Abstract
Stem cells are a versatile source for cell therapy. Their use is particularly significant for the treatment of neurological disorders for which no definitive conventional medical treatment is available. Neurological disorders are of diverse etiology and pathogenesis. Alzheimer’s disease (AD) is caused by abnormal protein deposits, leading to progressive dementia. Parkinson’s disease (PD) is due to the specific degeneration of the dopaminergic neurons causing motor and sensory impairment. Huntington’s disease (HD) includes a transmittable gene mutation, and any treatment should involve gene modulation of the transplanted cells. Multiple sclerosis (MS) is an autoimmune disorder affecting multiple neurons sporadically but induces progressive neuronal dysfunction. Amyotrophic lateral sclerosis (ALS) impacts upper and lower motor neurons, leading to progressive muscle degeneration. This shows the need to try to tailor different types of cells to repair the specific defect characteristic of each disease. In recent years, several types of stem cells were used in different animal models, including transgenic animals of various neurologic disorders. Based on some of the successful animal studies, some clinical trials were designed and approved. Some studies were successful, others were terminated and, still, a few are ongoing. In this manuscript, we aim to review the current information on both the experimental and clinical trials of stem cell therapy in neurological disorders of various disease mechanisms. The different types of cells used, their mode of transplantation and the molecular and physiologic effects are discussed. Recommendations for future use and hopes are highlighted.
1. Introduction
In the 21st century, stem cells have gained tremendous importance in the fields of medical research and therapy. Stem cells are recognized as body cells that have unique characteristics, including the ability of self-renewal and differentiation into several type of body cells []. They can remain undifferentiated (totipotent) and are capable of differentiating into several mature cells []. Stem cells can be categorized depending on their sources: embryonic stem cells, fetal stem cells, adult stem cells and induced pluripotent stem cells [].
In the medical field, stem cells have been authorized for use in bone marrow transplantation for the treatment of hematological malignancies and some inherited metabolic diseases. Recently, successful stem cell transplantation was reported to cure human immunodeficiency virus (HIV) infection []. Several experimental studies and/or clinical trials studied the use of different kinds of stem cells for the treatment of neurological disorders particularly the disorders lacking a definitive medical treatment. These include Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s Disease (HD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), temporal lobe epilepsy (TLE), neuropathic pain (NP), and brain ischemic stroke (BIS) [,].
The significance of stem cells is derived from their reported ability to replenish damaged cells and tissues as well as their anti-inflammatory and immune-modulatory properties. Stem cells can differentiate and replenish cells that have been weakened or destroyed, promoting neural tissue growth and development. Most of the neurological disorders are characterized by the widespread neuronal death and the extremely low regenerative potential of the brain. The treatment needs materials or cells that can cross the blood brain barrier (BBB). All these factors contribute to making stem cell therapy a viable option for treating chronic intractable neurologic diseases [,]
By combining additional drugs, the results of stem cell therapy may be improved []. Stem cell treatment, for example, in combination with erythropoietin, had synergetic effects on rat neurogenesis. To overcome the limitations of stem cell migration and inclusion in functional networks [,,], nanoparticle distribution systems are investigated. Since they cross the BBB and enter the target brain areas without affecting the surroundings, these nanoparticles are beneficial for drug and cell systems. Another choice for delivering and preserving stem cells in the transplant site is hydrophilic polymer encapsulation, thereby providing mechanical assistance in supply processes and increasing the proliferation and differentiation in hydrogels []. In recent research, gene therapy and neural development factors have also been used to extend the retention of AD and PD transplanted stem cells [].
Many recent trials have shown the value of peripheral stem cell treatments for acute stroke survivors, enabling minimally invasive cell therapy to become a practical alternative option. Additionally, there is consideration of the use of mesenchymal stem cells (MSCs) for supplying bioactive factors, such as brain-derived neurotrophic factors (BDNF), in treating neurologic disorders, such as HD [,,].
This review article aims to review the current research on the use of stem cells both experimentally and in clinical trials for the treatment of selected neurological disorders. The molecular mechanisms of stem cell regenerative actions will be detailed. The successful and promising therapeutic modalities will be emphasized.
2. Selection of Transplant Recipients
Cell transplantation requires several sets of issues that must be considered in preclinical and clinical trials []. The first is whether the disease causes brain cell death or initiates a transition in cell interactions. The next is the probability of systemic transplantation, which happens only if the blood–brain barrier (BBB) is known to be open. Another aspect is whether pathology causes an inflammatory response in relation to the condition itself. In this case, the transplanted cells can play not only an alternative role, but also an anti-inflammatory role. All critical issues must be considered for nearly all cells used in clinical trials [].
Given the above, it is not easy to choose the cells to be transplanted. Many various stem cell types play a possible therapeutic role in the treatment of neurological conditions. Cells must play either a substitutional or trophic role. There was a great focus on the substitution feature in earlier years of stem cell transplantation in neurological disorders. Many experimental studies in animal models showed that transplanted cells in the nerve tissue did not produce a physiological response [,]. If the neural dysfunction is still significant and the surgical approach takes place, the tissue’s reconstitution and hence the rebuilding of the injured neural pathways are very complex. Spinal cord damage is associated with the loss of motor neurons with long axons covered by the myelin sheath. In such circumstances, the transplanted cells must replenish neurons and glia; however, to exert their therapeutic activity, they must be capable of expanding their processes in the right direction. It is unlikely that this challenge will be completed with the current expertise, but a potential approach for transplant research in sophisticated tissues may be the fusion of transplant therapy and bioengineering (scaffold construction) [].
No biomarkers or successful medicines were able to slow down disease development, despite the billions in dollars of clinical trials and considerable advances in studying neurodegenerative mechanisms. This makes stem cell therapy for the treatment of neurodegenerative diseases a beneficial approach to be tested []. The first aim of stem cell therapy involves determining distinct neuronal subtypes and the recapitulation of a network of neural diseases similar to those lost. The development of environmental reinforcement in support of host neurons by the production of neurotrophic and scavenging toxic factors and the construction of an auxiliary neural network across the affected areas is another approach to the treatment of neurodegenerative disorders []. Another strategic approach is the synthesis of neuroprotective growth factors in the sites of diseases (e.g., glial-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), the insulin-like factor 1 growth factor (IGF-1), and VEGF).
One of the biggest concerns is immune rejection of transplanted fetal tissue or cells, which can trigger serious host responses []. Although the brain is considered immuno-privileged, few human leucocyte antigen cells remain matched to the haplotype, requiring immunosuppression in recipients to prevent cell-induced immune refusal. While there are a few exceptions, new technologies are necessary to increase donors’ and recipients’ compatibility and avoid further immune rejections [,].
3. Stem Cells in Alzheimer’s Disease (AD)
Alzheimer’s disease is a widespread chronic, pathologically marked neurodegenerative condition with ß-amyloid plaques and neurofibrillary tangles. Current alternatives to medication only relieve the symptoms without treating the illness, which is a significant problem that impacts the quality of life of patients and their care providers. Stem cell therapy may offer new opportunities for AD patients’ care. More and more research has shown that neural stem cells(NSCs) developed from embryonic stem cells (ESCs) were efficient as a treatment approach in AD models, showing changes both in vitro and in vivo [,]. Stem cells have the potential to differentiate from the brain extracellular matrix into neural cells, and they may restore neuroplasticity and neurogenesis via neurotrophic factors [].
The stem cell approach to treating AD was first examined in animal models []. Neural stem cells from neonatal rat brains were used to establish new cholinergic neurons and increased learning and memory in rats with AD []. Neuron-like embryonic stem cells were used to restore AD-damaged rat brains []. Lately, the most used cells in Alzheimer’s disease research were embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), brain-derived neural stem cells (NSCs), and induced pluripotent stem cells (iPSCs) [].
The basal forebrain cholinergic neurons (BFCNs) are critically implicated in memory and learning disorders, such as AD []. The ESCs possess the pluripotency potential, which is double-bladed. Although that pluripotency is a great advantage for ESCs, it is a considerable disadvantage, as it may lead to the differentiation of the ESCs into several directions, which ultimately can lead to the formation of teratomas and tumors. Moreover, there is a tendency for eliciting disturbed immune reactions and rejection on transplanting ESCs [,]. Therefore, although the ESCs showed promising results in rat models of AD and improved memory performance, it has limited clinical applications. Two types of ESCs have been used in AD research: mouse ESCs (mESCs) and human ESCs (hESCs). Both produced the differentiation into BFCNs when transplanted in mice models with AD.
The immune rejection that occurs using allogeneic hESCs can be mitigated by transplanting iPSCs. Therefore, stimulating the differentiation of autologous hiPSCs could be promising in diminishing the chances of such immune rejection. Nevertheless, there are many concerns regarding the safety of using the iPSCs including the potential risk of oncogenesis and teratoma formation, the safety of the long-term use, the reprogramming efficiency, and immunogenic liability. The partial reprogramming and the unstable genes might elicit an immunological reaction with iPSCs. There is a need to develop new methods and protocols to avoid the expression of the tumorigenic genes when using iPSCs [,]. The iPSCs can differentiate into different cell types, including neurons. In AD research, iPSCs can be used, for example, to investigate the inflammatory reaction, to induce macrophages that can express a protease that degrades beta-amyloid called neprilysin and to reprogram the fibroblast and hence identify the phenotype of the AD [,,].
Despite the ethical issues, the most widely used type of stem cells utilized in AD research is MSCs obtained from umbilical cord blood. This is attributed to the feasibility of obtaining umbilical cord blood after delivery [,]. Previous reports have shown that MSCs can improve the deficits in memory and learning in AD murine models. Boutajangout et al. reported that human umbilical cord mesenchymal stem cell (HUC-MSCs) xenografts improved cognitive decline and reduced the Amyloid burden in a mouse model of Alzheimer’s disease [].
Many mechanisms have been suggested to be involved in this process, including decreased beta-amyloid plaques, a dramatic decrease in β-secretase 1 (BACE-1) levels, reduced hyperphosphorylation of tau, and the reversal of the inflammatory process in the microglia as well as the enhancement of anti-inflammatory cytokines []. Additionally, the immunomodulation and anti-inflammatory effects of MSCs have been reported to occur via enhancing the neuroprotection and depressing the proinflammatory cytokines. Moreover, bone marrow MSCs have been found to stimulate the formation of extracellular vesicles and microvesicles. These vesicles, in turn, target the amyloid-beta [,]. Additionally, the evolved plasticity and neurotrophic decline, decreased tau phosphorylation, and neuroinflammation, and tau down-regulation are promising targets for stem cells. Results showed the transgenic mice survived without any harmful effects and showed increased memory. This was confirmed in another study, where synaptogenesis increased the mental capacity in mice [,]. The successful preliminary animal studies showed positive findings. Researchers grafted human umbilical mesenchymal stem cells obtained from donor cords, into AD mice. An anti-inflammatory and immune-modulatory reaction was simultaneously induced in the mice by this study, and the presence of M2-like microglia enhanced synapsin and raised A β levels in the brain, thereby decreasing amyloid accumulation []. This led to a clinical trial in 2015 using human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) on nine patients with mild-to-to-moderate AD. The hUCB-MSCs were stereotactically inserted into the hippocampus. The method of administration of the stem cells was stable and feasible, with no consequences. Better studies with larger sample size and placebo monitoring are needed to advance the hypothesis. The administration of stem cell therapy was safe but needs to be further checked for its therapeutic efficacy on AD pathogenesis. []. The outcome of some studies on Alzheimer’s patients is still unknown such as (NTC01547689, NTC02672306, NTC02054208, and NTC02600130 from Clinicaltrials.gov). Nevertheless, they are all experiments constrained by the variation of neurons affected by AD.
The biotechnology company Nature Cell has begun a new phase II clinical trial using a stem cell medicine for AD (AstroStem) consisting of autologous adipose tissue stem cells administered intravenously into 60 AD patients (200 million cells/injection) (NCT03117738).
4. Stem Cells in Parkinson’s Disease (PD)
Parkinson’s disease is a degenerative disorder characterized by nigrostriatal dopaminergic neuronal loss. There are about 10 million patients globally []. Till now, there have been no medications that stop the dopaminergic neuronal degeneration. The PD hallmarks represent the cytoplasmic accumulation of α-synuclein and synthesis of Lewy bodies in dopaminergic neurons affecting several regions of the central nervous system [,]. It causes motor impairments, such as muscular rigidity, bradykinesia, static tremors and postural instability []. In addition, PD patients presented with other manifestations, such as sleep and behavioral disorders and abnormal GIT motility []. Because no actual treatment can halt neuronal degeneration, stem cell transplantation is a promising therapy that can restore dopamine (DA) neurotransmission and replace the lost neurons.
Early studies in PD animal models transplanted with mesencephalic cells formed neuronal protrusions with dopamine formation [,,,,]. Additionally, human fetal ventral mesencephalic transplantation in PD patients in clinical studies declared the moderate improvement of PD symptoms [,,,,]. However, the postmortem investigation of patients that transplanted fetal ventral mesenchephalic (fVM) tissue grafts showed the presence of Lewy bodies in the transplanted cells [], suggesting that Lewy body pathology can spread from host to graft []. Additionally, graft-induced dyskinesia was a side effect of fVM transplantation. Indeed, most of successful results were achieved in PD cases below the age of 60, shown in Figure 1 [].
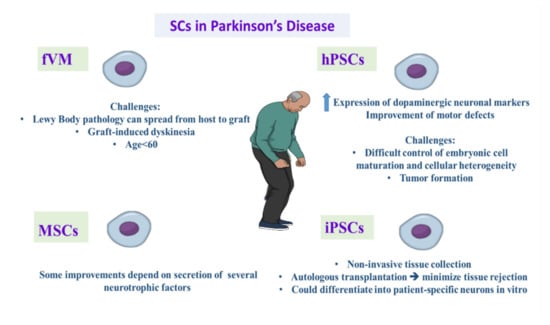
Figure 1.
Demonstrating the promising role and challenges of different types of stem cell therapy in treating Parkinson’s disease. iPSCs: induced pluripotent stem cells, hPSCs: human pluripotent stem cells, fVM: fetal ventral mesencephalic tissue, MSCs: mesenchymal stem cells.
Multiple technical problems appear before the use of fVM grafts in clinical practice. The first problem is the limited viability of grafted cells in the host striatum. Attempts were made to deal with this issue through the supply of several neurotrophic factors []. The second challenge is the minimal accessibility of human fetal cells, and the variations in protocols. Notably, immunosuppressant intake is required to avoid allograft-induced immune rejection. We need up to seven human fetal donor cells for each patient, and this creates actual ethical concerns [,].
To overcome these contests, the European consortium TRANSEURO, a multicenter for clinical trials, works to assess the feasibility and efficacy of human fetal cell transplantation in PD cases and provide more reliable results and more understanding of the potential therapeutic benefits [].
Human embryonic stem cells (hESCs) are pluripotent stem cells located in the inner layer of early embryonic blastocysts [], and they can differentiate into multiple cells via various differentiation protocols in vitro [,]. By using hESCs, there was a remarkable expression of dopaminergic neuronal markers together with improvement of motor defects [,]. However, the major challenge is difficult control embryonic cell maturation and cellular heterogeneity that result in poor results in clinical application [,]. Another major problem is the risk of tumor formation [,].
