Osteoprotegerin (OPG) Upregulation Activates Breast Stromal Fibroblasts and Enhances Their Pro-Carcinogenic Effects through the STAT3/IL-6 Signaling
Abstract
:1. Introduction
2. Materials Methods
2.1. Cells, Cell Culture and Reagents
2.2. RNA Purification and qRT-PCR
2.3. siRNA Transfection
2.4. Transfections with Plasmids
2.5. Cell Lysate Preparation and Immunoblotting
2.6. Cell Invasion, Migration, and Proliferation Assays
2.7. 3D Spheroid Assay
2.8. Three-Dimensional Cell Culture
2.9. Serum-Free Conditioned Media Preparation
2.10. ELISA Assays
2.11. Human Cytokine Antibody Array
2.12. Orthotopic Tumor Xenografts
2.13. Statistical Analysis and Quantification
3. Results
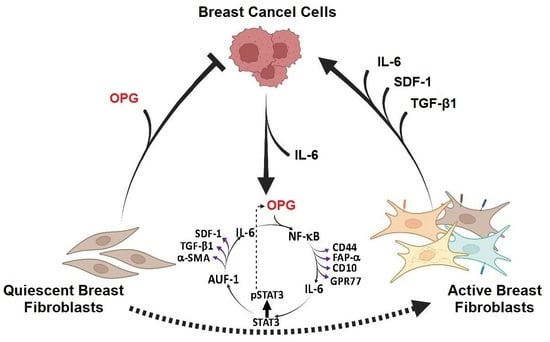
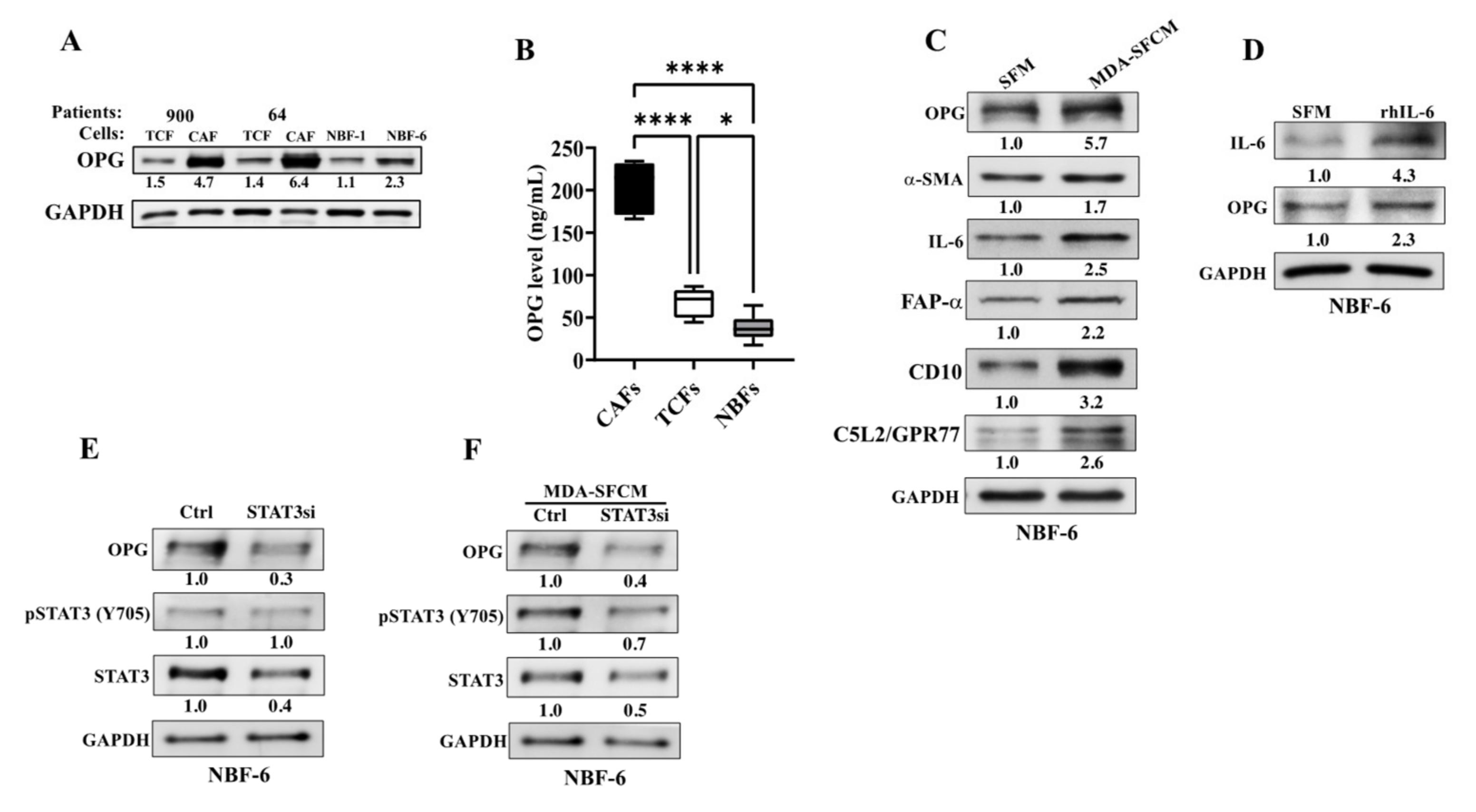
3.1. OPG Is Upregulated in Active Breast Cancer-Associated Fibroblasts
3.2. Breast Cancer Cells Upregulate OPG in Breast Stromal Fibroblasts in a Paracrine Manner through the IL-6/STAT3 Pathway
3.3. Ectopic Expression of OPG Activates Breast Stromal Fibroblasts through the NF-κB/STAT3/AUF1 Signaling Pathway
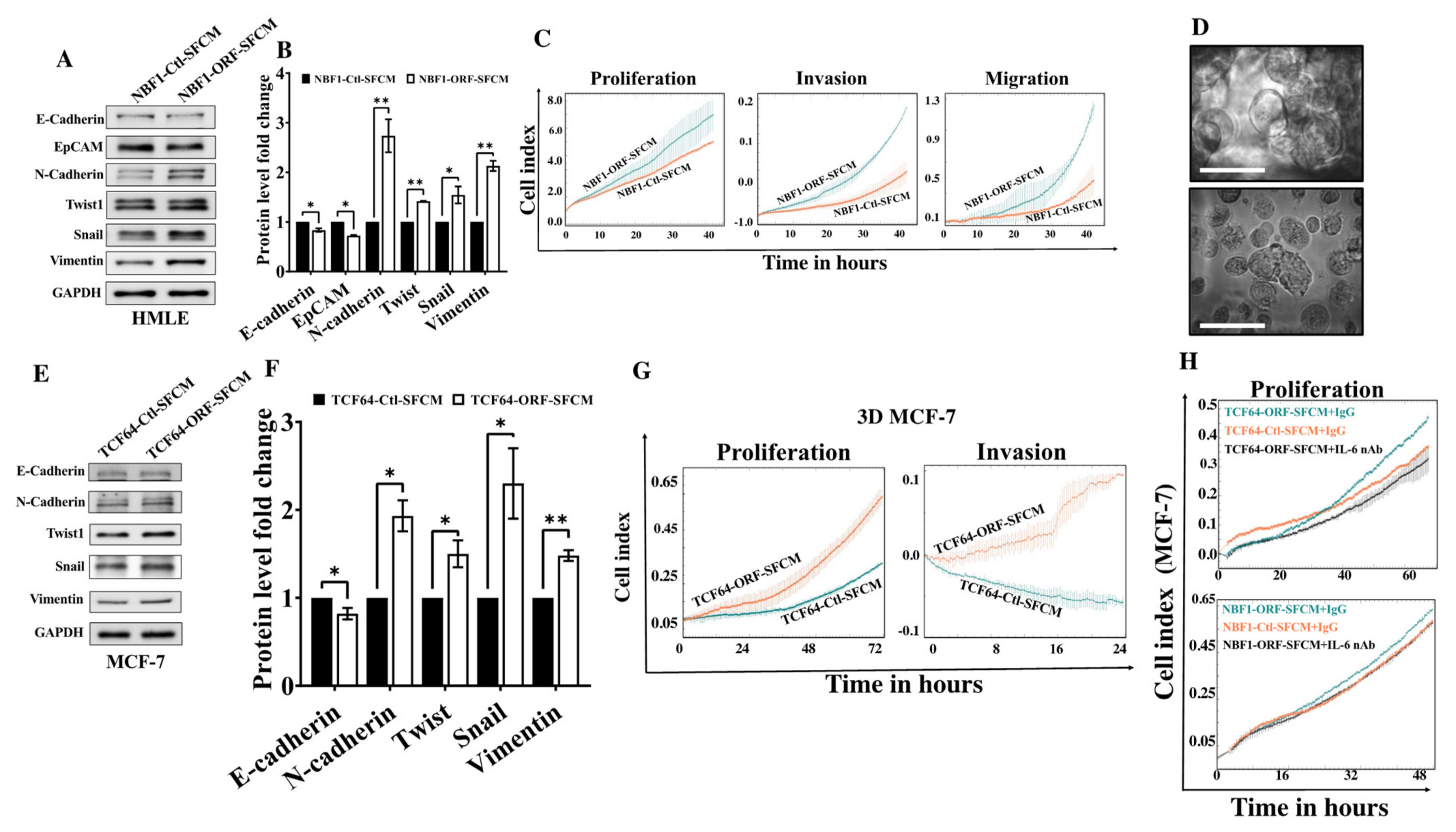
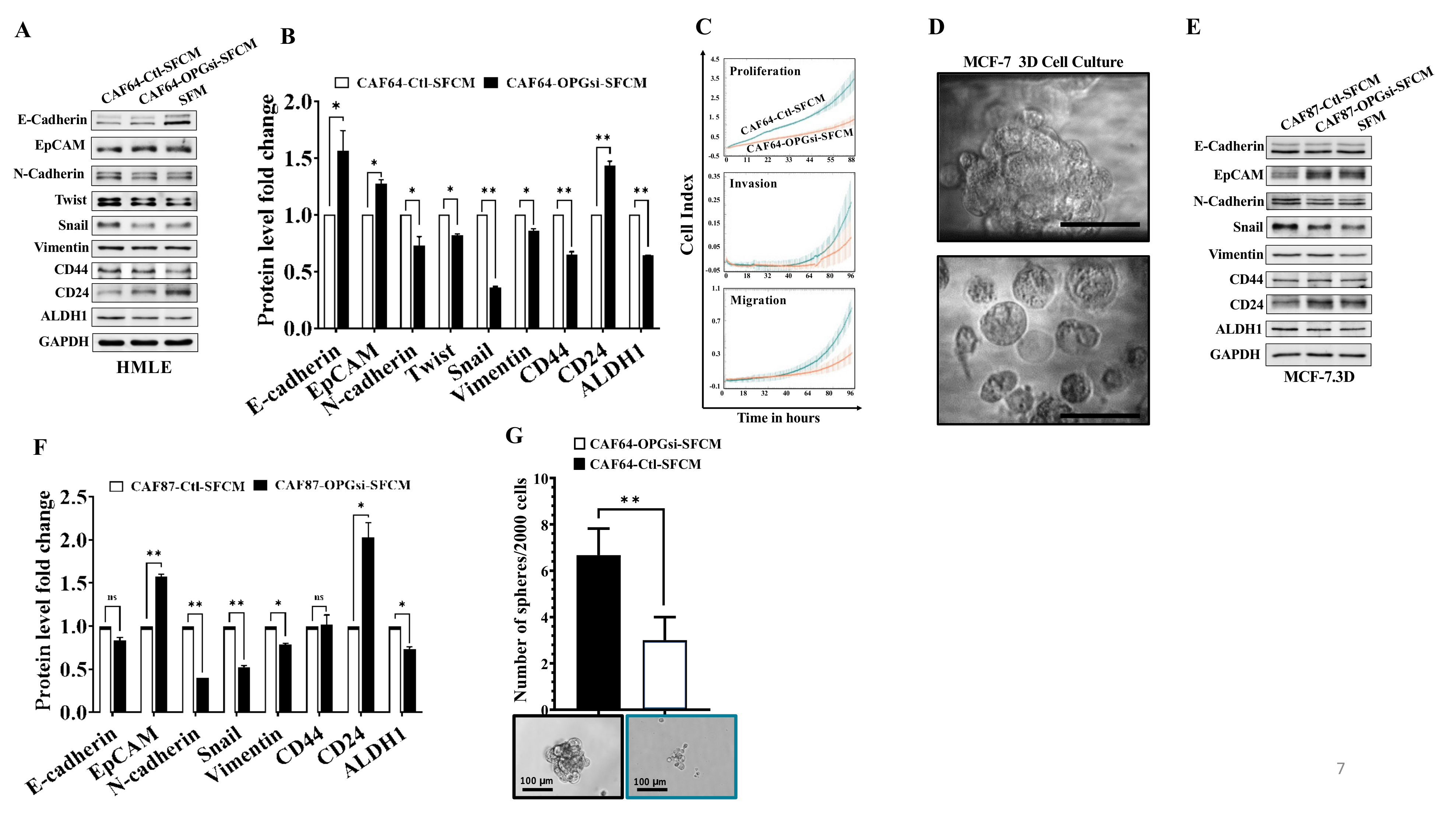
