HIF-1α Regulates Bone Homeostasis and Angiogenesis, Participating in the Occurrence of Bone Metabolic Diseases
Abstract
1. Introduction
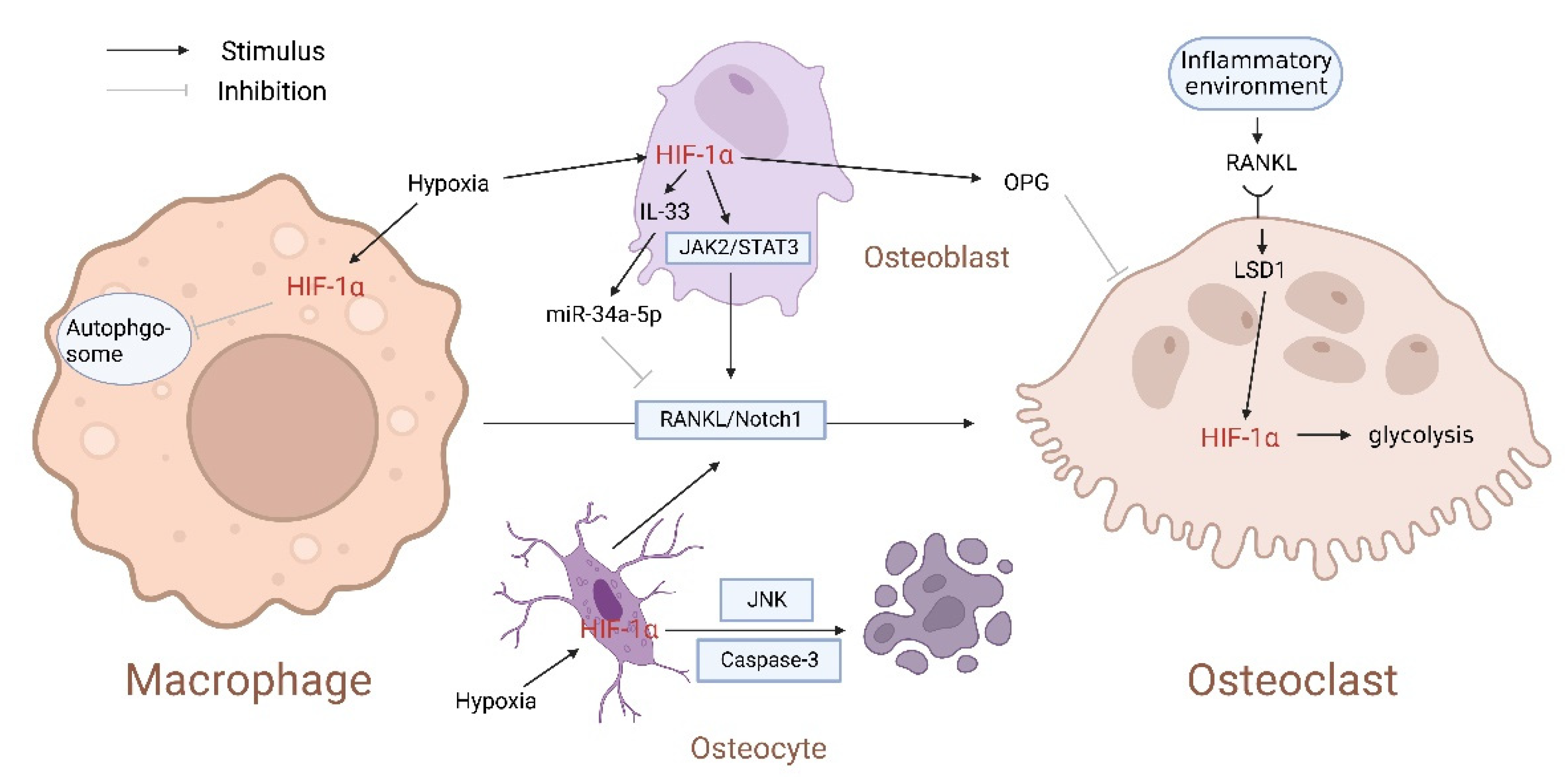
2. The Relationship between HIF-1α and Osteoclasts
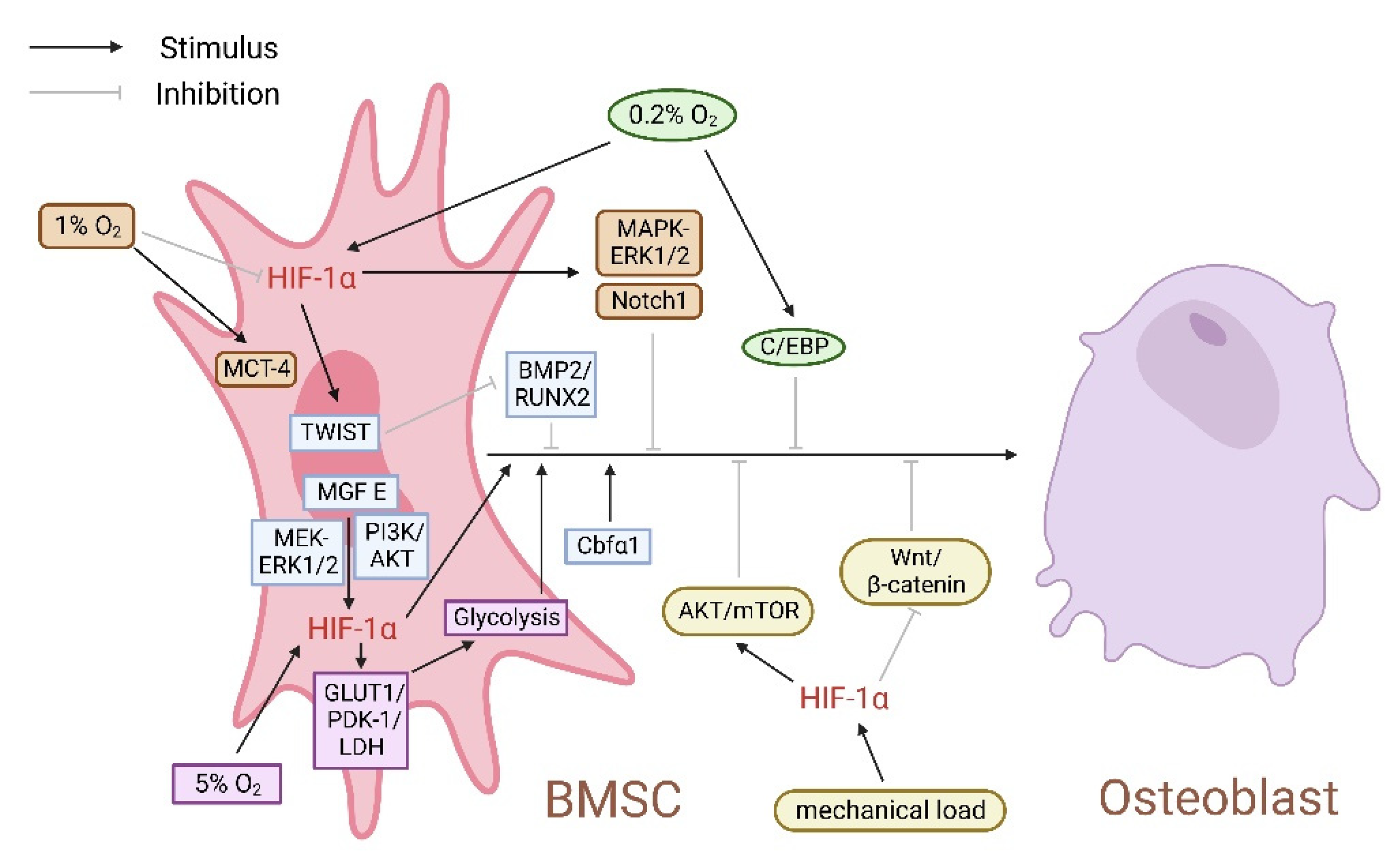
3. Hypoxia Affects the Expression of HIF-1α and Regulates the Osteogenic Activity of BMSCs
3.1. Hypoxia Dual-Directionally Regulates the Expression of HIF-1α, Affecting the Proliferation and Osteogenic Differentiation of BMSCs
3.2. HIF-1α Regulates Bone Homeostasis by Affecting Energy Metabolism
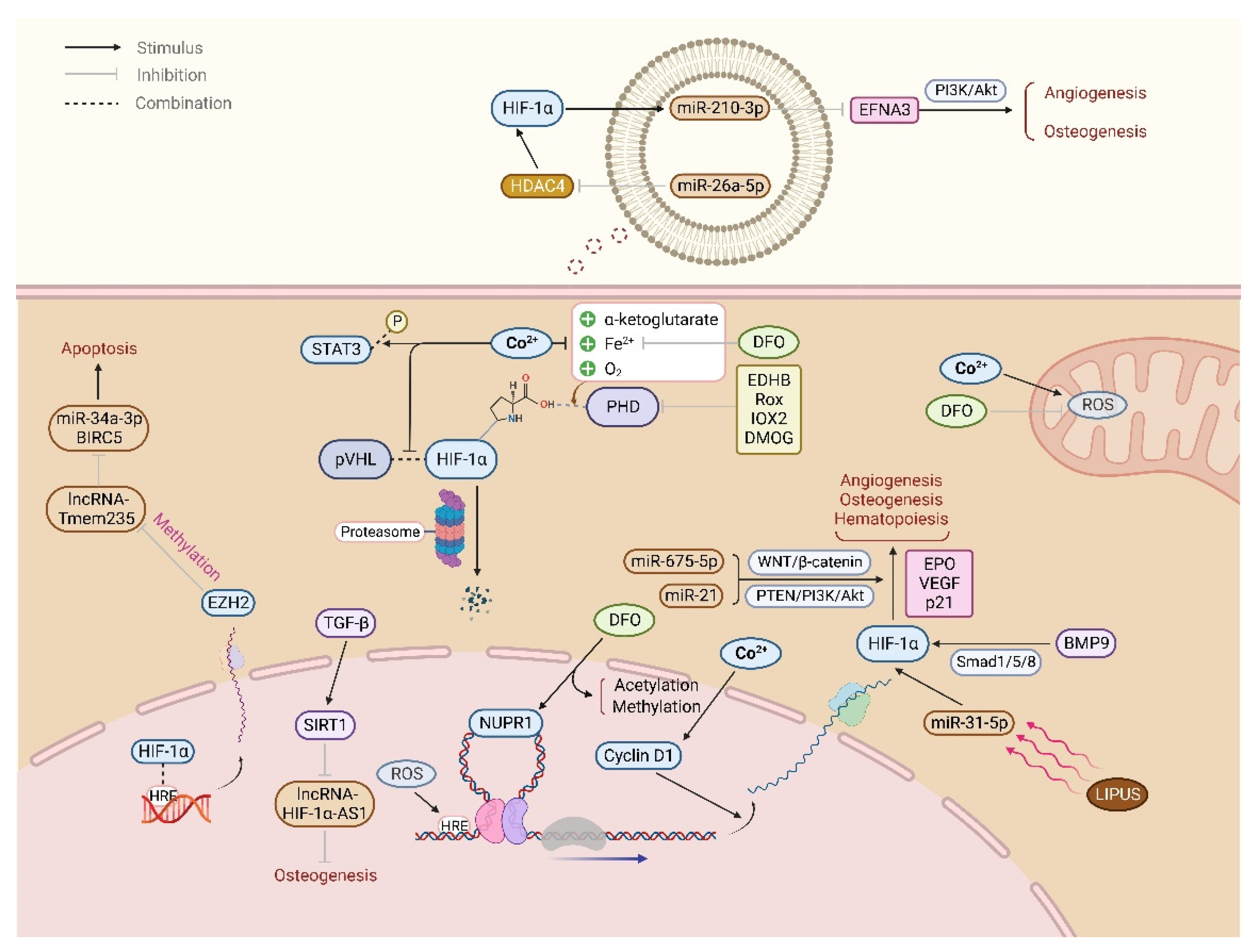
4. Inactivation of PHD/VHL Stabilizes HIF-1α, Promoting Angiogenesis and BMSCs Osteogenic Differentiation
4.1. PHD
4.2. VHL
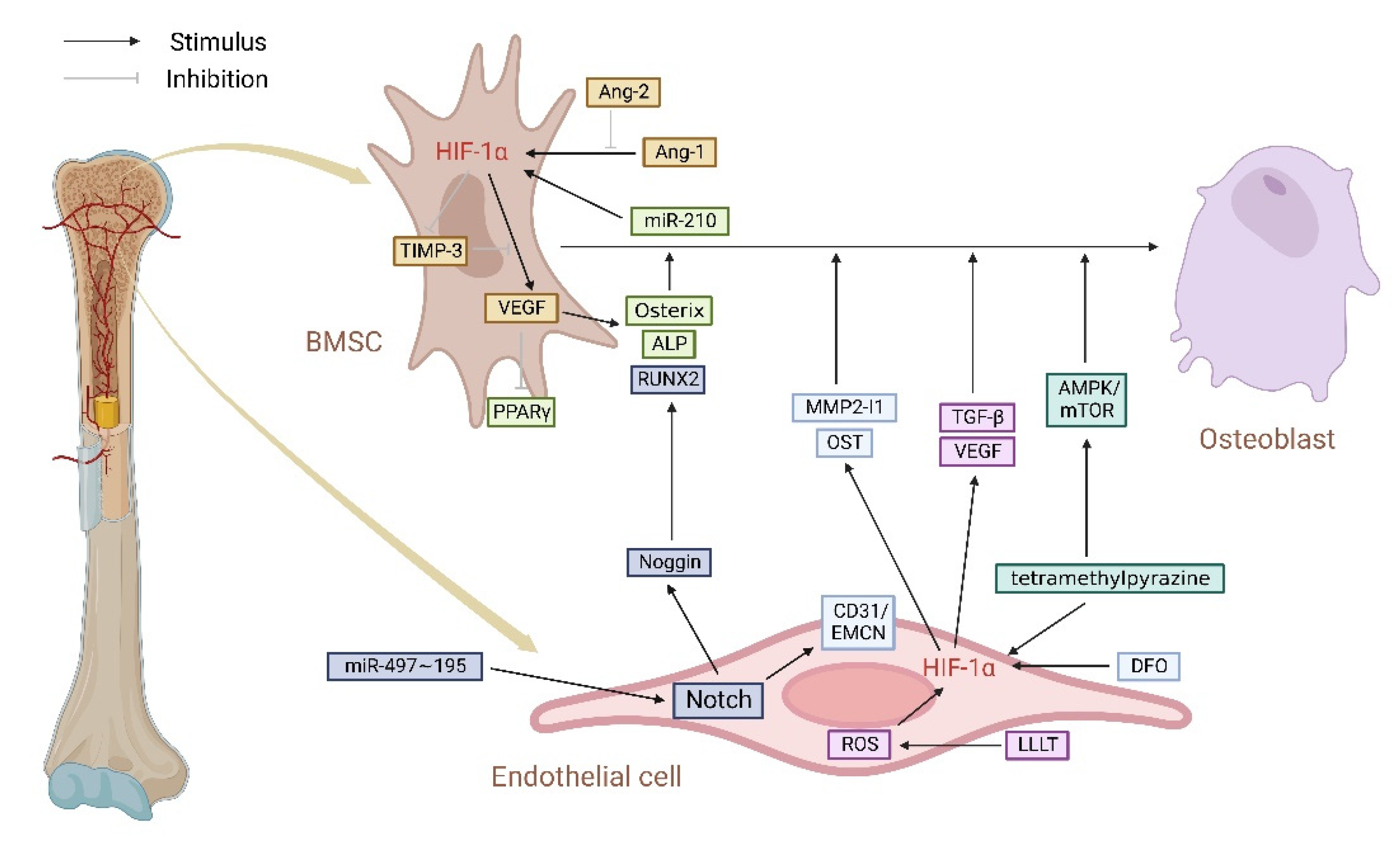
5. HIF-1α/VEGF Pathway Regulates Type H Vessels, Coupling of Angiogenesis and Osteogenesis
5.1. HIF-1α/VEGF Pathway Connects Type H ECs and Osteoblasts in the Skeletal System
5.2. HIF-1α/VEGF Pathway Regulates Type H Vessels in Various Bone Metabolic Disease Models
6. The Role of Non-Coding RNA (ncRNA) in HIF-1α Regulating Bone Homeostasis
6.1. Interaction between miRNA and HIF-1α Promotes Angiogenesis and BMSCs Osteogenic Differentiation, Preventing Bone Metabolism Diseases
6.2. Interaction between lncRNA and HIF-1α Regulates Apoptosis, Angiogenesis, and BMSCs Osteogenic Differentiation Involved in Bone Development and Regeneration
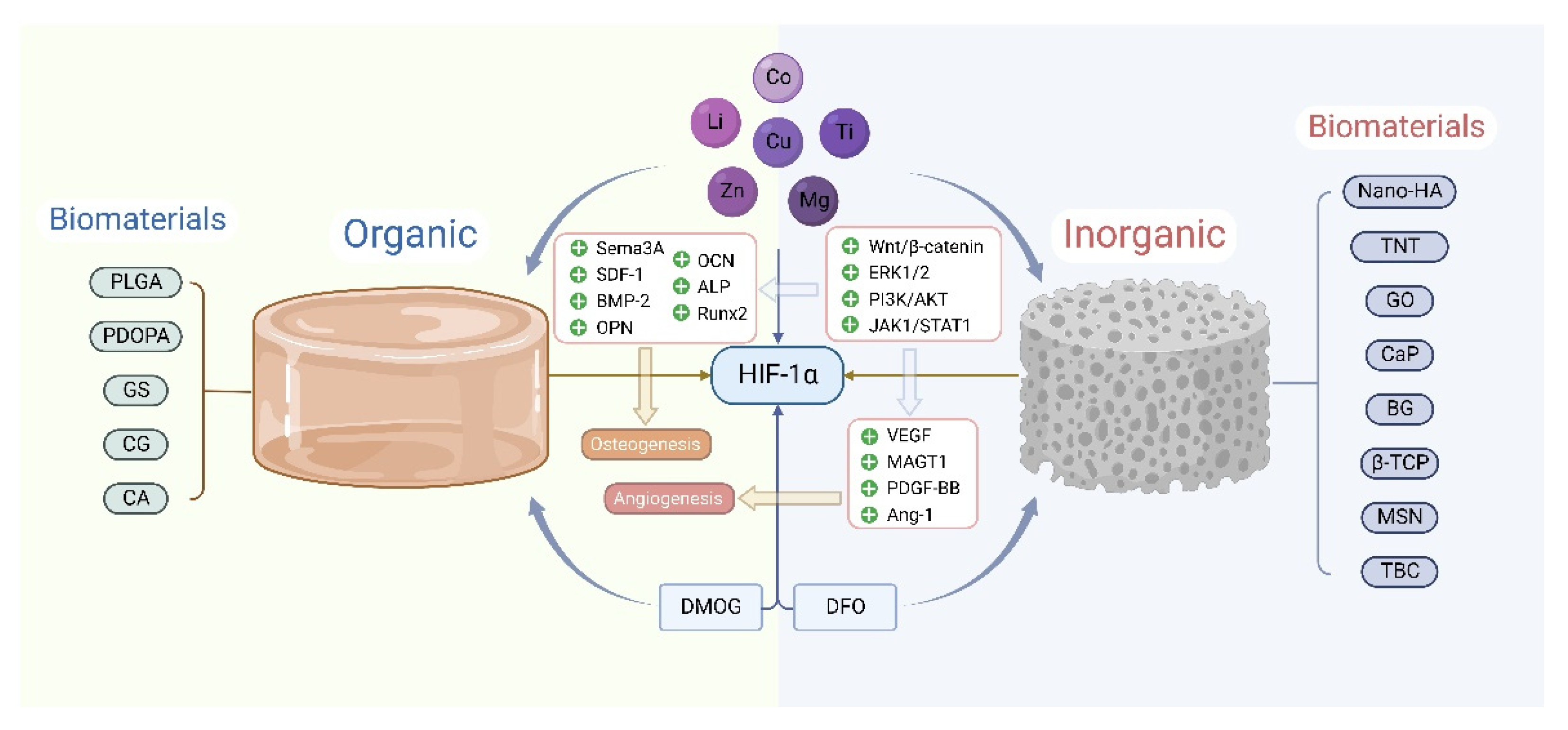
7. HIF-1α Involvement in the Bone Repair of Biomaterials
7.1. Biomaterials Doped with Trace Elements Upregulate HIF-1α, Promoting Angiogenesis and Osteogenesis
7.1.1. Copper
7.1.2. Cobalt
7.1.3. Other Trace Elements
7.2. Biomaterials Loaded with PHD Inhibitors Stabilize HIF-1α, Promoting Bone Defect Repair
7.2.1. DFO
7.2.2. DMOG
7.3. Application of Tissue Engineering Combined with Gene Mutation Technique to HIF-1α in Bone Defects Repair
8. The Role of Other Factors in Bone Homeostasis Regulation by HIF-1α
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palumbo, C.; Ferretti, M. The Osteocyte: From “Prisoner” to “Orchestrator”. J. Funct. Morphol. Kinesiol. 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, H.; Guo, J.; Yuan, Y.; Ni, G. Effects of BMSC-Derived EVs on Bone Metabolism. Pharmaceutics 2022, 14, 1012. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, Y.; Zhang, Q. Emerging Role of Extracellular Vesicles in Bone Remodeling. J. Dent. Res. 2018, 97, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, H.; Yuan, Y.; Wu, W. Biochemical Signals Mediate the Crosstalk between Cartilage and Bone in Osteoarthritis. BioMed. Res. Int. 2020, 2020, 5720360. [Google Scholar] [CrossRef] [PubMed]
- Heyman, S.N.; Rosen, S.; Rosenberger, C. Hypoxia-inducible factors and the prevention of acute organ injury. Crit. Care 2011, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.-W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Ohh, M.; Park, C.W.; Ivan, M.; Hoffman, M.A.; Kim, T.Y.; Huang, L.E.; Pavletich, N.; Chau, V.; Kaelin, W.G. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2000, 2, 423–427. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001, 13, 167–171. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Ocular neovascularization. J. Mol. Med. 2013, 9, 311–321. [Google Scholar] [CrossRef]
- Park, S.-Y.; Jang, W.-J.; Yi, E.-Y.; Jang, J.-Y.; Jung, Y.; Jeong, J.-W.; Kim, Y.-J. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J. Pineal Res. 2010, 48, 178–184. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Chang, C.-Y.; Kao, A.; Hsi, B.; Lee, S.-H.; Chen, Y.-H.; Wang, I.-J. Hypoxia-Induced Retinal Neovascularization in Zebrafish Embryos: A Potential Model of Retinopathy of Prematurity. PLoS ONE 2015, 10, e0126750. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, H.; Bu, J.; Li, X.; Chen, Z.; Xiao, T. Hypoxia-inducible factor-1 expression predicts osteosarcoma patients’ survival: A meta-analysis. Int. J. Biol. Markers 2016, 31, e229–e234. [Google Scholar] [CrossRef]
- Zhang, F.-J.; Luo, W.; Lei, G.-H. Role of HIF-1α and HIF-2α in osteoarthritis. Jt. Bone Spine 2015, 82, 144–147. [Google Scholar] [CrossRef]
- Fayed, H.A.; Barakat, B.M.; Elshaer, S.S.; Abdel-Naim, A.B.; Menze, E.T. Antiosteoporotic activities of isoquercitrin in ovariec-tomized rats: Role of inhibiting hypoxia inducible factor-1 alpha. Eur. J. Pharmacol. 2019, 865, 172785. [Google Scholar] [CrossRef]
- Chen, C.; Yan, S.; Geng, Z.; Wang, Z. Fracture repair by IOX2: Regulation of the hypoxia inducible factor-1α signaling path-way and BMSCs. Eur. J. Pharmacol. 2022, 921, 174864. [Google Scholar] [CrossRef]
- Li, D.; Hu, Q.; Tan, G.; Xie, X.; Yang, Z.; Kang, P. Erythropoietin Enhances Bone Repair Effects via the Hypoxia-Inducible Factor Signal Pathway in Glucocorticoid-Induced Osteonecrosis of the Femoral Head. Am. J. Med. Sci. 2018, 355, 597–606. [Google Scholar] [CrossRef]
- Park, I.-H.; Kim, K.-H.; Choi, H.-K.; Shim, J.-S.; Whang, S.-Y.; Hahn, S.J.; Kwon, O.-J.; Oh, I.-H. Constitutive stabilization of hypoxia-inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state. Exp. Mol. Med. 2013, 45, e44. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Arnett, T.R.; Gibbons, D.C.; Utting, J.C.; Orriss, I.R.; Hoebertz, A.; Rosendaal, M.; Meghji, S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J. Cell. Physiol. 2003, 196, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Yuan, Y.; Qian, Z.; Zhong, Z.; Lv, T.; Kuang, Y.; Yu, B. Hypoxia inhibits RANKL-induced ferritinophagy and protects osteoclasts from ferroptosis. Free Radic. Biol. Med. 2021, 169, 271–282. [Google Scholar] [CrossRef]
- Zhuang, Y.; Cheng, M.; Li, M.; Cui, J.; Huang, J.; Zhang, C.; Si, J.; Lin, K.; Yu, H. Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210-3p/EFNA3/PI3K pathway. Acta Biomater. 2022, 150, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, Y.; Wu, Q.; Ji, Y.-C.; Feng, Z.-F.; Kang, F.-W. HIF-1α facilitates osteocyte-mediated osteoclastogenesis by activating JAK2/STAT3 pathway in vitro. J. Cell. Physiol. 2019, 234, 21182–21192. [Google Scholar] [CrossRef] [PubMed]
- Hulley, P.A.; Bishop, T.; Vernet, A.; Schneider, J.E.; Edwards, J.R.; Athanasou, N.A.; Knowles, H.J. Hypoxia-inducible factor 1-alpha does not regulate osteoclastogenesis but enhances bone resorption activity via prolyl-4-hydroxylase 2. J. Pathol. 2017, 242, 322–333. [Google Scholar] [CrossRef]
- Doi, K.; Murata, K.; Ito, S.; Suzuki, A.; Terao, C.; Ishie, S.; Umemoto, A.; Murotani, Y.; Nishitani, K.; Yoshitomi, H.; et al. Role of Lysine-Specific Demethylase 1 in Metabolically Integrating Osteoclast Differentiation and Inflammatory Bone Resorption Through Hypoxia-Inducible Factor 1α and E2F1. Arthritis Rheumatol. 2022, 74, 948–960. [Google Scholar] [CrossRef]
- Song, X.; Tang, Y.; Zhu, J.; Tian, Y.; Song, Z.; Hu, X.; Hong, C.; Cai, Y.; Kang, F. HIF-1α induces hypoxic apoptosis of MLO-Y4 osteocytes via JNK/caspase-3 pathway and the apoptotic-osteocyte-mediated osteoclastogenesis in vitro. Tissue Cell 2020, 67, 101402. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Fritton, J.C.; Morgan, S.A.; Seref-Ferlengez, Z.; Basta-Pljakic, J.; Thi, M.M.; Suadicani, S.O.; Spray, D.C.; Majeska, R.J.; Schaffler, M.B. Pannexin-1 and P2X7-Receptor Are Required for Apoptotic Osteocytes in Fatigued Bone to Trigger RANKL Production in Neighboring Bystander Osteocytes. J. Bone Miner. Res. 2016, 31, 890–899. [Google Scholar] [CrossRef]
- Bondar, C.; Ormazabal, M.; Crivaro, A.; Ferreyra-Compagnucci, M.; Delpino, M.V.; Rozenfeld, P.A.; Mucci, J.M. Osteocyte Al-terations Induce Osteoclastogenesis in an In Vitro Model of Gaucher Disease. Int. J. Mol. Sci. 2017, 18, 112. [Google Scholar] [CrossRef]
