A Balance between Transmembrane-Mediated ER/Golgi Retention and Forward Trafficking Signals in Glycophorin-Anion Exchanger-1 Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Red Blood Cell Samples and Cultured Cells
2.2. Cloning of Glycophorin Transcripts
2.3. Transfection
2.4. Flow Cytometry and Monoclonal Antibodies (mAb)
2.5. Confocal Microscopy
3. Results
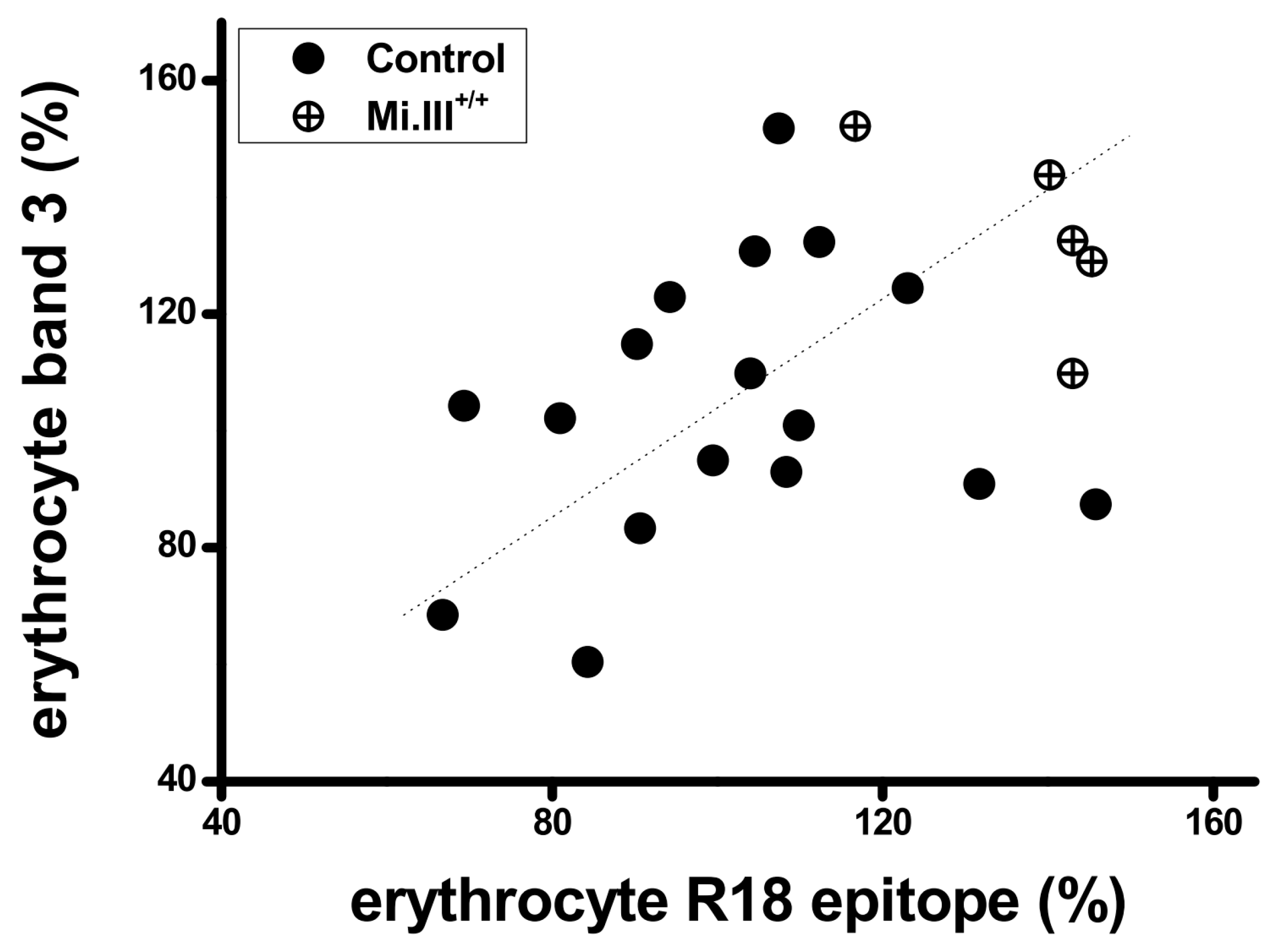
3.1. The Expression Levels of AE1 and the Glycophorin R18 Epitope were Quantitatively Directly Correlated on the Human Erythrocyte Membrane
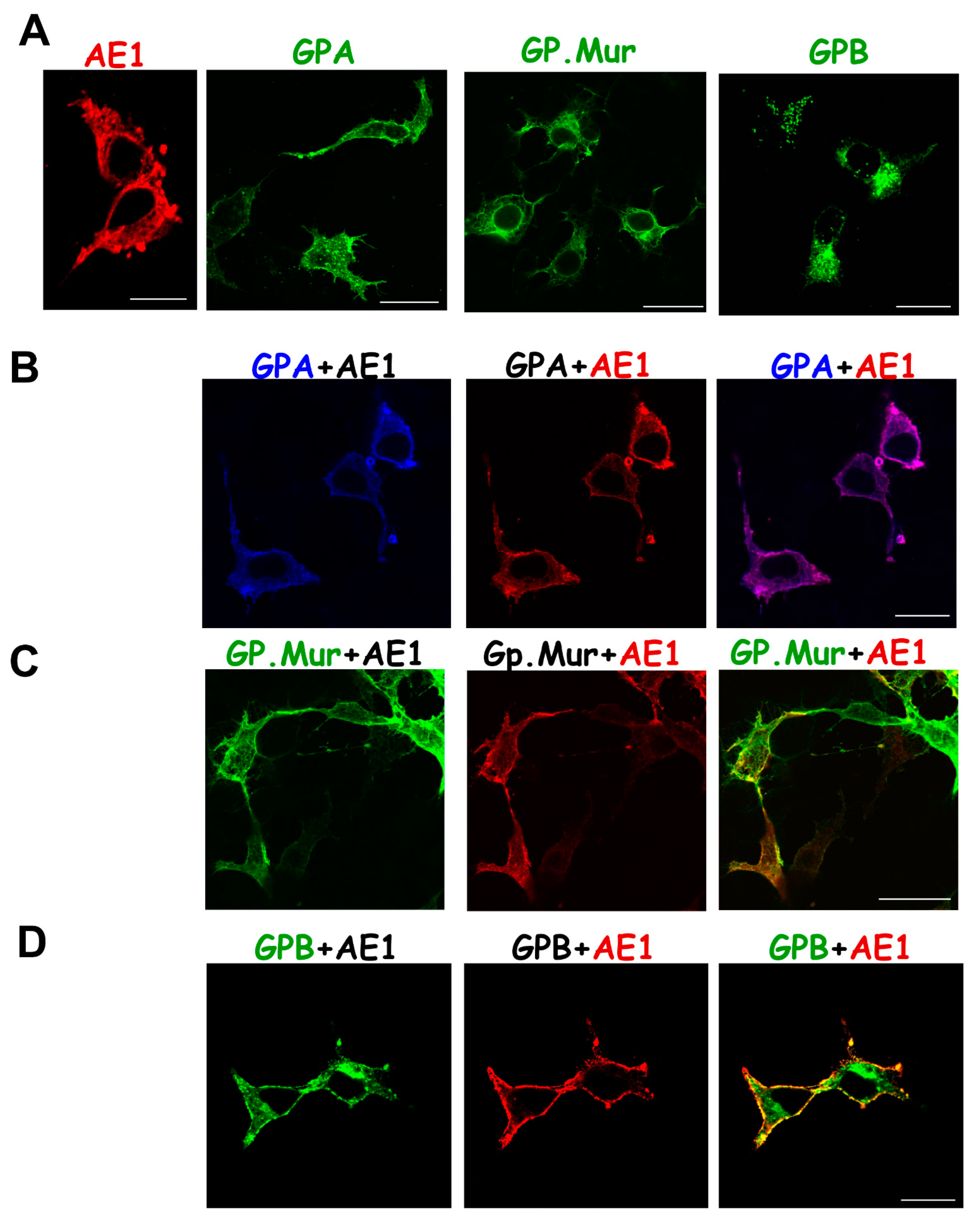
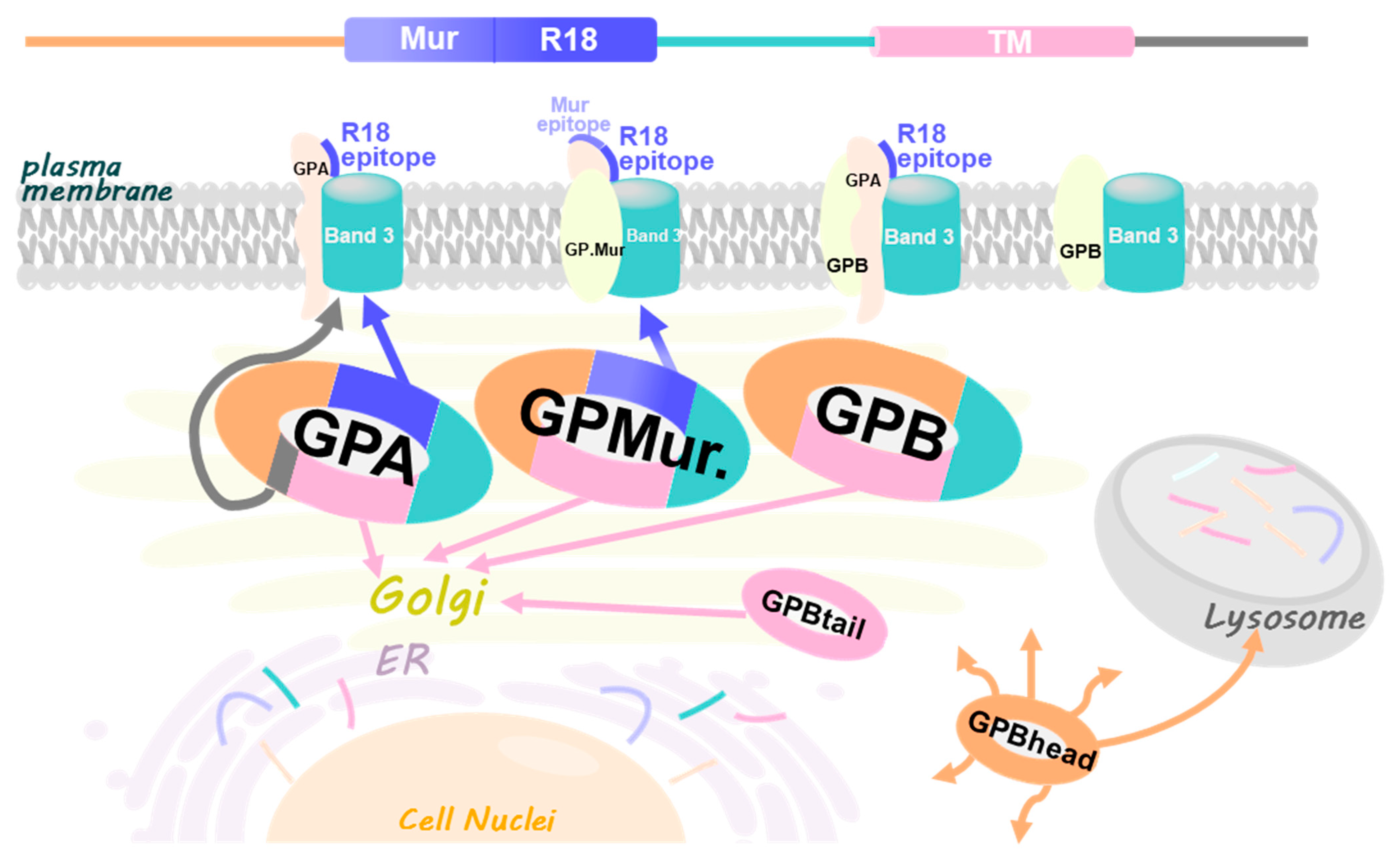
3.2. Differential Subcellular Localization Patterns between AE1 and GPA/GPB/GP.Mur
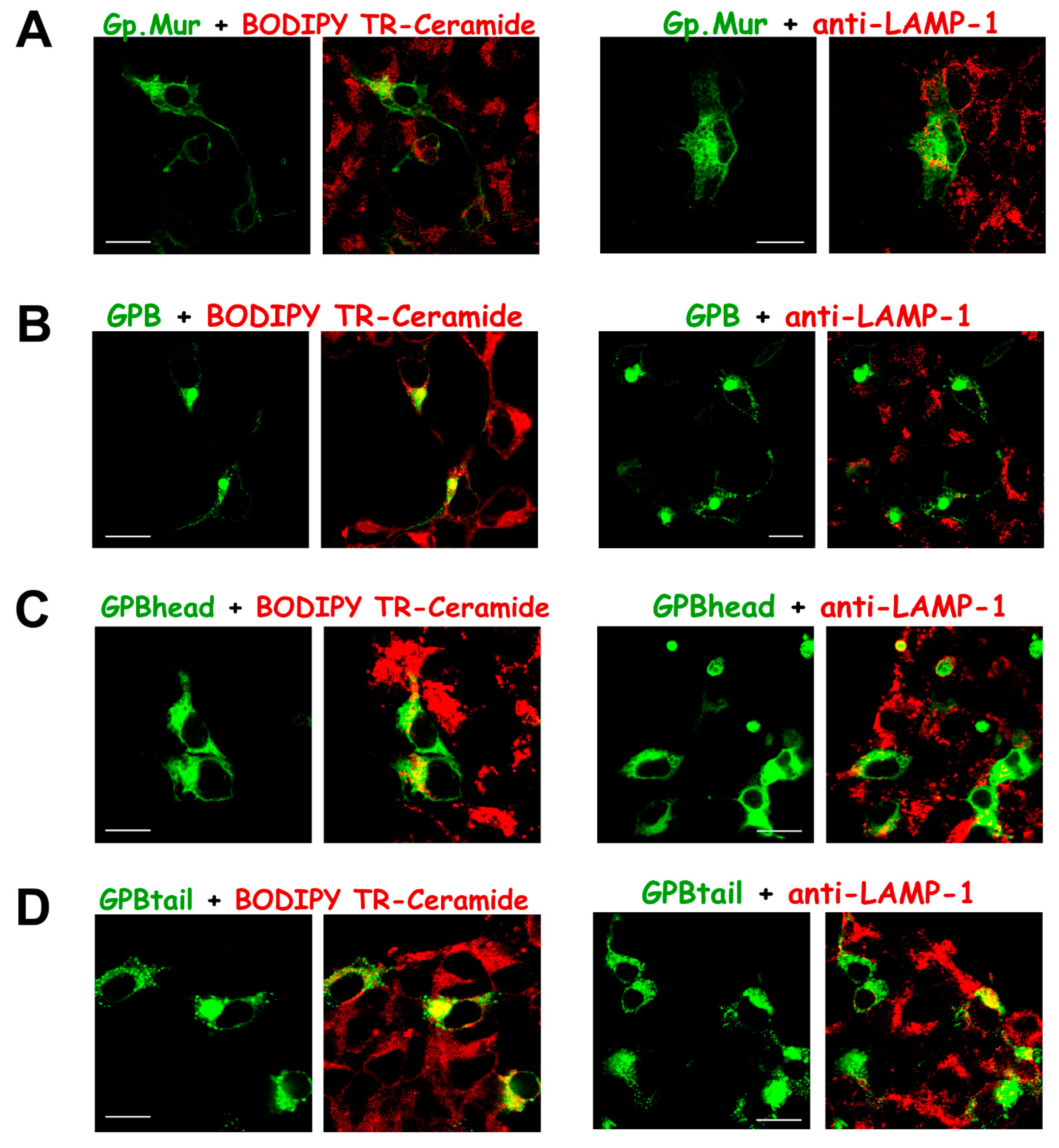
3.3. Substantial Localization of GPB in the ER and Golgi Apparatus Could Be Redirected to the Plasma Membrane upon AE1 Coexpression
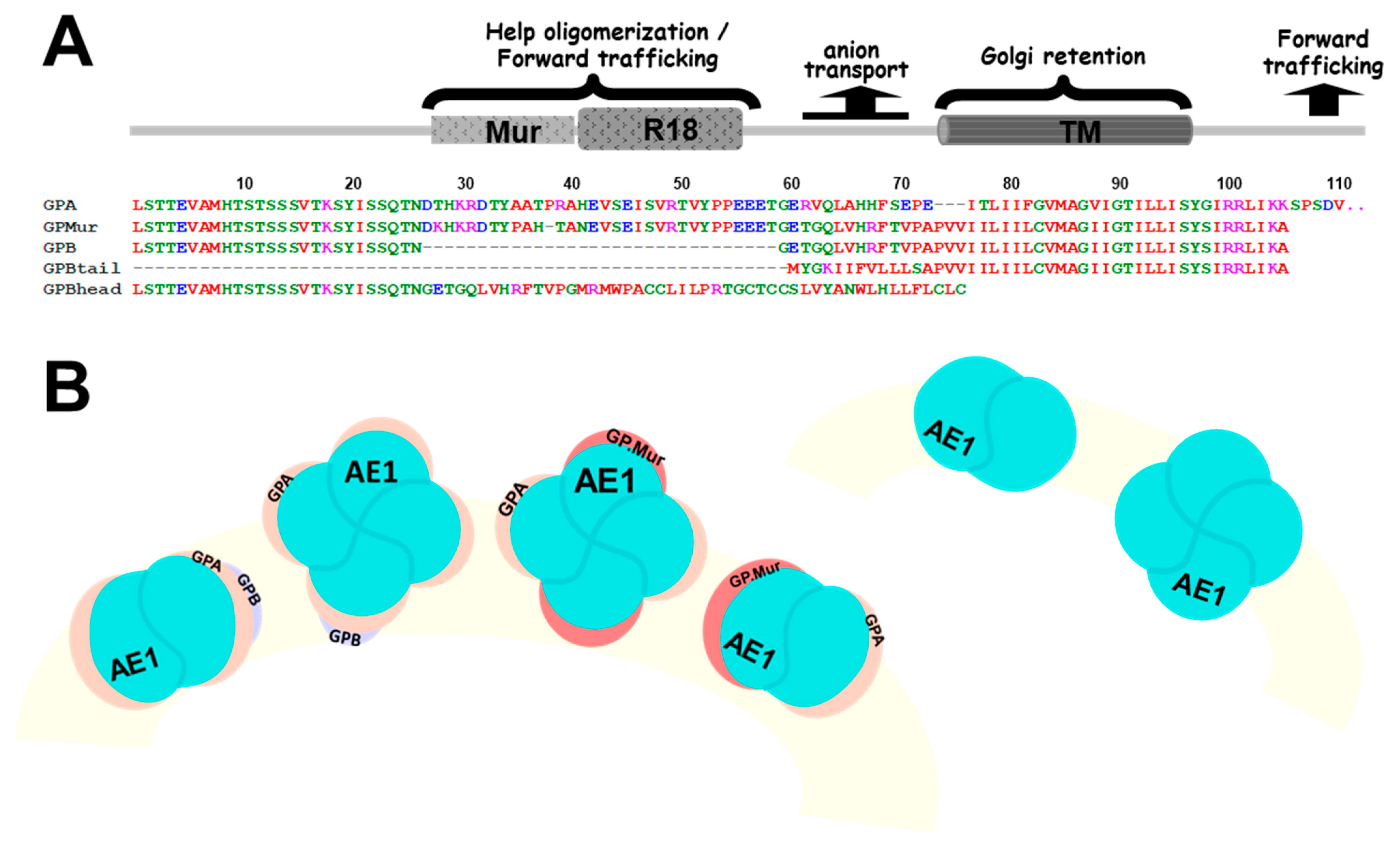
3.4. The Glycophorin Transmembrane Domain Encodes a Golgi Retention Signal
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cartron, J.P.; Rahuel, C. Human erythrocyte glycophorins: Protein and gene structure analyses. Transfus. Med. Rev. 1992, 6, 63–92. [Google Scholar] [CrossRef]
- Steck, T.L. The band 3 protein of the human red cell membrane: A review. J. Supramol. Struct. 1978, 8, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Steck, T.L.; Ramos, B.; Strapazon, E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry 1976, 15, 1153–1161. [Google Scholar] [CrossRef]
- Knauf, P.A.; Gasbjerg, P.K.; Brahm, J. The asymmetry of chloride transport at 38 degrees C in human red blood cell membranes. J. Gen. Physiol. 1996, 108, 577–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alper, S.L. The band 3-related anion exchanger (AE) gene family. Annu Rev. Physiol. 