The DNA Methylation in Neurological Diseases
Abstract
:1. Introduction
2. DNA Methylation in Neurodegenerative Diseases
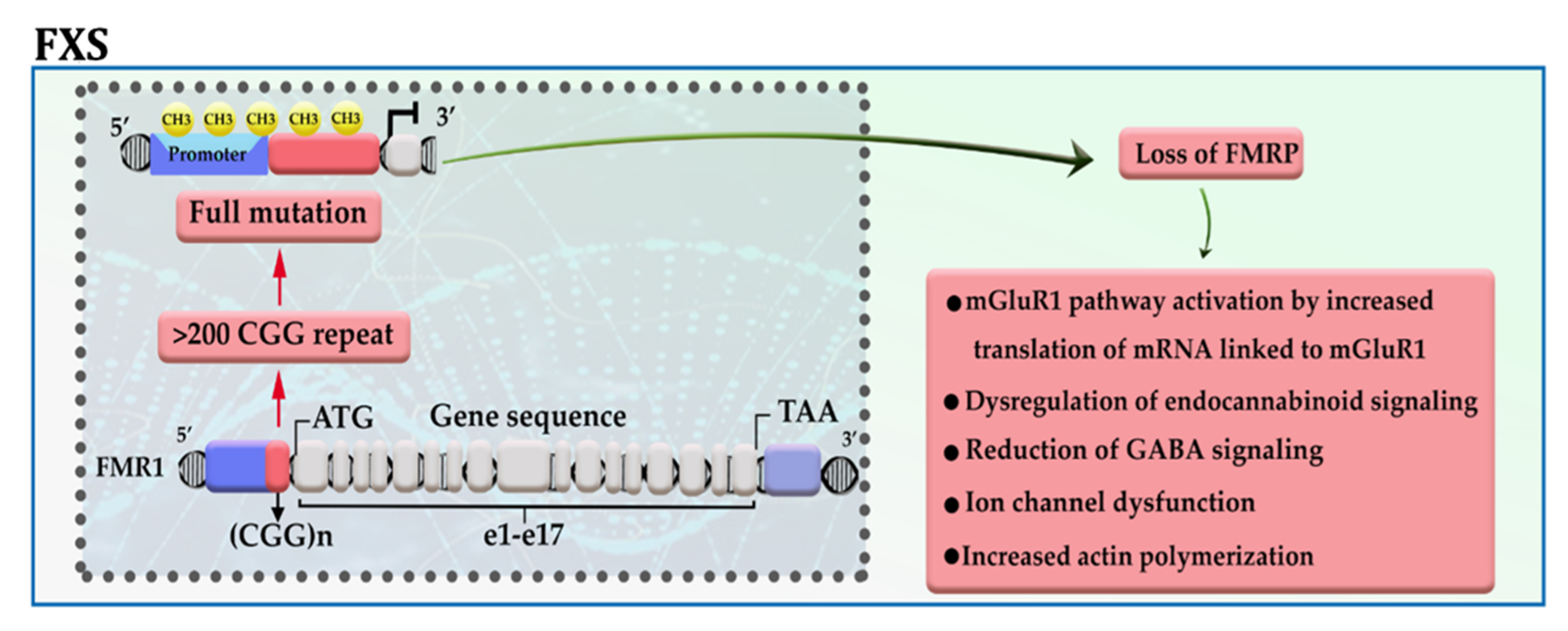
2.1. Fragile X Syndrome (FXS)
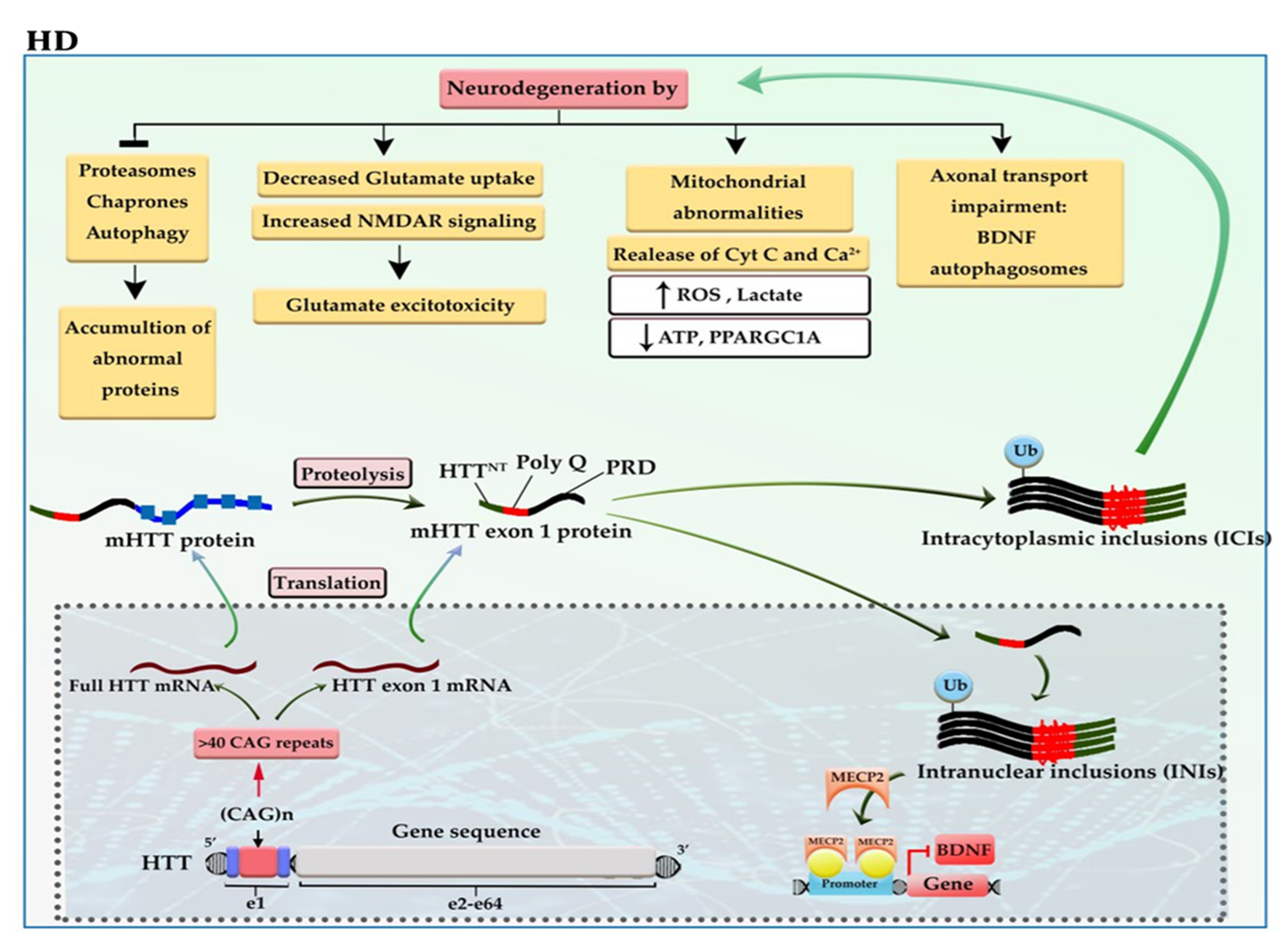
2.2. Huntington’s Disease (HD)
2.3. Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Dementia (FTD)
2.4. Alzheimer’s Disease (AD)
2.5. Parkinson’s Disease (PD)
3. DNA Methylation in Neurodevelopmental Disorders
3.1. Autism Spectrum Disorders (ASD)
3.2. Rett Syndrome
4. DNA Methylation in Neuropsychiatric Diseases
4.1. Schizophrenia (SZ)
4.2. Epilepsy
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5hmC | 5-hydroxymethylcytosine | LTP | Long-term potentiation |
| 5mC | 5-methylcytosine | MBD | Methyl-CpG-binding domain |
| AD | Alzheimer’s disease | mCA | Methylated CA |
| ADK | Adenosine kinase | mCAC | Methylated CAC |
| ADO | Adenosine | mCG | Methylated CpG |
| ADORA2A | Adenosine A2a receptor | MCI | Mild cognitive impairment |
| AHCY | Adenosylhomocysteinase | Met | Methionine |
| ALS | Amyotrophic lateral sclerosis | mGluRI | Metabotropic glutamate receptor group I |
| AMP | Adenosine monophosphate | mHTT | Mutant HTT |
| ASD | Autism spectrum disorders | mQTL | Methylation quantitative trait locus |
| Aβ | Amyloid beta | MTs | Microtubules |
| C9orf72 | Chromosome 9 open reading frame 72 | NCI | No cognitive impairment; |
| D-loop | Displacement loop | NFT | Neurofibrillary tangles |
| DMRs | Differentially methylated regions | NPCs | Neural progenitor cells |
| DNAm | DNA methylation | NTD | N-terminal domain |
| DNMTs | DNA methyltransferases | PB | Peripheral blood |
| DRPs | Dipeptide repeat proteins | PBMCs | Peripheral blood mononuclear cells |
| FMRP | Fragile X mental retardation protein | PHFs | Paired helical filaments |
| FTD | Frontotemporal dementia | pHRE | Pathogenic hexanucleotide G4C2 repeats expansion |
| FXS | Fragile X syndrome | Pol II | RNA polymerase II |
| G4C2 | GGGGCC | p-tau | Phosphorylated-tau |
| GABA | Gamma-aminobutyric acid | RAN | Repeat-associated non-ATG translation |
| GoF | Gain of function | RBPs | RNA binding proteins |
| Hcy | Homocysteine | SAH | S-adenosyl homocysteine |
| HD | Huntington’s disease | SAM | S-adenosyl methionine |
| HTT | Huntingtin gene | SNCA | α-synuclein |
| ICIs | Intracytoplasmic inclusions | SNP | Single-nucleotide polymorphism |
| INIs | Intranuclear inclusions | SZ | Schizophrenia |
| LAS | Lysosomal autophagy system | TET | Ten-eleven translocation |
| lncRNAs | Long non-coding RNAs | TFs | Transcription factors |
| LoF | Loss of function | TRD | Transcriptional repression domain |
| LTD | Long-term depression |
References
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, Z.D.; Meissner, A. DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Schübeler, D. Function and information content of DNA methylation. Nature 2015, 517, 321–326. [Google Scholar] [CrossRef]
- Unnikrishnan, A.; Freeman, W.M.; Jackson, J.; Wren, J.D.; Porter, H.; Richardson, A. The role of DNA methylation in epigenetics of aging. Pharmacol. Ther. 2018, 195, 172–185. [Google Scholar] [CrossRef]
- Straussman, R.; Nejman, D.; Roberts, D.; Steinfeld, I.; Blum, B.; Benvenisty, N.; Simon, I.; Yakhini, Z.; Cedar, H. Developmental programming of CpG island methylation profiles in the human genome. Nat. Struct. Mol. Biol. 2009, 16, 564–571. [Google Scholar] [CrossRef]
- Delgado-Morales, R.; Agís-Balboa, R.C.; Esteller, M.; Berdasco, M. Epigenetic mechanisms during ageing and neurogenesis as novel therapeutic avenues in human brain disorders. Clin. Epigenetics 2017, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dor, Y.; Cedar, H. Principles of DNA methylation and their implications for biology and medicine. Lancet 2018, 392, 777–786. [Google Scholar] [CrossRef]
- Jones, P.A.; Ohtani, H.; Chakravarthy, A.; De Carvalho, D.D. Epigenetic therapy in immune-oncology. Nat. Cancer 2019, 19, 151–161. [Google Scholar] [CrossRef]
- Verkerk, A.J.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.-H.; Kuhl, D.P.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.; et al. Identification of a gene (FMR1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991, 65, 905–914. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B., Jr.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L.; et al. Fragile X syndrome. Nat. Rev. Dis. Primers 2017, 3, 17065. [Google Scholar] [CrossRef]
- De Esch, C.E.; Ghazvini, M.; Loos, F.; Schelling-Kazaryan, N.; Widagdo, W.; Munshi, S.T.; van der Wal, E.; Douben, H.; Gunhanlar, N.; Kushner, S.; et al. Epigenetic Characterization of the FMR1 Promoter in Induced Pluripotent Stem Cells from Human Fibroblasts Carrying an Unmethylated Full Mutation. Stem Cell Rep. 2014, 3, 548–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.S.; Wu, H.; Krzisch, M.; Wu, X.; Graef, J.; Muffat, J.; Hnisz, D.; Li, C.H.; Yuan, B.; Xu, C.; et al. Rescue of Fragile X Syndrome Neurons by DNA Methylation Editing of the FMR1 Gene. Cell 2018, 172, 979–992.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caron, N.S.; Dorsey, E.R.; Hayden, M. Therapeutic approaches to Huntington disease: From the bench to the clinic. Nat. Rev. Drug Discov. 2018, 17, 729–750. [Google Scholar] [CrossRef]