- Boyce, B.F. Advances in the Regulation of Osteoclasts and Osteoclast Functions. J. Dent. Res. 2013, 92, 860–867. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Ann, E.-J.; Yoon, J.-H.; Mo, J.-S.; Kim, M.-Y.; Park, H.-S. Calcium/calmodulin-dependent protein kinase IV (CaMKIV) enhances osteoclast differentiation via the up-regulation of Notch1 protein stability. Biochim. Biophys. Acta 2013, 1833, 69–79. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L.; Chen, D. Osteoprotegerin, the bone protector, is a surprising target for beta-catenin signaling. Cell Metab. 2005, 2, 344–345. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, Y.; Yang, T.; Qi, J.; Zhang, L.; Deng, L. HIF-1α disturbs osteoblasts and osteoclasts coupling in bone remodeling by up-regulating OPG expression. In Vitro Cell. Dev. Biol.-Anim. 2015, 51, 808–814. [Google Scholar] [CrossRef]
- Kang, H.; Yang, K.; Xiao, L.; Guo, L.; Guo, C.; Yan, Y.; Qi, J.; Wang, F.; Ryffel, B.; Li, C.; et al. Osteoblast Hypoxia-Inducible Factor-1α Pathway Activation Restrains Osteoclastogenesis via the Interleukin-33-MicroRNA-34a-Notch1 Pathway. Front. Immunol. 2017, 8, 1312. [Google Scholar] [CrossRef]
- Walker, E.C.; McGregor, N.E.; Poulton, I.J.; Pompolo, S.; Allan, E.H.; Quinn, J.M.W.; Gillespie, M.T.; Martin, T.J.; Sims, N.A. Car-diotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J. Bone Miner. Res. 2008, 23, 2025–2032. [Google Scholar] [CrossRef]
- Richards, C.D.; Langdon, C.; Deschamps, P.; Pennica, D.; Shaughnessy, S.G. Stimulation of osteoclast differentiation in vitro by mouse oncostatin M, leukaemia inhibitory factor, cardiotrophin-1 and interleukin 6: Synergy with dexamethasone. Cytokine 2000, 12, 613–621. [Google Scholar] [CrossRef]
- Walker, E.C.; McGregor, N.E.; Poulton, I.J.; Solano, M.; Pompolo, S.; Fernandes, T.J.; Constable, M.J.; Nicholson, G.C.; Zhang, J.-G.; Nicola, N.A.; et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J. Clin. Investig. 2010, 120, 582–592. [Google Scholar] [CrossRef]
- Tian, Y.; Shao, Q.; Tang, Y.; Li, X.; Qi, X.; Jiang, R.; Liang, Y.; Kang, F. HIF-1α regulates osteoclast activation and mediates osteogenesis during mandibular bone repair via CT-1. Oral Dis. 2020, 28, 428–441. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, J.; Qiu, M.; Zhang, L.; Yang, K.; Chang, L.; Jia, P.; Qi, J.; Deng, L.; Li, C. Osteocytic HIF-1α Pathway Manipulates Bone Micro-structure and Remodeling via Regulating Osteocyte Terminal Differentiation. Front. Cell Dev. Biol. 2022, 9, 721561. [Google Scholar] [CrossRef]
- Pasarica, M.; Sereda, O.R.; Redman, L.M.; Albarado, D.C.; Hymel, D.T.; Roan, L.E.; Rood, J.C.; Burk, D.H.; Smith, S.R. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kawamoto, E.; Gaowa, A.; Park, E.J.; Shimaoka, M. Remodeling of Bone Marrow Niches and Roles of Exosomes in Leukemia. Int. J. Mol. Sci. 2021, 22, 1881. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, E.; Helenius, G.; Friberg, Ö.; Samano, N.; Frøbert, O.; Johansson, K. Bone marrow- and adipose tissue-derived mes-enchymal stem cells from donors with coronary artery disease; growth, yield, gene expression and the effect of oxygen con-centration. Scand. J. Clin. Lab. Investig. 2020, 80, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Chun, S.Y.; Ha, Y.-S.; Kim, D.H.; Kim, J.; Song, P.H.; Kim, H.T.; Yoo, E.S.; Kim, B.S.; Kwon, T.G. Hypoxia Enhances Cell Properties of Human Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2017, 14, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Weijers, E.M.; Van Den Broek, L.J.; Waaijman, T.; Van Hinsbergh, V.W.M.; Gibbs, S.; Koolwijk, P. The Influence of Hypoxia and Fibrinogen Variants on the Expansion and Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cells. Tissue Eng. Part A 2011, 17, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.D.; Andrade, P.Z.; Boura, J.S.; Abecasis, M.M.; da Silva, C.L.; Cabral, J.M.S. Ex vivo expansion of human mesen-chymal stem cells: A more effective cell proliferation kinetics and metabolism under hypoxia. J. Cell. Physiol. 2010, 223, 27–35. [Google Scholar]
- Pattappa, G.; Thorpe, S.D.; Jegard, N.C.; Heywood, H.K.; de Bruijn, J.D.; Lee, D.A. Continuous and uninterrupted oxygen ten-sion influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng Part C Methods 2013, 19, 68–79. [Google Scholar] [CrossRef]
- Yasui, Y.; Chijimatsu, R.; Hart, D.A.; Koizumi, K.; Sugita, N.; Shimomura, K.; Myoui, A.; Yoshikawa, H.; Nakamura, N. Prepara-tion of Scaffold-Free Tissue-Engineered Constructs Derived from Human Synovial Mesenchymal Stem Cells Under Low Oxy-gen Tension Enhances Their Chondrogenic Differentiation Capacity. Tissue Eng. Part A 2016, 22, 490–500. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, P.; Yu, C.; Wu, J. The Proliferation and Stemness of Peripheral Blood-Derived Mesenchymal Stromal Cells Were Enhanced by Hypoxia. Front. Endocrinol. 2022, 13, 873662. [Google Scholar] [CrossRef]
- Yang, M.; Liu, H.; Wang, Y.; Wu, G.; Qiu, S.; Liu, C.; Tan, Z.; Guo, J.; Zhu, L. Hypoxia reduces the osteogenic differentiation of peripheral blood mesenchymal stem cells by upregulating Notch-1 expression. Connect. Tissue Res. 2019, 60, 583–596. [Google Scholar] [CrossRef]
- Antebi, B.; Ii, L.A.R.; Walker, K.P.; Asher, A.M.; Kamucheka, R.M.; Alvarado, L.; Mohammadipoor, A.; Cancio, L.C. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef]
- Zhang, P.; Ha, N.; Dai, Q.; Zhou, S.; Yu, C.; Jiang, L. Hypoxia suppresses osteogenesis of bone mesenchymal stem cells via the extracellular signal-regulated 1/2 and p38-mitogen activated protein kinase signaling pathways. Mol. Med. Rep. 2017, 16, 5515–5522. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, J.; Dai, Y.; Cao, P.; Zhang, L.; Peng, S.; Zhou, Y.; Li, G.; Tang, J.; Xiang, J. HIF-1A and C/EBPs transcriptionally reg-ulate adipogenic differentiation of bone marrow-derived MSCs in hypoxia. Stem Cell Res. Ther. 2015, 6, 21. [Google Scholar] [CrossRef]
- Rabie, A.M.; Tang, G.H.; Hägg, U. Cbfa1 couples chondrocytes maturation and endochondral ossification in rat mandibular condylar cartilage. Arch. Oral Biol. 2003, 49, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.; de Bari, C. The regulation of differentiation in mesenchymal stem cells. Hum. Gene Ther. 2010, 21, 1226–1238. [Google Scholar] [CrossRef]
- Huang, J.; Deng, F.; Wang, L.; Xiang, X.R.; Zhou, W.W.; Hu, N.; Xu, L. Hypoxia induces osteogenesis-related activities and ex-pression of core binding factor alpha1 in mesenchymal stem cells. Tohoku J. Exp. Med. 2011, 224, 7–12. [Google Scholar] [CrossRef][Green Version]
- Kindrick, J.; Mole, D. Hypoxic Regulation of Gene Transcription and Chromatin: Cause and Effect. Int. J. Mol. Sci. 2020, 21, 8320. [Google Scholar] [CrossRef]
- Chen, Z.F.; Behringer, R.R. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995, 9, 686–699. [Google Scholar] [CrossRef]
- Isenmann, S.; Arthur, A.; Zannettino, A.C.; Turner, J.L.; Shi, S.; Glackin, C.A.; Gronthos, S. TWIST Family of Basic Helix-Loop-Helix Transcription Factors Mediate Human Mesenchymal Stem Cell Growth and Commitment. Stem Cells 2009, 27, 2457–2468. [Google Scholar] [CrossRef]
- Yang, D.-C.; Yang, M.-H.; Tsai, C.-C.; Huang, T.-F.; Chen, Y.-H.; Hung, S.-C. Hypoxia Inhibits Osteogenesis in Human Mesenchymal Stem Cells through Direct Regulation of RUNX2 by TWIST. PLoS ONE 2011, 6, e23965. [Google Scholar] [CrossRef]