The stem cell research was revolutionized after the reprogramming of human fibroblasts to pluripotent cells [,,]. iPSCs have the same characteristics as hESCs but have a relatively easier extraction process. The most important point is non-invasive tissue collection as it can be extracted from skin fibroblasts, mononuclear blood cells, and umbilical mesenchymal cells [,,,]. iPSCs could differentiate into patient-specific neurons in vitro [,,]. Additionally, the autologous transplantation of stem cells is important to minimize tissue rejection []. The efficacy of DA neurons derived from iPSCs was like that of hESCs [,]. Animal studies showed the effective improvement of symptoms after iPSCs-derived neuron transplantation in PD animal models [].
In a study on the PD monkey model, the transplantation of autologous iPSC-derived DA neurons exhibited an obvious improvement in motor functions without immunosuppression therapy []. MSCs were confirmed to affect the management of multiple diseases, including PD []. MSCs cause improved PD symptoms in PD mouse models. Some improvements depend on the secretion of several neurotrophic factors that minimize DA neuronal degeneration [,].
In 1987, Professor Madrazo headed a team that recognized neural grafting as a new method for substituting missing dopamine cells. Two young PD patients were autografted with adrenal medulla tissue into the brain, leading to improved PD symptoms, including tremors, rigidity, and akinesia. Cell-based neural transplant and treatment have since been regarded as potential treatments for PD as a suitable candidate for a focal degeneration disorder []. Pilot research was conducted two years later including 18 patients who confirmed the findings of Madrazo. However, the technique has been discontinued because of insufficient preclinical evidence and patients having mental disorders after surgery [].
These groundbreaking experimental studies are underway in stem cell therapy for Parkinson’s disease and have brought a new and unprecedented revolution to clinical trials. Thus, therapeutic translation instructions and treatment protocols were determined for the patients. With this sort of technology, in 2018, a Chinese team hoped to implant neural precursor cells that were obtained from human embryos into people with Parkinson’s disease. The research studied how the dopamine-producing neurons can progress to maturity. The world’s first neural stem cell transplant was completed in Australia by neurologist Dr. Andrew Evans of the Royal Melbourne Hospital and his colleagues. A few Australian patients were administered artificially generated, parthenogenically derived neural stem cells in a phase I safety study (NCT02452723). Furthermore, the iPSCs production by Takahashi and Yamanaka and personalized neural progenitor cells from stem cells give a great source of patient-specific and disease-specific neurons, avoiding many of the problems with hESC lines (on both ethical and environmental fronts) [,]. In light of the recent progress in iPSCs, it was concluded that the most critical iPSCs priorities are studying the human disease mechanisms, and that is why iPSCs studies are scarce in clinical trials [].
In 2017, a team of Japanese researchers found a safe and efficient method to create neurons that can make dopamine from patient-derived iPSCs cells. By the process of direct midbrain dopaminergic progenitor grafting into the monkeys, they treated them with 1-methyl-4-phenyl-1,2, 3,6-tetrahydropyridine (MPTP), which ablates nigral dopaminergic neurons to watch their behavior for two years to see how it will decrease their symptoms []. The researchers’ hopes were dramatically raised, as the monkeys recovered with stable motor functions and with no cancer evidence of tumor growth in both PD families. Therefore, these tests proved to these researchers that the technique could be used on people.
5. Stem Cells in Huntington’s Disease (HD)
Huntington’s disease is a degenerative autosomal dominant disorder characterized by GABAergic degeneration of medium spiny neurons (MSNs) in striatum, cerebral cortex, thalamus, and hypothalamus []. This degeneration leads to progressive deterioration of motor and cognitive functions []. HD represents multiple repeats of the trinucleotide CAG in the HD gene, causing increased expression of the Huntington (HTT) protein, which reduces brain-derived neurotrophic factor (BDNF) and causes medium spiny neurons (MSNs) [] protein processing abnormalities [] and improper mitochondrial function []. Despite the detection of the causative mutation more than 20 years ago, no effective treatment can halt HD consequences. Cell-based therapy is an attractive approach to treat HD disease. The principal scope is to restore the degenerated neurons and supply neurotropic support to avoid more deterioration; see Figure 2.
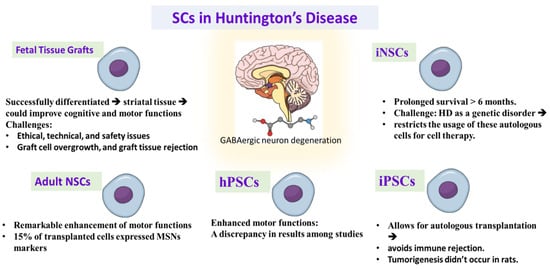
Figure 2.
Demonstrating the promising role and challenges of different types of stem cell therapy in treating Huntington’s disease. iNSC: induced neuronal stem cells, iPSCs: induced pluripotent stem cells, hPSCs: human pluripotent stem cells, NSCs: neuronal stem cells, MSNs: striatal medium spiny neurons.
Traditionally, embryonic, and fetal striatal tissue grafts were utilized in animal models of HD [,,]. These studies declared that the transplanted fetal tissue successfully differentiated into striatal tissue could improve cognitive and motor functions [,,,,,,,,]. The usage of fetal stem cell therapy in HD is faced with many obstacles, concerns, and ethical, technical, and safety issues. Some studies showed some side effects of fetal cell transplantation such as graft cell overgrowth, and graft tissue rejection [,]. While immunosuppressive treatments can limit these reactions, prolonged immunosuppression raises additional safety concerns [].
Neural stem cells were taken from a fetal brain. This procedure still needs the usage of aborted fetuses, but the ability to generate immortalized lines of hNSCs produces homogenous cell groups with lower tissue requirement. Ryu et al. stated that hNSCs transplantation to the HD rat model enhanced motor functions when the hNSCs were grafted before HD lesion, while cell transplantation after induction of HD lesion did not improve motor function []. The transplanted hNSCs displayed the endogenous secretion of BDNF, indicating that the motor enhancement may be caused partially by neurotrophic secretion by the transplanted cells. Another study declared significant improvement in the motor function and reduction of striatal atrophy after hNSCs transplantation in HD model with the existence or absence of the ciliary-derived neurotropic factor supply before transplantation, representing the role of transplanted cells in the production of further neurotrophic support []. Other studies demonstrated that hNSCs migrated into the striatum and enhanced motor functions when they were injected to the ventricles or intravenously in HD rats, giving attention to use this less invasive method in the clinical studies of HD patients [,].
Human pluripotent stem cells took a remarkable consideration as a cell-based therapy for neurodegenerative disorders. Early preclinical studies declared that hESC-derived neuronal stem cells enhanced motor functions in the HD model. However, the transplanted stem cells did not exhibit differentiation into MSNs [,]. To overcome this challenge, groups of hESCs differentiated into lateral ganglionic eminence progenitor cells or into striatal precursor cells were used to enhance cell MSNs’ specified differentiation [,,,,]. While several studies declared proper integration of the transplanted cells and improvement of motor functions [,], other studies did not achieve the same results [,]. These discrepancies may be due to variability in the cell maturation and the transplanted cell numbers between studies [,,].
Induced pluripotent stem cells are another a source to be used in cell replacement therapy of HD [,]. iPSCs have the advantage that they are collected from the patient, so they allow for autologous transplantation, which avoids immune rejection and the need for immunosuppressive therapy after transplantation [,]. In a study, iNSCs derived from fibroblasts were transplanted into the striatum, differentiated to neurons that expressed MSNs markers in mice []. Moreover, the transplanted cells exhibited prolonged survival for more than 6 months. However, the nature of HD as a genetic disorder restricts the usage of these autologous cells for cell therapy. Therefore, the genetic modification of the HD mutation becomes an important target before autologous cell therapy. There was a study that corrected the HD mutation in iPSCs before transplantation into HD mouse model []. Although this study did not show motor improvement, the transplanted cells were differentiated into neurons with the expression of MSNs markers.
Another promising study discussed the combination of stem cell and gene therapy in a transgenic model of HD in mice by using the neural progenitor cells of a rhesus monkey. Genetically adjusted NPCs showed a significant prolonged lifespan and enhancement of motor functions []. While there was no evidence of tumorigenesis after hNSC transplantation therapy in rats, the short lifespan of rat and mice models does not produce adequate observation of prolonged side effects []. Thus, it is important that these procedures are tried on other animals with a comparable lifespan to humans before using these remedies in HD patients.
An alternative method to obtain neural progenitor cells is by obtaining NSCs directly from neurogenic areas of the adult brain [,]. An initial study utilized NSCs extracted from the subventricular zone for transplantation into the striatum of the HD rat model. This procedure led to a remarkable enhancement of motor functions []. About 15% of transplanted cells expressed MSNs markers. Moreover, the handling of NSCs with lithium chloride before transplantation elevated the percentage of MSN neurons to 34% [].
6. Stem Cells in Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic lateral sclerosis is a degenerative disorder that affects motor neurons leading to atrophy and weakness of skeletal muscles []. Alteration in executive function occurs in about 50% of patients, and about 15% of patients represent frontotemporal dementia []. There is no real therapy for ALS. So, stem cells are an optimistic approach that may restore degenerated neurons. The transplanted stem cells and derived motor neurons require developing long axons, and synapse with endogenous neurons and muscles [,]. ALS is a lethal neurodegenerative disease, which affects both the upper and lower motor neurons. Only about 10 to 15% of the cases are familial, whilst the sporadic cases represent most cases. The mechanisms underlying ALS are still elusive and many previous reports documented a variety of proposed mechanisms. Amongst these mechanisms are dysfunction of mitochondria, oxidative stress, and glutamate toxicity. Therefore, the development of a therapeutic protocol that affects multiple suggested mechanisms could lead to a more favorable outcome [,].
There is only one documented drug for managing ALS, riluzole, in Europe, and there have been no other newly registered drugs since 1994. In Japan and the USA, a newly emerging drug, edaravone, has been registered []. Stem cell therapy in ALS disease is based on “neighborhood theory”, where the transplanted cells secrete neuroprotective substances that limit the process of neurodegeneration. Transplanted stem cells, also, differentiate into astrocytes and microglia, or into other neurons, which connect with the affected motor neurons [].
Most of the preclinical studies conducted on ALS models expressed superoxide dismutase 1 mutation (SOD1) []. SOD1 is the most prevalent mutation of ALS []. In an early study, the intravenous injection of umbilical cord blood delayed motor symptoms expression and prolonged survival in the ALS mice model [,]. Histological investigation showed that the injected cells infiltrated the regions of cerebral and spinal cord degeneration with the expression of neural biomarkers []. In addition, inflammatory cytokines were reduced in the degenerated areas []. In another study, grafted neurons from ESCs were differentiated into motor neurons and transplanted to the spinal cord of ALS rats []. Grafted rats showed improved motor functions but were exposed to paralysis later. The initial improvement of motor functions indicated that the engrafted motor neurons exhibit some benefits, probably by secretion of neurotropic factors.
Hematopoietic stem cells [] and glial restricted precursors [] improved motor functions in ALS rats. In addition, bone marrow transplantation from wild rats to ALS mice models delayed the progress of disease [], while intravenous injection of bone marrow-extracted stem cells to ALS mice prolonged lifespan []. The transplantation of olfactory ensheathing cells delayed the appearance of symptoms; these cells were differentiated into oligodendrocytes and astrocytes [], indicating that various cell types can alleviate ALS progress. The injection of mesenchymal stem cells to skeletal muscles of ALS rats produced a neuroprotective action via secretion of the glial-cell-derived neurotrophic factor [] or vascular endothelial growth factor [];
Another source of stem cells is adipose-derived MSCs. Preclinical studies showed the potential effect of adipose-derived MSCs in ALS mice model []. When adipose-derived MSCs were injected intravenously, the motor deterioration was slowed. Histological investigation of spinal cord detected a greater number of motor neurons in adipose-derived MSCs injected mice []. Additionally, the transplantation of NPCs into the spinal cord improved the disorder phenotypes in ALS mice, implicating the neuroprotective role of neurotrophins []. The transplantation of NPCs programmed to secrete GDNF, IGF-1, VEGF and brain-derived neurotrophic factor showed a variable level of success [,,].
iPSCs are another source of autologous cells that have a great promise for treating ALS []. The utilization of human fibroblast cells programmed into iPSCs and differentiated to NPCs showed that the transplanted cells integrated into the spinal cord []. Another study compared the use of single versus multiple bone marrow-derived hMSCs injections into the cerebrospinal fluid in the SOD1 transgenic mice model of ALS. The researchers found no effect of the single hMSCs transplantation, whilst the multiple injections of hMSCs could have therapeutic potential against ALS []. An interesting study where the researchers administered adipose-derived MSCs (ADMSCs) into (SOD1)-mutant transgenic mice early in the clinical course of the disease documented postponed motor disturbances as assessed by electrophysiological methods and clinically. Additionally, they investigated the underlying mechanisms of this improvement. They suggested that the neuroprotective effect of the ADMSCs either directly or indirectly is behind the long-lasting powerful positive effect on motor function. The direct effect was obtained via the upregulation of the glial-derived growth factor, which is produced by the ADMSCs, while the indirect effect was suggested to be via the modulation of the local glial cells secretomes to be neuroprotective [].
Despite that, stem-cell-based therapy in treating ALS is still in the beginning stages. Some clinical trials have been conducted in many countries around the world []. For example, a study that introduced Wharton’s jelly derived mesenchymal stem cells for patients with ALS reported the safety and effectiveness of this therapeutic approach. The study reported that the clinical and demographic characteristics, excluding sex, have no significant implications on the outcome. The study reported that the predictors for an effective outcome are female sex and good response to the first dose of therapy []. Another Chinese study introduced olfactory enhancing stem cells (OESCs) intracranially and documented preliminary outcomes about controlling or reversing the physiological derangements in ALS patients []. Nevertheless, other studies that utilized similar protocols showed contradicting results []. Therefore, the introduction of stem cell-based therapy for patients with ALS is still questionable and in need of further pre-clinical and clinical research before accepting it as a line of management of ALS.
7. Stem Cells in Multiple Sclerosis (MS)
Multiple sclerosis is an autoimmune disorder characterized by neuronal loss and demyelination []. Disease-modifying therapies for MS can reduce the severity of the disease. There is no successful therapy to stop the progress of the disorder and repair the present neural damage [,]. In the last two decades, stem cell therapy has been considered a potentially attractive method for MS.
Immunoablation therapy followed by autologous hematopoietic stem cells (aHSCs) transplantation was studied following the findings of the improvement of autoimmune phenoyptes in patients that undergo bone marrow transplantation for hematological malignancies [,]. In studies conducted on rats with experimental autoimmune encephalomyelitis (EAE), immunoablation followed by aHSCs promoted remission and avoided relapses [,].
The rationale of this approach is to remove the present immune system by using high-dose immunosuppressive therapy (HDIT), and develop a new intact immune system following aHSCs transplantation []. Earlier studies were directed to patients with advanced disease and higher disability [,,]. Recent studies concentrated on patients with relapsing–remitting MS (RRMS) with poor prognosis, showing that aHSCs transplantation is most successful in these patients [,,,]. A study carried out on patients with RRMS reported 5-year event-free survival of 69% of patients [].