- Yang, S.; Yang, H.; Huang, L.; Chen, L.; Qin, Z.; Li, S.; Li, X.-J. Lack of RAN-mediated toxicity in Huntington’s disease knock-in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 4411–4417. [Google Scholar] [CrossRef] [PubMed]
- Moumné, L.; Betuing, S.; Caboche, J. Multiple Aspects of Gene Dysregulation in Huntington’s Disease. Front. Neurol. 2013, 4, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarland, K.N.; Huizenga, M.N.; Darnell, S.B.; Sangrey, G.R.; Berezovska, O.; Cha, J.-H.J.; Outeiro, T.F.; Sadri-Vakili, G. MeCP2: A novel Huntingtin interactor. Hum. Mol. Genet. 2013, 23, 1036–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, C.W.; Yildirim, F.; Yap, Y.S.; Dalin, S.; Matthews, B.J.; Velez, P.J.; Labadorf, A.; Housman, D.E.; Fraenkel, E. Extensive changes in DNA methylation are associated with expression of mutant huntingtin. Proc. Natl. Acad. Sci. USA 2013, 110, 2354–2359. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Daito, T.; Sasaki, Y.; Chung, Y.H.; Xing, X.; Pondugula, S.; Swamidass, S.J.; Wang, T.; Kim, A.H.; Yano, H. Inhibition of DNA Methyltransferases Blocks Mutant Huntingtin-Induced Neurotoxicity. Sci. Rep. 2016, 6, 31022. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Yang, Y.; Lin, X.; Wang, J.-Q.; Wu, Y.-S.; Xie, W.; Wang, D.; Zhu, S.; Liao, Y.-Q.; Sun, Q.; et al. Genome-wide loss of 5-hmC is a novel epigenetic feature of Huntington’s disease. Hum. Mol. Genet. 2013, 22, 3641–3653. [Google Scholar] [CrossRef]
- Villar-Menéndez, I.; Blanch, M.; Tyebji, S.; Pereira-Veiga, T.; Albasanz, J.L.; Martín, M.; Ferrer, I.; Perez-Navarro, E.; Barrachina, M. Increased 5-Methylcytosine and Decreased 5-Hydroxymethylcytosine Levels are Associated with Reduced Striatal A2AR Levels in Huntington’s Disease. NeuroMolecular Med. 2013, 15, 295–309. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R.; Boeve, B.F.; Boxer, A.L.; Baker, M.; Rutherford, N.J.; Nicholson, A.M.; Finch, N.A.; Flynn, H.; Adamson, J.; et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardiman, O.; van den Berg, L.H.; Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Spina, S.; Miller, B.L. Frontotemporal dementia. Lancet 2015, 386, 1672–1682. [Google Scholar] [CrossRef] [Green Version]
- Xi, Z.; Zhang, M.; Bruni, A.; Maletta, R.G.; Colao, R.; Fratta, P.; Polke, J.M.; Sweeney, M.G.; Mudanohwo, E.; Nacmias, B.; et al. The C9orf72 repeat expansion itself is methylated in ALS and FTLD patients. Acta Neuropathol. 2015, 129, 715–727. [Google Scholar] [CrossRef]
- Balendra, R.; Isaacs, A.M. C9orf72-mediated ALS and FTD: Multiple pathways to disease. Nat. Rev. Neurol. 2018, 14, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.Y.; Russ, J.; Wu, K.; Neal, D.; Suh, E.; McNally, A.G.; Irwin, D.; Van Deerlin, V.M.; Lee, E.B. C9orf72 hypermethylation protects against repeat expansion-associated pathology in ALS/FTD. Acta Neuropathol. 2014, 128, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Russ, J.; Liu, E.Y.; Wu, K.; Neal, D.; Suh, E.; Irwin, D.; McMillan, C.; Harms, M.B.; Cairns, N.J.; Wood, E.M.; et al. Hypermethylation of repeat expanded C9orf72 is a clinical and molecular disease modifier. Acta Neuropathol. 2014, 129, 39–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, Z.; Zinman, L.; Moreno, D.; Schymick, J.; Liang, Y.; Sato, C.; Zheng, Y.; Ghani, M.; Dib, S.; Keith, J.; et al. Hypermethylation of the CpG Island Near the G4C2 Repeat in ALS with a C9orf72 Expansion. Am. J. Hum. Genet. 2013, 92, 981–989. [Google Scholar] [CrossRef] [Green Version]
- Stoccoro, A.; Smith, A.R.; Mosca, L.; Marocchi, A.; Gerardi, F.; Lunetta, C.; Cereda, C.; Gagliardi, S.; Lunnon, K.; Migliore, L.; et al. Reduced mitochondrial D-loop methylation levels in sporadic amyotrophic lateral sclerosis. Clin. Epigenetics 2020, 12, 137. [Google Scholar] [CrossRef]
- Banzhaf-Strathmann, J.; Claus, R.; Mücke, O.; Rentzsch, K.; Van Der Zee, J.; Engelborghs, S.; De Deyn, P.P.; Cruts, M.; Van Broeckhoven, C.; Plass, C.; et al. Promoter DNA methylation regulates progranulin expression and is altered in FTLD. Acta Neuropathol. Commun. 2013, 1, 16. [Google Scholar] [CrossRef]
- Chestnut, B.A.; Chang, Q.; Price, A.; Lesuisse, C.; Wong, M.; Martin, L.J. Epigenetic Regulation of Motor Neuron Cell Death through DNA Methylation. J. Neurosci. 2011, 31, 16619–16636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; McKeever, P.M.; Xi, Z.; Moreno, D.; Sato, C.; Bergsma, T.; McGoldrick, P.; Keith, J.; Robertson, J.; Zinman, L.; et al. DNA methylation age acceleration is associated with ALS age of onset and survival. Acta Neuropathol. 2020, 139, 943–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.D.; Smith, D.D.; Kish, S.J. Brain S-Adenosylmethionine Levels Are Severely Decreased in Alzheimer’s Disease. J. Neurochem. 2002, 67, 1328–1331. [Google Scholar] [CrossRef]
- Coppedè, F.; Tannorella, P.; Pezzini, I.; Migheli, F.; Ricci, G.; Ienco, E.C.; Piaceri, I.; Polini, A.; Nacmias, B.; Monzani, F.; et al. Folate, Homocysteine, Vitamin B12, and Polymorphisms of Genes Participating in One-Carbon Metabolism in Late-Onset Alzheimer’s Disease Patients and Healthy Controls. Antioxidants Redox Signal. 2012, 17, 195–204. [Google Scholar] [CrossRef]
- Carmo, S.D.; Hanzel, C.E.; Jacobs, M.L.; Machnes, Z.; Iulita, M.F.; Yang, J.; Yu, L.; Ducatenzeiler, A.; Danik, M.; Breuillaud, L.S.; et al. Rescue of Early bace-1 and Global DNA Demethylation by S-Adenosylmethionine Reduces Amyloid Pathology and Improves Cognition in an Alzheimer’s Model. Sci. Rep. 2016, 6, 34051. [Google Scholar] [CrossRef] [Green Version]