- Yu, H.; Yu, W.; Liu, Y.; Yuan, X.; Yuan, R.; Guo, Q. Expression of HIF-1α in cycling stretch-induced osteogenic differentiation of bone mesenchymal stem cells. Mol. Med. Rep. 2019, 20, 4489–4498. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, J.; Wu, X. Effect of Hypoxia-Inducible Factor-1α on Osteogenesis of Titanium Dioxide Nanotube Bone Marrow Mesenchymal Stem Cells with Different Diameters Under Periodic Tensile Stress. J. Biomed. Nanotechnol. 2022, 18, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, X.; Yu, H.; Yang, J.; Li, Y.; Yuan, X.; Guo, Q. HIF-1α-TWIST pathway restrains cyclic mechanical stretch-induced osteogenic differentiation of bone marrow mesenchymal stem cells. Connect. Tissue Res. 2019, 60, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.; Goldspink, G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with mus-cle satellite (stem) cell activation following local tissue damage. J. Physiol. 2003, 549, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Feng, W.; Wu, Y.; Lv, H.; Jia, Y.; Jiang, D. Mechano-growth factor accelerates the proliferation and osteogenic differen-tiation of rabbit mesenchymal stem cells through the PI3K/AKT pathway. BMC Biochem. 2015, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Lv, Y.; Xu, Z.; Yang, L.; Hao, X.; Afandi, R. MGF E peptide pretreatment improves the proliferation and osteogenic dif-ferentiation of BMSCs via MEK-ERK1/2 and PI3K-Akt pathway under severe hypoxia. Life Sci. 2017, 189, 52–62. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Y.; Li, Q.; Li, X.; Wang, W.; Ai, X.; Kuang, T.; Chen, X.; Zhang, Y.; Zhang, J.; et al. Rhodiola crenulata attenuates apoptosis and mitochondrial energy metabolism disorder in rats with hypobaric hypoxia-induced brain injury by regulating the HIF-1α/microRNA 210/ISCU1/2(COX10) signaling pathway. J. Ethnopharmacol. 2019, 241, 111801. [Google Scholar] [CrossRef]
- Hu, J.; Nie, Y.; Chen, S.; Xie, C.; Fan, Q.; Wang, Z.; Long, B.; Yan, G.; Zhong, Q.; Yan, X. Leucine reduces reactive oxygen species levels via an energy metabolism switch by activation of the mTOR-HIF-1α pathway in porcine intestinal epithelial cells. Int. J. Biochem. Cell Biol. 2017, 89, 42–56. [Google Scholar] [CrossRef]
- Saraswati, S.; Guo, Y.; Atkinson, J.; Young, P.P. Prolonged hypoxia induces monocarboxylate transporter-4 expression in mes-enchymal stem cells resulting in a secretome that is deleterious to cardiovascular repair. Stem Cells 2015, 33, 1333–1344. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Majore, I.; Kasper, C.; Hass, R. Effects of hypoxic culture conditions on umbilical cord-derived human mesen-chymal stem cells. Cell Commun. Signal. 2010, 8, 18. [Google Scholar] [CrossRef]
- Regan, J.N.; Lim, J.; Shi, Y.; Joeng, K.S.; Arbeit, J.M.; Shohet, R.V.; Long, F. Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc. Natl. Acad. Sci. USA 2014, 111, 8673–8678. [Google Scholar] [CrossRef]
- Xu, W.N.; Zheng, H.L.; Yang, R.Z.; Jiang, L.S.; Jiang, S.D. HIF-1alpha Regulates Glucocorticoid-Induced Osteoporosis Through PDK1/AKT/mTOR Signaling Pathway. Front. Endocrinol. 2019, 10, 922. [Google Scholar] [CrossRef]
- Dirckx, N.; Tower, R.J.; Mercken, E.M.; Vangoitsenhoven, R.; Moreau-Triby, C.; Breugelmans, T.; Nefyodova, E.; Cardoen, R.; Mathieu, C.; Van der Schueren, B.; et al. Vhl deletion in osteoblasts boosts cellular glycolysis and improves global glucose metabolism. J. Clin. Investig. 2018, 128, 1087–1105. [Google Scholar] [CrossRef]
- Shum, L.C.; White, N.S.; Mills, B.N.; Bentley, K.L.; Eliseev, R.A. Energy Metabolism in Mesenchymal Stem Cells During Osteo-genic Differentiation. Stem Cells Dev. 2016, 25, 114–122. [Google Scholar] [CrossRef]
- Ke, W.; Ma, L.; Wang, B.; Song, Y.; Luo, R.; Li, G.; Liao, Z.; Shi, Y.; Wang, K.; Feng, X.; et al. N-cadherin mimetic hydrogel enhances MSC chondrogenesis through cell metabolism. Acta Biomater. 2022, 150, 83–95. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Sanz-Ros, J.; Román-Domínguez, A.; Gimeno-Mallench, L.; Inglés, M.; Viña, J.; Borrás, C. Extracellular Vesicles from Healthy Cells Improves Cell Function and Stemness in Premature Senescent Stem Cells by miR-302b and HIF-1α Acti-vation. Biomolecules 2020, 10, 957. [Google Scholar] [CrossRef]
- Riddle, R.C.; Leslie, J.M.; Gross, T.S.; Clemens, T.L. Hypoxia-inducible Factor-1α Protein Negatively Regulates Load-induced Bone Formation*. J. Biol. Chem. 2011, 286, 44449–44456. [Google Scholar] [CrossRef]
- Zhao, H.; Yeersheng, R.; Xia, Y.; Kang, P.; Wang, W. Hypoxia Enhanced Bone Regeneration Through the HIF-1alpha/beta-Catenin Pathway in Femoral Head Osteonecrosis. Am. J. Med. Sci. 2021, 362, 78–91. [Google Scholar] [CrossRef]
- Sheng, H.; Lao, Y.; Zhang, S.; Ding, W.; Lu, D.; Xu, B. Combined Pharmacotherapy with Alendronate and Desferoxamine Regu-late the Bone Resorption and Bone Regeneration for Preventing Glucocorticoids-Induced Osteonecrosis of the Femoral Head. Biomed. Res. Int. 2020, 2020, 3120458. [Google Scholar] [CrossRef]
- Yuan, Y.; Hilliard, G.; Ferguson, T.; Millhorn, D.E. Cobalt Inhibits the Interaction between Hypoxia-inducible Factor-α and von Hippel-Lindau Protein by Direct Binding to Hypoxia-inducible Factor-α. J. Biol. Chem. 2003, 278, 15911–15916. [Google Scholar] [CrossRef]
- Hewitson, K.S.; McNeill, L.A.; Riordan, M.V.; Tian, Y.-M.; Bullock, A.N.; Welford, R.W.; Elkins, J.M.; Oldham, N.J.; Bhattacharya, S.; Gleadle, J.M.; et al. Hypoxia-inducible Factor (HIF) Asparagine Hydroxylase Is Identical to Factor Inhibiting HIF (FIH) and Is Related to the Cupin Structural Family. J. Biol. Chem. 2002, 277, 26351–26355. [Google Scholar] [CrossRef]
- Loboda, A.; Jazwa, A.; Wegiel, B.; Jozkowicz, A.; Dulak, J. Heme oxygenase-1-dependent and -independent regulation of angi-ogenic genes expression: Effect of cobalt protoporphyrin and cobalt chloride on VEGF and IL-8 synthesis in human microvas-cular endothelial cells. Cell Mol. Biol. 2005, 51, 347–355. [Google Scholar] [PubMed]
- Yu, X.; Wan, Q.; Cheng, G.; Cheng, X.; Zhang, J.; Pathak, J.L.; Li, Z. CoCl, a mimic of hypoxia, enhances bone marrow mesen-chymal stem cells migration and osteogenic differentiation via STAT3 signaling pathway. Cell Biol. Int. 2018, 42, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wan, Q.; Ye, X.; Cheng, Y.; Pathak, J.L.; Li, Z. Cellular hypoxia promotes osteogenic differentiation of mesenchymal stem cells and bone defect healing via STAT3 signaling. Cell. Mol. Biol. Lett. 2019, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Pacary, E.; Legros, H.; Valable, S.; Duchatelle, P.; Lecocq, M.; Petit, E.; Nicole, O.; Bernaudin, M. Synergistic effects of CoCl2 and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J. Cell Sci. 2006, 119, 2667–2678. [Google Scholar] [CrossRef]
- Fan, W.; Crawford, R.; Xiao, Y. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stro-mal cells in a tissue engineered periosteum model. Biomaterials 2010, 31, 3580–3589. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaf-folds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Jell, G.; O’Donnell, M.D.; Law, R.V.; Hill, R.G.; Stevens, M.M. Synthesis and characterization of hypoxia-mimicking bioactive glasses for skeletal regeneration. J. Mater. Chem. 2010, 20, 8854–8864. [Google Scholar] [CrossRef]
- Badat, M.; Kaya, B.; Telfer, P. Combination-therapy with concurrent deferoxamine and deferiprone is effective in treating re-sistant cardiac iron-loading in aceruloplasminaemia. Br. J. Haematol. 2015, 171, 430–432. [Google Scholar] [CrossRef]
- Smith, G.C.; Alpendurada, F.; Carpenter, J.P.; Alam, M.H.; Berdoukas, V.; Karagiorga, M.; Ladis, V.; Piga, A.; Aessopos, A.; Gotsis, E.D.; et al. Effect of deferiprone or deferoxamine on right ventricular function in thalassemia major patients with myocardial iron overload. J. Cardiovasc. Magn. Reson. 2011, 13, 34. [Google Scholar] [CrossRef]
- Sorond, F.A.; Tan, C.O.; LaRose, S.; Monk, A.D.; Fichorova, R.; Ryan, S.; Lipsitz, L.A. Deferoxamine, Cerebrovascular Hemody-namics, and Vascular Aging: Potential Role for Hypoxia-Inducible Transcription Factor-1-Regulated Pathways. Stroke 2015, 46, 2576–2583. [Google Scholar] [CrossRef][Green Version]
- Matsunaga, K.; Fujisawa, K.; Takami, T.; Burganova, G.; Sasai, N.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. NUPR1 acts as a pro-survival factor in human bone marrow-derived mesenchymal stem cells and is induced by the hypoxia mimetic reagent deferoxamine. J. Clin. Biochem. Nutr. 2019, 64, 209–216. [Google Scholar] [CrossRef]
