Breast Cancer MCF-7 Cells Acquire Heterogeneity during Successive Co-Culture with Hematopoietic and Bone Marrow-Derived Mesenchymal Stem/Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Source of Primary Cells
2.3. Nucleated Cell Isolation
2.4. RFP Tagging
2.5. Co-Culture with Bystander Cells
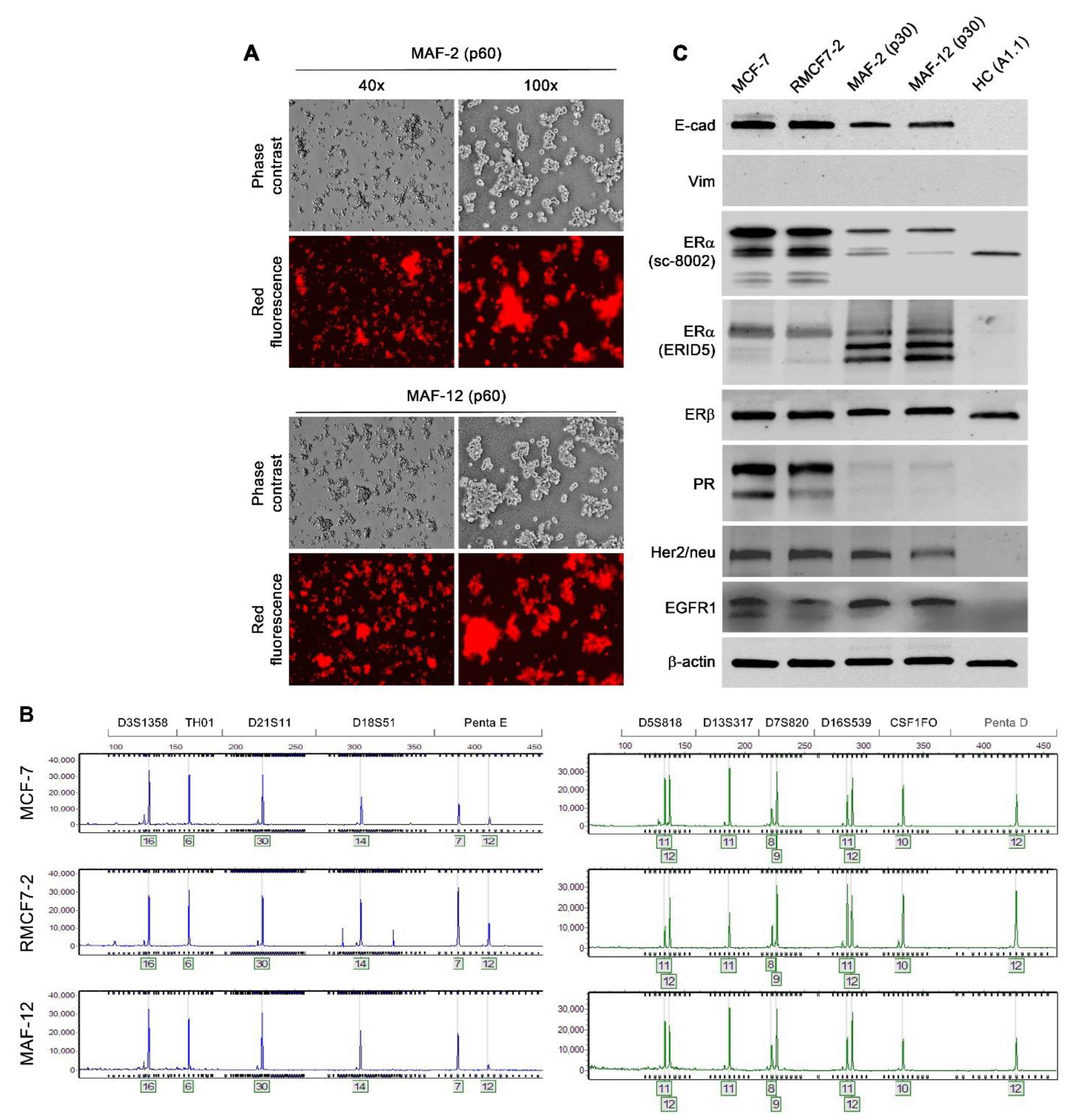
2.6. Genotyping for Cell Lineage Authentication
2.7. Western Blotting
2.8. Phenotypic Characterizations
2.9. Fluorescence Microscopic Analysis
3. Results
3.1. RFP-Tagging to Track the Fate of MCF-7 Cells
3.2. Epithelial RMCF7-2 Cells Adopting Suspension Growth upon Co-Culture with HCs
3.3. Stability of the Morphologic and Behavioral Changes
3.4. Co-Culture-Induced Marker Protein Expression Changes
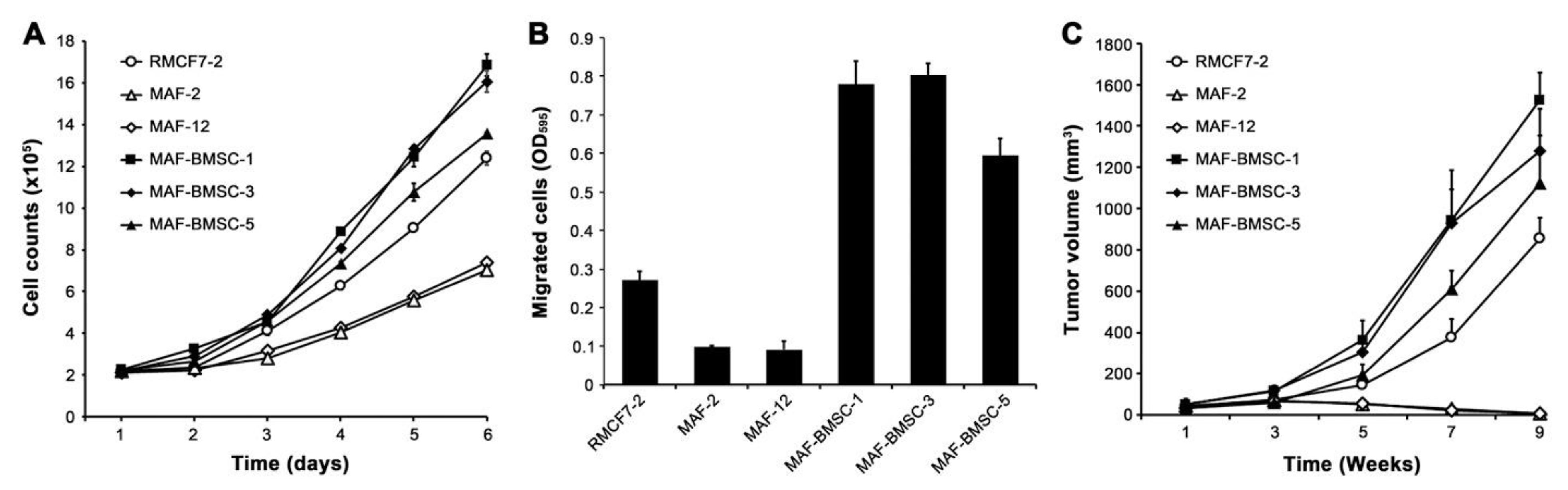
3.5. Resuming Attachment Growth through Co-Culture with hBM-MSCs
3.6. Stability of Red Fluorescent Cells after Resuming Attachment Growth
3.7. Acquired Aggressive Behaviors from Successive Co-Culture
4. Discussion
4.1. Interaction with HCs as a Mechanism of Circulating Tumor Cell (CTC) Formation
4.2. Phenotypic Changes Caused by Interaction with HCs and Lineage Plasticity in Cancer Cells
4.3. Interaction with HCs as a Model of Malignant Progression
4.4. The Mechanism of Cancer Cell Changes Caused by Interaction with HCs
4.5. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buerger, H.; Mommers, E.C.; Littmann, R.; Diallo, R.; Brinkschmidt, C.; Poremba, C.; Dockhorn-Dworniczak, B.; van Diest, P.J.; Bocker, W. Correlation of morphologic and cytogenetic parameters of genetic instability with chromosomal alterations in in situ carcinomas of the breast. Am. J. Clin. Pathol. 2000, 114, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Salamatian, V.; de Roquancourt, A.; Rigaut, J.P. Breast carcinoma, intratumour heterogeneity and histological grading, using geostatistics. Anal. Cell. Pathol. 2000, 20, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Almendro, V.; Kim, H.J.; Cheng, Y.K.; Gonen, M.; Itzkovitz, S.; Argani, P.; van Oudenaarden, A.; Sukumar, S.; Michor, F.; Polyak, K. Genetic and phenotypic diversity in breast tumor metastases. Cancer Res. 2014, 74, 1338–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinohara, K.; Polyak, K. Intratumoral Heterogeneity: More Than Just Mutations. Trends Cell. Biol. 2019, 29, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Januskeviciene, I.; Petrikaite, V. Heterogeneity of breast cancer: The importance of interaction between different tumor cell populations. Life Sci. 2019, 239, 117009. [Google Scholar] [CrossRef]