1991, 53, 549–564. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Ikeda, Y.; Abe, Y.; Kuma, H.; Kang, D.; Hamasaki, N.; Hirai, T. Structure of the membrane domain of human erythrocyte anion exchanger 1 revealed by electron crystallography. J. Mol. Biol 2010, 397, 179–189. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Fujii, T.; Abe, Y.; Hirai, T.; Kang, D.; Namba, K.; Hamasaki, N.; Mitsuoka, K. Helical image reconstruction of the outward-open human erythrocyte band 3 membrane domain in tubular crystals. J. Struct. Biol. 2010, 169, 406–412. [Google Scholar] [CrossRef]
- Low, P.S. Structure and function of the cytoplasmic domain of band 3: Center of erythrocyte membrane-peripheral protein interactions. Biochim. Biophys. Acta 1986, 864, 145–167. [Google Scholar] [CrossRef]
- Jay, D.G. Role of band 3 in homeostasis and cell shape. Cell 1996, 86, 853–854. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.; Mohandas, N.; Waugh, R.E. Integral protein linkage and the bilayer-skeletal separation energy in red blood cells. Biophys. J. 2008, 95, 1826–1836. [Google Scholar] [CrossRef]
- Hsu, K.; Chi, N.; Gucek, M.; Van Eyk, J.E.; Cole, R.N.; Lin, M.; Foster, D.B. Miltenberger blood group antigen type III (Mi.III) enhances the expression of band 3. Blood 2009, 114, 1919–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groves, J.D.; Tanner, M.J. Glycophorin A facilitates the expression of human band 3-mediated anion transport in Xenopus oocytes. J. Biol. Chem. 1992, 267, 22163–22170. [Google Scholar] [CrossRef]
- Groves, J.D.; Tanner, M.J. The effects of glycophorin A on the expression of the human red cell anion transporter (band 3) in Xenopus oocytes. J. Membr. Biol. 1994, 140, 81–88. [Google Scholar]