- Shadid, M.; Buonocore, G.; Groenendaal, F.; Moison, R.; Ferrali, M.; Berger, H.M.; van Bel, F. Effect of deferoxamine and allopu-rinol on non-protein-bound iron concentrations in plasma and cortical brain tissue of newborn lambs following hypoxia-ischemia. Neurosci. Lett. 1998, 248, 5–8. [Google Scholar] [CrossRef]
- Bartolome, S.; Dhillon, N.K.; Buch, S.; Casillan, A.J.; Wood, J.G.; O’Brien-Ladner, A.R. Deferoxamine mimics the pattern of hy-poxia-related injury at the microvasculature. Shock 2009, 31, 481–485. [Google Scholar] [CrossRef]
- Hou, Z.; Nie, C.; Si, Z.; Ma, Y. Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia-inducible factor-1α. Diabetes Res. Clin. Pract. 2013, 101, 62–71. [Google Scholar] [CrossRef]
- Xie, P.; Yang, L.; Talaiti, A.; Wu, J.J.; Yu, J.; Yu, T.; Wang, H.Y.; Huang, B.; Wu, Q.; Maimaitili, Y.; et al. Deferoxamine-activated hypoxia-inducible factor-1 restores cardioprotective effects of sevoflurane postconditioning in diabetic rats. Acta Physiol. 2017, 221, 98–114. [Google Scholar] [CrossRef]
- Choi, C.W.; Lee, J.; Lee, H.J.; Park, H.-S.; Chun, Y.-S.; Kim, B.I. Deferoxamine Improves Alveolar and Pulmonary Vascular De-velopment by Upregulating Hypoxia-inducible Factor-1α in a Rat Model of Bronchopulmonary Dysplasia. J. Korean Med. Sci. 2015, 30, 1295–1301. [Google Scholar] [CrossRef]
- Du, R.; Zhao, J.; Wen, Y.; Zhu, Y.; Jiang, L. Deferoxamine enhances the migration of dental pulp cells via hypoxia-inducible factor 1α. Am. J. Transl. Res. 2021, 13, 4780–4787. [Google Scholar]
- Oses, C.; Olivares, B.; Ezquer, M.; Acosta, C.; Bosch, P.; Donoso, M.; Léniz, P.; Ezquer, F. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS ONE 2017, 12, e0178011. [Google Scholar] [CrossRef]
- Nouri, F.; Salehinejad, P.; Nematollahi-Mahani, S.N.; Kamarul, T.; Zarrindast, M.R.; Sharifi, A.M. Deferoxamine Precondition-ing of Neural-Like Cells Derived from Human Wharton’s Jelly Mesenchymal Stem Cells as a Strategy to Promote Their Toler-ance and Therapeutic Potential: An In Vitro Study. Cell Mol. Neurobiol. 2016, 36, 689–700. [Google Scholar] [CrossRef]
- Groenendaal, F.; Shadid, M.; McGowan, J.E.; Mishra, O.P.; van Bel, F. Effects of deferoxamine, a chelator of free iron, on NA(+), K(+)-ATPase activity of cortical brain cell membrane during early reperfusion after hypoxia-ischemia in newborn lambs. Pediatr. Res. 2000, 48, 560–564. [Google Scholar] [CrossRef]
- Vrtačnik, P.; Marc, J.; Ostanek, B. Hypoxia mimetic deferoxamine influences the expression of histone acetylation- and DNA methylation-associated genes in osteoblasts. Connect. Tissue Res. 2015, 56, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Donneys, A.; Weiss, D.M.; Deshpande, S.S.; Ahsan, S.; Tchanque-Fossuo, C.N.; Sarhaddi, D.; Levi, B.; Goldstein, S.A.; Buchman, S.R. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone 2013, 52, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Farberg, A.S.; Jing, X.L.; Monson, L.A.; Donneys, A.; Tchanque-Fossuo, C.N.; Deshpande, S.S.; Buchman, S.R. Deferoxamine reverses radiation induced hypovascularity during bone regeneration and repair in the murine mandible. Bone 2012, 50, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Stefanowski, J.; Pfeiffenberger, M.; Wolter, A.; Damerau, A.; Hemmati-Sadeghi, S.; Haag, R.; Hauser, A.E.; Lohning, M.; Duda, G.N.; et al. MIF does only marginally enhance the pro-regenerative capaci-ties of DFO in a mouse-osteotomy-model of compromised bone healing conditions. Bone 2022, 154, 116247. [Google Scholar] [CrossRef] [PubMed]
- Messer, J.G.; Cooney, P.T.; Kipp, D.E. Iron chelator deferoxamine alters iron-regulatory genes and proteins and suppresses osteoblast phenotype in fetal rat calvaria cells. Bone 2010, 46, 1408–1415. [Google Scholar] [CrossRef]
- Chen, B.; Yan, Y.-L.; Liu, C.; Bo, L.; Li, G.; Wang, H.; Xu, Y.-J. Therapeutic Effect of Deferoxamine on Iron Overload-Induced Inhibition of Osteogenesis in a Zebrafish Model. Calcif. Tissue Res. 2014, 94, 353–360. [Google Scholar] [CrossRef]
- Chung, J.H.; Kim, Y.S.; Noh, K.; Lee, Y.M.; Chang, S.W.; Kim, E.C. Deferoxamine promotes osteoblastic differentiation in hu-man periodontal ligament cells via the nuclear factor erythroid 2-related factor-mediated antioxidant signaling pathway. J. Periodontal Res. 2014, 49, 563–573. [Google Scholar] [CrossRef]
- Lee, K.E.; Mo, S.; Lee, H.-S.; Jeon, M.; Song, J.S.; Choi, H.-J.; Cho, H.; Kang, C.-M. Deferoxamine Reduces Inflammation and Os-teoclastogenesis in Avulsed Teeth. Int. J. Mol. Sci. 2021, 22, 8225. [Google Scholar]
- Guo, C.; Yang, K.; Yan, Y.; Yan, D.; Cheng, Y.; Yan, X.; Qian, N.; Zhou, Q.; Chen, B.; Jiang, M.; et al. SF-deferoxamine, a bone-seeking angiogenic drug, prevents bone loss in estrogen-deficient mice. Bone 2019, 120, 156–165. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, W.; Ding, C.; Yao, G.; Zhao, H.; Wu, S. Deferoxamine inhibits iron-uptake stimulated osteoclast differentiation by suppressing electron transport chain and MAPKs signaling. Toxicol. Lett. 2019, 313, 50–59. [Google Scholar] [CrossRef]
- Fan, L.; Li, J.; Yu, Z.; Dang, X.; Wang, K. Hypoxia-inducible factor prolyl hydroxylase inhibitor prevents steroid-associated oste-onecrosis of the femoral head in rabbits by promoting angiogenesis and inhibiting apoptosis. PLoS ONE 2014, 9, e107774. [Google Scholar] [CrossRef]
- Li, L.; Li, A.; Zhu, L.; Gan, L.; Zuo, L. Roxadustat promotes osteoblast differentiation and prevents estrogen deficiency-induced bone loss by stabilizing HIF-1α and activating the Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 2022, 17, 286. [Google Scholar] [CrossRef]
- Deppe, J.; Popp, T.; Egea, V.; Steinritz, D.; Schmidt, A.; Thiermann, H.; Weber, C.; Ries, C. Impairment of hypoxia-induced HIF-1α signaling in keratinocytes and fibroblasts by sulfur mustard is counteracted by a selective PHD-2 inhibitor. Arch. Toxicol. 2015, 90, 1141–1150. [Google Scholar] [CrossRef]
- Peng, J.; Lai, Z.G.; Fang, Z.L.; Xing, S.; Hui, K.; Hao, C.; Jin, Q.; Qi, Z.; Shen, W.J.; Dong, Q.N.; et al. Dimethyloxalyl-glycine prevents bone loss in ovariectomized C57BL/6J mice through enhanced angiogenesis and osteogenesis. PLoS ONE 2014, 9, e112744. [Google Scholar] [CrossRef]
- Zhou, B.; Ge, T.; Zhou, L.; Jiang, L.; Zhu, L.; Yao, P.; Yu, Q. Dimethyloxalyl Glycine Regulates the HIF-1 Signaling Pathway in Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2020, 16, 702–710. [Google Scholar] [CrossRef]
- Woo, K.M.; Jung, H.-M.; Oh, J.-H.; Rahman, S.U.; Kim, S.M.; Baek, J.-H.; Ryoo, H.-M. Synergistic effects of dimethyloxalylglycine and butyrate incorporated into α-calcium sulfate on bone regeneration. Biomaterials 2015, 39, 1–14. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Z.; Wei, J.; Yu, Y.; Luo, J.; Zhou, J.; Li, Y.; Zheng, X.; Tang, W.; Liu, L.; et al. Repair of Critical-Sized Mandible Defects in Aged Rat Using Hypoxia Preconditioned BMSCs with Up-regulation of Hif-1α. Int. J. Biol. Sci. 2018, 14, 449–460. [Google Scholar] [CrossRef]
- Wang, X.; Wei, L.; Li, Q.; Lai, Y. HIF-1α protects osteoblasts from ROS-induced apoptosis. Free Radic. Res. 2022, 56, 143–153. [Google Scholar] [CrossRef]
- Abu-Shahba, A.G.; Gebraad, A.; Kaur, S.; Paananen, R.O.; Peltoniemi, H.; Seppanen-Kaijansinkko, R.; Mannerstrom, B. Proan-giogenic Hypoxia-Mimicking Agents Attenuate Osteogenic Potential of Adipose Stem/Stromal Cells. Tissue Eng. Regen. Med. 2020, 17, 477–493. [Google Scholar] [CrossRef]
- Ding, H.; Gao, Y.-S.; Wang, Y.; Hu, C.; Sun, Y.; Zhang, C. Dimethyloxaloylglycine Increases the Bone Healing Capacity of Adipose-Derived Stem Cells by Promoting Osteogenic Differentiation and Angiogenic Potential. Stem Cells Dev. 2014, 23, 990–1000. [Google Scholar] [CrossRef]