- Karaayvaz, M.; Cristea, S.; Gillespie, S.M.; Patel, A.P.; Mylvaganam, R.; Luo, C.C.; Specht, M.C.; Bernstein, B.E.; Michor, F.; Ellisen, L.W. Unravelling subclonal heterogeneity and aggressive disease states in TNBC through single-cell RNA-seq. Nat. Commun. 2018, 9, 3588. [Google Scholar] [CrossRef] [Green Version]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [Green Version]
- Navin, N.; Kendall, J.; Troge, J.; Andrews, P.; Rodgers, L.; McIndoo, J.; Cook, K.; Stepansky, A.; Levy, D.; Esposito, D.; et al. Tumour evolution inferred by single-cell sequencing. Nature 2011, 472, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Polyak, K. Heterogeneity in breast cancer. J. Clin. Investig. 2011, 121, 3786–3788. [Google Scholar] [CrossRef] [Green Version]
- Mizejewski, G.J. Breast cancer, metastasis, and the microenvironment: Disabling the tumor cell-to-stroma communication network. J. Cancer Metastasis Treat. 2019, 5, 35. [Google Scholar] [CrossRef]
- Nishida-Aoki, N.; Gujral, T.S. Emerging approaches to study cell-cell interactions in tumor microenvironment. Oncotarget 2019, 10, 785–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhawan, A.; Friedrichs, J.; Bonin, M.V.; Bejestani, E.P.; Werner, C.; Wobus, M.; Chavakis, T.; Bornhauser, M. Breast cancer cells compete with hematopoietic stem and progenitor cells for intercellular adhesion molecule 1-mediated binding to the bone marrow microenvironment. Carcinogenesis 2016, 37, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, R.N.; Psaila, B.; Lyden, D. Bone marrow cells in the ‘pre-metastatic niche’: Within bone and beyond. Cancer Metastasis Rev. 2006, 25, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Qian, B.Z.; Pollard, J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015, 15, 73–86. [Google Scholar] [CrossRef] [Green Version]
- DeNardo, D.G.; Andreu, P.; Coussens, L.M. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010, 29, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Waaijer, S.J.H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schroder, C.P. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, R.H.; Reagan, M.R.; Anderson, K.; Kaplan, D.L.; Rosenblatt, M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2010, 70, 10044–10050. [Google Scholar] [CrossRef] [Green Version]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Lazennec, G.; Jorgensen, C. Concise review: Adult multipotent stromal cells and cancer: Risk or benefit? Stem Cells 2008, 26, 1387–1394. [Google Scholar] [CrossRef]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thalmann, G.N.; Anezinis, P.E.; Chang, S.M.; Zhau, H.E.; Kim, E.E.; Hopwood, V.L.; Pathak, S.; von Eschenbach, A.C.; Chung, L.W. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994, 54, 2577–2581. [Google Scholar] [PubMed]