- Tomita, M.; Furthmayr, H.; Marchesi, V.T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry 1978, 17, 4756–4770. [Google Scholar] [CrossRef] [PubMed]
- Gahmberg, C.G.; Ekblom, M.; Andersson, L.C. Differentiation of human erythroid cells is associated with increased O-glycosylation of the major sialoglycoprotein, glycophorin A. Proc. Natl. Acad. Sci. USA 1984, 81, 6752–6756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takabatake, N.; Okamura, M.; Yokoyama, N.; Ikehara, Y.; Akimitsu, N.; Arimitsu, N.; Hamamoto, H.; Sekimizu, K.; Suzuki, H.; Igarashi, I. Glycophorin A-knockout mice, which lost sialoglycoproteins from the red blood cell membrane, are resistant to lethal infection of Babesia rodhaini. Vet. Parasitol. 2007, 148, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Arimitsu, N.; Akimitsu, N.; Kotani, N.; Takasaki, S.; Kina, T.; Hamamoto, H.; Kamura, K.; Sekimizu, K. Glycophorin A requirement for expression of O-linked antigens on the erythrocyte membrane. Genes Cells 2003, 8, 769–777. [Google Scholar] [CrossRef]
- Kudo, S.; Fukuda, M. Structural organization of glycophorin A and B genes: Glycophorin B gene evolved by homologous recombination at Alu repeat sequences. Proc. Natl. Acad. Sci. USA 1989, 86, 4619–4623. [Google Scholar] [CrossRef] [Green Version]
- Salinas, N.D.; Paing, M.M.; Tolia, N.H. Critical glycosylated residues in exon three of erythrocyte glycophorin A engage Plasmodium falciparum EBA-175 and define receptor specificity. MBio 2014, 5, e01606–e01614. [Google Scholar] [CrossRef] [Green Version]
- Jennings, M.L. Cell physiology and molecular mechanism of anion transport by erythrocyte band 3/AE1. Am. J. Physiol. Cell Physiol. 2021, 321, C1028–C1059. [Google Scholar] [CrossRef]