- Weng, T.; Xie, Y.; Huang, J.; Luo, F.; Yi, L.; He, Q.; Chen, D.; Chen, L. Inactivation of Vhl in osteochondral progenitor cells causes high bone mass phenotype and protects against age-related bone loss in adult mice. J. Bone Miner. Res. 2014, 29, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Jiang, W.; Phillips, F.M.; Haydon, R.C.; Peng, Y.; Zhou, L.; Luu, H.H.; An, N.; Breyer, B.; Vanichakarn, P.; et al. Osteogenic Activity of the Fourteen Types of Human Bone Morphogenetic Proteins (BMPS). J. Bone Jt. Surg. 2003, 85, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Celeste, A.J.; Kong, F.M.; Jirtle, R.L.; Rosen, V.; Thies, R.S. Bone morphogenetic protein-9 binds to liver cells and stimu-lates proliferation. Endocrinology 1995, 136, 4293–4297. [Google Scholar] [CrossRef] [PubMed]
- Castonguay, R.; Werner, E.D.; Matthews, R.G.; Presman, E.; Mulivor, A.W.; Solban, N.; Sako, D.; Pearsall, R.S.; Underwood, K.W.; Seehra, J.; et al. Soluble Endoglin Specifically Binds Bone Morphogenetic Proteins 9 and 10 via Its Orphan Domain, Inhibits Blood Vessel Formation, and Suppresses Tumor Growth. J. Biol. Chem. 2011, 286, 30034–30046. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.I.; Pardali, E.; Thorikay, M.; Anderberg, C.; Hawinkels, L.; Goumans, M.-J.; Seehra, J.; Heldin, C.-H.; Dijke, P.T.; Pietras, K. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J. Exp. Med. 2010, 207, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Jiang, D.; Huang, E.; Liu, X.; Li, R.; Liang, X.; Kim, S.H.; Chen, X.; Gao, J.L.; Zhang, H.; et al. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal pro-genitor cells. J. Cell Sci. 2013, 126, 532–541. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, C.; Deng, L.; Liu, X.; Cao, X.; Gilbert, S.R.; Bouxsein, M.L.; Faugere, M.-C.; Guldberg, R.E.; Gerstenfeld, L.C.; et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteo-genesis during skeletal development. J. Clin. Investig. 2007, 117, 1616–1626. [Google Scholar] [CrossRef]
- Zhang, L.-F.; Qi, J.; Zuo, G.; Jia, P.; Shen, X.; Shao, J.; Kang, H.; Yang, H.; Deng, L. Osteoblast-secreted factors promote prolifera-tion and osteogenic differentiation of bone marrow stromal cells via VEGF/heme-oxygenase-1 pathway. PLoS ONE 2014, 9, e99946. [Google Scholar]
- Zhao, Q.; Shen, X.; Zhang, W.; Zhu, G.; Qi, J.; Deng, L. Mice with increased angiogenesis and osteogenesis due to conditional activation of HIF pathway in osteoblasts are protected from ovariectomy induced bone loss. Bone 2012, 50, 763–770. [Google Scholar] [CrossRef]
- Zuo, G.-L.; Zhang, L.-F.; Qi, J.; Kang, H.; Jia, P.; Chen, H.; Shen, X.; Guo, L.; Zhou, H.-B.; Wang, J.-S.; et al. Activation of HIFa Pathway in Mature Osteoblasts Disrupts the Integrity of the Osteocyte/Canalicular Network. PLoS ONE 2015, 10, e0121266. [Google Scholar] [CrossRef]
- Loots, G.G.; Robling, A.G.; Chang, J.C.; Murugesh, D.K.; Bajwa, J.; Carlisle, C.; Manilay, J.O.; Wong, A.; Yellowley, C.E.; Genetos, D.C. Vhl deficiency in osteocytes produces high bone mass and hematopoietic defects. Bone 2018, 116, 307–314. [Google Scholar] [CrossRef]
- Mangiavini, L.; Merceron, C.; Araldi, E.; Khatri, R.; Gerard-O’Riley, R.; Wilson, T.L.; Rankin, E.B.; Giaccia, A.J.; Schipani, E. Loss of VHL in mesenchymal progenitors of the limb bud alters multiple steps of endochondral bone development. Dev. Biol. 2014, 393, 124–136. [Google Scholar] [CrossRef][Green Version]
- Pfander, D.; Kobayashi, T.; Knight, M.C.; Zelzer, E.; Chan, D.A.; Olsen, B.R.; Giaccia, A.J.; Johnson, R.S.; Haase, V.H.; Schipani, E. Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development 2004, 131, 2497–2508. [Google Scholar] [CrossRef]
- Rivera, J.C.; Madaan, A.; Zhou, T.E.; Chemtob, S. Review of the mechanisms and therapeutic avenues for retinal and choroidal vascular dysfunctions in retinopathy of prematurity. Acta Paediatr. 2016, 105, 1421–1433. [Google Scholar] [CrossRef]
- Rubio, R.G.; Adamis, A.P. Ocular Angiogenesis: Vascular Endothelial Growth Factor and Other Factors. Basic Sci. Retin. 2015, 55, 28–37. [Google Scholar] [CrossRef]
- Palazon, A.; Tyrakis, P.A.; Macias, D.; Veliça, P.; Rundqvist, H.; Fitzpatrick, S.; Vojnovic, N.; Phan, A.T.; Loman, N.; Hedenfalk, I.; et al. An HIF-1α/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell 2017, 32, 669–683.e5. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, X.; Hu, Y.; Yang, B.; Tsui, C.-K.; Yu, S.; Lu, L.; Liang, X. Melatonin attenuated retinal neovascularization and neu-roglial dysfunction by inhibition of HIF-1α-VEGF pathway in oxygen-induced retinopathy mice. J. Pineal Res. 2018, 64, e12473. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Wang, L.; Chen, S.; Tian, B.; Huang, K.; Corrigan, C.J.; Ying, S.; Wang, W.; Wang, C. IL-33 Initiates Vascular Remodelling in Hypoxic Pulmonary Hypertension by up-Regulating HIF-1α and VEGF Expression in Vascular Endothelial Cells. eBioMedicine 2018, 33, 196–210. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, C.; Chen, C.; Zong, Y.; Feng, H.; Liu, D.; Feng, W.; Zhao, J.; Lu, A. CCL19 suppresses angiogenesis through promot-ing miR-206 and inhibiting Met/ERK/Elk-1/HIF-1α/VEGF-A pathway in colorectal cancer. Cell Death Dis. 2018, 9, 974. [Google Scholar] [CrossRef]
- Fan, J.; Lv, H.; Li, J.; Che, Y.; Xu, B.; Tao, Z.; Jiang, W. Roles of Nrf2/HO-1 and HIF-1α/VEGF in lung tissue injury and repair following cerebral ischemia/reperfusion injury. J. Cell. Physiol. 2019, 234, 7695–7707. [Google Scholar] [CrossRef]
- Lin, C.-J.; Lan, Y.-M.; Ou, M.-Q.; Ji, L.-Q.; Lin, S.-D. Expression of miR-217 and HIF-1α/VEGF pathway in patients with diabetic foot ulcer and its effect on angiogenesis of diabetic foot ulcer rats. J. Endocrinol. Investig. 2019, 42, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Jiang, C.; Xu, L.; Chen, D.; Liu, H.; Xu, Y.; Ma, K.; Wang, M. Ginsenoside protects against AKI via activation of HIF-1α and VEGF-A in the kidney-brain axis. Int. J. Mol. Med. 2020, 45, 939–946. [Google Scholar] [CrossRef]
- Hepp, M.; Werion, A.; De Greef, A.; Goyet, C.D.V.D.; de Bournonville, M.; Behets, C.; Lengelé, B.; Daumerie, C.; Mourad, M.; Ludgate, M.; et al. Oxidative Stress-Induced Sirtuin1 Downregulation Correlates to HIF-1α, GLUT-1, and VEGF-A Upregulation in Th1 Autoimmune Hashimoto’s Thyroiditis. Int. J. Mol. Sci. 2021, 22, 3806. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-T.; Yang, T.; Hou, X.-Q.; Wu, H.-Y.; Feng, J.-T.; Ou, B.-J.; Cai, S.-J.; Li, J.; Mei, Z.-G. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomed. Pharmacother. 2019, 113, 108759. [Google Scholar] [CrossRef] [PubMed]
- Westra, J.; Molema, G.; Kallenberg, C.G.M. Hypoxia-Inducible Factor-1 as Regulator of Angiogenesis in Rheumatoid Arthritis—Therapeutic Implications. Curr. Med. Chem. 2010, 17, 254–263. [Google Scholar] [CrossRef]
- Veale, D.J.; Orr, C.; Fearon, U. Cellular and molecular perspectives in rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 343–354. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Brown, N.J.; Jones, R.; Lewis, C.E.; Mujamammi, A.H.; Muthana, M.; Seed, M.P.; Barker, M.D. A peptide derived from TIMP-3 inhibits multiple angiogenic growth factor receptors and tumour growth and inflammatory arthritis in mice. Angiogenesis 2013, 17, 207–219. [Google Scholar] [CrossRef]
- Chai, M.; Gu, C.; Shen, Q.; Liu, J.; Zhou, Y.; Jin, Z.; Xiong, W.; Zhou, Y.; Tan, W. Hypoxia alleviates dexamethasone-induced inhi-bition of angiogenesis in cocultures of HUVECs and rBMSCs via HIF-1alpha. Stem Cell Res. Ther. 2020, 11, 343. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014, 507, 376–380. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, F.; Zhang, P.; Wang, H.; Qu, Z.; Jia, P.; Yao, Z.; Shen, G.; Li, G.; Zhao, G.; et al. Human type H vessels are a sensitive biomarker of bone mass. Cell Death Dis. 2017, 8, e2760. [Google Scholar] [CrossRef]
