetic Retinopathy. J. Diabetes Res. 2018, 2018, 8546423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Phone, A.; Lamy, R.; Ma, D.; Laotaweerungsawat, S.; Chen, Y.; Zhao, T.; Ma, W.; Zhang, F.; Psaras, C.; et al. Correlation of Aqueous, Vitreous, and Plasma Cytokine Levels in Patients With Proliferative Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustavsson, C.; Agardh, C.-D.; Agardh, E. Profile of Intraocular Tumour Necrosis Factor-α and Interleukin-6 in Diabetic Subjects with Different Degrees of Diabetic Retinopathy. Acta Ophthalmol. 2013, 91, 445–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitoma, H.; Horiuchi, T.; Tsukamoto, H.; Ueda, N. Molecular Mechanisms of Action of Anti-TNF-α Agents—Comparison among Therapeutic TNF-α Antagonists. Cytokine 2018, 101, 56–63. [Google Scholar] [CrossRef]
- Sfikakis, P.P.; Markomichelakis, N.; Theodossiadis, G.P.; Grigoropoulos, V.; Katsilambros, N.; Theodossiadis, P.G. Regression of Sight-Threatening Macular Edema in Type 2 Diabetes Following Treatment with the Anti-Tumor Necrosis Factor Monoclonal Antibody Infliximab. Diabetes Care 2005, 28, 445–447. [Google Scholar] [CrossRef] [Green Version]
- Mesquida, M.; Drawnel, F.; Lait, P.J.; Copland, D.A.; Stimpson, M.L.; Llorenç, V.; Sainz de la Maza, M.; Adan, A.; Widmer, G.; Strassburger, P.; et al. Modelling Macular Edema: The Effect of IL-6 and IL-6R Blockade on Human Blood–Retinal Barrier Integrity In Vitro. Transl. Vis. Sci. Technol. 2019, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Valle, M.L.; Dworshak, J.; Sharma, A.; Ibrahim, A.S.; Al-Shabrawey, M.; Sharma, S. Inhibition of Interleukin-6 Trans-Signaling Prevents Inflammation and Endothelial Barrier Disruption in Retinal Endothelial Cells. Exp. Eye Res. 2019, 178, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Yun, J.-H.; Cho, C.S.; Kim, J.H.; Kim, J.H.; Cho, C.-H. Interaction between Microglia and Retinal Pigment Epithelial Cells Determines the Integrity of Outer Blood-Retinal Barrier in Diabetic Retinopathy. Glia 2019, 67, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.C.; Anderson, M.E.; Moots, R.J. The Many Faces of Interleukin-6: The Role of IL-6 in Inflammation, Vasculopathy, and Fibrosis in Systemic Sclerosis. Int. J. Rheumatol. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Pro-Inflammatory Activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S. Interleukin-6 Trans-Signaling: A Pathway With Therapeutic Potential for Diabetic Retinopathy. Front. Physiol. 2021, 12, 689429. [Google Scholar] [CrossRef]
- Ohsugi, Y.; Kishimoto, T. The Recombinant Humanized Anti-IL-6 Receptor Antibody Tocilizumab, an Innovative Drug for the Treatment of Rheumatoid Arthritis. Expert Opin. Biol. Ther. 2008, 8, 669–681. [Google Scholar] [CrossRef]
- Noda, K.; Nakao, S.; Zandi, S.; Engelstädter, V.; Mashima, Y.; Hafezi-Moghadam, A. Vascular Adhesion Protein-1 Regulates Leukocyte Transmigration Rate in the Retina during Diabetes. Exp. Eye Res. 2009, 89, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, M.; Noda, K.; Fukuhara, J.; Kanda, A.; Kase, S.; Saito, W.; Ozawa, Y.; Mochizuki, S.; Kimura, S.; Mashima, Y.; et al. Soluble Vascular Adhesion Protein-1 Accumulates in Proliferative Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2012, 53, 4055. [Google Scholar] [CrossRef]