MSCs were reported as a hopeful cell therapy for neurodegenerative disorders [,]. These potential results may be caused by paracrine signaling and the integration of MSCs in the damaged tissues []. When BM-MSCs were administered to EAE mice model, there was a diminution of the symptom’s severity, decrease in the infiltration of immune cells, and decrease in demyelination and axonal injury [,,,,,]. These results were shown when MSCs were injected intraventricularly, intravenously and intraperitoneally [,,]. MSCs might influence differentiation of neuronal stem cell and induce axonal remyelination []. Histopathological analysis of EAE mice infused with MSCs reported the repairing of white matter, and enhanced axonal integration in diseased tissues [].
Neuronal stem cells (NSCs) had a potential therapeutic effect as they migrated to the demyelinating tissues and differentiated into oligodendrocytes []. A major challenge is the fewer NSCs available to be used as an endogenous cell therapy. IVT-transplanted NPCs to EAE mice migrated into the injured white matter and differentiated to oligodendrocytes. However, the direct effect on myelin remyelination is still unclear [].
Human embryonic stem cells (hESCs) differentiate to neural cell lineage, and this has attracted consideration as a potential approach to manage MS []. The transplantation of hESC-derived neural progenitors caused an improvement of symptoms in EAE mice. Histological analysis declared the integration of the transplanted neural progenitors in brain tissues. The improvement might be due to neuroprotective mechanisms [].
Studies declared that oligodendrocyte precursor-derived induced pluripotent stem cells (iPSC) ameliorated the features of EAE []. This therapeutic effect was mostly due to the neuroprotective influence rather than remyelination. However, recent studies suggest the potential for malignancy. So, more research studies should be performed to overcome this challenge [].
Multiple sclerosis typically presents with relapsing-remitting illness (RRMS), which is discrete and self-limited. However, after the remission of clinical symptoms, the damage that occurred in the brain does not resolve. Usually, it takes about 10–20 years for the RRMS to turn into secondary progressive MS (SPMS). Three major factors interplay to achieve the best efficacy of aHSCs transplantation in patients with MS. These factors are the selection of the patient, choosing the transplant regimen, and the experience of the working team []. In this section, we will focus on the patient section.
Previous reports documented that autologous stem cell transplantation (ASCT) began as pilot clinical began trials (Phase I/II) in Greece in 1995 for MS patients; in 2010, there were reports for 400 patients around the globe treated with ASCT []. Accumulating pieces of evidence point to the increased efficacy of the aHSCT in patients with RRMS when compared to patients with primary progressive MS (PPMS) or secondary progressive MS [,].
aHSCT should be introduced when there is an expected severe case of MS and should be done so early in the course of the disease, before irreversible neural damage occurs []. A variety of demographic and disease-related characters influence the outcome of the therapy by aHSCT. It was found that the patients with young age, patients with active inflammatory disease, patients with low scores of Kurtzke’s Expanded Disability Status Scale (EDSS) and patients without other co-morbidities show the better outcome of therapy by aHSCT [,,]. The previously mentioned factors are interlinked to each other. For instance, when the patient has a shorter disease duration, this means that he/she is in the RRMS phase, which in turn points to better EDSS scores. It was found that patients with the active inflammatory stage of MS have better outcomes compared to patients with indolent inflammation. This is attributed to the ability of aHSCT to rapidly cease the inflammatory cells; see Figure 3 [,].
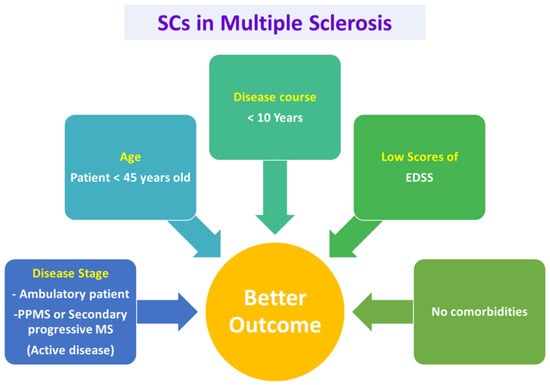
Figure 3.
Selection criteria for patients of MS to be treated with aHSCs for a better outcome. Based on the EBMT and previous reports, Patients with early active disease stages, younger than 45 years old, with disease course lesser than 10 years and low scores of EDSS and no comorbidities are found to have better outcome on transplantation of autologous stem cells. EDSS: Kurtzke’s Expanded Disability Status Scale, EBMT: European Group for Blood and Marrow Transplantation.
The gadolinium enhancement leads to the breaking down of the blood–brain barrier and hence allows the conditioned regimen to penetrate the CNS and, therefore, enhance the process of eliminating the autoreactive immune cells. The European Group for Blood and Marrow Transplantation (EBMT) published recommended guidelines for choosing patients who are eligible for aHSCT in 2012. They recommended aHSCT for patients who have RRMS and can ambulate independently, yet they suffered from two clinical relapses associated with MRI evidence of concurrent illness during the previous year in spite of the conventional disease-modifying therapies. Nevertheless, the individuals suffering from the inability to ambulate due to rapid progression of disability or sometimes those who have clear clinical or MRI evidence of progressive disease might be eligible for aHSCT. However, the outcome will be less favorable. Currently, there is a suggestion to use aHSCT in patients younger than 45 years old and with a disease course of less than 10 years [,,]. Collectively, it is apparent that aHSCT is beneficial in treating MS during the different stages of the disease, however, for achieving a favorable outcome, the patient should be selected according to the recommendations of EBMT.
8. Stem Cells in Temporal Lobe Epilepsy (TLE)
Epilepsy is a neurological disorder which affects about 70 million patients all over the world []. TLE is the most prevalent type of epilepsy characterized by repeated seizures created in the structures of temporal lobe such as amygdala and hippocampus []. The loss of GABA-ergic neurons and synaptic changes were noticed in the hippocampus in patients and animal models with TLE [], resulting in overall increased neuronal excitatory tone []. The current therapy of TLE mainly depends on anticonvulsant therapy or surgical interventions. About 1/3 of epileptic patients exhibit poor outcome when anticonvulsant therapy is used alone []. For refractory TLE, surgical intervention with temporal lobectomy is performed. However, the surgery results in reduced cognitive functions. So, there is an urgent requirement to find out a new therapeutic way for treatment of TLE. In the past years, different stem types of cells were used in preclinical studies to treat epilepsy in animal models.
Studies revealed that transplantation of ESCs could replace the damaged neurons and produce inhibitory mediators such as GABA which reduce neuronal excitability in epilepsy []. Another study reported that the transplanted ESCs into the hippocampi of status epilepticus mice model were differentiated to mature neurons [].
As GABA has a role in reduction of excitability thus inhibition of seizures [], transplantation of fetal GABA-ergic cells to the cerebral tissues had been studied in several epilepsy models. They hypothesized that elevation of GABA neurotransmitter level could inhibit seizure exposure [,]. The transplantation of fetal neural cells in epileptic rats minimized the severity of convulsive seizures, replaced degenerated neurons and repaired the damaged neural circuits [].
Recent studies focused on fetal medial ganglionic eminence (MGE) cells for the treatment of TLE because MGE is the source of most striatal and hippocampal neurons []. The transplantation of fetal MGE precursor cells led to migration and synaptic formation in pyramidal neurons in the brain [], and reduction in seizures number in mice [,]. The intravenous injection of fetal neural stem cells provided a remarkable inhibition in seizure rate and severity []. In addition, hippocampal precursor cells transplantation enhanced memory and learning defects in epilepticus status [].
MSCs exhibited a neuroprotective property by releasing several neurotrophic factors and immunomodulation [] which could inhibit seizure activity []. In a study, bone marrow MSCs transplantation decreased the chemical and histological alterations, restored neurotransmitters normal level, and reduced apoptotic and inflammatory marker levels in an epileptic rat model []. Another study revealed that adipose-extracted mesenchymal cell transplantation enhanced the release of neurotrophic markers and reduced seizure activity in an epileptic rat model [].
9. Stem Cells in Neuropathic Pain (NP)
Neuropathic pain is a frequent complaint and usually occurs in diabetic patients with female predominance. Unfortunately, the current pharmacological therapies for neuropathic pain are mainly symptomatic, not fully effective, and have many side effects. Amongst these drugs are pregabalin (known commercially as Lyrica), tricyclic antidepressants, and opioids [,,]. The administration of a combination of the available drugs may give a better outcome. However, the side effects remain to occur. Therefore, shifting toward stem cell therapy for neuropathic pain could provide a good chance for a better cure by affecting the underlying mechanisms of neuropathic pain instead of solely treating the symptoms; see Figure 4 and Figure 5 [].
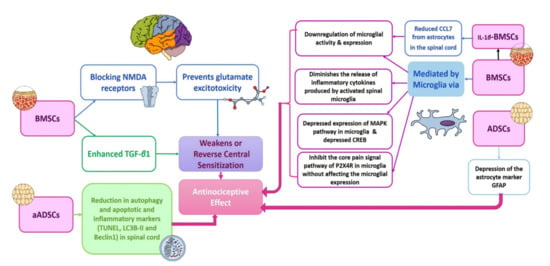
Figure 4.
Proposed mechanisms for central NP alleviation via different types of stem cell therapy. Based on previous research, there are three main mechanisms for the antinociceptive effects of stem cell therapy. These mechanisms encompass the modulation of the glial cells (microglia and astrocytes) functions, weakening or reversal of central sensitization and reduction in autophagy, apoptosis and inflammatory markers in the spinal cord. Each of these main mechanisms are mediated via different ways according to the type of stem cells investigated. It is obvious that BMSCs act via different mechanisms to induce the analgesic effect. Additionally, conditioning of the BMSCs via IL-1β adds to these mechanisms. The ADSCs act mainly via depressing the astrocyte markers. Moreover, the transplantation of autologous ADSCs reduces the autophagy, apoptotic and inflammatory markers in the spinal cord. ADSCs: adipose-derived stem cells; aADSCs: autologous adipose-derived stem cells; BMSCs: bone-marrow-derived stem cells; CCL7: chemokine (C-C Motif) ligand 7; CREB: cAMP response element-binding protein; GFAP: autoimmune glial fibrillary acid protein; IL-1β BMSCs: IL-1β conditioned bone-marrow-derived stem cells; LC3-BII: autophagy marker light chain 3; MAPK: mitogen-activated protein kinase; NMDA: N-methyl-D-aspartate receptor; P2X4R: purinergic 2X4 receptor; TGF- β1: transforming growth factor beta 1; TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
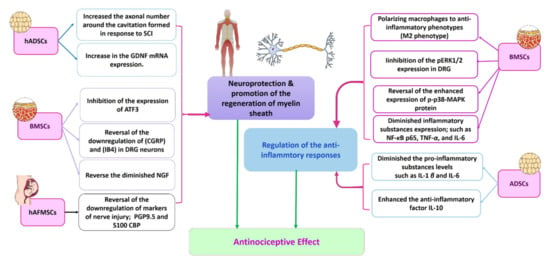
Figure 5.
Proposed mechanisms involved in peripheral NP alleviation by stem cell therapy. Based on previous research, it is clear that there are two main mechanisms by which the different types of stem cells counteract hyperalgesia. These mechanisms are regulation of the anti-inflammatory responses and neuroprotection and promotion of the regeneration of myelin sheath. BMSCs act via the two mechanisms through a variety of pathways that varied according to the methodologies of the previous research. The ADSCs seem to regulate the anti-inflammatory responses, whilst the hADSCs work through the neuroprotective mechanism. The hAFMSCs act mainly via neuroprotection [,,]. ADSCs: adipose-derived stem cells; ATF3: activating transcription factor 3; BMSCs: bone marrow derived stem cells; CGRP: calcitonin gene-related peptide CREB: cAMP response element-binding protein; DRG: dorsal root ganglion; GDNF: glial-derived neurotrophic factor; hADSCs: human adipose derived stem cells; hAFMSCs: amniotic fluid-derived mesenchymal stem cells; IB4: isolectin B4; IL: interleukin; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B-cell transcription factor; NGF: nerve growth factor; pERK½: phosphorylated extracellular-regulated kinase ½; PGP9.5: protein gene product 9.5; p-p38-MAPK protein MAPK: mitogen-activated protein kinase; S100-CBP: S100 calcium-binding protein; TNF: tumor necrosis factor; SCI: spinal cord injury.
Among the crucial mechanisms of NP is the central sensitization, in which there is an enhancement in neuronal excitability. After nerve injury, there is an enhanced release of the excitatory neurotransmitter glutamate and upregulation of the N-methyl-d-aspartate (NMDA) receptors in the spinal cord. Studies showed that intravenous injection of bone marrow mesenchymal stem cells (BMSCs) inhibited the expression of NMDA receptors and hence prevented glutamate excitotoxicity [,].
One of the important mechanisms that lead to pain sensation in neuropathy is the glial cells (astrocytes, oligodendrocytes, and microglia) activation. Within 24 h post-injury, the microglia become activated and continue for about 12 weeks. This is followed by the release of cytokines from the microglia and astrocytes, along with the upregulation of the glutamate and glucocorticoids. This in turn can produce spinal cord excitation and therefore contribute to hypersensitivity and pain sensation. BMSCs were found to lower the microglial activity when injected intrathecally in case of non-compressive disc herniation. Additionally, it can lower the release of the inflammatory cytokines by the spinal cord-activated microglia and therefore affect behavioral hypersensitivity in nerve root pain [,,]. Other studies found that intravenously administered ADSCs can decrease the expression of the astrocyte marker, GFAP.
Furthermore, amongst the mechanisms that modulate the effect of transplanted stem cells in NP are depressed apoptosis, autophagy, and promoting nerve recovery. It has been found that the expression of the apoptotic marker, TUNNEL, and LC3B-II and Beclin1 in the spinal cord are depressed in a burn fat model of neuropathic pain upon subcutaneous transplantation of adipose stem cells [].
In addition to the previously mentioned central mechanisms that modulate the action of stem cells in neuropathic pain, there are many peripheral mechanisms, including powerful immunosuppressive and anti-inflammatory effects, enhanced expression of glial-derived neurotrophic factor (GDNF), enhancement of neurogenesis, neuronal growth, and myelin formation [,].
10. Stem Cells in Brain Ischemic Stroke (BIS)
It is generally stated that the transplantation of neural stem cells is an alternative therapy to replace damaged or dead neural cells or improve the self-repair system after brain ischemic stroke (BIS) []. In 2010, the first transplantation of a neural stem cell on clinical patients—phase I—with BIS was performed []. It was reported that after transplantation of the exogenous neural stem cells, the patients’ neurological function was improved without any unfavorable reaction. The number of clinical studies were few; however, there is a large number of preclinical animal studies [,,,]. These studies stated that neural stem cell transplantation therapy could enhance the functional outcomes as well as the histological outcomes where the infraction volume is considerably decreased. It has been reported that, essentially, the transplantation is focusing in two directions; the first strategy is to replace the dead cells, and the second strategy is to improve the self-repair system by mediating the neural network reconstruction. Moreover, it has been found that the degree of progress in function of BIS animal model has an effect on the injection time of neural stem cells and the source of stem cells [].

Table 1.
Summary of the reviewed neurological diseases and the types of cells used in experimental studies and/or clinical trials.