- García-Martín, A.; Acitores, A.; Maycas, M.; Villanueva-Peñacarrillo, M.L.; Esbrit, P. Src kinases mediate VEGFR2 transactiva-tion by the osteostatin domain of PTHrP to modulate osteoblastic function. J. Cell. Biochem. 2013, 114, 1404–1413. [Google Scholar] [CrossRef]
- Wu, D.; Liu, L.; Fu, S.; Zhang, J. Osteostatin improves the Osteogenic differentiation of mesenchymal stem cells and enhances angiogenesis through HIF-1α under hypoxia conditions in vitro. Biochem. Biophys. Res. Commun. 2022, 606, 100–107. [Google Scholar] [CrossRef]
- Gao, B.; Lin, X.; Jing, H.; Fan, J.; Ji, C.; Jie, Q.; Zheng, C.; Wang, D.; Xu, X.; Hu, Y.; et al. Local delivery of tetra-methylpyrazine eliminates the senescent phenotype of bone marrow mesenchymal stromal cells and creates an anti-inflammatory and angiogenic environment in aging mice. Aging Cell 2018, 17, e12741. [Google Scholar] [CrossRef]
- Liu, X.-D.; Cai, F.; Liu, L.; Zhang, Y.; Yang, A.-L. microRNA-210 is involved in the regulation of postmenopausal osteoporosis through promotion of VEGF expression and osteoblast differentiation. Biol. Chem. 2015, 396, 339–347. [Google Scholar] [CrossRef]
- Yang, M.; Li, C.-J.; Sun, X.; Guo, Q.; Xiao, Y.; Su, T.; Tu, M.-L.; Peng, H.; Lu, Q.; Liu, Q.; et al. MiR-497∼195 cluster regulates angiogenesis during coupling with osteogenesis by maintaining endothelial Notch and HIF-1α activity. Nat. Commun. 2017, 8, 16003. [Google Scholar] [CrossRef]
- Weinstein, R.S.; Hogan, E.A.; Borrelli, M.J.; Liachenko, S.; O’Brien, C.A.; Manolagas, S.C. The Pathophysiological Sequence of Glucocorticoid-Induced Osteonecrosis of the Femoral Head in Male Mice. Endocrinology 2017, 158, 3817–3831. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, K.; Wang, C.; Xue, D.; Pan, Z. The Effect of MMP-2 Inhibitor 1 on Osteogenesis and Angiogenesis During Bone Regeneration. Front. Cell Dev. Biol. 2021, 8, 596783. [Google Scholar] [CrossRef]
- Yamauchi, N.; Taguchi, Y.; Kato, H.; Umeda, M. High-power, red-light-emitting diode irradiation enhances proliferation, osteogenic differentiation, and mineralization of human periodontal ligament stem cells via ERK signaling pathway. J. Periodontol. 2018, 89, 351–360. [Google Scholar] [CrossRef]
- Sarvestani, F.K.; Dehno, N.S.; Nazhvani, S.D.; Bagheri, M.H.; Abbasi, S.; Khademolhosseini, Y.; Gorji, E. Effect of low-level laser therapy on fracture healing in rabbits. Laser Ther. 2017, 26, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Escudero, J.S.B.; Perez, M.G.B.; Rosso, M.P.D.O.; Buchaim, D.V.; Pomini, K.T.; Campos, L.M.G.; Audi, M.; Buchaim, R.L. Photobiomodulation therapy (PBMT) in bone repair: A systematic review. Injury 2019, 50, 1853–1867. [Google Scholar] [CrossRef]
- Chen, C.; Yan, S.; Qiu, S.; Geng, Z.; Wang, Z. HIF/Ca2+/NO/ROS is critical in roxadustat treating bone fracture by stimulating the proliferation and migration of BMSCs. Life Sci. 2021, 264, 118684. [Google Scholar] [CrossRef] [PubMed]
- Migliario, M.; Pittarella, P.; Fanuli, M.; Rizzi, M.; Renò, F. Laser-induced osteoblast proliferation is mediated by ROS production. Lasers Med. Sci. 2014, 29, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, L.; Kou, N.; Bai, Y.; Zhang, Y.; Lu, Y.; Gao, L.; Wang, F. Low level laser therapy promotes bone regeneration by cou-pling angiogenesis and osteogenesis. Stem Cell Res. Ther. 2021, 12, 432. [Google Scholar] [CrossRef]
- Li, Z.; Yang, B.; Weng, X.; Tse, G.; Chan, M.T.V.; Wu, W.K.K. Emerging roles of MicroRNAs in osteonecrosis of the femoral head. Cell Prolif. 2017, 51, e12405. [Google Scholar] [CrossRef]
- Yang, C.; Liu, X.; Zhao, K.; Zhu, Y.; Hu, B.; Zhou, Y.; Wang, M.; Wu, Y.; Zhang, C.; Xu, J.; et al. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone regeneration in critical size defects. Stem Cell Res. Ther. 2019, 10, 65. [Google Scholar] [CrossRef]
- Costa, V.; Raimondi, L.; Conigliaro, A.; Salamanna, F.; Carina, V.; de Luca, A.; Bellavia, D.; Alessandro, R.; Fini, M.; Giavaresi, G. Hypoxia-inducible factor 1A may regulate the commitment of mesenchymal stromal cells toward angio-osteogenesis by mir-na-675-5P. Cytotherapy 2017, 19, 1412–1425. [Google Scholar] [CrossRef]
- Costa, V.; Carina, V.; Conigliaro, A.; Raimondi, L.; De Luca, A.; Bellavia, D.; Salamanna, F.; Setti, S.; Alessandro, R.; Fini, M.; et al. miR-31-5p Is a LIPUS-Mechanosensitive MicroRNA that Targets HIF-1α Signaling and Cytoskeletal Proteins. Int. J. Mol. Sci. 2019, 20, 1569. [Google Scholar] [CrossRef]
- Zhang, D.; Du, J.; Yu, M.; Suo, L. Urine-derived stem cells-extracellular vesicles ameliorate diabetic osteoporosis through HDAC4/HIF-1α/VEGFA axis by delivering microRNA-26a-5p. Cell Biol. Toxicol. 2022. [Google Scholar] [CrossRef]
- Li, Z.; Huang, C.; Yang, B.; Hu, W.; Chan, M.T.; Wu, W.K.K. Emerging roles of long non-coding RNAs in osteonecrosis of the femoral head. Am. J. Transl. Res. 2020, 12, 5984–5991. [Google Scholar]
- Tian, Y.; Shao, Q.; Gu, J.; Tang, Y.; Bie, M.; Zhou, Y.; Cheng, C.; Liang, Y.; Zhang, Q.; Kang, F. LncRNA-mRNA Expression Pro-files of Osteoclast After Conditional Knockout HIF-1α. Front. Genet. 2022, 13, 909095. [Google Scholar] [CrossRef]
- Zhang, Z.-C.; Tang, C.; Dong, Y.; Zhang, J.; Yuan, T.; Tao, S.-C.; Li, X.-L. Targeting the long noncoding RNA MALAT1 blocks the pro-angiogenic effects of osteosarcoma and suppresses tumour growth. Int. J. Biol. Sci. 2017, 13, 1398–1408. [Google Scholar] [CrossRef]
- Saigusa, N.; Hirai, H.; Tada, Y.; Kawakita, D.; Nakaguro, M.; Tsukahara, K.; Kano, S.; Ozawa, H.; Kondo, T.; Okami, K.; et al. The Role of the EZH2 and H3K27me3 Expression as a Predictor of Clinical Outcomes in Salivary Duct Carcinoma Patients: A Large-Series Study with Emphasis on the Relevance to the Combined Androgen Blockade and HER2-Targeted Therapy. Front. Oncol. 2022, 11, 779882. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, H.; Peng, W.; Wang, L.; Wang, T.; Xie, Z.; Zhang, J.; Dong, W.; Zheng, X.; Liu, G.; et al. Hy-poxic condition induced H3K27me3 modification of the LncRNA Tmem235 promoter thus supporting apoptosis of BMSCs. Apoptosis 2022, 27, 762–777. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Tang, C.; Chen, W. Upregulation of long non-coding RNA HIF 1α-anti-sense 1 induced by transforming growth factor-β-mediated targeting of sirtuin 1 promotes osteoblastic differentiation of human bone marrow stromal cells. Mol. Med. Rep. 2015, 12, 7233–7238. [Google Scholar] [CrossRef]
- Tan, Z.; Zhou, B.; Zheng, J.; Huang, Y.; Zeng, H.; Xue, L.; Wang, D. Lithium and Copper Induce the Osteogenesis-Angiogenesis Coupling of Bone Marrow Mesenchymal Stem Cells via Crosstalk between Canonical Wnt and HIF-1alpha Signaling Path-ways. Stem Cells Int. 2021, 2021, 6662164. [Google Scholar] [CrossRef]
- Li, J.-Y.; Wang, T.-T.; Li, C.; Wang, Z.-F.; Li, S.; Ma, L.; Zheng, L.-L. Semaphorin 3A-hypoxia inducible factor 1 subunit alpha co-overexpression enhances the osteogenic differentiation of induced pluripotent stem cells-derived mesenchymal stem cells in vitro. Chin. Med. J. 2020, 133, 301–309. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Li, C.; Wang, Z.; Wang, P.; Zheng, L. Sema3A and HIF1alpha co-overexpressed iPSC-MSCs/HA scaffold facili-tates the repair of calvarial defect in a mouse model. J. Cell Physiol. 2020, 235, 6754–6766. [Google Scholar] [CrossRef]