- Chouliaras, L.; Mastroeni, D.; Delvaux, E.; Grover, A.; Kenis, G.; Hof, P.R.; Steinbusch, H.W.; Coleman, P.D.; Rutten, B.P.; Hove, D.L.V.D. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients. Neurobiol. Aging 2013, 34, 2091–2099. [Google Scholar] [CrossRef] [Green Version]
- De Jager, P.L.; Srivastava, G.; Lunnon, K.; Burgess, J.; Schalkwyk, L.C.; Yu, L.; Eaton, M.L.; Keenan, B.T.; Ernst, J.; McCabe, C.; et al. Alzheimer’s disease: Early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat. Neurosci. 2014, 17, 1156–1163. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.G.; Pishva, E.; Shireby, G.; Smith, A.R.; Roubroeks, J.A.Y.; Hannon, E.; Wheildon, G.; Mastroeni, D.; Gasparoni, G.; Riemenschneider, M.; et al. A meta-analysis of epigenome-wide association studies in Alzheimer’s disease highlights novel differentially methylated loci across cortex. Nat. Commun. 2021, 12, 3517. [Google Scholar] [CrossRef]
- Sanchez-Mut, J.V.; Aso, E.; Heyn, H.; Matsuda, T.; Bock, C.; Ferrer, I.; Esteller, M. Promoter hypermethylation of the phosphatase DUSP22 mediates PKA-dependent TAU phosphorylation and CREB activation in Alzheimer’s disease. Hippocampus 2014, 24, 363–368. [Google Scholar] [CrossRef]
- Yu, L.; Chibnik, L.B.; Srivastava, G.P.; Pochet, N.; Yang, J.; Xu, J.; Kozubek, J.; Obholzer, N.; Leurgans, S.E.; Schneider, J.A.; et al. Association of Brain DNA Methylation in SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1 with Pathological Diagnosis of Alzheimer Disease. JAMA Neurol. 2015, 72, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mano, T.; Nagata, K.; Nonaka, T.; Tarutani, A.; Imamura, T.; Hashimoto, T.; Bannai, T.; Koshi-Mano, K.; Tsuchida, T.; Ohtomo, R.; et al. Neuron-specific methylome analysis reveals epigenetic regulation and tau-related dysfunction of BRCA1 in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, E9645–E9654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Mut, J.V.; Heyn, H.; Silva, B.A.; Dixsaut, L.; Esparcia, P.G.; Vidal, E.; Sayols, S.; Glauser, L.; Monteagudo-Sánchez, A.; Perez-Tur, J.; et al. PM20D1 is a quantitative trait locus associated with Alzheimer’s disease. Nat. Med. 2018, 24, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Arosio, B.; D’Addario, C.; Galimberti, D.; Gussago, C.; Pucci, M.; Casati, M.; Fenoglio, C.; Abbate, C.; Rossi, P.D.; et al. Gene promoter methylation and expression of Pin1 differ between patients with frontotemporal dementia and Alzheimer’s disease. J. Neurol. Sci. 2016, 362, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.-X.; Xu, Q.; Hu, Y.-C.; Song, C.-Y.; Guo, J.-F.; Shen, L.; Wang, C.-R.; Yu, R.-L.; Yan, X.-X.; Tang, B.-S. Hypomethylation of SNCA in blood of patients with sporadic Parkinson’s disease. J. Neurol. Sci. 2013, 337, 123–128. [Google Scholar] [CrossRef]
- Moran, S.; Martínez-Cardús, A.; Sayols, S.; Musulén, E.; Balañá, C.; Estival-Gonzalez, A.; Moutinho, C.; Heyn, H.; Diaz-Lagares, A.; de Moura, M.C.; et al. Epigenetic profiling to classify cancer of unknown primary: A multicentre, retrospective analysis. Lancet Oncol. 2016, 17, 1386–1395. [Google Scholar] [CrossRef]
- Desplats, P.; Spencer, B.; Coffee, E.; Patel, P.; Michael, S.; Patrick, C.; Adame, A.; Rockenstein, E.; Masliah, E. α-Synuclein Sequesters Dnmt1 from the Nucleus. J. Biol. Chem. 2011, 286, 9031–9037. [Google Scholar] [CrossRef] [Green Version]
- Kaut, O.; Schmitt, I.; Wüllner, U. Genome-scale methylation analysis of Parkinson’s disease patients’ brains reveals DNA hypomethylation and increased mRNA expression of cytochrome P450 2E1. Neurogenetics 2012, 13, 87–91. [Google Scholar] [CrossRef]
- Schmitt, I.; Kaut, O.; Khazneh, H.; Deboni, L.; Ahmad, A.; Berg, D.; Klein, C.; Fröhlich, H.; Wüllner, U. L-dopa increases α-synuclein DNA methylation in Parkinson’s disease patients in vivo and in vitro. Mov. Disord. 2015, 30, 1794–1801. [Google Scholar] [CrossRef]
- Figueroa-Romero, C.; Hur, J.; Bender, D.E.; Delaney, C.E.; Cataldo, M.D.; Smith, A.L.; Yung, R.; Ruden, D.M.; Callaghan, B.C.; Feldman, E.L. Identification of Epigenetically Altered Genes in Sporadic Amyotrophic Lateral Sclerosis. PLoS ONE 2012, 7, e52672. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D.; D’Addario, C.; Dell’Osso, B.; Fenoglio, C.; Marcone, A.; Cerami, C.; Cappa, S.; Palazzo, M.C.; Arosio, B.; Mari, D.; et al. Progranulin gene (GRN) promoter methylation is increased in patients with sporadic frontotemporal lobar degeneration. Neurol. Sci. 2012, 34, 899–903. [Google Scholar] [CrossRef]
- Li, P.; Marshall, L.; Oh, G.; Jakubowski, J.L.; Groot, D.; He, Y.; Wang, T.; Petronis, A.; Labrie, V. Epigenetic dysregulation of enhancers in neurons is associated with Alzheimer’s disease pathology and cognitive symptoms. Nat. Commun. 2019, 10, 2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Garrido, N.; Cruz, F.; Egea, R.R.; Simon, C.; Sadler-Riggleman, I.; Beck, D.; Nilsson, E.; Ben Maamar, M.; Skinner, M.K. Sperm DNA methylation epimutation biomarker for paternal offspring autism susceptibility. Clin. Epigenetics 2021, 13, 6. [Google Scholar] [CrossRef]