- Wu, T.T.; Sikes, R.A.; Cui, Q.; Thalmann, G.N.; Kao, C.; Murphy, C.F.; Yang, H.; Zhau, H.E.; Balian, G.; Chung, L.W. Establishing human prostate cancer cell xenografts in bone: Induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int. J. Cancer 1998, 77, 887–894. [Google Scholar] [CrossRef]
- Wang, R.X.; Chu, C.Y.; Zhau, H.E.; Chung, L.W.K. Using A Spaceflight Three-Dimensional Microenvironment to Probe Cancer-Stromal Interactions. In Effect of Spaceflight and Spaceflight Analogue Culture of Human and Microbial Cells: Novel Insights into Disease Mechsnisms; Nickerson, C.A., Pellis, N.R., Ott, C.M., Eds.; Springer: New York, NY, USA, 2016; pp. 131–150. [Google Scholar]
- Wang, R.; Xu, J.; Juliette, L.; Castilleja, A.; Love, J.; Sung, S.Y.; Zhau, H.E.; Goodwin, T.J.; Chung, L.W. Three-dimensional co-culture models to study prostate cancer growth, progression, and metastasis to bone. Semin. Cancer Biol. 2005, 15, 353–364. [Google Scholar] [CrossRef]
- Josson, S.; Matsuoka, Y.; Chung, L.W.; Zhau, H.E.; Wang, R. Tumor-stroma co-evolution in prostate cancer progression and metastasis. Semin. Cell Dev. Biol. 2010, 21, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; He, H.; Xie, Z.; Qian, W.; Zhau, H.E.; Chung, L.W.; Marshall, F.F.; Wang, R. Matched pairs of human prostate stromal cells display differential tropic effects on LNCaP prostate cancer cells. Vitr. Cell Dev. Biol. Anim. 2010, 46, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Lewis, M.S.; Lyu, J.; Zhau, H.E.; Pandol, S.J.; Chung, L.W.K. Cancer-stromal cell fusion as revealed by fluorescence protein tracking. Prostate 2020, 80, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Sun, X.; Wang, C.Y.; Hu, P.; Chu, C.Y.; Liu, S.; Zhau, H.E.; Chung, L.W. Spontaneous cancer-stromal cell fusion as a mechanism of prostate cancer androgen-independent progression. PLoS ONE 2012, 7, e42653. [Google Scholar] [CrossRef] [Green Version]
- Rhee, H.W.; Zhau, H.E.; Pathak, S.; Multani, A.S.; Pennanen, S.; Visakorpi, T.; Chung, L.W. Permanent phenotypic and genotypic changes of prostate cancer cells cultured in a three-dimensional rotating-wall vessel. In Vitro Cell Dev. Biol. Anim. 2001, 37, 127–140. [Google Scholar] [CrossRef]
- Yin, L.; Hu, P.; Shi, X.; Qian, W.; Zhau, H.E.; Pandol, S.J.; Lewis, M.S.; Chung, L.W.K.; Wang, R. Cancer cell’s neuroendocrine feature can be acquired through cell-cell fusion during cancer-neural stem cell interaction. Sci. Rep. 2020, 10, 1216. [Google Scholar] [CrossRef]