- Wang, Z.; Han, T.; Zhu, H.; Tang, J.; Guo, Y.; Jin, Y.; Wang, Y.; Chen, G.; Gu, N.; Wang, C. Potential Osteoinductive Effects of Hydroxyapatite Nanoparticles on Mesenchymal Stem Cells by Endothelial Cell Interaction. Nanoscale Res. Lett. 2021, 16, 67. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Z.; Wei, Z.; Li, D.; Luo, Y.; Kang, P. Copper-Lithium-Doped Nanohydroxyapatite Modulates Mesenchymal Stem Cells Homing to Treat Glucocorticoids-Related Osteonecrosis of the Femoral Head. Front. Bioeng. Biotechnol. 2022, 10, 916562. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lei, Y.; Hu, Q.; Li, D.; Zhao, H.; Kang, P. Porous copper- and lithium-doped nano-hydroxyapatite composite scaffold promotes angiogenesis and bone regeneration in the repair of glucocorticoids-induced osteonecrosis of the femoral head. Biomed. Mater. 2021, 16, 065012. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huifang, L.; Zhao, J.; Yang, Z.; Xie, X.; Wei, Z.; Li, D.; Kang, P. Porous lithium-doped hydroxyapatite scaffold seeded with hypoxia-preconditioned bone-marrow mesenchymal stem cells for bone-tissue regeneration. Biomed. Mater. 2018, 13, 055002. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, J.; Zhang, W.; Qian, H.; She, W.; Pan, H.; Wen, J.; Zhang, X.; Liu, X.; Jiang, X. Antibacterial property, angiogenic and osteogenic activity of Cu-incorporated TiO2 coating. J. Mater. Chem. B 2014, 2, 6738–6748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chang, Q.; Xu, L.; Li, G.; Yang, G.; Ding, X.; Wang, X.; Cui, D.; Jiang, X. Graphene Oxide-Copper Nanocomposite-Coated Porous CaP Scaffold for Vascularized Bone Regeneration via Activation of Hif-1α. Adv. Healthc. Mater. 2016, 5, 1299–1309. [Google Scholar] [CrossRef]
- Wang, X.; Wu, C.; Qi, H.; Tian, M.; Xie, H.; Wang, Y.; Gu, Z.; Peng, X.; Yu, X. Introducing copper and collagen (via poly(DOPA)) coating to activate inert ceramic scaffolds for excellent angiogenic and osteogenic capacity. RSC Adv. 2018, 8, 15575–15586. [Google Scholar] [CrossRef]
- Zhao, H.; Liang, G.; Liang, W.; Li, Q.; Huang, B.; Li, A.; Qiu, D.; Jin, D. In vitro and in vivo evaluation of the pH-neutral bioac-tive glass as high performance bone grafts. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111249. [Google Scholar] [CrossRef]
- Dai, Q.; Li, Q.; Gao, H.; Yao, L.; Lin, Z.; Li, D.; Zhu, S.; Liu, C.; Yang, Z.; Wang, G.; et al. 3D printing of Cu-doped bioactive glass composite scaffolds promotes bone regeneration through activating the HIF-1α and TNF-α pathway of hUVECs. Biomater. Sci. 2021, 9, 5519–5532. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Jiang, J.; Lv, F.; Chang, J.; Chen, S.; Wu, C. An osteogenesis/angiogenesis-stimulation artificial ligament for anterior cruciate ligament reconstruction. Acta Biomater. 2017, 54, 399–410. [Google Scholar] [CrossRef]
- Kulanthaivel, S.; Roy, B.; Agarwal, T.; Giri, S.; Pramanik, K.; Pal, K.; Ray, S.S.; Maiti, T.K.; Banerjee, I. Cobalt doped proangiogen-ic hydroxyapatite for bone tissue engineering application. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 648–658. [Google Scholar] [CrossRef]
- Shah, K.; Dunning, M.; Gartland, A.; Wilkinson, J. Distinct Concentration-Dependent Molecular Pathways Regulate Bone Cell Responses to Cobalt and Chromium Exposure from Joint Replacement Prostheses. Int. J. Mol. Sci. 2021, 22, 5225. [Google Scholar] [CrossRef]
- Deng, Z.; Lin, B.; Jiang, Z.; Huang, W.; Li, J.; Zeng, X.; Wang, H.; Wang, D.; Zhang, Y. Hypoxia-Mimicking Cobalt-Doped Borosil-icate Bioactive Glass Scaffolds with Enhanced Angiogenic and Osteogenic Capacity for Bone Regeneration. Int. J. Biol. Sci. 2019, 15, 1113–1124. [Google Scholar] [CrossRef]
- Littmann, E.; Autefage, H.; Solanki, A.K.; Kallepitis, C.; Jones, J.R.; Alini, M.; Peroglio, M.; Stevens, M.M. Cobalt-containing bio-active glasses reduce human mesenchymal stem cell chondrogenic differentiation despite HIF-1α stabilization. J. Eur. Ceram. Soc. 2018, 38, 877–886. [Google Scholar] [CrossRef]
- Perez, R.; Kim, J.-H.; Buitrago, J.O.; Wall, I.B.; Kim, H.-W. Novel therapeutic core–shell hydrogel scaffolds with sequential delivery of cobalt and bone morphogenetic protein-2 for synergistic bone regeneration. Acta Biomater. 2015, 23, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, E.; Partap, S.; Azevedo, M.M.; Jell, G.; Stevens, M.M.; O’Brien, F.J. Hypoxia-mimicking bioactive glass/collagen gly-cosaminoglycan composite scaffolds to enhance angiogenesis and bone repair. Biomaterials 2015, 52, 358–366. [Google Scholar] [CrossRef]
- Kulanthaivel, S.; Agarwal, T.; Rathnam, V.S.; Pal, K.; Banerjee, I. Cobalt doped nano-hydroxyapatite incorporated gum tragacanth-alginate beads as angiogenic-osteogenic cell encapsulation system for mesenchymal stem cell based bone tissue engineering. Int. J. Biol. Macromol. 2021, 179, 101–115. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Rizvi, S.F.A.; Wasim, B.; Usman, S.; Borges, K.J.J.; Sahibdad, I.; Salim, A.; Khan, I. Zinc and hypoxic preconditioning: A strategy to enhance the functionality and therapeutic potential of bone marrow-derived mesenchymal stem cells. Mol. Cell. Biochem. 2022. [Google Scholar] [CrossRef]
- Gao, P.; Fan, B.; Yu, X.; Liu, W.; Wu, J.; Shi, L.; Yang, D.; Tan, L.; Wan, P.; Hao, Y.; et al. Biofunc-tional magnesium coated Ti6Al4V scaffold enhances osteogenesis and angiogenesis and for orthopedic application. Bioact. Mater. 2020, 5, 680–693. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, Z.; Lu, H.; Liu, Z.; Shang, P.; Zhang, J.; Yin, W.; Chu, H.; Yuan, R.; Ye, Y.; et al. Impact of High-Altitude Hypoxia on Early Osseointegration With Bioactive Titanium. Front. Physiol. 2021, 12, 689807. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Qiao, Y.; Peng, F.; Xia, C.; Qian, S.; Wang, T.; Sun, J.; Liu, X. Si-doped porous TiO2 coatings enhanced in vitro angiogenic behavior of human umbilical vein endothelial cells. Colloids Surf. B Biointerfaces 2017, 159, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Monte, F.; Cebe, T.; Ripperger, D.; Ighani, F.; Kojouharov, H.; Chen, B.M.; Kim, H.K.W.; Aswath, P.B.; Varanasi, V.G. Ionic silicon improves endothelial cells’ survival under toxic oxidative stress by overexpressing angiogenic markers and antioxidant enzymes. J. Tissue Eng. Regen. Med. 2018, 12, 2203–2220. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wei, Q.; Cao, B.; Cheng, X.; Tian, J.; Pu, H.; Yusufu, A.; Cao, L. A multifunctional bioactive material that stimulates oste-ogenesis and promotes the vascularization bone marrow stem cells and their resistance to bacterial infection. PLoS ONE 2017, 12, e0172499. [Google Scholar]
- Jia, P.; Chen, H.; Kang, H.; Qi, J.; Zhao, P.; Jiang, M.; Guo, L.; Zhou, Q.; Qian, N.D.; Zhou, H.B.; et al. Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angio-genesis and osteogenesis. J. Biomed. Mater. Res. A 2016, 104, 2515–2527. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Deng, L.; Qiu, S.; Deng, R. New bone formation in a true bone ceramic scaffold loaded with desferrioxamine in the treatment of segmental bone defect: A preliminary study. J. Orthop. Sci. 2012, 17, 289–298. [Google Scholar] [CrossRef]
- Stewart, R.; Goldstein, J.; Eberhardt, A.; Chu, G.T.-M.G.; Gilbert, S. Increasing Vascularity to Improve Healing of a Segmental Defect of the Rat Femur. J. Orthop. Trauma 2011, 25, 472–476. [Google Scholar] [CrossRef]
- Ran, Q.; Yu, Y.; Chen, W.; Shen, X.; Mu, C.; Yuan, Z.; Tao, B.; Hu, Y.; Yang, W.; Cai, K. Deferoxamine loaded titania nanotubes substrates regulate osteogenic and angiogenic differentiation of MSCs via activation of HIF-1α signaling. Mater. Sci. Eng. C 2018, 91, 44–54. [Google Scholar] [CrossRef]
- Li, H.; Luo, B.; Wen, W.; Zhou, C.; Tian, L.; Ramakrishna, S. Deferoxamine immobilized poly(D,L-lactide) membrane via poly-dopamine adhesive coating: The influence on mouse embryo osteoblast precursor cells and human umbilical vein endothelial cells. Mater. Sci. Engineering. C Mater. Biol. Appl. 2017, 70, 701–709. [Google Scholar] [CrossRef]
- Ding, H.; Chen, S.; Song, W.-Q.; Gao, Y.-S.; Guan, J.-J.; Wang, Y.; Sun, Y.; Zhang, C.-Q. Dimethyloxaloylglycine improves angio-genic activity of bone marrow stromal cells in the tissue-engineered bone. Int. J. Biol. Sci. 2014, 10, 746–756. [Google Scholar] [CrossRef]
- Jahangir, S.; Hosseini, S.; Mostafaei, F.; Sayahpour, F.A.; Eslaminejad, M.B. 3D-porous β-tricalcium phosphate–alginate–gelatin scaffold with DMOG delivery promotes angiogenesis and bone formation in rat calvarial defects. J. Mater. Sci. Mater. Med. 2018, 30, 1. [Google Scholar] [CrossRef]