- Tékus, V.; Horváth, Á.I.; Csekő, K.; Szabadfi, K.; Kovács-Valasek, A.; Dányádi, B.; Deres, L.; Halmosi, R.; Sághy, É.; Varga, Z.V.; et al. Protective Effects of the Novel Amine-Oxidase Inhibitor Multi-Target Drug SZV 1287 on Streptozotocin-Induced Beta Cell Damage and Diabetic Complications in Rats. Biomed. Pharmacother. 2021, 134, 111105. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Kulkarni, Y.A. Vascular Adhesion Protein-1 and Microvascular Diabetic Complications. Pharmacol. Rep. 2022, 74, 40–46. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Sepah, Y.J.; Berger, B.; Brown, D.; Do, D.V.; Garcia-Hernandez, A.; Patel, S.; Rahhal, F.M.; Shildkrot, Y.; Renfurm, R.W.; et al. Primary Outcomes of the VIDI Study: Phase 2, Double-Masked, Randomized, Active-Controlled Study of ASP8232 for Diabetic Macular Edema. Int. J. Retina Vitr. 2019, 5, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, P.; Knaus, E.E. Evolution of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Cyclooxygenase (COX) Inhibition and Beyond. J. Pharm. Pharm. Sci. 2008, 11, 81s–110s. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.; Francis, P. Ophthalmic Utility of Topical Bromfenac, a Twice-Daily Nonsteroidal Anti-Inflammatory Agent. Expert Opin. Pharmacother. 2009, 10, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, B.I.; Onyekwuluje, A. Topical Ophthalmic NSAIDs: A Discussion with Focus on Nepafenac Ophthalmic Suspension. Clin. Ophthalmol. 2008, 2, 355–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinna, A.; Blasetti, F.; Ricci, G.D.; Boscia, F. Bromfenac Eyedrops in the Treatment of Diabetic Macular Edema: A Pilot Study. Eur. J. Ophthalmol. 2017, 27, 326–330. [Google Scholar] [CrossRef]
- Callanan, D.; Williams, P. Topical Nepafenac in the Treatment of Diabetic Macular Edema. Clin. Ophthalmol. 2008, 2, 689–692. [Google Scholar] [CrossRef] [Green Version]
- Howaidy, A.; Eldaly, Z.H.; Anis, M.; Othman, T.M. Prophylaxis of Macular Edema after Cataract Surgery in Diabetic Patients, Topical Nepafenac versus Intravitreal Ranibizumab. Eur. J. Ophthalmol. 2022, 32, 205–212. [Google Scholar] [CrossRef]
- Naftali Ben Haim, L.; Moisseiev, E. Drug Delivery via the Suprachoroidal Space for the Treatment of Retinal Diseases. Pharmaceutics 2021, 13, 967. [Google Scholar] [CrossRef]
- Ranta, V.-P.; Mannermaa, E.; Lummepuro, K.; Subrizi, A.; Laukkanen, A.; Antopolsky, M.; Murtomäki, L.; Hornof, M.; Urtti, A. Barrier Analysis of Periocular Drug Delivery to the Posterior Segment. J. Control. Release 2010, 148, 42–48. [Google Scholar] [CrossRef]
- Barakat, M.R.; Wykoff, C.C.; Gonzalez, V.; Hu, A.; Marcus, D.; Zavaleta, E.; Ciulla, T.A. Suprachoroidal CLS-TA plus Intravitreal Aflibercept for Diabetic Macular Edema: A Randomized, Double-Masked, Parallel-Design, Controlled Study. Ophthalmol. Retina 2021, 5, 60–70. [Google Scholar] [CrossRef]
- Fachinger, G.; Deutsch, U.; Risau, W. Functional Interaction of Vascular Endothelial-Protein-Tyrosine Phosphatase with the Angiopoietin Receptor Tie-2. Oncogene 1999, 18, 5948–5953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, R.M.; Neiweem, A.E.; Kansara, V.; Harris, A.; Ciulla, T.A. Tie-2/Angiopoietin Pathway Modulation as a Therapeutic Strategy for Retinal Disease. Expert Opin. Investig. Drugs 2019, 28, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Mirando, A.C.; Shen, J.; Silva, R.L.E.; Chu, Z.; Sass, N.C.; Lorenc, V.E.; Green, J.J.; Campochiaro, P.A.; Popel, A.S.; Pandey, N.B. A Collagen IV-Derived Peptide Disrupts A5β1 Integrin and Potentiates Ang2/Tie2 Signaling. JCI Insight 2019, 4, 122043. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; Eichenbaum, D.A.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. Efficacy, Durability, and Safety of Intravitreal Faricimab with Extended Dosing up to Every 16 Weeks in Patients with Diabetic Macular Oedema (YOSEMITE and RHINE): Two Randomised, Double-Masked, Phase 3 Trials. Lancet 2022, 399, 10326. [Google Scholar] [CrossRef]