- Sanders, S.J.; He, X.; Willsey, A.J.; Ercan-Sencicek, A.G.; Samocha, K.E.; Cicek, A.E.; Murtha, M.T.; Bal, V.H.; Bishop, S.L.; Dong, S.; et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron 2015, 87, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Krumm, N.; Turner, T.; Baker, C.; Vives, L.; Mohajeri, K.; Witherspoon, K.; Raja, A.; Coe, B.P.; Stessman, H.; He, Z.-X.; et al. Excess of rare, inherited truncating mutations in autism. Nat. Genet. 2015, 47, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Cukier, H.N.; Rabionet, R.; Konidari, I.; Rayner-Evans, M.Y.; Baltos, M.L.; Wright, H.H.; Abramson, R.K.; Martin, E.R.; Cuccaro, M.L.; Pericak-Vance, M.A.; et al. Novel variants identified in methyl-CpG-binding domain genes in autistic individuals. Neurogenetics 2009, 11, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Talkowski, M.; Mullegama, S.V.; Rosenfeld, J.A.; van Bon, B.W.; Shen, Y.; Repnikova, E.A.; Gastier-Foster, J.; Thrush, D.L.; Kathiresan, S.; Ruderfer, D.; et al. Assessment of 2q23.1 Microdeletion Syndrome Implicates MBD5 as a Single Causal Locus of Intellectual Disability, Epilepsy, and Autism Spectrum Disorder. Am. J. Hum. Genet. 2011, 89, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Liu, Z.; Mao, W.; Wang, X.; Zheng, X.; Chen, S.; Cao, B.; Huang, S.; Zhang, X.; Zhou, T.; et al. Locus-specific DNA methylation of Mecp2 promoter leads to autism-like phenotypes in mice. Cell Death Dis. 2020, 11, 85. [Google Scholar] [CrossRef]
- Zhubi, A.; Chen, Y.; Guidotti, A.; Grayson, D.R. Epigenetic regulation of RELN and GAD1 in the frontal cortex (FC) of autism spectrum disorder (ASD) subjects. Int. J. Dev. Neurosci. 2017, 62, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Nardone, S.; Sams, D.S.; Reuveni, E.; Getselter, D.; Oron, O.; Karpuj, M.; Elliott, E. DNA methylation analysis of the autistic brain reveals multiple dysregulated biological pathways. Transl. Psychiatry 2014, 4, e433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramaswami, G.; Won, H.; Gandal, M.J.; Haney, J.; Wang, J.C.; Wong, C.C.Y.; Sun, W.; Prabhakar, S.; Mill, J.; Geschwind, D.H. Integrative genomics identifies a convergent molecular subtype that links epigenomic with transcriptomic differences in autism. Nat. Commun. 2020, 11, 4873. [Google Scholar] [CrossRef] [PubMed]
- Nardone, S.; Sams, D.S.; Zito, A.; Reuveni, E.; Elliott, E. Dysregulation of Cortical Neuron DNA Methylation Profile in Autism Spectrum Disorder. Cereb. Cortex 2017, 27, 5739–5754. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S.V.; Ellis, S.E.; Bakulski, K.M.; Sheppard, B.; Croen, L.A.; Hertz-Picciotto, I.; Newschaffer, C.J.; Feinberg, A.P.; Arking, D.E.; Ladd-Acosta, C.; et al. Cross-tissue integration of genetic and epigenetic data offers insight into autism spectrum disorder. Nat. Commun. 2017, 8, 1011. [Google Scholar] [CrossRef] [Green Version]
- Kundakovic, M.; Gudsnuk, K.; Herbstman, J.B.; Tang, D.; Perera, F.P.; Champagne, F.A. DNA methylation of BDNF as a biomarker of early-life adversity. Proc. Natl. Acad. Sci. USA 2014, 112, 6807–6813. [Google Scholar] [CrossRef] [Green Version]
- Kimura, R.; Nakata, M.; Funabiki, Y.; Suzuki, S.; Awaya, T.; Murai, T.; Hagiwara, M. An epigenetic biomarker for adult high-functioning autism spectrum disorder. Sci. Rep. 2019, 9, 13662. [Google Scholar] [CrossRef] [Green Version]
- Homs, A.; Codina-Solà, M.; Rodríguez-Santiago, B.; Villanueva, C.M.; Monk, D.; Cuscó, I.; A Pérez-Jurado, L. Genetic and epigenetic methylation defects and implication of the ERMN gene in autism spectrum disorders. Transl. Psychiatry 2016, 6, e855. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Li, Z.; Wang, Y.; Li, X.; Yang, X.; Zhan, X.; Huang, Y.; Gao, Z.; Zhang, M.; Sun, C.; et al. Genome-Wide DNA Methylation Analysis Reveals Epigenetic Pattern of SH2B1 in Chinese Monozygotic Twins Discordant for Autism Spectrum Disorder. Front. Neurosci. 2019, 13, 712. [Google Scholar] [CrossRef] [Green Version]
- Hu, V.W.; Hong, Y.; Xu, M.; Shu, H.T. Altered DNA methylation in a severe subtype of idiopathic autism: Evidence for sex differences in affected metabolic pathways. Autism 2020, 25, 887–910. [Google Scholar] [CrossRef]
- Zhu, Y.; E Mordaunt, C.; Yasui, D.H.; Marathe, R.; Coulson, R.L.; Dunaway, K.W.; Jianu, J.M.; Walker, C.K.; Ozonoff, S.; Hertz-Picciotto, I.; et al. Placental DNA methylation levels at CYP2E1 and IRS2 are associated with child outcome in a prospective autism study. Hum. Mol. Genet. 2019, 28, 2659–2674. [Google Scholar] [CrossRef] [PubMed]
- Ip, J.P.K.; Mellios, N.; Sur, M. Rett syndrome: Insights into genetic, molecular and circuit mechanisms. Nat. Rev. Neurosci. 2018, 19, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.-T.; Zoghbi, H.Y.; Rosenmund, C. MeCP2 Controls Excitatory Synaptic Strength by Regulating Glutamatergic Synapse Number. Neuron 2007, 56, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schanen, N.C.; Kurczynski, T.W.; Brunelle, D.; Woodcock, M.M.; Dure, L.S.; Percy, A.K. Neonatal encephalopathy in two boys in families with recurrent Rett syndrome. J. Child Neurol. 1998, 13, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Christodoulou, J.; Clarke, A.J.; Bahi-Buisson, N.; Leonard, H.; Bailey, M.E.S.; Schanen, N.C.; Zappella, M.; et al. Rett syndrome: Revised diagnostic criteria and nomenclature. Ann. Neurol. 2010, 68, 944–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neul, J.L.; Fang, P.; Barrish, J.; Lane, J.; Caeg, E.B.; Smith, E.O.; Zoghbi, H.; Percy, A.; Glaze, D.G. Specific mutations in Methyl-CpG-Binding Protein 2 confer different severity in Rett syndrome. Neurology 2008, 70, 1313–1321. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.L.; Veenstra, G.J.C.; Wade, P.A.; Vermaak, D.; Kass, S.U.; Landsberger, N.; Strouboulis, J.; Wolffe, A.P. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 1998, 19, 187–191. [Google Scholar] [CrossRef]