- Shi, M.; Zhou, Y.; Shao, J.; Chen, Z.; Song, B.; Chang, J.; Wu, C.; Xiao, Y. Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater. 2015, 21, 178–189. [Google Scholar] [CrossRef]
- Qi, X.; Liu, Y.; Ding, Z.-Y.; Cao, J.-Q.; Huang, J.-H.; Zhang, J.-Y.; Jia, W.-T.; Wang, J.; Liu, C.-S.; Li, X.-L. Synergistic effects of di-methyloxallyl glycine and recombinant human bone morphogenetic protein-2 on repair of critical-sized bone defects in rats. Sci. Rep. 2017, 7, 42820. [Google Scholar] [CrossRef]
- Min, Z.; Shichang, Z.; Chen, X.; Yufang, Z.; Changqing, Z. 3D-printed dimethyloxallyl glycine delivery scaffolds to improve angiogenesis and osteogenesis. Biomater. Sci. 2015, 3, 1236–1244. [Google Scholar] [CrossRef]
- Jin, X.; Han, D.; Tao, J.; Huang, Y.; Zhou, Z.; Zhang, Z.; Qi, X.; Jia, W. Dimethyloxallyl Glycine-Incorporated Borosilicate Bioac-tive Glass Scaffolds for Improving Angiogenesis and Osteogenesis in Critical-Sized Calvarial Defects. Curr. Drug Deliv. 2019, 16, 565–576. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Chang, J.; Xiao, Y. Delivery of dimethyloxallyl glycine in mesoporous bioactive glass scaffolds to improve angiogenesis and osteogenesis of human bone marrow stromal cells. Acta Biomater. 2013, 9, 9159–9168. [Google Scholar] [CrossRef]
- Zou, D.; Han, W.; You, S.; Ye, D.; Wang, L.; Wang, S.; Zhao, J.; Zhang, W.; Jiang, X.; Zhang, X.; et al. In vitro study of en-hanced osteogenesis induced by HIF-1alpha-transduced bone marrow stem cells. Cell Prolif. 2011, 44, 234–243. [Google Scholar] [CrossRef]
- Li, H.; Liu, D.; Li, C.; Zhou, S.; Tian, D.; Xiao, D.; Zhang, H.; Gao, F.; Huang, J. Exosomes secreted from mutant-HIF-1α-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol. Int. 2017, 41, 1379–1390. [Google Scholar] [CrossRef]
- Ying, C.; Wang, R.; Wang, Z.; Tao, J.; Yin, W.; Zhang, J.; Yi, C.; Qi, X.; Han, D. BMSC-Exosomes Carry Mutant HIF-1α for Improving Angiogenesis and Osteogenesis in Critical-Sized Calvarial Defects. Front. Bioeng. Biotechnol. 2020, 8, 565561. [Google Scholar] [CrossRef]
- Zou, D.; Zhang, Z.; He, J.; Zhu, S.; Wang, S.; Zhang, W.; Zhou, J.; Xu, Y.; Huang, Y.; Wang, Y.; et al. Repairing critical-sized calvarial defects with BMSCs modified by a constitutively active form of hypoxia-inducible factor-1α and a phosphate cement scaffold. Biomaterials 2011, 32, 9707–9718. [Google Scholar] [CrossRef]
- Zou, D.; Zhang, Z.; Ye, D.; Tang, A.; Deng, L.; Han, W.; Zhao, J.; Wang, S.; Zhang, W.; Zhu, C.; et al. Repair of Critical-Sized Rat Calvarial Defects Using Genetically Engineered BMSCs Overexpressing HIF-1α. Stem Cells 2011, 29, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Zhang, Z.; He, J.; Zhang, K.; Ye, D.; Han, W.; Zhou, J.; Wang, Y.; Li, Q.; Liu, X.; et al. Blood vessel formation in the tissue-engineered bone with the constitutively active form of HIF-1α mediated BMSCs. Biomaterials 2011, 33, 2097–2108. [Google Scholar] [CrossRef]
- West, X.Z.; Malinin, N.L.; Merkulova, A.A.; Tischenko, M.; Kerr, B.A.; Borden, E.C.; Podrez, E.A.; Salomon, R.G.; Byzova, T.V. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 2010, 467, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gu, X.; Dong, J.; Zhu, C.; Cai, Z.; He, D.; Yang, C.; Xu, L.; Zheng, J. The use of TLR2 modified BMSCs for enhanced bone regeneration in the inflammatory micro-environment. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Yarden, Y. EGF–ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef]
- Genetos, D.C.; Rao, R.R.; Vidal, M.A. Betacellulin inhibits osteogenic differentiation and stimulates proliferation through HIF-1α. Cell Tissue Res. 2010, 340, 81–89. [Google Scholar] [CrossRef]
- Li, Y.; Yang, F.; Gao, M.; Gong, R.; Jin, M.; Liu, T.; Sun, Y.; Fu, Y.; Huang, Q.; Zhang, W.; et al. miR-149-3p Regulates the Switch between Adipogenic and Os-teogenic Differentiation of BMSCs by Targeting FTO, Molecular Therapy. Nucleic Acids 2019, 17, 590–600. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, C.; Liu, Y.; Chen, Z.; Liu, W.; Yang, F.; Zeng, F.; Guo, Q. Demethylase FTO promotes mechanical stress induced osteogenic differentiation of BMSCs with up-regulation of HIF-1α. Mol. Biol. Rep. 2022, 49, 2777–2784. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Han, Y.; Zhang, W.; Xin, W.; Zheng, X.; Zhang, J. Icariin promotes the migration of bone marrow stromal cells via the SDF-1α/HIF-1α/CXCR4 pathway. Drug Des. Dev. Ther. 2018, 12, 4023–4031. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Tian, K.; Tang, Z.-H.; Chen, X.-J.; Bian, Z.-X.; Wang, Y.-T.; Lu, J.-J. Phytochemistry and Pharmacology of Cartha-mus tinctorius L. Am. J. Chin. Med. 2016, 44, 197–226. [Google Scholar] [CrossRef]
- Tang, Z.; Xie, H.; Jiang, S.; Cao, S.; Pu, Y.; Zhou, B.; Zhang, X.; Xiong, H. Safflower yellow promotes angiogenesis through p-VHL/ HIF-1alpha/VEGF signaling pathway in the process of osteogenic differentiation. Biomed. Pharmacother. 2018, 107, 1736–1743. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Yang, Y.; Zhang, W.; Luo, L.; Han, F.; Guan, H.; Tao, K.; Hu, D. Curcumin pretreatment protects against hypoxia/reoxgenation injury via improvement of mitochondrial function, destabilization of HIF-1α and activation of Epac1-Akt pathway in rat bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2018, 109, 1268–1275. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, J.; Chen, Y.; Jin, X.; Li, Z.; Wen, X.; Xia, Q.; Wang, Y. Salidroside improves angiogenesis-osteogenesis coupling by regulating the HIF-1α/VEGF signalling pathway in the bone environment. Eur. J. Pharmacol. 2020, 884, 173394. [Google Scholar] [CrossRef]
- Pauly, S.; Luttosch, F.; Morawski, M.; Haas, N.P.; Schmidmaier, G.; Wildemann, B. Simvastatin locally applied from a biode-gradable coating of osteosynthetic implants improves fracture healing comparable to BMP-2 application. Bone 2009, 45, 505–511. [Google Scholar] [CrossRef]
- Fukui, T.; Ii, M.; Shoji, T.; Matsumoto, T.; Mifune, Y.; Kawakami, Y.; Akimaru, H.; Kawamoto, A.; Kuroda, T.; Saito, T.; et al. Therapeutic effect of local administration of low-dose simvastatin-conjugated gelatin hydrogel for fracture healing. J. Bone Miner. Res. 2012, 27, 1118–1131. [Google Scholar] [CrossRef]
- Rojbani, H.; Nyan, M.; Ohya, K.; Kasugai, S. Evaluation of the osteoconductivity of α-tricalcium phosphate, β-tricalcium phosphate, and hydroxyapatite combined with or without simvastatin in rat calvarial defect. J. Biomed. Mater. Res. Part A 2011, 98, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Yueyi, C.; Xiaoguang, H.; Jingying, W.; Quansheng, S.; Jie, T.; Xin, F.; Yingsheng, X.; Chunli, S. Calvarial defect healing by re-cruitment of autogenous osteogenic stem cells using locally applied simvastatin. Biomaterials 2013, 34, 9373–9380. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Wu, P.; Yu, F.; Luo, G.; Qing, L.; Tang, J. HIF-1α Regulates Bone Homeostasis and Angiogenesis, Participating in the Occurrence of Bone Metabolic Diseases. Cells 2022, 11, 3552. https://doi.org/10.3390/cells11223552
Chen W, Wu P, Yu F, Luo G, Qing L, Tang J. HIF-1α Regulates Bone Homeostasis and Angiogenesis, Participating in the Occurrence of Bone Metabolic Diseases. Cells. 2022; 11(22):3552. https://doi.org/10.3390/cells11223552
Chicago/Turabian StyleChen, Wei, Panfeng Wu, Fang Yu, Gaojie Luo, Liming Qing, and Juyu Tang. 2022. "HIF-1α Regulates Bone Homeostasis and Angiogenesis, Participating in the Occurrence of Bone Metabolic Diseases" Cells 11, no. 22: 3552. https://doi.org/10.3390/cells11223552
APA StyleChen, W., Wu, P., Yu, F., Luo, G., Qing, L., & Tang, J. (2022). HIF-1α Regulates Bone Homeostasis and Angiogenesis, Participating in the Occurrence of Bone Metabolic Diseases. Cells, 11(22), 3552. https://doi.org/10.3390/cells11223552