Table 2.
A summary of recent studies reported the paracrine effect of stem cells secretomes, exosomes, microvesicles, and extracellular vesicles on neurological disorders.
11. Strategies to Enhance Cell Survival after Transplantation: Hypoxic Preconditioning and Genetic Modification
Recently, stem cell research included several creative strategies for treating neural disorders. These strategies aimed to resolve the problems of cell survival, differentiation, and engraftment rate after stem cell transplantation. They include preconditioning with different methods, such as hypoxia, drugs, or growth factors. They also include genetic modifications, upregulating the expression of specific growth factors, pro-survival, or anti-apoptotic genes of the stem cells before transplantation [,].
It has been reported that preconditioned neural stem cells with hypoxia before transplantation produced a significant increase in the rate of survival, proliferation, and differentiation as well as decrease in the number of apoptotic cells [,]. In addition, it also showed a significant increase in the biological function of transplanted cells []. The oxygen level of 1.5 to 5.3% was found to be efficient to increase proliferation of neural stem cells and differentiation into functional neuron cells []. The postulated mechanism is the activation of hypoxia-inducible factor1 (HIF1) transcriptional complex [,]. It comprises alpha (HIF1-α) and beta (HIF1-β) subunits. In mammals, it is the master regulator that facilitates important homeostatic responses to low oxygen levels. In the presence of oxygen, the HIF1-α is downregulated through hydroxylation by prolyl-4-hydroxylase and factor-inhibiting HIF enzymes [,]. These two hydroxylases inhibit the activation of the HIF system. Meanwhile, under hypoxic condition, HIF1-α will translocate to the nucleus and form dimerization with HIF1-β subunits. Then, the activated HIF system is attached to the hypoxia response elements, leading to activation of the expression of several essential genes, such as erythropoietin (EPO), vascular endothelial growth factor (VEGF) and glucose transporter 1 (GLUT-1) []. Those genes have a fundamental role in improving neural stem cell survival and treating ischemic stroke disorders.
Another way to improve cell survival and therapeutic outcomes after transplantation is to genetically modify the stem cell. The neural stem cell is genetically modified prior transplantation to overexpress neurotrophic support genes, such as brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), VEGF, EPO and nerve growth factor (NGF). These produce enhanced survival rates and improved proliferation and neural differentiation [,,,]. In addition, they show immunomodulatory action and increase the expression of antioxidant and survival genes such as Bcl-2 [,]. Gretchen et al. (2018) used a genetically modified human cortical-derived neural stem cell to overproduce GDNF before transplantation into a rat cortex. These cells were able to differentiate into astrocytes producing GDNF []. Hypoxic preconditioning and genetically modified neural stem cell transplantation characterize a therapeutic strategy for neural disorders.
12. Conclusions and Future Perspectives
Previous studies indicated that stem cell therapy for neurological diseases may be successful in experimental disease models and in certain limited clinical trials. The selection of the most suitable cell type that suits a particular disease is a challenge. This is due to the diverse neuropathological effects of these diseases. Despite the drawbacks and complexities of stem cell treatment, the future of neurodegenerative disorders is still an exciting approach. It remains impractical and far to use stem cells in neurodegenerative diseases to substitute missing neurons and incorporate them into the original neural circuitry []. However, it is more practical and feasible in the short-term to use stem cells to provide therapeutic factors and inhibit disease progression. More information on the correct delivery strategy and immunosuppression, graft survival, and effectiveness will be provided by the continuing and prospective clinical trials. The development of new cellular origins and the further development of successful combination approaches to treating neuropathic disorders was possible through best practices in stem cell therapies. The use of ESCs and iPSCs, though pluripotent, may be complicated by teratomas and /or cancer. MSCs, whether derived from bone marrow or adipocytes, possess immense plasticity, but their mode of action and duration of survival in the host is questionable. Human umbilical cord stem cells provided some improvement in cognitive function in AD. The grafting of fetal and/or adrenal medullary tissues in PD had positive impact on the disease symptoms, but the patients suffered from neuropsychiatric complications. Additionally, the use of fetal tissue from aborted fetuses creates ethical concerns in different disciplines. In addition, long-term immunosuppression is needed after tissue transplantation. Combining the transplanted cells with certain drugs, such as erythropoietin, proved to be useful in experimental neurogenesis. Nanoparticle distribution systems cross the BBB and enter the target brain areas without affecting the surroundings; these nanoparticles are beneficial for facilitating the delivery of drugs and cell systems. Stem cells could be encapsulated in hydrophilic polymers, thus helping delivery and preservation in the transplant site []. Gene therapy and neural development factors have also been used to extend the retention of AD and PD transplanted stem cells []. Cell preconditioning with growth factors, exposure to hypoxia, and genetic modification proved to be useful at the experimental level. The idea is to provide a better fate and function to the transplanted cells. The combination and preconditioning therapies are gaining momentum and it seems that they will dominate the future work in this field.
Author Contributions
M.A.Z., S.S. and G.M.A. conceptualized, wrote, and edited the manuscript. H.O.A., S.M.Y., G.I.A., N.H.A., S.A., K.S.A.G., S.A.B. and A.J. performed the literature survey and drafted and edited the manuscript. B.S.A. ideated the scheme and performed artwork. S.M.Y. designed the figures and graphical abstract. N.H.A. and S.A. drafted the tables. H.M.A., G.I.A. and A.J. significantly helped in revision and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by the Institutional Fund Projects under grant no. IFPDP-65-22 which is supported by Ministry of Education and Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia.
Acknowledgments
Authors gratefully acknowledge technical and financial support from Ministry of Education and Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
Abbreviations
| AD | Alzheimer’s Disease |
| ADSCs | Adipose Derived Stem Cells |
| aHSCT | Autologous Hematopoietic Stem cells |
| ALS | Amyotrophic Lateral Sclerosis |
| ASC-CCM | Adipose Tissue-Derived Mesenchymal Stem Cells |
| ASCT | Autologous Stem Cell Transplantation |
| BACE 1 | Beta-secretase 1 |
| BBB | Blood-Brain Barrier |
| BDNF | Brain-Derived Neurotrophic Factors |
| BIS | Brain Ischemic Stroke |
| BM-MSCs | Bone Marrow Mesenchymal Stem Cells |
| CNS | Central Nervous System |
| DA | Dopamine |
| EAE | Experimental Autoimmune Encephalomyelitis |
| EBMT | European Group for Blood and Marrow Transplantation |
| EDSS | Expanded Disability Status Scale |
| EPO | Erythropoietin |
| ESCs | Embryonic Stem Cells |
| fVM | Fetal Ventral Mesenchephalic |
| GABA | Gamma-Aminobutyric Acid |
| GDNF | Glial-Derived Neurotrophic Factor |
| GIT | Gastrointestinal |
| GLUT-1 | Glucose Transporter 1 |
| HD | Huntington’s Disease |
| HDIT | High-Dose Immunosuppressive Therapy |
| hESCs | Human Embryonic Stem Cells |
| HGF | Hepatocyte Growth Factor |
| HIF | Hypoxia-Inducible Factor1 |
| HTT | Huntingtin |
| hUCB-MSCs | Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells |
| HUC-MSCs | Human Umbilical Cord Mesenchymal Stem Cell |
| iPSCs | induced Pluripotent Stem Cells |
| LC3B-II | Autophagy Marker Light Chain 3 |
| MGE | Medial Ganglionic Eminence |
| MS | Multiple Sclerosis |
| MSCs | Mesenchymal Stem Cells |
| MSN | Medium Spiny Neuron |
| NGF | Nerve Growth Factor |
| NMDA | N-methyl-D-aspartate receptor |
| NP | Neuropathic Pain |
| NSCs | Neural Stem Cells |
| OESCs | Olfactory Enhancing Stem Cells |
| PD | Parkinson’s Disease |
| PPMS | Primary Progressive Multiple Sclerosis |
| RRMS | Relapsing–Remitting Multiple Sclerosis |
| SOD1 | Superoxide Dismutase 1 |
| SPMS | Secondary Progressive Multiple Sclerosis |
| TGF | Transforming Growth Factor |
| TIMP-1 | Tissue Inhibitor of Metalloproteinase Type 1 |
| TLE | Temporal Lobe Epilepsy |
| TSG-6 | TNFα-stimulated gene-6 |
| TUNNEL | Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling |
| VEGF | Vascular Endothelial Growth Factor |
References
- Hui, H.; Tang, Y.; Hu, M.; Zhao, X. Stem Cells: General Features and Characteristics; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Mitalipov, S.; Wolf, D. Totipotency, pluripotency and nuclear reprogramming. Adv. Biochem Eng. Biotechnol 2009, 114, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Peppa, D.; Hill, A.L.; Gálvez, C.; Salgado, M.; Pace, M.; McCoy, L.E.; Griffith, S.A.; Thornhill, J.; Alrubayyi, A.; et al. Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: A case report. Lancet HIV 2020, 7, e340–e347. [Google Scholar] [CrossRef]
- Biehl, J.K.; Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009, 24, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Conese, M.; Piro, D.; Carbone, A.; Castellani, S.; Di Gioia, S. Hematopoietic and Mesenchymal Stem Cells for the Treatment of Chronic Respiratory Diseases: Role of Plasticity and Heterogeneity. Sci. World J. 2014, 2014, 859817. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Photobiomodulation for Alzheimer’s Disease: Has the Light Dawned? Photonics 2019, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Cucullo, L. Regenerative Stem Cell Therapy for Neurodegenerative Diseases: An Overview. Int. J. Mol. Sci. 2021, 22, 2153. [Google Scholar] [CrossRef]
- Ritfeld, G.J.; Roos, R.A.; Oudega, M. Stem cells for central nervous system repair and rehabilitation. PMR 2011, 3, S117–S122. [Google Scholar] [CrossRef]
- Carballo-Molina, O.A.; Sánchez-Navarro, A.; López-Ornelas, A.; Lara-Rodarte, R.; Salazar, P.; Campos-Romo, A.; Ramos-Mejía, V.; Velasco, I. Semaphorin 3C released from a biocompatible hydrogel guides and promotes axonal growth of rodent and human dopaminergic neurons. Tissue Eng. Part. A 2016, 22, 850–861. [Google Scholar] [CrossRef]
- Garcia-Bennett, A.E.; König, N.; Abrahamsson, N.; Kozhevnikova, M.; Zhou, C.; Trolle, C.; Pankratova, S.; Berezin, V.; Kozlova, E.N. In vitro generation of motor neuron precursors from mouse embryonic stem cells using mesoporous nanoparticles. Nanomedicine 2014, 9, 2457–2466. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Rajesh, R. Nerve growth factor-loaded heparinized cationic solid lipid nanoparticles for regulating membrane charge of induced pluripotent stem cells during differentiation. Mater. Sci. Eng. C 2017, 77, 680–689. [Google Scholar] [CrossRef]
- Zhu, W.; George, J.K.; Sorger, V.J.; Zhang, L.G. 3D printing scaffold coupled with low level light therapy for neural tissue regeneration. Biofabrication 2017, 9, 025002. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xing, H.; Wang, Y.; Guo, Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol. Ther. -Nucleic Acids 2021, 23, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Anderson, J.D.; Yu, A.S.; Annett, G.; Fink, K.D.; Nolta, J.A. Engineered BDNF producing cells as a potential treatment for neurologic disease. Expert Opin. Biol. Ther. 2016, 16, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.D.; Deng, P.; Gutierrez, J.; Anderson, J.S.; Torrest, A.; Komarla, A.; Kalomoiris, S.; Cary, W.; Anderson, J.D.; Gruenloh, W. Allele-specific reduction of the mutant huntingtin allele using transcription activator-like effectors in human huntington’s disease fibroblasts. Cell Transplant. 2016, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Nolta, J.A. “Next-Generation” Mesenchymal Stem or Stromal Cells for the In Vivo Delivery of Bioactive Factors: Progressing Toward the Clinic. Transfusion 2016, 56, 15S–17S. [Google Scholar] [CrossRef] [PubMed]
- Adami, R.; Scesa, G.; Bottai, D. Stem cell transplantation in neurological diseases: Improving effectiveness in animal models. Front. Cell Dev. Biol. 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.; Biziato, D.; Brambilla, E.; Donegà, M.; Alfaro-Cervello, C.; Snider, S.; Salani, G.; Pucci, F.; Comi, G.; Garcia-Verdugo, J.M.; et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain A J. Neurol. 2012, 135, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Nizzardo, M.; Simone, C.; Rizzo, F.; Ruggieri, M.; Salani, S.; Riboldi, G.; Faravelli, I.; Zanetta, C.; Bresolin, N.; Comi, G.P. Minimally invasive transplantation of iPSC-derived ALDHhiSSCloVLA4+ neural stem cells effectively improves the phenotype of an amyotrophic lateral sclerosis model. Hum. Mol. Genet. 2014, 23, 342–354. [Google Scholar] [CrossRef]
- Karussis, D.; Petrou, P.; Kassis, I. Clinical experience with stem cells and other cell therapies in neurological diseases. J. Neurol. Sci. 2013, 324, 1–9. [Google Scholar] [CrossRef]
- Lunn, J.S.; Sakowski, S.A.; Hur, J.; Feldman, E.L. Stem cell technology for neurodegenerative diseases. Ann. Neurol. 2011, 70, 353–361. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Z.-n.; Westenskow, P.D.; Todorova, D.; Hu, Z.; Lin, T.; Rong, Z.; Kim, J.; He, J.; Wang, M. Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell 2015, 17, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, C.; Xuan, A.; Xu, L.; Bao, G.; Liu, F.; Fang, J.; Long, D. Treatment efficacy of NGF nanoparticles combining neural stem cell transplantation on Alzheimer’s disease model rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 3608. [Google Scholar] [CrossRef] [PubMed]