- Lyst, M.J.; Ekiert, R.; Ebert, D.H.; Merusi, C.; Nowak, J.; Selfridge, J.; Guy, J.; Kastan, N.R.; Robinson, N.D.; de Lima Alves, F.; et al. Rett syndrome mutations abolish the interaction of MeCP2 with the NCoR/SMRT co-repressor. Nat. Neurosci. 2013, 16, 898–902. [Google Scholar] [CrossRef] [Green Version]
- Gabel, H.W.; Kinde, B.; Stroud, H.; Gilbert, C.S.; Harmin, D.A.; Kastan, N.R.; Hemberg, M.; Ebert, D.H.; Greenberg, M.E. Disruption of DNA-methylation-dependent long gene repression in Rett syndrome. Nature 2015, 522, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Lagger, S.; Connelly, J.C.; Schweikert, G.; Webb, S.; Selfridge, J.; Ramsahoye, B.H.; Yu, M.; He, C.; Sanguinetti, G.; Sowers, L.C.; et al. MeCP2 recognizes cytosine methylated tri-nucleotide and di-nucleotide sequences to tune transcription in the mammalian brain. PLoS Genet. 2017, 13, e1006793. [Google Scholar] [CrossRef]
- Mellén, M.; Ayata, P.; Dewell, S.; Kriaucionis, S.; Heintz, N. MeCP2 Binds to 5hmC Enriched within Active Genes and Accessible Chromatin in the Nervous System. Cell 2012, 151, 1417–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reif, A.; Fritzen, S.; Finger, M.; Strobel, A.; Lauer, M.S.; Schmitt, A.; Lesch, K.-P. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry 2006, 11, 514–522. [Google Scholar] [CrossRef]
- Ruzicka, W.B.; Zhubi, A.; Veldic, M.; Grayson, D.R.; Costa, E.; Guidotti, A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol. Psychiatry 2007, 12, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Zhubi, A.; Veldic, M.; Puri, N.; Kadriu, B.; Caruncho, H.; Loza, I.; Sershen, H.; Lajtha, A.; Smith, R.; Guidotti, A.; et al. An upregulation of DNA-methyltransferase 1 and 3a expressed in telencephalic GABAergic neurons of schizophrenia patients is also detected in peripheral blood lymphocytes. Schizophr. Res. 2009, 111, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matrisciano, F.; Tueting, P.; Dalal, I.; Kadriu, B.; Grayson, D.R.; Davis, J.M.; Nicoletti, F.; Guidotti, A. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology 2012, 68, 184–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, R.; Davis, K.N.; Li, C.; Shin, J.H.; Gao, Y.; Jaffe, A.; Gondré-Lewis, M.C.; Weinberger, D.R.; Kleinman, J.E.; Hyde, T.M. GAD1 alternative transcripts and DNA methylation in human prefrontal cortex and hippocampus in brain development, schizophrenia. Mol. Psychiatry 2017, 23, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Jossin, Y. Reelin Functions, Mechanisms of Action and Signaling Pathways During Brain Development and Maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Beffert, U.; Weeber, E.J.; Durudas, A.; Qiu, S.; Masiulis, I.; Sweatt, J.D.; Li, W.-P.; Adelmann, G.; Frotscher, M.; Hammer, R.E.; et al. Modulation of Synaptic Plasticity and Memory by Reelin Involves Differential Splicing of the Lipoprotein Receptor Apoer2. Neuron 2005, 47, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Gao, R. Common Mechanisms of Excitatory and Inhibitory Imbalance in Schizophrenia and Autism Spectrum Disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef]
- Hannon, E.; Spiers, H.; Viana, J.; Pidsley, R.; Burrage, J.; Murphy, T.M.; Troakes, C.; Turecki, G.; O’Donovan, M.C.; Schalkwyk, L.C.; et al. Methylation QTLs in the developing brain and their enrichment in schizophrenia risk loci. Nat. Neurosci. 2015, 19, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Kobow, K.; Kaspi, A.; Harikrishnan, K.N.; Kiese, K.; Ziemann, M.; Khurana, I.; Fritzsche, I.; Hauke, J.; Hahnen, E.; Coras, R.; et al. Deep sequencing reveals increased DNA methylation in chronic rat epilepsy. Acta Neuropathol. 2013, 126, 741–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Wang, L.; Zhang, Y.; Zhao, F.-H.; Luo, J.; Xiao, Z.; Chen, G.-J.; Wang, X.-F. Increased Expression of DNA methyltransferase 1 and 3a in Human Temporal Lobe Epilepsy. J. Mol. Neurosci. 2011, 46, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, X.; Wang, L.; Fu, X.; Zhou, J.X.; Zhu, B.; Luo, J.; Wang, X.; Xiao, Z. Association of RASgrf1 methylation with epileptic seizures. Oncotarget 2017, 8, 46286–46297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobow, K.; Jeske, I.; Hildebrandt, M.; Hauke, J.; Hahnen, E.; Buslei, R.; Buchfelder, M.; Weigel, D.; Stefan, H.; Kasper, B.; et al. Increased Reelin Promoter Methylation Is Associated With Granule Cell Dispersion in Human Temporal Lobe Epilepsy. J. Neuropathol. Exp. Neurol. 2009, 68, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Miller-Delaney, S.F.; Bryan, K.; Das, S.; McKiernan, R.C.; Bray, I.M.; Reynolds, J.P.; Gwinn, R.; Stallings, R.L.; Henshall, D.C. Differential DNA methylation profiles of coding and non-coding genes define hippocampal sclerosis in human temporal lobe epilepsy. Brain 2014, 138, 616–631. [Google Scholar] [CrossRef] [Green Version]