- Shirley, M. Faricimab: First Approval. Drugs 2022, 82, 825–830. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Peters, K.G. Targeting Tie2 for Treatment of Diabetic Retinopathy and Diabetic Macular Edema. Curr. Diab. Rep. 2016, 16, 126. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Khanani, A.; Singer, M.; Patel, S.; Boyer, D.; Dugel, P.; Kherani, S.; Withers, B.; Gambino, L.; Peters, K.; et al. Enhanced Benefit in Diabetic Macular Edema from AKB-9778 Tie2 Activation Combined with Vascular Endothelial Growth Factor Suppression. Ophthalmology 2016, 123, 1722–1730. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Y.; Xu, J.-Y.; Zhang, J.; Sinclair, S.H.; Yanoff, M.; Xu, G.; Li, W.; Xu, G.-T. ERK- and Akt-Dependent Neuroprotection by Erythropoietin (EPO) against Glyoxal-AGEs via Modulation of Bcl-XL, Bax, and BAD. Investig. Ophthalmol. Vis. Sci. 2010, 51, 35–46. [Google Scholar] [CrossRef]
- Hu, L.-M.; Luo, Y.; Zhang, J.; Lei, X.; Shen, J.; Wu, Y.; Qin, M.; Unver, Y.B.; Zhong, Y.; Xu, G.-T.; et al. EPO Reduces Reactive Gliosis and Stimulates Neurotrophin Expression in Muller Cells. Front. Biosci.-Elite 2011, 3, 1541–1555. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hu, L.-M.; Xu, G.; Wu, Y.; Shen, J.; Luo, Y.; Zhong, Y.; Sinclair, S.H.; Yanoff, M.; Li, W.; et al. Anti-VEGF Effects of Intravitreal Erythropoietin in Early Diabetic Retinopathy. Front. Biosci.-Elite 2010, 2, 912–927. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Zhang, J.; Shen, J.; Hu, L.-M.; Wu, Y.; Mou, L.; Xu, G.; Li, W.; Xu, G.-T. EPO Attenuates Inflammatory Cytokines by Muller Cells in Diabetic Retinopathy. Front. Biosci. Elite Ed. 2011, 3, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Kang, D.; Zhang, C.; Lou, H.; Sun, C.; Yang, Q.; Lu, L.; Xu, G.-T.; Zhang, J.; Wang, F. Erythropoietin Protects Retinal Cells in Diabetic Rats Through Upregulating ZnT8 via Activating ERK Pathway and Inhibiting HIF-1α Expression. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8166–8178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Xu, H.; Wang, F.; Xu, G.; Sinha, D.; Wang, J.; Xu, J.-Y.; Tian, H.; Gao, F.; Li, W.; et al. Erythropoietin Exerts a Neuroprotective Function against Glutamate Neurotoxicity in Experimental Diabetic Retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8208–8222. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, H.; Zhang, C.; Xie, H.; Yang, Q.; Li, W.; Tian, H.; Lu, L.; Xu, J.-Y.; Xu, G.; et al. Erythropoietin Maintains VE-Cadherin Expression and Barrier Function in Experimental Diabetic Retinopathy via Inhibiting VEGF/VEGFR2/Src Signaling Pathway. Life Sci. 2020, 259, 118273. [Google Scholar] [CrossRef]
- Li, W.; Sinclair, S.H.; Xu, G.-T. Effects of Intravitreal Erythropoietin Therapy for Patients with Chronic and Progressive Diabetic Macular Edema. Ophthalmic Surg. Lasers Imaging Retin. 2010, 41, 18–25. [Google Scholar] [CrossRef]
- Ahmet, I.; Tae, H.-J.; Juhaszova, M.; Riordon, D.R.; Boheler, K.R.; Sollott, S.J.; Brines, M.; Cerami, A.; Lakatta, E.G.; Talan, M.I. A Small Nonerythropoietic Helix B Surface Peptide Based upon Erythropoietin Structure Is Cardioprotective against Ischemic Myocardial Damage. Mol. Med. 2011, 17, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Brines, M.; Patel, N.S.A.; Villa, P.; Brines, C.; Mennini, T.; De Paola, M.; Erbayraktar, Z.; Erbayraktar, S.; Sepodes, B.; Thiemermann, C.; et al. Nonerythropoietic, Tissue-Protective Peptides Derived from the Tertiary Structure of Erythropoietin. Proc. Natl. Acad. Sci. USA 2008, 105, 10925–10930. [Google Scholar] [CrossRef] [Green Version]