- Hallett, P.J.; Cooper, O.; Sadi, D.; Robertson, H.; Mendez, I.; Isacson, O. Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell Rep. 2014, 7, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jia, Y.; Liu, J.; Zhai, J.; Cao, N.; Yue, W.; He, H.; Pei, X. Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease. Cell Biol. Int. 2017, 41, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H. Embryonic neural stem cell transplantation for Alzheimer’s disease. Chin. J. Tissue Eng. Res. 2016, 20, 4805–4810. [Google Scholar]
- Enciu, A.M.; Nicolescu, M.I.; Manole, C.G.; Mureşanu, D.F.; Popescu, L.M.; Popescu, B.O. Neuroregeneration in neurodegenerative disorders. BMC Neurol. 2011, 11, 1–7. [Google Scholar] [CrossRef]
- Kang, J.M.; Yeon, B.K.; Cho, S.J.; Suh, Y.H. Stem Cell Therapy for Alzheimer’s Disease: A Review of Recent Clinical Trials. J. Alzheimer’s Dis. JAD 2016, 54, 879–889. [Google Scholar] [CrossRef]
- Xuan, A.; Luo, M.; Ji, W.; Long, D. Effects of engrafted neural stem cells in Alzheimer’s disease rats. Neurosci. Lett. 2009, 450, 167–171. [Google Scholar] [CrossRef]
- Hoveizi, E.; Mohammadi, T.; Moazedi, A.A.; Zamani, N.; Eskandary, A. Transplanted neural-like cells improve memory and Alzheimer-like pathology in a rat model. Cytotherapy 2018, 20, 964–973. [Google Scholar] [CrossRef]
- Liu, M.; Li, K.; Wang, Y.; Zhao, G.; Jiang, J. Stem cells in the treatment of neuropathic pain: Research progress of mechanism. Stem Cells Int 2020, 2020, 8861251. [Google Scholar] [CrossRef]
- Liu, Y.; Weick, J.P.; Liu, H.; Krencik, R.; Zhang, X.; Ma, L.; Zhou, G.M.; Ayala, M.; Zhang, S.C. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat. Biotechnol 2013, 31, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Oh, S.H.; Pi, L.; Hatch, H.M.; Shupe, T.; Petersen, B.E. Teratoma formation leads to failure of treatment for type I diabetes using embryonic stem cell-derived insulin-producing cells. Am. J. Pathol. 2005, 166, 1781–1791. [Google Scholar] [CrossRef]
- Jin, X.; Lin, T.; Xu, Y. Stem Cell Therapy and Immunological Rejection in Animal Models. Curr. Mol. Pharmacol. 2016, 9, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Pareja, E.; Gómez-Lechón, M.J. Clinical Application of Pluripotent Stem Cells: An Alternative Cell-Based Therapy for Treating Liver Diseases? Transplantation 2016, 100, 2548–2557. [Google Scholar] [CrossRef]
- Israel, M.A.; Yuan, S.H.; Bardy, C.; Reyna, S.M.; Mu, Y.; Herrera, C.; Hefferan, M.P.; Van Gorp, S.; Nazor, K.L.; Boscolo, F.S. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 2012, 482, 216–220. [Google Scholar] [CrossRef]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013, 3, 1–11. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 1–14. [Google Scholar] [CrossRef]
- Boutajangout, A.; Noorwali, A.; Atta, H.; Wisniewski, T. Human Umbilical Cord Stem Cell Xenografts Improve Cognitive Decline and Reduce the Amyloid Burden in a Mouse Model of Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 104–111. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.K.; Lee, H.; Carter, J.E.; Chang, J.W.; Oh, W.; Yang, Y.S.; Suh, J.G.; Lee, B.H.; Jin, H.K.; et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol. Aging 2012, 33, 588–602. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yang, L.P.; Zhao, L. Stem cell therapy for Alzheimer’s disease. World J. Stem Cells 2020, 12, 787–802. [Google Scholar] [CrossRef]
- Yang, H.; Yang, H.; Xie, Z.; Wei, L.; Bi, J. Systemic transplantation of human umbilical cord derived mesenchymal stem cells-educated T regulatory cells improved the impaired cognition in AbetaPPswe/PS1dE9 transgenic mice. PLoS ONE 2013, 8, e69129. [Google Scholar] [CrossRef][Green Version]
- Ager, R.R.; Davis, J.L.; Agazaryan, A.; Benavente, F.; Poon, W.W.; LaFerla, F.M.; Blurton-Jones, M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus 2015, 25, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-S.; Jung, K.; Kim, I.-S.; Lee, H.; Kim, M.; Yun, S.; Hwang, K.; Shin, J.E.; Park, K.I. Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol. Neurodegener. 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xie, Z.H.; Wei, L.F.; Yang, H.N.; Yang, S.N.; Zhu, Z.Y.; Wang, P.; Zhao, C.P.; Bi, J.Z. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem Cell Res. Ther. 2013, 4, 1–14. [Google Scholar] [CrossRef]
- Kim, H.J.; Seo, S.W.; Chang, J.W.; Lee, J.I.; Kim, C.H.; Chin, J.; Choi, S.J.; Kwon, H.; Yun, H.J.; Lee, J.M. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: A phase 1 clinical trial. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2015, 1, 95–102. [Google Scholar] [CrossRef]
- Osborn, T.M.; Hallett, P.J.; Schumacher, J.M.; Isacson, O. Advantages and recent developments of autologous cell therapy for Parkinson’s disease patients. Front. Cell. Neurosci. 2020, 14, 58. [Google Scholar] [CrossRef]
- More, S.V.; Kumar, H.; Kim, I.S.; Song, S.-Y.; Choi, D.-K. Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediat. Inflamm. 2013, 2013, 952375. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Glial reactions in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 474–483. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G.; Del Tredici, K.; Braak, H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013, 9, 13. [Google Scholar] [CrossRef]
- Olanow, C.W.; Stern, M.B.; Sethi, K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology 2009, 72, S1–S136. [Google Scholar] [CrossRef]
- Dunnett, S.; Bjorklund, A.; Schmidt, R.; Stenevi, U.; Iversen, S. Intracerebral grafting of neuronal cell suspensions. V. Behavioural recovery in rats with bilateral 6-OHDA lesions following implantation of nigral cell suspensions. Acta Physiol Scand. Suppl 1983, 522, 39–47. [Google Scholar] [PubMed]
- Brundin, P.; Nilsson, O.; Strecker, R.; Lindvall, O.; Åstedt, B.; Björklund, A. Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson’s disease. Exp. Brain Res. 1986, 65, 235–240. [Google Scholar] [CrossRef]
- Redmond, D.E.; Roth, R.; Elsworth, J.; Sladek JR, J.; Collier, T.; Deutch, A.; Haber, S. Fetal neuronal grafts in monkeys given methylphenyltetrahydropyridine. Lancet 1986, 327, 1125–1127. [Google Scholar] [CrossRef]
- Strömberg, I.; Bygdeman, M.; Goldstein, M.; Seiger, Å.; Olson, L. Human fetal substantia nigra grafted to the dopamine-denervated striatum of immunosuppressed rats: Evidence for functional reinnervation. Neurosci. Lett. 1986, 71, 271–276. [Google Scholar] [CrossRef]
- Bakay, R.A.; Barrow, D.L.; Fiandaca, M.S.; Iuvone, P.M.; Schiff, A.; Collins, D.C. Biochemical and Behavioral Correction of MPTP Parkinson-like Syndrome by Fetal Cell Transplantation a. Ann. New York Acad. Sci. 1987, 495, 623–638. [Google Scholar] [CrossRef]
- Lindvall, O.; Rehncrona, S.; Gustavii, B.; Brundin, P.; Astedt, B.; Widner, H.; Lindholm, T.; Björklund, A.; Leenders, K.; Rothwell, J. Fetal dopamine-rich mesencephalic grafts in Parkinson’s disease. Lancet 1988, 2, 1483–1484. [Google Scholar] [CrossRef]
- Madrazo, I.; Leon, V.; Torres, C.; Aguilera, M.; Varela, G.; Alvarez, F.; Fraga, A.; Drucker-Colin, R.; Ostrosky, F.; Skurovich, M. Transplantation of fetal substantia nigra and adrenal medulla to the caudate nucleus in two patients with Parkinson’s disease. New Engl. J. Med. 1988, 318, 51. [Google Scholar]
- Freed, C.R.; Breeze, R.E.; Rosenberg, N.L.; Schneck, S.A.; Wells, T.H.; Barrett, J.N.; Grafton, S.T.; Huang, S.; Eidelberg, D.; Rottenberg, D.A. Transplantation of human fetal dopamine cells for Parkinson’s disease: Results at 1 year. Arch. Neurol. 1990, 47, 505–512. [Google Scholar] [CrossRef]
- Freed, C.R.; Greene, P.E.; Breeze, R.E.; Tsai, W.-Y.; DuMouchel, W.; Kao, R.; Dillon, S.; Winfield, H.; Culver, S.; Trojanowski, J.Q. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. New Engl. J. Med. 2001, 344, 710–719. [Google Scholar] [CrossRef]
- Olanow, C.W.; Goetz, C.G.; Kordower, J.H.; Stoessl, A.J.; Sossi, V.; Brin, M.F.; Shannon, K.M.; Nauert, G.M.; Perl, D.P.; Godbold, J. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003, 54, 403–414. [Google Scholar] [CrossRef]
- Kordower, J.H.; Chu, Y.; Hauser, R.A.; Freeman, T.B.; Olanow, C.W. Lewy body–like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008, 14, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.; Kordower, J.H. Neuropathology in transplants in Parkinson’s disease: Implications for disease pathogenesis and the future of cell therapy. Prog. Brain Res. 2012, 200, 221–241. [Google Scholar] [PubMed]
- Ma, Y.; Tang, C.; Chaly, T.; Greene, P.; Breeze, R.; Fahn, S.; Freed, C.; Dhawan, V.; Eidelberg, D. Dopamine cell implantation in Parkinson’s disease: Long-term clinical and 18F-FDOPA PET outcomes. J. Nucl. Med. 2010, 51, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, H.-Y. How to improve the survival of the fetal ventral mesencephalic cell transplanted in Parkinson’s disease? Neurosci. Bull. 2007, 23, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, J.A.; Studer, L. Moving stem cells to the clinic: Potential and limitations for brain repair. Neuron 2015, 86, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Barker, R. TRANSEURO consortium. Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat. Med. 2019, 25, 1045–1053. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Itsykson, P.; Turetsky, T.; Pera, M.F.; Reinhartz, E.; Itzik, A.; Ben-Hur, T. Neural progenitors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1134–1140. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Wernig, M.; Duncan, I.D.; Brüstle, O.; Thomson, J.A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001, 19, 1129–1133. [Google Scholar] [CrossRef]
- Grealish, S.; Diguet, E.; Kirkeby, A.; Mattsson, B.; Heuer, A.; Bramoulle, Y.; Van Camp, N.; Perrier, A.L.; Hantraye, P.; Björklund, A. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell 2014, 15, 653–665. [Google Scholar] [CrossRef]
- Kirkeby, A.; Nolbrant, S.; Tiklova, K.; Heuer, A.; Kee, N.; Cardoso, T.; Ottosson, D.R.; Lelos, M.J.; Rifes, P.; Dunnett, S.B. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson’s disease. Cell Stem Cell 2017, 20, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Lee, Y.-E.; Kim, J.Y.; Chung, S.; Cho, Y.H.; Kim, D.-S.; Kang, S.-M.; Lee, H.; Kim, M.-H.; Kim, J.-H. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 3392–3397. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.; Opitz, T.; Steinbeck, J.A.; Ladewig, J.; Brüstle, O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc. Natl. Acad. Sci. USA 2009, 106, 3225–3230. [Google Scholar] [CrossRef] [PubMed]
- Brederlau, A.; Correia, A.S.; Anisimov, S.V.; Elmi, M.; Paul, G.; Roybon, L.; Morizane, A.; Bergquist, F.; Riebe, I.; Nannmark, U. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: Effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells 2006, 24, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Okita, K.; Nakagawa, M.; Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2007, 2, 3081. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R. Induced pluripotent stem cell lines derived from human somatic cells. Obstet. Gynecol. Surv. 2008, 63, 154–155. [Google Scholar] [CrossRef][Green Version]
- Pulecio, J.; Nivet, E.; Sancho-Martinez, I.; Vitaloni, M.; Guenechea, G.; Xia, Y.; Kurian, L.; Dubova, I.; Bueren, J.; Laricchia-Robbio, L. Conversion of human fibroblasts into monocyte-like progenitor cells. Stem Cells 2014, 32, 2923–2938. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, P.H.; Bang, O.Y.; Lee, G.; Ahn, Y.H. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson’s disease. J. Neurochem. 2008, 107, 141–151. [Google Scholar] [CrossRef]
- Biju, K.; Santacruz, R.A.; Chen, C.; Zhou, Q.; Yao, J.; Rohrabaugh, S.L.; Clark, R.A.; Roberts, J.L.; Phillips, K.A.; Imam, S.Z. Bone marrow-derived microglia-based neurturin delivery protects against dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. Neurosci. Lett. 2013, 535, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Song, B.; Zhang, H.; Wang, Y.; Wang, N.; Ji, Y.; Qi, J.; Chandra, A.; Yang, B.; Zhang, Y. Transplantation of human neuro-epithelial-like stem cells derived from induced pluripotent stem cells improves neurological function in rats with experimental intracerebral hemorrhage. Neurosci. Lett. 2013, 548, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 2009, 136, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Beevers, J.E.; Caffrey, T.M.; Wade-Martins, R. Induced pluripotent stem cell (iPSC)-derived dopaminergic models of Parkinson’s disease. Biochem. Soc. Trans. 2013, 41, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Sison, S.; Vermilyea, S.; Emborg, M.; Ebert, A. Using patient-derived induced pluripotent stem cells to identify Parkinson’s disease-relevant phenotypes. Curr. Neurol. Neurosci. Rep. 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Hallett, P.J.; Deleidi, M.; Astradsson, A.; Smith, G.A.; Cooper, O.; Osborn, T.M.; Sundberg, M.; Moore, M.A.; Perez-Torres, E.; Brownell, A.-L. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell 2015, 16, 269–274. [Google Scholar] [CrossRef]
- Doi, D.; Samata, B.; Katsukawa, M.; Kikuchi, T.; Morizane, A.; Ono, Y.; Sekiguchi, K.; Nakagawa, M.; Parmar, M.; Takahashi, J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2014, 2, 337–350. [Google Scholar] [CrossRef]
- Kikuchi, T.; Morizane, A.; Doi, D.; Magotani, H.; Onoe, H.; Hayashi, T.; Mizuma, H.; Takara, S.; Takahashi, R.; Inoue, H. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017, 548, 592–596. [Google Scholar] [CrossRef]
- Wakeman, D.R.; Hiller, B.M.; Marmion, D.J.; McMahon, C.W.; Corbett, G.T.; Mangan, K.P.; Ma, J.; Little, L.E.; Xie, Z.; Perez-Rosello, T. Cryopreservation maintains functionality of human iPSC dopamine neurons and rescues parkinsonian phenotypes in vivo. Stem Cell Rep. 2017, 9, 149–161. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Mesenchymal stem cell therapy in Parkinson’s disease animal models. Curr. Res. Transl. Med. 2017, 65, 51–60. [Google Scholar] [CrossRef]