- Corbett, J.D.; Golan, D.E. Band 3 and glycophorin are progressively aggregated in density-fractionated sickle and normal red blood cells. Evidence from rotational and lateral mobility studies. J. Clin. Investig. 1993, 91, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, H.; Hanada, T.; Lutchman, M.; Sahr, K.E.; Palek, J.; Hanspal, M.; Chishti, A.H. Complete deficiency of glycophorin A in red blood cells from mice with targeted inactivation of the band 3 (AE1) gene. Blood 1998, 91, 2146–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, A.J.; Reithmeier, R.A. Interaction of anion exchanger 1 and glycophorin A in human erythroleukaemic K562 cells. Biochem. J. 2009, 421, 345–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telen, M.J.; Chasis, J.A. Relationship of the human erythrocyte Wrb antigen to an interaction between glycophorin A and band 3. Blood 1990, 76, 842–848. [Google Scholar] [CrossRef] [Green Version]
- Bruce, L.J.; Ring, S.M.; Anstee, D.J.; Reid, M.E.; Wilkinson, S.; Tanner, M.J. Changes in the blood group Wright antigens are associated with a mutation at amino acid 658 in human erythrocyte band 3: A site of interaction between band 3 and glycophorin A under certain conditions. Blood 1995, 85, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Hsu, K.; Lin, Y.C.; Lee, T.Y.; Lin, M. Miltenberger blood group antigen subtype III (Mi.III) supports Wr(b) expression. Vox Sang. 2011, 100, 389–394. [Google Scholar] [CrossRef]
- Peters, L.L.; Shivdasani, R.A.; Liu, S.C.; Hanspal, M.; John, K.M.; Gonzalez, J.M.; Brugnara, C.; Gwynn, B.; Mohandas, N.; Alper, S.L.; et al. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell 1996, 86, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Southgate, C.D.; Chishti, A.H.; Mitchell, B.; Yi, S.J.; Palek, J. Targeted disruption of the murine erythroid band 3 gene results in spherocytosis and severe haemolytic anaemia despite a normal membrane skeleton. Nat. Genet. 1996, 14, 227–230. [Google Scholar] [CrossRef]