- Williams-Karnesky, R.L.; Sandau, U.S.; Lusardi, T.; Lytle, N.K.; Farrell, J.M.; Pritchard, E.M.; Kaplan, D.L.; Boison, D. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J. Clin. Investig. 2013, 123, 3552–3563. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Cao, Y.; Long, H.; Luo, Z.; Li, S.; Deng, N.; Wang, J.; Lu, X.; Wang, T.; Ning, S.; et al. Genome-Wide DNA Methylation Patterns Analysis of Noncoding RNAs in Temporal Lobe Epilepsy Patients. Mol. Neurobiol. 2017, 55, 793–803. [Google Scholar] [CrossRef]
- Auta, J.; Smith, R.; Dong, E.; Tueting, P.; Sershen, H.; Boules, S.; Lajtha, A.; Davis, J.; Guidotti, A. DNA-methylation gene network dysregulation in peripheral blood lymphocytes of schizophrenia patients. Schizophr. Res. 2013, 150, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Hannon, E.; Dempster, E.; Viana, J.; Burrage, J.; Smith, A.R.; Macdonald, R.; Clair, D.S.; Mustard, C.; Breen, G.; Therman, S.; et al. An integrated genetic-epigenetic analysis of schizophrenia: Evidence for co-localization of genetic associations and differential DNA methylation. Genome Biol. 2016, 17, 176. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zang, Z.; Braun, U.; Schwarz, K.; Harneit, A.; Kremer, T.; Ma, R.; Schweiger, J.; Moessnang, C.; Geiger, L.; et al. Association of a Reproducible Epigenetic Risk Profile for Schizophrenia With Brain Methylation and Function. JAMA Psychiatry 2020, 77, 628. [Google Scholar] [CrossRef]
- Wockner, L.F.; Morris, C.P.; Noble, E.P.; Lawford, B.R.; Whitehall, V.L.J.; Young, R.; Voisey, J. Brain-specific epigenetic markers of schizophrenia. Transl. Psychiatry 2015, 5, e680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins-Chen, A.T.; Boks, M.P.; Vinkers, C.H.; Kahn, R.S.; Levine, M.E. Schizophrenia and Epigenetic Aging Biomarkers: Increased Mortality, Reduced Cancer Risk, and Unique Clozapine Effects. Biol. Psychiatry 2020, 88, 224–235. [Google Scholar] [CrossRef] [PubMed]
- De Nijs, L.; Choe, K.; Steinbusch, H.; Schijns, O.E.M.G.; Dings, J.; Hove, D.L.A.V.D.; Rutten, B.P.F.; Hoogland, G. DNA methyltransferase isoforms expression in the temporal lobe of epilepsy patients with a history of febrile seizures. Clin. Epigenetics 2019, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Long, H.-Y.; Feng, L.; Kang, J.; Luo, Z.-H.; Xiao, W.-B.; Long, L.-L.; Yan, X.-X.; Zhou, L.; Xiao, B. Blood DNA methylation pattern is altered in mesial temporal lobe epilepsy. Sci. Rep. 2017, 7, 43810. [Google Scholar] [CrossRef] [Green Version]
- Hannon, E.; Lunnon, K.; Schalkwyk, L.; Mill, J. Interindividual methylomic variation across blood, cortex, and cerebellum: Implications for epigenetic studies of neurological and neuropsychiatric phenotypes. Epigenetics 2015, 10, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Farré, P.; Jones, M.J.; Meaney, M.J.; Emberly, E.; Turecki, G.; Kobor, M.S. Concordant and discordant DNA methylation signatures of aging in human blood and brain. Epigenetics Chromatin 2015, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Braun, P.R.; Han, S.; Hing, B.; Nagahama, Y.; Gaul, L.N.; Heinzman, J.T.; Grossbach, A.J.; Close, L.; Dlouhy, B.J.; Howard, M.; et al. Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl. Psychiatry 2019, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, P.; Luthman, H.; E McGuigan, F.; E Akesson, K. Epigenome-wide cross-tissue correlation of human bone and blood DNA methylation—can blood be used as a surrogate for bone? Epigenetics 2020, 16, 92–105. [Google Scholar] [CrossRef]
- Walton, E.; Hass, J.; Liu, J.; Roffman, J.L.; Bernardoni, F.; Roessner, V.; Kirsch, M.; Schackert, G.; Calhoun, V.; Ehrlich, S. Correspondence of DNA Methylation Between Blood and Brain Tissue and Its Application to Schizophrenia Research. Schizophr. Bull. 2015, 42, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Bahado-Singh, R.O.; Radhakrishna, U.; Gordevičius, J.; Aydas, B.; Yilmaz, A.; Jafar, F.; Imam, K.; Maddens, M.; Challapalli, K.; Metpally, R.P.; et al. Artificial Intelligence and Circulating Cell-Free DNA Methylation Profiling: Mechanism and Detection of Alzheimer’s Disease. Cells 2022, 11, 1744. [Google Scholar] [CrossRef]




| Cell/Tissue Type | Main Findings | Ref. | |
|---|---|---|---|
| Neurodegenerative Diseases | |||
| HD | Brain tissues from HD patients and HCs | Increased 5mC, together with reduced 5hmC levels, were detected in the 5’UTR region of the ADORA2A gene in the putamen of HD patients compared to HCs. | [20] |
| ALS | Postmortem brain and spinal cord samples from sporadic ALS and HCs | DNMT1 and DNMT3A were upregulated in the motor cortex and spinal cord motor neurons of patients with sporadic ALS compared to HCs. 5-mC was detected in the motor cortex of ALS but not in HCs. | [31] |
| Postmortem frozen spinal cord samples and WB from sporadic ALS and HCs | Global 5-mC and 5-hmC were increased in the spinal cord, but not WB of patients with sporadic ALS compared to HCs. | [51] | |
| DNA blood from patients with ALS and HCs | C9orf72 promoter hypermethylation was associated with reduced disease duration before death in patients with C9-ALS. | [28] | |
| PBMCs from sporadic ALS patients, HCs, and familial ALS patients with SOD1- or C9orf72-mutant | The hypomethylation of the mitochondrial D-loop region, together with increased mtDNA copy number, could represent compensatory mechanisms to counteract mitochondrial impairment in SOD1-mutant and sporadic ALS patients. | [29] | |