- McVicar, C.M.; Hamilton, R.; Colhoun, L.M.; Gardiner, T.A.; Brines, M.; Cerami, A.; Stitt, A.W. Intervention With an Erythropoietin-Derived Peptide Protects Against Neuroglial and Vascular Degeneration During Diabetic Retinopathy. Diabetes 2011, 60, 2995–3005. [Google Scholar] [CrossRef] [Green Version]
- Simó, R.; Hernández, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; García-Arumí, J.; et al. Effects of Topically Administered Neuroprotective Drugs in Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Diabetes 2019, 68, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Grauslund, J.; Frydkjaer-Olsen, U.; Peto, T.; Fernández-Carneado, J.; Ponsati, B.; Hernández, C.; Cunha-Vaz, J.; Simó, R.; EUROCONDOR. Topical Treatment With Brimonidine and Somatostatin Causes Retinal Vascular Dilation in Patients With Early Diabetic Retinopathy From the EUROCONDOR. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2257–2262. [Google Scholar] [CrossRef] [Green Version]
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Urner, S.; Ho, F.; Jha, J.C.; Ziegler, D.; Jandeleit-Dahm, K. NADPH Oxidase Inhibition: Preclinical and Clinical Studies in Diabetic Complications. Antioxid. Redox Signal. 2020, 33, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-Berka, J.L.; Deliyanti, D.; Rana, I.; Miller, A.G.; Agrotis, A.; Armani, R.; Szyndralewiez, C.; Wingler, K.; Touyz, R.M.; Cooper, M.E.; et al. NADPH Oxidase, NOX1, Mediates Vascular Injury in Ischemic Retinopathy. Antioxid. Redox Signal. 2014, 20, 2726–2740. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabrawey, M.; Rojas, M.; Sanders, T.; Behzadian, A.; El-Remessy, A.; Bartoli, M.; Parpia, A.K.; Liou, G.; Caldwell, R.B. Role of NADPH Oxidase in Retinal Vascular Inflammation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3239–3244. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.J.; Yu, Q.; Chen, K.; Mahadev, K.; Zhang, S.X. Inhibition of Reactive Oxygen Species by Lovastatin Downregulates Vascular Endothelial Growth Factor Expression and Ameliorates Blood-Retinal Barrier Breakdown in Db/Db Mice: Role of NADPH Oxidase 4. Diabetes 2010, 59, 1528–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clementi, M.E.; Pizzoferrato, M.; Bianchetti, G.; Brancato, A.; Sampaolese, B.; Maulucci, G.; Tringali, G. Cytoprotective Effect of Idebenone through Modulation of the Intrinsic Mitochondrial Pathway of Apoptosis in Human Retinal Pigment Epithelial Cells Exposed to Oxidative Stress Induced by Hydrogen Peroxide. Biomedicines 2022, 10, 503. [Google Scholar] [CrossRef]
- Clementi, M.E.; Maulucci, G.; Bianchetti, G.; Pizzoferrato, M.; Sampaolese, B.; Tringali, G. Cytoprotective Effects of Punicalagin on Hydrogen-Peroxide-Mediated Oxidative Stress and Mitochondrial Dysfunction in Retinal Pigment Epithelium Cells. Antioxidants 2021, 10, 192. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells 2022, 11, 3362. https://doi.org/10.3390/cells11213362
Zhang J, Zhang J, Zhang C, Zhang J, Gu L, Luo D, Qiu Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells. 2022; 11(21):3362. https://doi.org/10.3390/cells11213362
Chicago/Turabian StyleZhang, Jingfa, Jingxiang Zhang, Chaoyang Zhang, Jingting Zhang, Limin Gu, Dawei Luo, and Qinghua Qiu. 2022. "Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications" Cells 11, no. 21: 3362. https://doi.org/10.3390/cells11213362
APA StyleZhang, J., Zhang, J., Zhang, C., Zhang, J., Gu, L., Luo, D., & Qiu, Q. (2022). Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells, 11(21), 3362. https://doi.org/10.3390/cells11213362