- Bruce, L.J.; Groves, J.D.; Okubo, Y.; Thilaganathan, B.; Tanner, M.J. Altered band 3 structure and function in glycophorin A- and B-deficient (MkMk) red blood cells. Blood 1994, 84, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Bruce, L.J.; Pan, R.J.; Cope, D.L.; Uchikawa, M.; Gunn, R.B.; Cherry, R.J.; Tanner, M.J. Altered structure and anion transport properties of band 3 (AE1, SLC4A1) in human red cells lacking glycophorin A. J. Biol. Chem. 2004, 279, 2414–2420. [Google Scholar] [CrossRef] [Green Version]
- Williamson, R.C.; Toye, A.M. Glycophorin A: Band 3 aid. Blood Cells Mol. Dis. 2008, 41, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bruce, L.J.; Beckmann, R.; Ribeiro, M.L.; Peters, L.L.; Chasis, J.A.; Delaunay, J.; Mohandas, N.; Anstee, D.J.; Tanner, M.J. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 2003, 101, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Young, M.T.; Tanner, M.J. Distinct regions of human glycophorin A enhance human red cell anion exchanger (band 3; AE1) transport function and surface trafficking. J. Biol. Chem. 2003, 278, 32954–32961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smythe, J.S.; Spring, F.A.; Gardner, B.; Parsons, S.F.; Judson, P.A.; Anstee, D.J. Monoclonal antibodies recognizing epitopes on the extracellular face and intracellular N-terminus of the human erythrocyte anion transporter (band 3) and their application to the analysis of South East Asian ovalocytes. Blood 1995, 85, 2929–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, M.J.; Poole, J.; Anstee, D.J. An application of immunoblotting in the classification of the Miltenberger series of blood group antigens. Transfusion 1989, 29, 106–112. [Google Scholar] [CrossRef]