3.4. OPG Upregulation Promotes the Paracrine Pro-EMT Effect of Breast Stromal Fibroblasts in an IL-6-Dependent Manner
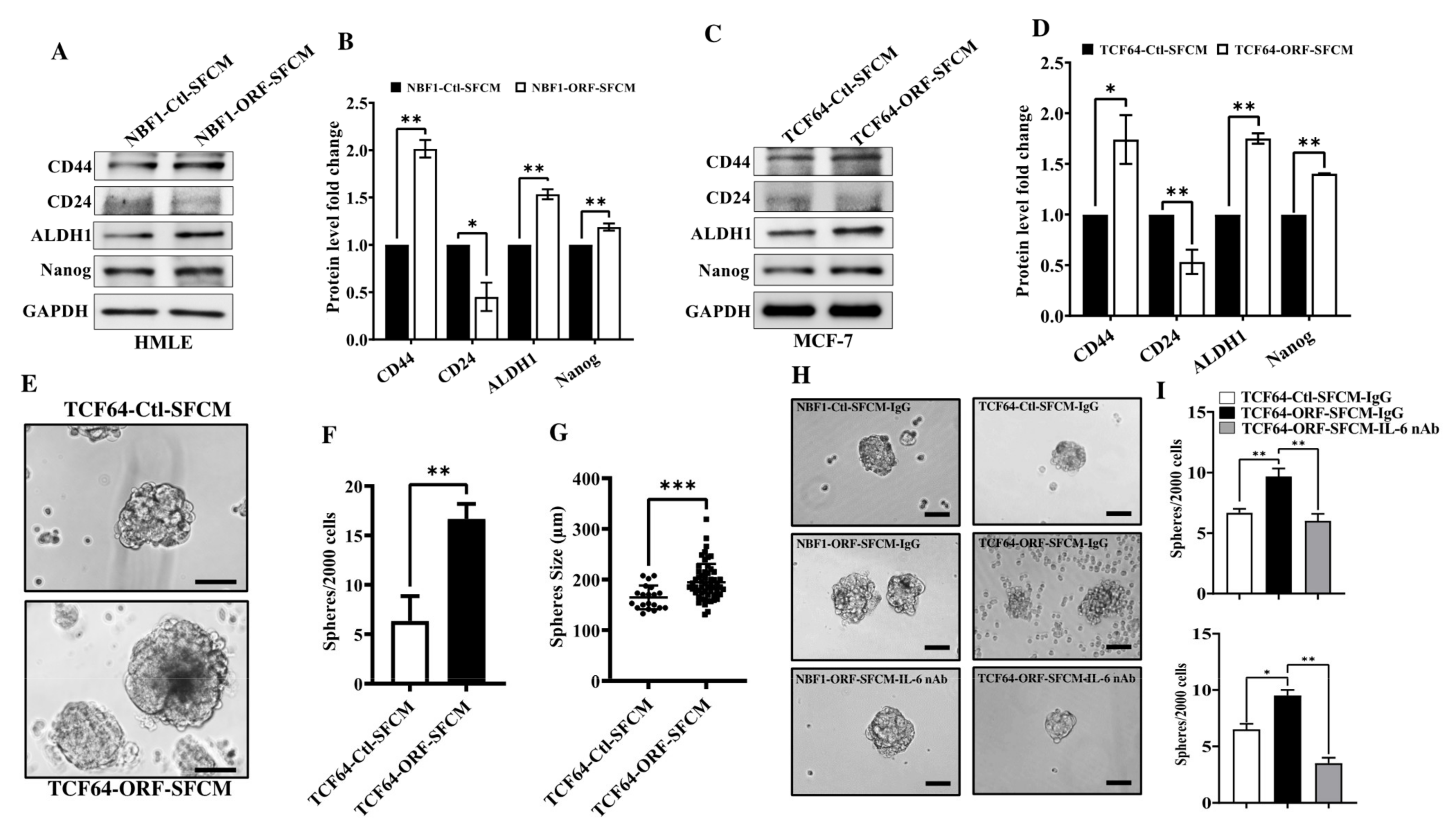
3.5. OPG Upregulation Promotes the Paracrine Pro-Stemness Effect of Breast Stromal Fibroblasts in an IL-6-Dependent Manner
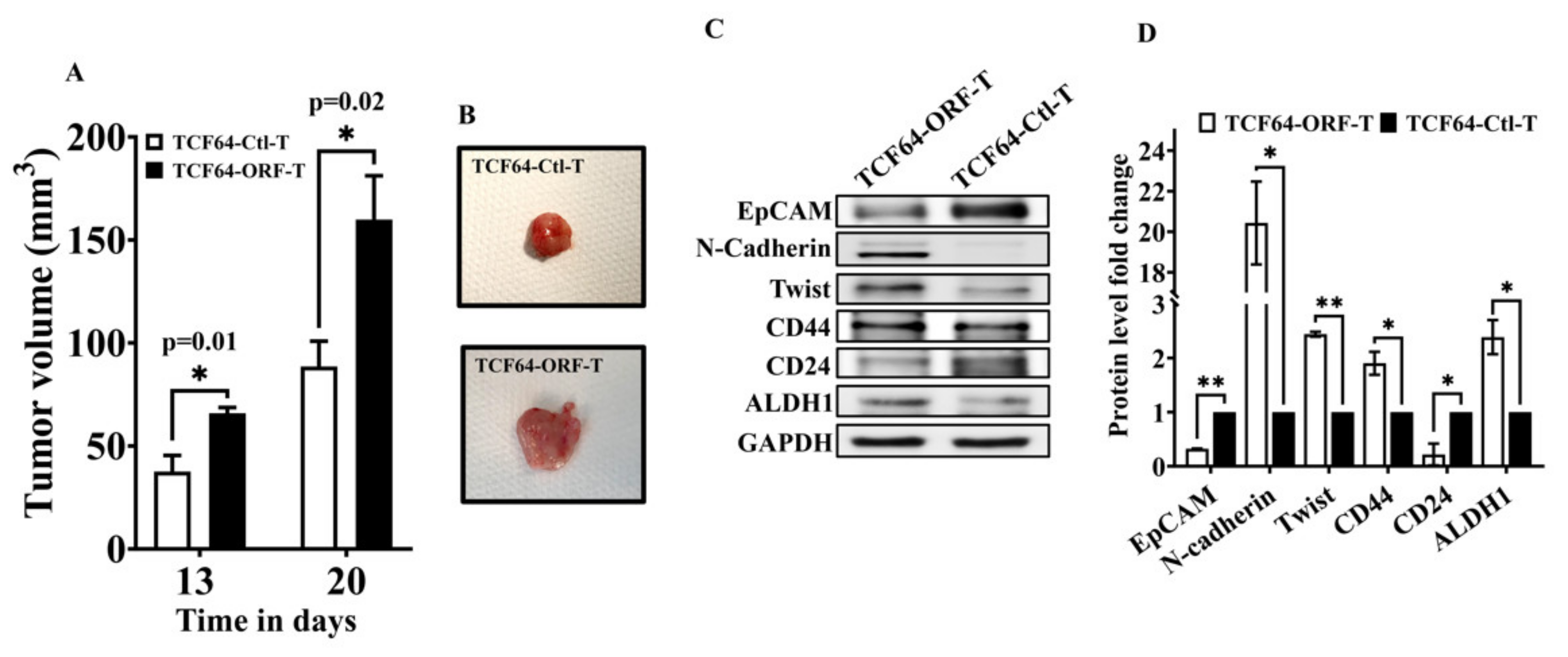
3.6. OPG Upregulation in Breast Stromal Fibroblasts Enhances Their Paracrine Pro-Carcinogenic Effects In Vivo
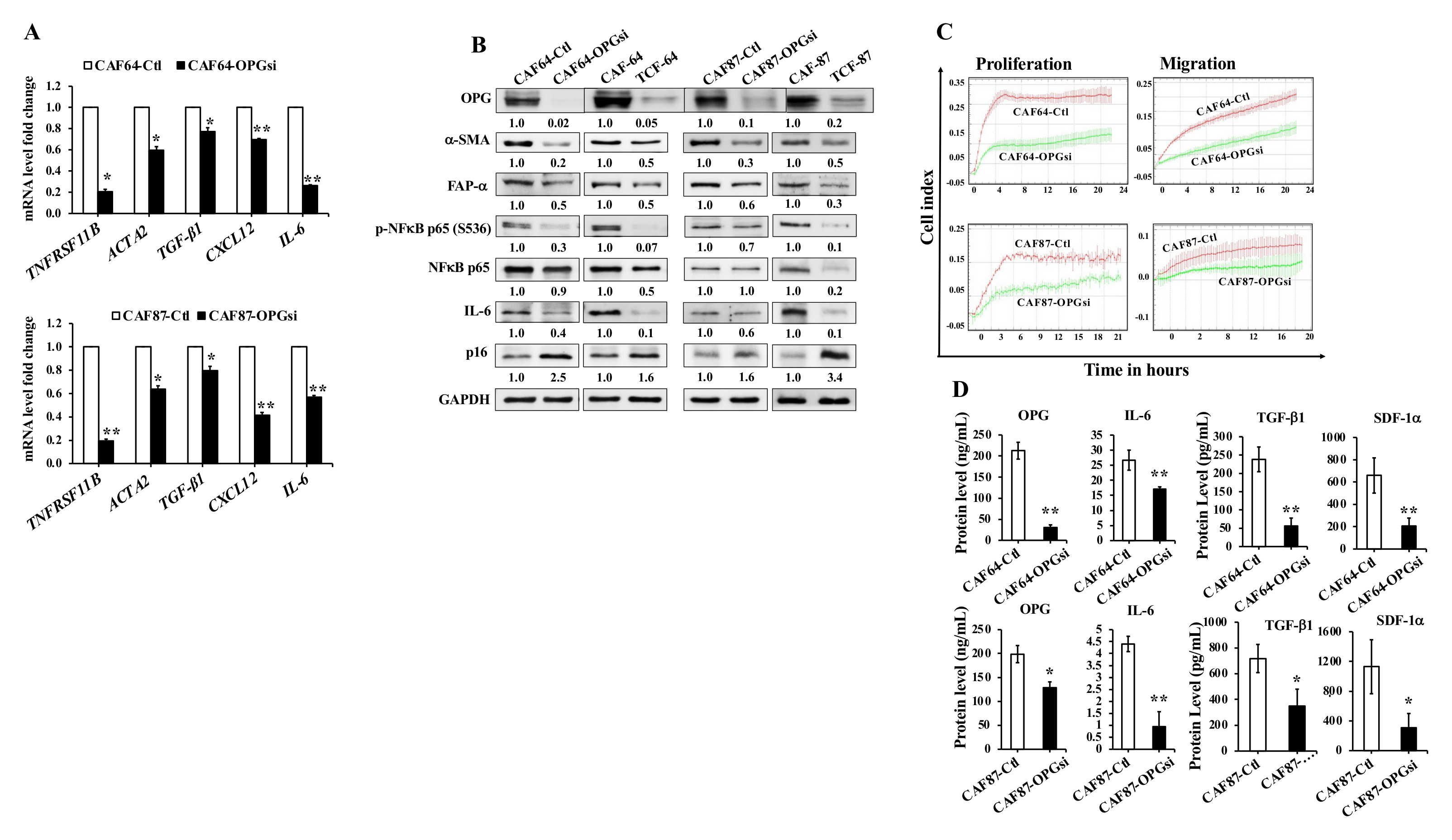
3.7. OPG Downregulation Suppresses Active Breast Stromal Fibroblasts
3.8. OPG Downregulation Suppresses the Paracrine Pro-Carcinogenic Effects of Active CAFs
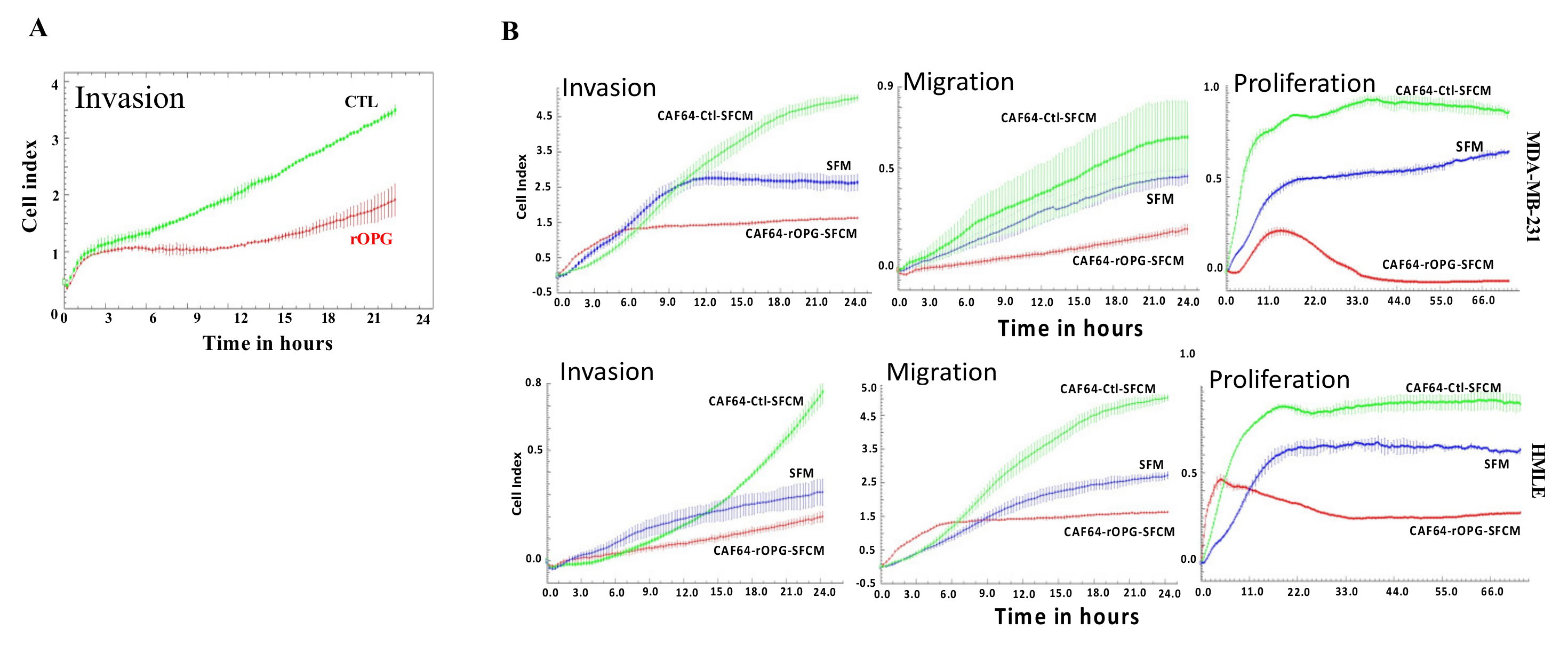
3.9. Human Recombinant OPG Suppresses the Paracrine Pro-Carcinogenic Effects of Active CAFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Twigger, A.J.; Khaled, W.T. Mammary gland development from a single cell ‘omics view. Semin. Cell Dev. Biol. 2021, 114, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alraouji, N.N.; Hendrayani, S.F.; Ghebeh, H.; Al-Mohanna, F.H.; Aboussekhra, A. Osteoprotegerin (OPG) mediates the anti-carcinogenic effects of normal breast fibroblasts and targets cancer stem cells through inhibition of the beta-catenin pathway. Cancer Lett. 2021, 520, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Sadlonova, A.; Mukherjee, S.; Bowe, D.B.; Gault, S.R.; Dumas, N.A.; Van Tine, B.A.; Frolova, N.; Page, G.P.; Welch, D.R.; Novak, L.; et al. Human breast fibroblasts inhibit growth of the MCF10AT xenograft model of proliferative breast disease. Am. J. Pathol. 2007, 170, 1064–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadlonova, A.; Novak, Z.; Johnson, M.R.; Bowe, D.B.; Gault, S.R.; Page, G.P.; Thottassery, J.V.; Welch, D.R.; Frost, A.R. Breast fibroblasts modulate epithelial cell proliferation in three-dimensional in vitro co-culture. Breast Cancer Res. 2005, 7, R46–R59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.e10. [Google Scholar] [CrossRef]
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018, 172, 841–856.e16. [Google Scholar] [CrossRef] [PubMed]
- Pelon, F.; Bourachot, B.; Kieffer, Y.; Magagna, I.; Mermet-Meillon, F.; Bonnet, I.; Costa, A.; Givel, A.M.; Attieh, Y.; Barbazan, J.; et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat. Commun. 2020, 11, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Kwon, B.S.; Wang, S.; Udagawa, N.; Haridas, V.; Lee, Z.H.; Kim, K.K.; Oh, K.O.; Greene, J.; Li, Y.; Su, J.; et al. TR1, a new member of the tumor necrosis factor receptor superfamily, induces fibroblast proliferation and inhibits osteoclastogenesis and bone resorption. FASEB J. 1998, 12, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Infante, M.; Fabi, A.; Cognetti, F.; Gorini, S.; Caprio, M.; Fabbri, A. RANKL/RANK/OPG system beyond bone remodeling: Involvement in breast cancer and clinical perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 12. [Google Scholar] [CrossRef] [Green Version]
- Holen, I.; Cross, S.S.; Neville-Webbe, H.L.; Cross, N.A.; Balasubramanian, S.P.; Croucher, P.I.; Evans, C.A.; Lippitt, J.M.; Coleman, R.E.; Eaton, C.L. Osteoprotegerin (OPG) expression by breast cancer cells in vitro and breast tumours in vivo—A role in tumour cell survival? Breast Cancer Res. Treat. 2005, 92, 207–215. [Google Scholar] [CrossRef]
- Goswami, S.; Sharma-Walia, N. Osteoprotegerin rich tumor microenvironment: Implications in breast cancer. Oncotarget 2016, 7, 42777–42791. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.L.; Thomas-Mudge, R.J.; Elliott, J.; Hards, D.K.; Sims, N.A.; Slavin, J.; Martin, T.J.; Gillespie, M.T. Osteoprotegerin overexpression by breast cancer cells