| Blood and CNS tissues from sporadic ALS patients | Blood/CNS-based DNAm-age acceleration may be used as a marker to predict the age of onset and survival in ALS patients. | [32] | |
| ALS, FTD | Brain or blood samples from C9-ALS/FTD patients and HCs | The hypermethylation of G4C2 repeat expansion occurs in about 97% of C9-ALS/FTD patients with >50 repeats. It was found in both blood and brain tissues for the same individual, suggesting its potential use as a biomarker. | [24] |
| FTD | Brain or blood samples from C9-FTD patients and non-carrier family members | C9orf72 promoter hypermethylation was associated with longer survival in patients with C9-FTD. | [27] |
| Brain samples from HCs, FTD, AD, and PD patients | Promoter hypermethylation and associated silencing of the GRN gene were detected in patients with FTD compared to HCs, AD, and PD samples. | [30] | |
| PBMCs from FTD patients and HCs | Promoter hypermethylation associated with a reduced mRNA expression of the GRN gene was found in PB of patients with FTD compared to HCs. | [52] | |
| AD | Postmortem human brains from AD patients, donors with NCI and MCI | The greater methylation levels at specific CpG sites of the BACE1 gene promoter were associated with higher tangle density and lower β-amyloid load among persons with AD dementia than subjects with NCI or MCI. | [36] |
| Neurons of postmortem brain samples from AD patients and HCs | Promoter hypomethylation and increased mRNA and protein expression of BRCA1 was detected in the neurons of hippocampal and entorhinal cortex from AD patients compared to HCs. | [42] | |
| PFC neurons of Postmortem human brains from AD patients and HCs | Hypomethylation of the enhancers in the DSCAML1 gene, which targets the BACE1 promoter, caused the overexpression of BACE1 in AD patients; and was correlated with an increase in Aβ plaques, NFTs, and cognitive decline. | [53] | |
| PBMCs from AD patients, FTD donors, and HCs | PIN1 hypermethylation can serve as a useful predictive biomarker to distinguish AD from FTD. | [44] | |
| PBMCs from HCs, young obese females, or AD donors | Methylation levels of genes involved in AD pathogenesis, such as APP, BACE1, LRP1, and SORL1, can serve as prognostic biomarkers in obese individuals. | NCT02868905 | |
| PD | PBMCs from sporadic PD patients and HCs | The methylation status of SNCA intron-1 can be used as an early diagnostic marker for PD. | [46] |
| Postmortem human brains from PD patients and HCs | Promoter hypomethylation and the increased activity of CYP2E1 may contribute to the degeneration of dopaminergic neurons by the formation of toxic metabolites. | [49] | |
| Cell/Tissue Type | Main Findings | Ref. |
|---|---|---|
| ASD | ||
| Postmortem brain tissues from ASD patients and HCs | Increased MECP2 interaction with RELN and GAD1 gene promoters triggers the reduction of Reelin and GAD67 expression in the CB and FC of patients with ASD compared to HCs. | [61] |
| Postmortem brain tissues from ASD patients and HCs | Hypomethylation and overexpression of immune-related genes (such as C1Q, C3, ITGB2, and TNF-α) were observed in the PFC of ASD compared to HCs. | [62] |
| Postmortem tissues from ASD patients and HCs | Hypomethylation of CpG sites in the promoters of immune genes leads to an upregulated immune process in the convergent subtype. | [63] |
| Frozen brain samples from ASD and HCs | A total of 58 ASD-associated DMRs were enriched for genomic regions of neuronal, GABAergic, and immune system genes. | [64] |
| Cord, blood, and brain tissues from ASD and HCs | ASD-associated meQTLs across the genome were enriched for immune-related pathways in the cord, blood, and brain tissues of children with ASD. | [65] |
| PBMCs from children with ASD and HCs | Methylation and expression levels of BDNF in blood samples from children with ASD can use as a diagnostic biomarker. | [66] |
| PB from adults with high-functioning ASD and HCs | Hypermethylation of a CpG site (cg20793532) in the PPP2R2C promoter can serve as a blood biomarker for identifying adult patients with high-functioning ASD. | [67] |
| Blood DNA from male ASD patients and HCs | Most of the 700 DMCpGs (587; 83.9%) in ASD cases showed relative hypomethylation compared to HCs. Hypomethylation and overexpression of ERMN contribute to ASD susceptibility and can be altered by both rare SNPs at the CG position and mutations. | [68] |
| WB samples from ASD-discordant MZ twins, ASD-concordant MZ twins, and a set of pairs of sporadic case-control | A total of 2,397 DAGs were associated with neurotrophin signaling pathway in ASD-discordant MZ twins. The aberrant methylation of SH2B1 was identified in the ASD-discordant, ASD-concordant MZ twins, and sporadic cases compared to controls. | [69] |
| Lymphoblastoid cells from idiopathic ASD and unaffected sex-matched siblings | DAGs were associated with synaptogenesis, semaphorin, and mTOR pathways in idiopathic ASD compared to unaffected sex-matched siblings. | [70] |
| Placenta samples stored from children later diagnosed with ASD compared to typically developing controls | A total of 400 DMRs can distinguish placentas stored from children later diagnosed with ASD relative to typically developing controls. Methylation levels of two DMRs, mapping on CYP2E1 and IRS2, can serve as a useful predictive biomarker for ASD risk in placenta samples. | [71] |