- D’Angelo, M.; Cimini, A.; Castelli, V. Insights into the effects of mesenchymal stem cell-derived secretome in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 5241. [Google Scholar] [CrossRef] [PubMed]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Madrazo, I.; Drucker-Colín, R.; Díaz, V.; Martínez-Mata, J.; Torres, C.; Becerril, J.J. Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson’s disease. New Engl. J. Med. 1987, 316, 831–834. [Google Scholar] [CrossRef]
- Barker, R.A.; Drouin-Ouellet, J.; Parmar, M. Cell-based therapies for Parkinson disease—Past insights and future potential. Nat. Rev. Neurol. 2015, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.A.; de Beaufort, I. Scientific and ethical issues related to stem cell research and interventions in neurodegenerative disorders of the brain. Prog. Neurobiol. 2013, 110, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Scudellari, M. How iPS cells changed the world. Nat. News 2016, 534, 310. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef]
- Dayalu, P.; Albin, R.L. Huntington disease: Pathogenesis and treatment. Neurol. Clin. 2015, 33, 101–114. [Google Scholar] [CrossRef]
- Zuccato, C.; Marullo, M.; Conforti, P.; MacDonald, M.E.; Tartari, M.; Cattaneo, E. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain Pathol. 2008, 18, 225–238. [Google Scholar] [CrossRef]
- Sakahira, H.; Breuer, P.; Hayer-Hartl, M.K.; Hartl, F.U. Molecular chaperones as modulators of polyglutamine protein aggregation and toxicity. Proc. Natl. Acad. Sci. USA 2002, 99, 16412–16418. [Google Scholar] [CrossRef]
- Guo, X.; Disatnik, M.-H.; Monbureau, M.; Shamloo, M.; Mochly-Rosen, D.; Qi, X. Inhibition of mitochondrial fragmentation diminishes Huntington’s disease–associated neurodegeneration. J. Clin. Investig. 2013, 123, 5371–5388. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.L.; Rayment, F.D.; ToRRES, E.M.; Baker, H.F.; Ridley, R.M.; Dunnett, S.B. Functional integration of striatal allografts in a primate model of Huntington’s disease. Nat. Med. 1998, 4, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Dunnett, S.B.; Nathwani, F.; Björklund, A. The integration and function of striatal grafts. Prog. Brain Res. 2000, 127, 345–380. [Google Scholar] [PubMed]
- Deckel, A.W.; Moran, T.H.; Coyle, J.T.; Sanberg, P.R.; Robinson, R.G. Anatomical predictors of behavioral recovery following fetal striatal transplants. Brain Res. 1986, 365, 249–258. [Google Scholar] [CrossRef]
- Isacson, O.; Dunnett, S.B.; Björklund, A. Graft-induced behavioral recovery in an animal model of Huntington disease. Proc. Natl. Acad. Sci. USA 1986, 83, 2728–2732. [Google Scholar] [CrossRef]
- Pritzel, M.; Isacson, O.; Brundin, P.; Wiklund, L.; Björklund, A. Afferent and efferent connections of striatal grafts implanted into the ibotenic acid lesioned neostriatum in adult rats. Exp. Brain Res. 1986, 65, 112–126. [Google Scholar] [CrossRef]
- Sirinathsinghji, D.; Dunnett, S.; Isacson, O.; Clarke, D.; Kendrick, K.; Björklund, A. Striatal grafts in rats with unilateral neostriatal lesions—II. In vivo monitoring of GABA release in globus pallidus and substantia nigra. Neuroscience 1988, 24, 803–811. [Google Scholar] [CrossRef]
- Palfi, S.; Condé, F.; Riche, D.; Brouillet, E.; Dautry, C.; Mittoux, V.; Chibois, A.; Peschanski, M.; Hantraye, P. Fetal striatal allografts reverse cognitive deficits in a primate model of Huntington disease. Nat. Med. 1998, 4, 963–966. [Google Scholar] [CrossRef]
- Nakao, N.; Itakura, T. Fetal tissue transplants in animal models of Huntington’s disease: The effects on damaged neuronal circuitry and behavioral deficits. Prog. Neurobiol. 2000, 61, 313–338. [Google Scholar] [CrossRef]
- Freeman, T.B.; Hauser, R.A.; Willing, A.E.; Zigova, T.; Sanberg, P.R.; Saporta, S. Transplantation of human fetal striatal tissue in Huntington’s disease: Rationale for clinical studies. In Neural Transplantation in Neurodegenerative Disease: Current Status and New Directions; Novartis Foundation Symposia Series; Chadwick, D.J., Goode, J.A., Eds.; Novartis Foundation: Basel, Switzerland, 2000; pp. 129–144. [Google Scholar]
- Klein, A.; Lane, E.L.; Dunnett, S.B. Brain repair in a unilateral rat model of huntington’s disease: New insights into impairment and restoration of forelimb movement patterns. Cell Transplant. 2013, 22, 1735–1751. [Google Scholar] [CrossRef]
- Hauser, R.A.; Furtado, S.; Cimino, C.R.; Delgado, H.; Eichler, S.; Schwartz, S.; Scott, D.; Nauert, G.; Soety, E.; Sossi, V. Bilateral human fetal striatal transplantation in Huntington’s disease. Neurology 2002, 58, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Krystkowiak, P.; Gaura, V.; Labalette, M.; Rialland, A.; Remy, P.; Peschanski, M.; Bachoud-Lévi, A.-C. Alloimmunisation to donor antigens and immune rejection following foetal neural grafts to the brain in patients with Huntington’s disease. PLoS ONE 2007, 2, e166. [Google Scholar] [CrossRef] [PubMed]
- Benraiss, A.; Goldman, S.A. Cellular therapy and induced neuronal replacement for Huntington’s disease. Neurotherapeutics 2011, 8, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Kim, J.; Cho, S.J.; Hatori, K.; Nagai, A.; Choi, H.B.; Lee, M.C.; McLarnon, J.G.; Kim, S.U. Proactive transplantation of human neural stem cells prevents degeneration of striatal neurons in a rat model of Huntington disease. Neurobiol. Dis. 2004, 16, 68–77. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.L.; Behrstock, S.P.; Chen, E.Y.; Jakel, R.J.; Siegel, I.; Svendsen, C.N.; Kordower, J.H. Human neural stem cell transplants improve motor function in a rat model of Huntington’s disease. J. Comp. Neurol. 2004, 475, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-T.; Chu, K.; Park, J.-E.; Lee, K.; Kang, L.; Kim, S.U.; Kim, M. Intravenous administration of human neural stem cells induces functional recovery in Huntington’s disease rat model. Neurosci. Res. 2005, 52, 243–249. [Google Scholar] [CrossRef]
- Lee, S.-T.; Park, J.-E.; Lee, K.; Kang, L.; Chu, K.; Kim, S.U.; Kim, M.; Roh, J.-K. Noninvasive method of immortalized neural stem-like cell transplantation in an experimental model of Huntington’s disease. J. Neurosci. Methods 2006, 152, 250–254. [Google Scholar] [CrossRef]
- Joannides, A.J.; Webber, D.J.; Raineteau, O.; Kelly, C.; Irvine, K.-A.; Watts, C.; Rosser, A.E.; Kemp, P.J.; Blakemore, W.F.; Compston, A. Environmental signals regulate lineage choice and temporal maturation of neural stem cells from human embryonic stem cells. Brain 2007, 130, 1263–1275. [Google Scholar] [CrossRef]
- Song, J.; Lee, S.-T.; Kang, W.; Park, J.-E.; Chu, K.; Lee, S.-e.; Hwang, T.; Chung, H.; Kim, M. Human embryonic stem cell-derived neural precursor transplants attenuate apomorphine-induced rotational behavior in rats with unilateral quinolinic acid lesions. Neurosci. Lett. 2007, 423, 58–61. [Google Scholar] [CrossRef]
- Ma, L.; Hu, B.; Liu, Y.; Vermilyea, S.C.; Liu, H.; Gao, L.; Sun, Y.; Zhang, X.; Zhang, S.-C. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell 2012, 10, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Carri, A.D.; Onorati, M.; Lelos, M.J.; Castiglioni, V.; Faedo, A.; Menon, R.; Camnasio, S.; Vuono, R.; Spaiardi, P.; Talpo, F. Developmentally coordinated extrinsic signals drive human pluripotent stem cell differentiation toward authentic DARPP-32+ medium-sized spiny neurons. Development 2013, 140, 301–312. [Google Scholar] [CrossRef]
- Aubry, L.; Bugi, A.; Lefort, N.; Rousseau, F.; Peschanski, M.; Perrier, A.L. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc. Natl. Acad. Sci. USA 2008, 105, 16707–16712. [Google Scholar] [CrossRef]
- Arber, C.; Precious, S.V.; Cambray, S.; Risner-Janiczek, J.R.; Kelly, C.; Noakes, Z.; Fjodorova, M.; Heuer, A.; Ungless, M.A.; Rodríguez, T.A. Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development 2015, 142, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Nicoleau, C.; Varela, C.; Bonnefond, C.; Maury, Y.; Bugi, A.; Aubry, L.; Viegas, P.; Bourgois-Rocha, F.; Peschanski, M.; Perrier, A.L. Embryonic stem cells neural differentiation qualifies the role of Wnt/β-Catenin signals in human telencephalic specification and regionalization. Stem Cells 2013, 31, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Nasonkin, I.; Mahairaki, V.; Xu, L.; Hatfield, G.; Cummings, B.J.; Eberhart, C.; Ryugo, D.K.; Maric, D.; Bar, E.; Koliatsos, V.E. Long-term, stable differentiation of human embryonic stem cell-derived neural precursors grafted into the adult mammalian neostriatum. Stem Cells 2009, 27, 2414–2426. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.; Lee, N.; Li, J.Y.; Park, I.H.; Park, K.S.; Moon, J.; Shim, S.H.; Choi, C.; Chang, D.J.; Kwon, J. Neuronal properties, in vivo effects, and pathology of a Huntington’s disease patient-derived induced pluripotent stem cells. Stem Cells 2012, 30, 2054–2062. [Google Scholar] [CrossRef]
- An, M.C.; Zhang, N.; Scott, G.; Montoro, D.; Wittkop, T.; Mooney, S.; Melov, S.; Ellerby, L.M. Genetic correction of Huntington’s disease phenotypes in induced pluripotent stem cells. Cell Stem Cell 2012, 11, 253–263. [Google Scholar] [CrossRef]
- Mattis, V.B.; Svendsen, C.N. Modeling Huntington׳ s disease with patient-derived neurons. Brain Res. 2017, 1656, 76–87. [Google Scholar] [CrossRef]
- Chen, Y.; Carter, R.L.; Cho, I.K.; Chan, A.W. Cell-based therapies for Huntington’s disease. Drug Discov. Today 2014, 19, 980–984. [Google Scholar] [CrossRef]
- Victor, M.B.; Richner, M.; Hermanstyne, T.O.; Ransdell, J.L.; Sobieski, C.; Deng, P.-Y.; Klyachko, V.A.; Nerbonne, J.M.; Yoo, A.S. Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron 2014, 84, 311–323. [Google Scholar] [CrossRef]
- Cho, I.K.; Hunter, C.E.; Ye, S.; Pongos, A.L.; Chan, A.W.S. Combination of stem cell and gene therapy ameliorates symptoms in Huntington’s disease mice. NPJ Regen. Med. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Vazey, E.M.; Dottori, M.; Jamshidi, P.; Tomas, D.; Pera, M.F.; Horne, M.; Connor, B. Comparison of Transplant Efficiency between Spontaneously Derived and Noggin-Primed Human Embryonic Stem Cell Neural Precursors in the Quinolinic Acid Rat Model of Huntington’s Disease; SAGE Publications: Los Angeles, CA, USA, 2010. [Google Scholar]
- Vazey, E.M.; Chen, K.; Hughes, S.M.; Connor, B. Transplanted adult neural progenitor cells survive, differentiate and reduce motor function impairment in a rodent model of Huntington’s disease. Exp. Neurol. 2006, 199, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Goutman, S.A. Diagnosis and clinical management of amyotrophic lateral sclerosis and other motor neuron disorders. CONTINUUM Lifelong Learn. Neurol. 2017, 23, 1332–1359. [Google Scholar] [CrossRef]
- Ringholz, G.; Appel, S.H.; Bradshaw, M.; Cooke, N.; Mosnik, D.; Schulz, P. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 2005, 65, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Boulis, N.M.; Federici, T.; Glass, J.D.; Lunn, J.S.; Sakowski, S.A.; Feldman, E.L. Translational stem cell therapy for amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2012, 8, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Maragakis, N.J. Stem cells and the ALS neurologist. Amyotroph. Lateral Scler. 2010, 11, 417–423. [Google Scholar] [CrossRef]
- Lunn, J.S.; Sakowski, S.A.; Feldman, E.L. Concise review: Stem cell therapies for amyotrophic lateral sclerosis: Recent advances and prospects for the future. Stem Cells 2014, 32, 1099–1109. [Google Scholar] [CrossRef]
- Barczewska, M.; Maksymowicz, S.; Zdolinska-Malinowska, I.; Siwek, T.; Grudniak, M. Umbilical Cord Mesenchymal Stem Cells in Amyotrophic Lateral Sclerosis: An Original Study. Stem Cell Rev. Rep. 2020, 16, 922–932. [Google Scholar] [CrossRef]
- Cho, H.; Shukla, S. Role of Edaravone as a Treatment Option for Patients with Amyotrophic Lateral Sclerosis. Pharm. 2020, 14, 29. [Google Scholar] [CrossRef]
- Goutman, S.A.; Chen, K.S.; Feldman, E.L. Recent advances and the future of stem cell therapies in amyotrophic lateral sclerosis. Neurotherapeutics 2015, 12, 428–448. [Google Scholar] [CrossRef]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic lateral sclerosis. New Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ende, N. The potential for the use of mononuclear cells from human umbilical cord blood in the treatment of amyotrophic lateral sclerosis in SOD1 mice. J. Med. 2000, 31, 21–30. [Google Scholar] [PubMed]
- Ende, N.; Weinstein, F.; Chen, R.; Ende, M. Human umbilical cord blood effect on sod mice (amyotrophic lateral sclerosis). Life Sci. 2000, 67, 53–59. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Willing, A.E.; Zigova, T.; Saporta, S.; Justen, E.B.; Lane, J.C.; Hudson, J.E.; Chen, N.; Davis, C.D.; Sanberg, P.R. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: Distribution, migration, and differentiation. J. Hematotherapy Stem Cell Res. 2003, 12, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Garbuzova-Davis, S.; Sanberg, C.D.; Kuzmin-Nichols, N.; Willing, A.E.; Gemma, C.; Bickford, P.C.; Miller, C.; Rossi, R.; Sanberg, P.R. Human umbilical cord blood treatment in a mouse model of ALS: Optimization of cell dose. PLoS ONE 2008, 3, e2494. [Google Scholar] [CrossRef]
- López-González, R.; Kunckles, P.; Velasco, I. Transient recovery in a rat model of familial amyotrophic lateral sclerosis after transplantation of motor neurons derived from mouse embryonic stem cells. Cell Transplant. 2009, 18, 1171–1181. [Google Scholar] [CrossRef]
- Cabanes, C.; Bonilla, S.; Tabares, L.; Martínez, S. Neuroprotective effect of adult hematopoietic stem cells in a mouse model of motoneuron degeneration. Neurobiol. Dis. 2007, 26, 408–418. [Google Scholar] [CrossRef]