enhances orthotopic and osseous tumor growth and contrasts with that delivered therapeutically. Cancer Res. 2006, 66, 3620–3628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerts, D.; Chopra, C.; Connelly, L. Osteoprotegerin: Relationship to Breast Cancer Risk and Prognosis. Front. Oncol. 2020, 10, 462. [Google Scholar] [CrossRef]
- Hawsawi, N.M.; Ghebeh, H.; Hendrayani, S.F.; Tulbah, A.; Al-Eid, M.; Al-Tweigeri, T.; Ajarim, D.; Alaiya, A.; Dermime, S.; Aboussekhra, A. Breast carcinoma-associated fibroblasts and their counterparts display neoplastic-specific changes. Cancer Res. 2008, 68, 2717–2725. [Google Scholar] [CrossRef] [Green Version]
- Al-Mohanna, M.A.; Al-Khalaf, H.H.; Al-Yousef, N.; Aboussekhra, A. The p16INK4a tumor suppressor controls p21WAF1 induction in response to ultraviolet light. Nucleic Acids Res. 2007, 35, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrayani, S.F.; Al-Khalaf, H.H.; Aboussekhra, A. The Cytokine IL-6 Reactivates Breast Stromal Fibroblasts through Transcription Factor STAT3-dependent Up-regulation of the RNA-binding Protein AUF1. J. Biol. Chem. 2014, 289, 30962–30976. [Google Scholar] [CrossRef] [Green Version]