- Reid, M.E.; Lisowska, E.; Blanchard, D. Coordinator’s report: Glycophorin/band 3 and associated antigens. Transfus. Clin. Biol. 1997, 4, 57–64. [Google Scholar] [CrossRef]
- Gardner, B.; Parsons, S.F.; Merry, A.H.; Anstee, D.J. Epitopes on sialoglycoprotein alpha: Evidence for heterogeneity in the molecule. Immunology 1989, 68, 283–289. [Google Scholar]
- Huang, C.H.; Blumenfeld, O.O. Molecular genetics of human erythrocyte MiIII and MiVI glycophorins. Use of a pseudoexon in construction of two delta-alpha-delta hybrid genes resulting in antigenic diversification. J. Biol. Chem. 1991, 266, 7248–7255. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Burton, N.M.; Bruce, L.J. Modelling the structure of the red cell membrane. Biochem. Cell Biol. 2011, 89, 200–215. [Google Scholar] [CrossRef]
- Banfield, D.K. Mechanisms of protein retention in the Golgi. Cold Spring Harb. Perspect. Biol. 2011, 3, a005264. [Google Scholar] [CrossRef] [PubMed]
- Gahmberg, C.G.; Myllyla, G.; Leikola, J.; Pirkola, A.; Nordling, S. Absence of the major sialoglycoprotein in the membrane of human En(a--) erythrocytes and increased glycosylation of band 3. J. Biol. Chem. 1976, 251, 6108–6116. [Google Scholar] [CrossRef]
- Tanner, M.J.; Jenkins, R.E.; Anstee, D.J.; Clamp, J.R. Abnormal carbohydrate composition of the major penetrating membrane protein of En(a-) human erythrocytes. Biochem. J. 1976, 155, 701–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahr, W.; Moulds, J.; Unger, P.; Kordowicz, M. The Dantu erythrocyte phenotype of the NE variety. I. Dodecylsulfate polyacrylamide gel electrophoretic studies. Blut 1987, 55, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.; Lee, T.Y.; Chao, H.P.; Chan, Y.S.; Lin, Y.C.; Lin, M. Expression of the Rh/RhAG complex is reduced in Mi.III erythrocytes. Vox Sang. 2012, 102, 221–227. [Google Scholar] [CrossRef]