| Cell/Tissue Type | Main Findings | Ref. | |
|---|---|---|---|
| Neuropsychiatric Diseases | |||
| SZ | Human Brain tissue from SZ patients and HCs | Increased DNMT1 expression and subsequently elevated DNA methylation levels were detected in SZ patients compared to HCs. | [83] |
| Brain tissue and PBL from SZ patients and HCs | The mRNA expression of DNMT1 and DNMT3A was increased in both brain tissue and PBL of SZ patients compared to HCs. | [84] | |
| PBL from SZ patients and HCs | The mRNA expression of DNMT1, TET1, GCortR, and BDNF was increased in PBL of SZ patients compared to HCs. | [98] | |
| Human fetal and adult brain tissue | >16,000 fetal brain mQTLs were identified. Fetal brain-specific mQTLs were enriched among SZ-associated SNPs identified in a recent study. | [90] | |
| Blood and brain tissue from SZ-discordant MZ twins, SZ patients, HCs | 25 DMPs associated with SZ (p-value < 10−7). The seven meQTLs were enriched for schizophrenia risk variants in both brain and blood samples. | [99] | |
| Genome-wide DNA methylation data from WB samples and postmortem DLPFC samples from SZ patients and HCs | Blood PMS signature can distinguish SZ patients from HCs and several other major neuropsychiatric disorders, enriched for methylation differences detected in DLPFC postmortem samples and was correlated with altered functional DLPFC-HC coupling during working memory and biological pathways with synaptic function. | [100] | |
| Postmortem PFC brain tissue from SZ patients and HCs (the results from three independent studies) | The seven DMRs identified in near CERS3, DPPA5, PRDM9, DDX43, REC8, LY6G5C genes, and a region on chromosome 10 across all three PFC brain data sets may play an important role in the pathogenesis and progression of SZ patients. | [101] | |
| Genome-wide DNA methylation data of WB samples from SZ patients and HCs | Accelerations in 3 mortality clocks in SZ may result from smoking and 6 age-associated proteins. 2 mitotic clocks were decelerated in SZ related to NK and CD8+ T cells and may be a biological basis for reduced cancer risk. Chronological age clocks were decelerated in patients treated with clozapine. | [102] | |
| Postmortem brain tissue of SZ patients and HCs | The methylation levels of two CpG sites within the 5′ UTR of GAD1 were significantly associated with SZ-risk SNP rs3749034 and GAD25 expression in DLPFC. The expression of full-length GAD1 transcript encoding GAD67 was significantly higher in DLPFC of SZ patients who died through suicide. | [86] | |
| TLE | Brain tissue of TLE patients and HCs | The expression of DNMT1 and DNMT3A was increased in TLE patients relative to HCs, especially in NeuN+ neurons, but not GFAP+ astrocytes. | [92] |
| Postmortem brain tissue of TLE patients with and without FS and HCs | The levels of DNMT3A1 and DNMT3A2 isoforms were decreased in the hippocampus of TLE patients with FS relative to HCs and other TLE groups. Increased levels of DNMT1, DNMT3A1, and global DNA methylation were found in the neocortex of all TLE patients compared to HCs. | [103] | |
| Postmortem hippocampus from TLE patients and HCs | 81.5% of 146 differentially methylated protein-coding gene promoters were hypermethylated in TLE patients relative to HCs, and these genes are related to development, neuron remodeling, and neuron maturation. Four differentially methylated lncRNAs and 13 methylation-sensitive miRNAs were identified. miR-876-3p was associated with WG1 hippocampal sclerosis. | [95] | |
| Postmortem hippocampus from TLE patients with and without GCD, and HCs | RELN promoter methylation was higher in TLE patients than in HCs. Increased methylation of the RELN promoter was associated with GCD among TLE patients. | [94] | |
| PB DNAs of TLE patients and HCs | 85% and 87% of differentially methylated miRNA and lncRNA promoters were hypermethylated in TLE patients compared to HCs. The aberrantly methylated miRNAs and lncRNAs were correlated to drug metabolism, ion channel activity, MAPK- and neurotrophin signaling pathways. | [97] | |
| WB of MLTE patients and HCs | 216 DAGs, with 52 sites involved in hypo- and 164 sites hypermethylation, related to pathways involved in drug metabolism, anion binding, growth regulation, oxidoreductase activity, and skeletal development, with the most distinct ones including CYP3A43, CYP3A4, CYP2C9, CLCA4, CLCN6, and SLC34A2. | [104] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younesian, S.; Yousefi, A.-M.; Momeny, M.; Ghaffari, S.H.; Bashash, D. The DNA Methylation in Neurological Diseases. Cells 2022, 11, 3439. https://doi.org/10.3390/cells11213439
Younesian S, Yousefi A-M, Momeny M, Ghaffari SH, Bashash D. The DNA Methylation in Neurological Diseases. Cells. 2022; 11(21):3439. https://doi.org/10.3390/cells11213439
Chicago/Turabian StyleYounesian, Samareh, Amir-Mohammad Yousefi, Majid Momeny, Seyed H. Ghaffari, and Davood Bashash. 2022. "The DNA Methylation in Neurological Diseases" Cells 11, no. 21: 3439. https://doi.org/10.3390/cells11213439
APA StyleYounesian, S., Yousefi, A.-M., Momeny, M., Ghaffari, S. H., & Bashash, D. (2022). The DNA Methylation in Neurological Diseases. Cells, 11(21), 3439. https://doi.org/10.3390/cells11213439