- Kinugasa, Y.; Matsui, T.; Takakura, N. CD44 expressed on cancer-associated fibroblasts is a functional molecule supporting the stemness and drug resistance of malignant cancer cells in the tumor microenvironment. Stem. Cells 2014, 32, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chang, Q.; Xin, M.; Wang, Q.; Li, H.; Qian, J. Endogenous bone morphogenetic protein 2 plays a role in vascular smooth muscle cell calcification induced by interleukin 6 in vitro. Int. J. Immunopathol. Pharmacol. 2017, 30, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.T.; Geerts, D.; Roseman, K.; Renaud, A.; Connelly, L. Osteoprotegerin mediates tumor-promoting effects of Interleukin-1beta in breast cancer cells. Mol. Cancer 2017, 16, 27. [Google Scholar] [CrossRef] [Green Version]
- Takai, H.; Kanematsu, M.; Yano, K.; Tsuda, E.; Higashio, K.; Ikeda, K.; Watanabe, K.; Yamada, Y. Transforming growth factor-beta stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J. Biol. Chem. 1998, 273, 27091–27096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Chen, T.; Zhang, Y.; Lyu, L.; Zhang, B.; Huang, C.; Zhou, X.; Wu, Y.; Li, Z. MiR-30c-5p regulates adventitial progenitor cells differentiation to vascular smooth muscle cells through targeting OPG. Stem Cell Res. Ther. 2021, 12, 67. [Google Scholar] [CrossRef]