- Salomao, M.; Zhang, X.; Yang, Y.; Lee, S.; Hartwig, J.H.; Chasis, J.A.; Mohandas, N.; An, X. Protein 4.1R-dependent multiprotein complex: New insights into the structural organization of the red blood cell membrane. Proc. Natl. Acad. Sci. USA 2008, 105, 8026–8031. [Google Scholar] [CrossRef] [Green Version]
- Satchwell, T.J.; Toye, A.M. Band 3, an essential red blood cell hub of activity. Haematologica 2021, 106, 2792–2793. [Google Scholar] [CrossRef]
- Vallese, F.; Kim, K.; Yen, L.Y.; Johnston, J.D.; Noble, A.J.; Cali, T.; Clarke, O.B. Architecture of the human erythrocyte ankyrin-1 complex. Nat. Struct. Mol. Biol. 2022, 29, 706–718. [Google Scholar] [CrossRef]
- Patterson, S.T.; Li, J.; Kang, J.A.; Wickrema, A.; Williams, D.B.; Reithmeier, R.A. Loss of specific chaperones involved in membrane glycoprotein biosynthesis during the maturation of human erythroid progenitor cells. J. Biol. Chem. 2009, 284, 14547–14557. [Google Scholar] [CrossRef] [Green Version]
- Groves, J.D.; Tanner, M.J. Role of N-glycosylation in the expression of human band 3-mediated anion transport. Mol. Membr. Biol. 1994, 11, 31–38. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, K.N.; Lai, J.C.; Chen, L.Y.; Kuo, M.S.; Liao, C.C.; Hsu, K. Influence of hemoglobin on blood pressure among people with GP.Mur blood type. J. Med. Assoc. 2022, 121, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.S.; Chuang, C.H.; Cheng, H.C.; Lin, H.R.; Wang, J.S.; Hsu, K. Different Involvement of Band 3 in Red Cell Deformability and Osmotic Fragility—A Comparative GP.Mur Erythrocyte Study. Cells 2021, 10, 3369. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.; Liu, Y.; Tseng, W.; Huang, K.; Liu, C.; Chen, L.; Lee, H.; Lin, H.; Tseng, K.; Yeh, H. Comodulation of NO-dependent vasodilation by erythroid band 3 and hemoglobin: A GP.Mur athlete study. Front. Cardiovasc. Med. 2021, 8, 740100. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K. Exploring the Potential Roles of Band 3 and Aquaporin-1 in Blood CO2 Transport-Inspired by Comparative Studies of Glycophorin B-A-B Hybrid Protein GP.Mur. Front. Physiol. 2018, 9, 733. [Google Scholar] [CrossRef] [Green Version]
- Vince, J.W.; Reithmeier, R.A. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte Cl−/HCO3− exchanger. J. Biol. Chem. 1998, 273, 28430–28437. [Google Scholar] [CrossRef] [Green Version]
- Tanner, M.J. The structure and function of band 3 (AE1): Recent developments. Mol. Membr. Biol. 1997, 14, 155–165. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, K.; Lee, T.-Y.; Lin, J.-Y.; Chen, P.-L. A Balance between Transmembrane-Mediated ER/Golgi Retention and Forward Trafficking Signals in Glycophorin-Anion Exchanger-1 Interaction. Cells 2022, 11, 3512. https://doi.org/10.3390/cells11213512
Hsu K, Lee T-Y, Lin J-Y, Chen P-L. A Balance between Transmembrane-Mediated ER/Golgi Retention and Forward Trafficking Signals in Glycophorin-Anion Exchanger-1 Interaction. Cells. 2022; 11(21):3512. https://doi.org/10.3390/cells11213512
Chicago/Turabian StyleHsu, Kate, Ting-Ying Lee, Jian-Yi Lin, and Pin-Lung Chen. 2022. "A Balance between Transmembrane-Mediated ER/Golgi Retention and Forward Trafficking Signals in Glycophorin-Anion Exchanger-1 Interaction" Cells 11, no. 21: 3512. https://doi.org/10.3390/cells11213512
APA StyleHsu, K., Lee, T. -Y., Lin, J. -Y., & Chen, P. -L. (2022). A Balance between Transmembrane-Mediated ER/Golgi Retention and Forward Trafficking Signals in Glycophorin-Anion Exchanger-1 Interaction. Cells, 11(21), 3512. https://doi.org/10.3390/cells11213512