- Lepore, A.C.; Rauck, B.; Dejea, C.; Pardo, A.C.; Rao, M.S.; Rothstein, J.D.; Maragakis, N.J. Focal transplantation–based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat. Neurosci. 2008, 11, 1294–1301. [Google Scholar] [CrossRef]
- Ohnishi, S.; Ito, H.; Suzuki, Y.; Adachi, Y.; Wate, R.; Zhang, J.; Nakano, S.; Kusaka, H.; Ikehara, S. Intra-bone marrow-bone marrow transplantation slows disease progression and prolongs survival in G93A mutant SOD1 transgenic mice, an animal model mouse for amyotrophic lateral sclerosis. Brain Res. 2009, 1296, 216–224. [Google Scholar] [CrossRef]
- Corti, S.; Nizzardo, M.; Nardini, M.; Donadoni, C.; Salani, S.; Simone, C.; Falcone, M.; Riboldi, G.; Govoni, A.; Bresolin, N. Systemic transplantation of c-kit+ cells exerts a therapeutic effect in a model of amyotrophic lateral sclerosis. Hum. Mol. Genet. 2010, 19, 3782–3796. [Google Scholar] [CrossRef]
- Martin, L.J.; Liu, Z. Adult olfactory bulb neural precursor cell grafts provide temporary protection from motor neuron degeneration, improve motor function, and extend survival in amyotrophic lateral sclerosis mice. J. Neuropathol. Exp. Neurol. 2007, 66, 1002–1018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suzuki, M.; McHugh, J.; Tork, C.; Shelley, B.; Hayes, A.; Bellantuono, I.; Aebischer, P.; Svendsen, C.N. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol. Ther. 2008, 16, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Krakora, D.; Mulcrone, P.; Meyer, M.; Lewis, C.; Bernau, K.; Gowing, G.; Zimprich, C.; Aebischer, P.; Svendsen, C.N.; Suzuki, M. Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Mol. Ther. 2013, 21, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Marconi, S.; Bonaconsa, M.; Scambi, I.; Squintani, G.; Rui, W.; Turano, E.; Ungaro, D.; D’agostino, S.; Barbieri, F.; Angiari, S. Systemic treatment with adipose-derived mesenchymal stem cells ameliorates clinical and pathological features in the amyotrophic lateral sclerosis murine model. Neuroscience 2013, 248, 333–343. [Google Scholar] [CrossRef]
- Ciervo, Y.; Ning, K.; Jun, X.; Shaw, P.J.; Mead, R.J. Advances, challenges and future directions for stem cell therapy in amyotrophic lateral sclerosis. Mol. Neurodegener. 2017, 12, 1–22. [Google Scholar] [CrossRef]
- Corti, S.; Locatelli, F.; Papadimitriou, D.; Del Bo, R.; Nizzardo, M.; Nardini, M.; Donadoni, C.; Salani, S.; Fortunato, F.; Strazzer, S. Neural stem cells LewisX+ CXCR4+ modify disease progression in an amyotrophic lateral sclerosis model. Brain 2007, 130, 1289–1305. [Google Scholar] [CrossRef]
- Klein, S.M.; Behrstock, S.; McHugh, J.; Hoffmann, K.; Wallace, K.; Suzuki, M.; Aebischer, P.; Svendsen, C.N. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum. Gene Ther. 2005, 16, 509–521. [Google Scholar] [CrossRef]
- Hwang, D.; Lee, H.; Park, I.; Seok, J.; Kim, B.; Joo, I.; Kim, S. Intrathecal transplantation of human neural stem cells overexpressing VEGF provide behavioral improvement, disease onset delay and survival extension in transgenic ALS mice. Gene Ther. 2009, 16, 1234–1244. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.-T.; Yun, S.; Kim, I.-S.; Lee, J.; Lee, I.-S.; Park, K.I. Growth factor-expressing human neural progenitor cell grafts protect motor neurons but do not ameliorate motor performance and survival in ALS mice. Exp. Mol. Med. 2009, 41, 487–500. [Google Scholar] [CrossRef]
- Robinton, D.A.; Daley, G.Q. The promise of induced pluripotent stem cells in research and therapy. Nature 2012, 481, 295–305. [Google Scholar] [CrossRef]
- Sareen, D.; Gowing, G.; Sahabian, A.; Staggenborg, K.; Paradis, R.; Avalos, P.; Latter, J.; Ornelas, L.; Garcia, L.; Svendsen, C.N. Human induced pluripotent stem cells are a novel source of neural progenitor cells (iNPCs) that migrate and integrate in the rodent spinal cord. J. Comp. Neurol. 2014, 522, 2707–2728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, C.; Teng, J.J.; Zhao, R.L.; Song, Y.Q.; Zhang, C. Multiple administrations of human marrow stromal cells through cerebrospinal fluid prolong survival in a transgenic mouse model of amyotrophic lateral sclerosis. Cytotherapy 2009, 11, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, H.; Zhang, J.; Zhang, F.; Liu, Y.; Xi, H.; Wang, H.; Gu, Z.; Song, Y.; Li, Y.; et al. Short-term outcome of olfactory ensheathing cells transplantation for treatment of amyotrophic lateral sclerosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2007, 21, 961–966. [Google Scholar]
- Frischer, J.M.; Bramow, S.; Dal-Bianco, A.; Lucchinetti, C.F.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Lassmann, H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009, 132, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Reingold, S.; Steiner, J.; Polman, C.; Cohen, J.; Freedman, M.; Kappos, L.; Thompson, A.; Wolinsky, J. The challenge of follow-on biologics for treatment of multiple sclerosis. Neurology 2009, 73, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Carter, J.L. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clin. Proc. 2014, 89, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Fassas, A.; Anagnostopoulos, A.; Kazis, A.; Kapinas, K.; Sakellari, I.; Kimiskidis, V.; Tsompanakou, A. Peripheral blood stem cell transplantation in the treatment of progressive multiple sclerosis: First results of a pilot study. Bone Marrow Transplant. 1997, 20, 631–638. [Google Scholar] [CrossRef]
- Snowden, J.; Patton, W.; O’Donnell, J.; Hannah, E.; Hart, D. Prolonged remission of longstanding systemic lupus erythematosus after autologous bone marrow transplant for non-Hodgkin’s lymphoma. Bone Marrow Transplant. 1997, 19, 1247–1250. [Google Scholar] [CrossRef]
- Van Gelder, M.; Van Bekkum, D. Treatment of relapsing experimental autoimmune encephalomyelitis in rats with allogeneic bone marrow transplantation from a resistant strain. Bone Marrow Transplant. 1995, 16, 343–351. [Google Scholar]
- Van Gelder, M.; Van Bekkum, D. Effective treatment of relapsing experimental autoimmune encephalomyelitis with pseudoautologous bone marrow transplantation. Bone Marrow Transplant. 1996, 18, 1029–1034. [Google Scholar]
- Muraro, P.A.; Robins, H.; Malhotra, S.; Howell, M.; Phippard, D.; Desmarais, C.; Sousa, A.d.P.A.; Griffith, L.M.; Lim, N.; Nash, R.A. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J. Clin. Investig. 2014, 124, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Saccardi, R.; Kozak, T.; Bocelli-Tyndall, C.; Fassas, A.; Kazis, A.; Havrdova, E.; Carreras, E.; Saiz, A.; Löwenberg, B.t.; te Boekhorst, P.A. Autologous stem cell transplantation for progressive multiple sclerosis: Update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult. Scler. J. 2006, 12, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.D.; Kraft, G.H.; Wundes, A.; Guan, Q.; Maravilla, K.R.; Gooley, T.A.; McSweeney, P.A.; Pavletic, S.Z.; Openshaw, H.; Storb, R. Autologous hematopoietic cell transplantation following high-dose immunosuppressive therapy for advanced multiple sclerosis: Long-term results. Bone Marrow Transplant. 2012, 47, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Mancardi, G.; Sormani, M.; Di Gioia, M.; Vuolo, L.; Gualandi, F.; Amato, M.; Capello, E.; Currò, D.; Uccelli, A.; Bertolotto, A. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: The Italian multi-centre experience. Mult. Scler. J. 2012, 18, 835–842. [Google Scholar] [CrossRef]
- Burt, R.K.; Shah, S.J.; Dill, K.; Grant, T.; Gheorghiade, M.; Schroeder, J.; Craig, R.; Hirano, I.; Marshall, K.; Ruderman, E. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): An open-label, randomised phase 2 trial. Lancet 2011, 378, 498–506. [Google Scholar] [CrossRef]
- Krasulová, E.; Trněný, M.; Kozák, T.; Vacková, B.; Pohlreich, D.; Kemlink, D.; Kobylka, P.; Kovářová, I.; Lhotáková, P.; Havrdová, E. High-dose immunoablation with autologous haematopoietic stem cell transplantation in aggressive multiple sclerosis: A single centre 10-year experience. Mult. Scler. J. 2010, 16, 685–693. [Google Scholar] [CrossRef]
- Fassas, A.; Kimiskidis, V.; Sakellari, I.; Kapinas, K.; Anagnostopoulos, A.; Tsimourtou, V.; Sotirakoglou, K.; Kazis, A. Long-term results of stem cell transplantation for MS: A single-center experience. Neurology 2011, 76, 1066–1070. [Google Scholar] [CrossRef]
- Nash, R.A.; Hutton, G.J.; Racke, M.K.; Popat, U.; Devine, S.M.; Steinmiller, K.C.; Griffith, L.M.; Muraro, P.A.; Openshaw, H.; Sayre, P.H. High-dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS. Neurology 2017, 88, 842–852. [Google Scholar] [CrossRef]
- Uccelli, A.; Laroni, A.; Freedman, M.S. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011, 10, 649–656. [Google Scholar] [CrossRef]
- Rice, C.M.; Kemp, K.; Wilkins, A.; Scolding, N.J. Cell therapy for multiple sclerosis: An evolving concept with implications for other neurodegenerative diseases. Lancet 2013, 382, 1204–1213. [Google Scholar] [CrossRef]
- Wilkins, A.; Kemp, K.; Ginty, M.; Hares, K.; Mallam, E.; Scolding, N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009, 3, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lennon, D.P.; Eaton, V.; Maier, K.; Caplan, A.I.; Miller, S.D.; Miller, R.H. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 2009, 57, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Zappia, E.; Casazza, S.; Pedemonte, E.; Benvenuto, F.; Bonanni, I.; Gerdoni, E.; Giunti, D.; Ceravolo, A.; Cazzanti, F.; Frassoni, F. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005, 106, 1755–1761. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Chen, J.; Cui, Y.; Lu, M.; Elias, S.B.; Mitchell, J.B.; Hammill, L.; Vanguri, P.; Chopp, M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp. Neurol. 2005, 195, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Gerdoni, E.; Gallo, B.; Casazza, S.; Musio, S.; Bonanni, I.; Pedemonte, E.; Mantegazza, R.; Frassoni, F.; Mancardi, G.; Pedotti, R. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child. Neurol. Soc. 2007, 61, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.; Hares, K.; Mallam, E.; Heesom, K.J.; Scolding, N.; Wilkins, A. Mesenchymal stem cell-secreted superoxide dismutase promotes cerebellar neuronal survival. J. Neurochem. 2010, 114, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Pavlovska, G.; Glover, C.P.; Uney, J.B.; Wraith, D.; Scolding, N.J. Human mesenchymal stem cells abrogate experimental allergic encephalomyelitis after intraperitoneal injection, and with sparse CNS infiltration. Neurosci. Lett. 2008, 448, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Kassis, I.; Grigoriadis, N.; Gowda-Kurkalli, B.; Mizrachi-Kol, R.; Ben-Hur, T.; Slavin, S.; Abramsky, O.; Karussis, D. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch. Neurol. 2008, 65, 753–761. [Google Scholar] [CrossRef]
- Rivera, F.J.; Couillard-Despres, S.; Pedre, X.; Ploetz, S.; Caioni, M.; Lois, C.; Bogdahn, U.; Aigner, L. Mesenchymal stem cells instruct oligodendrogenic fate decision on adult neural stem cells. Stem Cells 2006, 24, 2209–2219. [Google Scholar] [CrossRef]
- Picard-Riera, N.; Decker, L.; Delarasse, C.; Goude, K.; Nait-Oumesmar, B.; Liblau, R.; Pham-Dinh, D.; Baron-Van Evercooren, A. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc. Natl. Acad. Sci. USA 2002, 99, 13211–13216. [Google Scholar] [CrossRef]
- Ben-Hur, T.; Einstein, O.; Mizrachi-Kol, R.; Ben-Menachem, O.; Reinhartz, E.; Karussis, D.; Abramsky, O. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia 2003, 41, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Izrael, M.; Zhang, P.; Kaufman, R.; Shinder, V.; Ella, R.; Amit, M.; Itskovitz-Eldor, J.; Chebath, J.; Revel, M. Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol. Cell. Neurosci. 2007, 34, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Aharonowiz, M.; Einstein, O.; Fainstein, N.; Lassmann, H.; Reubinoff, B.; Ben-Hur, T. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS ONE 2008, 3, e3145. [Google Scholar] [CrossRef] [PubMed]
- Laterza, C.; Merlini, A.; De Feo, D.; Ruffini, F.; Menon, R.; Onorati, M.; Fredrickx, E.; Muzio, L.; Lombardo, A.; Comi, G. iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nat. Commun. 2013, 4, 1–16. [Google Scholar] [CrossRef]
- Okano, H.; Nakamura, M.; Yoshida, K.; Okada, Y.; Tsuji, O.; Nori, S.; Ikeda, E.; Yamanaka, S.; Miura, K. Steps toward safe cell therapy using induced pluripotent stem cells. Circ. Res. 2013, 112, 523–533. [Google Scholar] [CrossRef]
- Das, J.; Sharrack, B.; Snowden, J.A. Autologous Haematopoietic Stem Cell Transplantation in Multiple Sclerosis: A Review of Current Literature and Future Directions for Transplant Haematologists and Oncologists. Curr Hematol Malig Rep. 2019, 14, 127–135. [Google Scholar] [CrossRef]
- Casanova, B.; Jarque, I.; Gascón, F.; Hernández-Boluda, J.C.; Pérez-Miralles, F.; de la Rubia, J.; Alcalá, C.; Sanz, J.; Mallada, J.; Cervelló, A. Autologous hematopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: Comparison with secondary progressive multiple sclerosis. Neurol. Sci. 2017, 38, 1213–1221. [Google Scholar] [CrossRef]
- Fassas, A.; Passweg, J.; Anagnostopoulos, A.; Kazis, A.; Kozak, T.; Havrdova, E.; Carreras, E.; Graus, F.; Kashyap, A.; Openshaw, H. Hematopoietic stem cell transplantation for multiple sclerosis. J. Neurol. 2002, 249, 1088–1097. [Google Scholar] [CrossRef]
- Burman, J.; Iacobaeus, E.; Svenningsson, A.; Lycke, J.; Gunnarsson, M.; Nilsson, P.; Vrethem, M.; Fredrikson, S.; Martin, C.; Sandstedt, A.; et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: The Swedish experience. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1116–1121. [Google Scholar] [CrossRef]
- Rastegar, F.; Shenaq, D.; Huang, J.; Zhang, W.; Zhang, B.Q.; He, B.C.; Chen, L.; Zuo, G.W.; Luo, Q.; Shi, Q.; et al. Mesenchymal stem cells: Molecular characteristics and clinical applications. World J. Stem Cells 2010, 2, 67–80. [Google Scholar] [CrossRef]