- Yang, X.; Lin, Y.; Shi, Y.; Li, B.; Liu, W.; Yin, W.; Dang, Y.; Chu, Y.; Fan, J.; He, R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016, 76, 4124–4135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Lu, Y.; Su, A.; Chen, J.; Li, J.; Zhou, B.; Liu, X.; Xia, Q.; Li, Y.; Li, J.; et al. A CD10-OGP Membrane Peptolytic Signaling Axis in Fibroblasts Regulates Lipid Metabolism of Cancer Stem Cells via SCD1. Adv. Sci. 2021, 8, e2101848. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, D.; Xi, L.; Chen, Y.; Fu, L.; Sun, K.; Yin, J.; Li, X.; Liu, S.; Qin, Y.; et al. Primed atypical ductal hyperplasia-associated fibroblasts promote cell growth and polarity changes of transformed epithelium-like breast cancer MCF-7 cells via miR-200b/c-IKKbeta signaling. Cell Death Dis. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Jin, P.; Xu, S.; Zhang, T.; Yang, X.; Li, X.; Wei, X.; Sun, C.; Chen, G.; Ma, D.; et al. Dicer reprograms stromal fibroblasts to a pro-inflammatory and tumor-promoting phenotype in ovarian cancer. Cancer Lett. 2018, 415, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Lyu, Y.L.; Cai, L. NF-kappaB affects proliferation and invasiveness of breast cancer cells by regulating CD44 expression. PLoS ONE 2014, 9, e106966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, Y.; Huang, Z.; Chen, X.; Zhang, B. The roles of osteoprotegerin in cancer, far beyond a bone player. Cell Death Discov. 2022, 8, 252. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Nasrallah, H.K.; Al-Ansari, M.M.; Aboussekhra, A. Osteoprotegerin (OPG) Upregulation Activates Breast Stromal Fibroblasts and Enhances Their Pro-Carcinogenic Effects through the STAT3/IL-6 Signaling. Cells 2022, 11, 3369. https://doi.org/10.3390/cells11213369
Al-Nasrallah HK, Al-Ansari MM, Aboussekhra A. Osteoprotegerin (OPG) Upregulation Activates Breast Stromal Fibroblasts and Enhances Their Pro-Carcinogenic Effects through the STAT3/IL-6 Signaling. Cells. 2022; 11(21):3369. https://doi.org/10.3390/cells11213369
Chicago/Turabian StyleAl-Nasrallah, Huda K., Mysoon M. Al-Ansari, and Abdelilah Aboussekhra. 2022. "Osteoprotegerin (OPG) Upregulation Activates Breast Stromal Fibroblasts and Enhances Their Pro-Carcinogenic Effects through the STAT3/IL-6 Signaling" Cells 11, no. 21: 3369. https://doi.org/10.3390/cells11213369