- Snowden, J.A.; Saccardi, R.; Allez, M.; Ardizzone, S.; Arnold, R.; Cervera, R.; Denton, C.; Hawkey, C.; Labopin, M.; Mancardi, G.; et al. Haematopoietic SCT in severe autoimmune diseases: Updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2012, 47, 770–790. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.A.; Sharrack, B.; Akil, M.; Kiely, D.G.; Lobo, A.; Kazmi, M.; Muraro, P.A.; Lindsay, J.O. Autologous haematopoietic stem cell transplantation (aHSCT) for severe resistant autoimmune and inflammatory diseases –A guide for the generalist. Clin. Med. 2018, 18, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Sadr, S.S.; Javanbakht, J.; Javidan, A.N.; Ghaffarpour, M.; Khamse, S.; Naghshband, Z. Descriptive epidemiology: Prevalence, incidence, sociodemographic factors, socioeconomic domains, and quality of life of epilepsy: An update and systematic review. Arch. Med. Sci. AMS 2018, 14, 717. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Zenteno, J.F.; Hernández-Ronquillo, L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res. Treat. 2012, 2012, 630853. [Google Scholar] [CrossRef]
- Maglóczky, Z. Sprouting in human temporal lobe epilepsy: Excitatory pathways and axons of interneurons. Epilepsy Res. 2010, 89, 52–59. [Google Scholar] [CrossRef]
- Morimoto, K.; Fahnestock, M.; Racine, R.J. Kindling and status epilepticus models of epilepsy: Rewiring the brain. Prog. Neurobiol. 2004, 73, 1–60. [Google Scholar] [CrossRef]
- Kalilani, L.; Sun, X.; Pelgrims, B.; Noack-Rink, M.; Villanueva, V. The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia 2018, 59, 2179–2193. [Google Scholar] [CrossRef]
- Rüschenschmidt, C.; Koch, P.G.; Brüstle, O.; Beck, H. Functional Properties of ES Cell–Derived Neurons Engrafted into the Hippocampus of Adult Normal and Chronically Epileptic Rats. Epilepsia 2005, 46, 174–183. [Google Scholar] [CrossRef]
- Carpentino, J.E.; Hartman, N.W.; Grabel, L.B.; Naegele, J.R. Region-specific differentiation of embryonic stem cell-derived neural progenitor transplants into the adult mouse hippocampus following seizures. J. Neurosci. Res. 2008, 86, 512–524. [Google Scholar] [CrossRef]
- Roper, S.N.; Steindler, D.A. Stem cells as a potential therapy for epilepsy. Exp. Neurol. 2013, 244, 59–66. [Google Scholar] [CrossRef]
- Hattiangady, B.; Rao, M.S.; Shetty, A.K. Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy. Exp. Neurol. 2008, 212, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Baraban, S.C.; Southwell, D.G.; Estrada, R.C.; Jones, D.L.; Sebe, J.Y.; Alfaro-Cervello, C.; García-Verdugo, J.M.; Rubenstein, J.L.; Alvarez-Buylla, A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1. 1 mutant mice. Proc. Natl. Acad. Sci. USA 2009, 106, 15472–15477. [Google Scholar] [CrossRef] [PubMed]
- Marín, O. Human cortical interneurons take their time. Cell Stem Cell 2013, 12, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Dolado, M.; Calcagnotto, M.E.; Karkar, K.M.; Southwell, D.G.; Jones-Davis, D.M.; Estrada, R.C.; Rubenstein, J.L.; Alvarez-Buylla, A.; Baraban, S.C. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J. Neurosci. 2006, 26, 7380–7389. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.F.; Girskis, K.M.; Rubenstein, J.L.; Alvarez-Buylla, A.; Baraban, S.C. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat. Neurosci. 2013, 16, 692–697. [Google Scholar] [CrossRef]
- Chu, K.; Kim, M.; Jung, K.-H.; Jeon, D.; Lee, S.-T.; Kim, J.; Jeong, S.-W.; Kim, S.U.; Lee, S.K.; Shin, H.-S. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004, 1023, 213–221. [Google Scholar] [CrossRef]
- Shetty, A.K. Progress in cell grafting therapy for temporal lobe epilepsy. Neurotherapeutics 2011, 8, 721–735. [Google Scholar] [CrossRef]
- Costa-Ferro, Z.S.; Vitola, A.S.; Pedroso, M.F.; Cunha, F.B.; Xavier, L.L.; Machado, D.C.; Soares, M.B.; Ribeiro-dos-Santos, R. Prevention of seizures and reorganization of hippocampal functions by transplantation of bone marrow cells in the acute phase of experimental epilepsy. Seizure 2010, 19, 84–92. [Google Scholar] [CrossRef]
- Jeon, D.; Chu, K.; Lee, S.T.; Jung, K.H.; Kang, K.M.; Ban, J.J.; Kim, S.; Seo, J.S.; Won, C.H.; Kim, M. A cell-free extract from human adipose stem cells protects mice against epilepsy. Epilepsia 2011, 52, 1617–1626. [Google Scholar] [CrossRef]
- Salem, N.A.; El-Shamarka, M.; Khadrawy, Y.; El-Shebiney, S. New prospects of mesenchymal stem cells for ameliorating temporal lobe epilepsy. Inflammopharmacology 2018, 26, 963–972. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Pan, X.; Zhang, Y.; Lin, L.; Wu, Y.; Huang, Y.; He, H. Adipose-derived stem cell transplantation improves learning and memory via releasing neurotrophins in rat model of temporal lobe epilepsy. Brain Res. 2021, 1750, 147121. [Google Scholar] [CrossRef] [PubMed]
- Yousof, S.M.; ElSayed, D.A.; El-Baz, A.A.; Sallam, H.S.; Abbas, F. Combined Treatment of Adipose Derived-Mesenchymal Stem Cells and Pregabalin Is Superior to Monotherapy for the Treatment of Neuropathic Pain in Rats. Stem Cells Int. 2021, 2021, 8847110. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Cruccu, G.; Baron, R.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; Nurmikko, T.; European Federation of Neurological, S. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J. Neurol 2010, 17, 1113-e88. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N. Pharmacotherapy of neuropathic pain: Time to rewrite the rulebook? Pain Manag. 2016, 6, 1–3. [Google Scholar] [CrossRef]
- Antonucci, I.; Stuppia, L.; Kaneko, Y.; Yu, S.; Tajiri, N.; Bae, E.C.; Chheda, S.H.; Weinbren, N.L.; Borlongan, C.V. Amniotic fluid as a rich source of mesenchymal stromal cells for transplantation therapy. Cell Transplant. 2011, 20, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, F.; Amin, B.; Ghorbani, A.; Ghazavi, H.; Ghasemi, F.; Sadri, K.; Mehri, S.; Sadeghnia, H.; Hosseinzadeh, H. New approach for the treatment of neuropathic pain: Fibroblast growth factor 1 gene-transfected adipose-derived mesenchymal stem cells. Eur. J. Pain 2018, 22, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, W.; Liu, X.; Xi, Y.; Yu, J.; Yang, X.; Ye, X. Bone mesenchymal stem cells attenuate radicular pain by inhibiting microglial activation in a rat noncompressive disk herniation model. Cell Tissue Res. 2018, 374, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Wu, S.H.; Lee, S.S.; Lin, Y.N.; Kuo, Y.R.; Chai, C.Y.; Huang, S.H. Autologous Adipose-Derived Stem Cells Reduce Burn-Induced Neuropathic Pain in a Rat Model. Int J. Mol. Sci 2017, 19, 34. [Google Scholar] [CrossRef]
- Tuder, R.M.; Weinberg, A.; Panajotopoulos, N.; Kalil, J. Cytomegalovirus infection amplifies class I major histocompatibility complex expression on cultured human endothelial cells. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 1994, 13, 129–138. [Google Scholar]
- Chakravarthy, K.; Chen, Y.; He, C.; Christo, P.J. Stem cell therapy for chronic pain management: Review of uses, advances, and adverse effects. Pain Physician 2017, 20, 293–305. [Google Scholar] [CrossRef]
- Huh, Y.; Ji, R.-R.; Chen, G. Neuroinflammation, bone marrow stem cells, and chronic pain. Front. Immunol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.L.; Zhu, Z.H.; Wang, Y.Z. Neural stem cell transplantation therapy for brain ischemic stroke: Review and perspectives. World J. Stem Cells 2019, 11, 817–830. [Google Scholar] [CrossRef]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar] [CrossRef]
- Vu, Q.; Xie, K.; Eckert, M.; Zhao, W.; Cramer, S.C. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology 2014, 82, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.S.; Sena, E.S.; Egan, K.J.; Antonic, A.; Koblar, S.A.; Howells, D.W.; MacLeod, M.R. Stem cell-based therapy for experimental stroke: A systematic review and meta-analysis. Int. J. Stroke 2012, 7, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, G.; Gu, Y.; Guo, X. Meta-Analysis and Systematic Review of Neural Stem Cells therapy for experimental ischemia stroke in preclinical studies. Sci. Rep. 2016, 6, 32291. [Google Scholar] [CrossRef] [PubMed]
- Samper Agrelo, I.; Schira-Heinen, J.; Beyer, F.; Groh, J.; Bütermann, C.; Estrada, V.; Poschmann, G.; Bribian, A.; Jadasz, J.J.; Lopez-Mascaraque, L. Secretome analysis of mesenchymal stem cell factors fostering oligodendroglial differentiation of neural stem cells in vivo. Int. J. Mol. Sci. 2020, 21, 4350. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.; Makaroff, L. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Jha, K.A.; Pentecost, M.; Lenin, R.; Gentry, J.; Klaic, L.; Del Mar, N.; Reiner, A.; Yang, C.H.; Pfeffer, L.M.; Sohl, N. TSG-6 in conditioned media from adipose mesenchymal stem cells protects against visual deficits in mild traumatic brain injury model through neurovascular modulation. Stem Cell Res. Ther. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Donders, R.; Bogie, J.F.; Ravanidis, S.; Gervois, P.; Vanheusden, M.; Maree, R.; Schrynemackers, M.; Smeets, H.J.; Pinxteren, J.; Gijbels, K. Human Wharton’s jelly-derived stem cells display a distinct immunomodulatory and proregenerative transcriptional signature compared to bone marrow-derived stem cells. Stem Cells Dev. 2018, 27, 65–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Su, Y.; Su, L.; Lan, X.; Wu, D.; Han, S.; Li, J.; Kvederis, L.; Corey, S. Hypoxia conditioning enhances neuroprotective effects of aged human bone marrow mesenchymal stem cell-derived conditioned medium against cerebral ischemia in vitro. Brain Res. 2019, 1725, 146432. [Google Scholar] [CrossRef]
- Hu, N.-W.; Corbett, G.T.; Moore, S.; Klyubin, I.; O’Malley, T.T.; Walsh, D.M.; Livesey, F.J.; Rowan, M.J. Extracellular forms of Aβ and tau from iPSC models of Alzheimer’s disease disrupt synaptic plasticity. Cell Rep. 2018, 23, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Mokarizadeh, A.; Delirezh, N.; Morshedi, A.; Mosayebi, G.; Farshid, A.-A.; Mardani, K. Microvesicles derived from mesenchymal stem cells: Potent organelles for induction of tolerogenic signaling. Immunol. Lett. 2012, 147, 47–54. [Google Scholar] [CrossRef] [PubMed]
- De Godoy, M.A.; Saraiva, L.M.; de Carvalho, L.R.; Vasconcelos-dos-Santos, A.; Beiral, H.J.; Ramos, A.B.; de Paula Silva, L.R.; Leal, R.B.; Monteiro, V.H.; Braga, C.V. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. J. Biol. Chem. 2018, 293, 1957–1975. [Google Scholar] [CrossRef]
- Raziyeva, K.; Smagulova, A.; Kim, Y.; Smagul, S.; Nurkesh, A.; Saparov, A. Preconditioned and genetically modified stem cells for myocardial infarction treatment. Int. J. Mol. Sci. 2020, 21, 7301. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef] [PubMed]
- Wakai, T.; Narasimhan, P.; Sakata, H.; Wang, E.; Yoshioka, H.; Kinouchi, H.; Chan, P.H. Hypoxic preconditioning enhances neural stem cell transplantation therapy after intracerebral hemorrhage in mice. J. Cereb Blood Flow Metab 2016, 36, 2134–2145. [Google Scholar] [CrossRef]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.-A.; Wei, L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [CrossRef]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef]
- Zhu, L.-L.; Wu, L.-Y.; Yew, D.T.; Fan, M. Effects of hypoxia on the proliferation and differentiation of NSCs. Mol. Neurobiol. 2005, 31, 231–242. [Google Scholar] [CrossRef]
- Othman, F.A.; Tan, S.C. Preconditioning strategies to enhance neural stem cell-based therapy for ischemic stroke. Brain Sci. 2020, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zhang, J.; Chen, X.; Wan, J.; Wang, J.; Zhang, P.; Liu, Y. Hypoxia stimulates neural stem cell proliferation by increasing HIF-1α expression and activating Wnt/β-catenin signaling. Cell. Mol. Biol. 2017, 63, 12. [Google Scholar] [CrossRef]
- Lisy, K.; Peet, D. Turn me on: Regulating HIF transcriptional activity. Cell Death Differ. 2008, 15, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Shibasaki, F. Hypoxia-Inducible Factor as an Angiogenic Master Switch. Front. Pediatrics 2015, 3, 33. [Google Scholar] [CrossRef]
- Abati, E.; Bresolin, N.; Comi, G.P.; Corti, S. Preconditioning and cellular engineering to increase the survival of transplanted neural stem cells for motor neuron disease therapy. Mol. Neurobiol. 2019, 56, 3356–3367. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.-J.; Lee, N.; Choi, C.; Jeon, I.; Oh, S.-H.; Shin, D.A.; Hwang, T.-S.; Lee, H.J.; Kim, S.U.; Moon, H. Therapeutic effect of BDNF-overexpressing human neural stem cells (HB1. F3. BDNF) in a rodent model of middle cerebral artery occlusion. Cell Transplant. 2013, 22, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-h.; Wang, R.-z.; Li, G.-l.; Wei, J.-j.; Li, Z.-j.; Feng, M.; Kang, J.; Du, W.-c.; Ma, W.-b.; Li, Y.-n. Transplantation of neural stem cells modified by human neurotrophin-3 promotes functional recovery after transient focal cerebral ischemia in rats. Neurosci. Lett. 2008, 444, 227–230. [Google Scholar] [CrossRef]
- Chen, B.; Gao, X.-Q.; Yang, C.-X.; Tan, S.-K.; Sun, Z.-L.; Yan, N.-H.; Pang, Y.-G.; Yuan, M.; Chen, G.-J.; Xu, G.-T. Neuroprotective effect of grafting GDNF gene-modified neural stem cells on cerebral ischemia in rats. Brain Res. 2009, 1284, 1–11. [Google Scholar] [CrossRef]
- Bernstock, J.D.; Peruzzotti-Jametti, L.; Ye, D.; Gessler, F.A.; Maric, D.; Vicario, N.; Lee, Y.-J.; Pluchino, S.; Hallenbeck, J.M. Neural stem cell transplantation in ischemic stroke: A role for preconditioning and cellular engineering. J. Cereb. Blood Flow Metab. 2017, 37, 2314–2319. [Google Scholar] [CrossRef]
- Thomsen, G.M.; Avalos, P.; Ma, A.A.; Alkaslasi, M.; Cho, N.; Wyss, L.; Vit, J.-P.; Godoy, M.; Suezaki, P.; Shelest, O. Transplantation of neural progenitor cells expressing glial cell line-derived neurotrophic factor into the motor cortex as a strategy to treat amyotrophic lateral sclerosis. Stem Cells 2018, 36, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, M.; Rezanejadbardaji, H.; Irfan-Maqsood, M.; Mokhtari, M.J.; Naderi-Meshkin, H. Stem cell therapy for neurodegenerative diseases: Strategies for regeneration against degeneration. Cell Ther. Regen. Med. J. 2016, 1, 3. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).