- Mrdenovic, S.; Zhang, Y.; Wang, R.; Yin, L.; Chu, G.C.; Yin, L.; Lewis, M.; Heffer, M.; Zhau, H.E.; Chung, L.W.K. Targeting Burkitt lymphoma with a tumor cell-specific heptamethine carbocyanine-cisplatin conjugate. Cancer 2019, 125, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Larson, B.L.; Smith, J.R.; Pochampally, R.; Cui, J.G.; Prockop, D.J. Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells 2002, 20, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Nissen, N.N.; Zhang, Y.; Shao, C.; Chu, C.Y.; Huynh, C.; Posadas, E.M.; Tomlinson, J.S.; Lewis, M.S.; Pandol, S.J. Circulating Fatty Objects and Their Preferential Presence in Pancreatic Cancer Patient Blood Samples. Front. Physiol. 2022, 13, 827531. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yang, X.; Davidson, A.J.; Wu, D.; Marshall, F.F.; Chung, L.W.; Zhau, H.E.; Wang, R. Progressive epithelial to mesenchymal transitions in ARCaP E prostate cancer cells during xenograft tumor formation and metastasis. Prostate 2010, 70, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Zhang, L.; Zhang, X.; Moreno, J.; Celluzzi, C.; Tondravi, M.; Shi, Y. Regulation of activation-induced receptor activator of NF-kappaB ligand (RANKL) expression in T cells. Eur. J. Immunol. 2002, 32, 1090–1098. [Google Scholar] [CrossRef]
- Wang, R.; Chu, G.C.; Wang, X.; Wu, J.B.; Hu, P.; Multani, A.S.; Pathak, S.; Zhau, H.E.; Chung, L.W.K. Establishment and characterization of a prostate cancer cell line from a prostatectomy specimen for the study of cellular interaction. Int. J. Cancer 2019, 145, 2249–2259. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Xu, J.; Saramaki, O.; Visakorpi, T.; Sutherland, W.M.; Zhou, J.; Sen, B.; Lim, S.D.; Mabjeesh, N.; Amin, M.; et al. PrLZ, a novel prostate-specific and androgen-responsive gene of the TPD52 family, amplified in chromosome 8q21.1 and overexpressed in human prostate cancer. Cancer Res. 2004, 64, 1589–1594. [Google Scholar] [CrossRef] [Green Version]
- Saati, T.A.; Clamens, S.; Cohen-Knafo, E.; Faye, J.C.; Prats, H.; Coindre, J.M.; Wafflart, J.; Caveriviere, P.; Bayard, F.; Delsol, G. Production of monoclonal antibodies to human estrogen-receptor protein (ER) using recombinant ER (RER). Int. J. Cancer 1993, 55, 651–654. [Google Scholar] [CrossRef]
- Yin, L.; Li, Q.; Mrdenovic, S.; Chu, G.C.; Wu, B.J.; Bu, H.; Duan, P.; Kim, J.; You, S.; Lewis, M.S.; et al. KRT13 promotes stemness and drives metastasis in breast cancer through a plakoglobin/c-Myc signaling pathway. Breast Cancer Res. 2022, 24, 7. [Google Scholar] [CrossRef]
- Wang, R.; Chu, G.C.Y.; Mrdenovic, S.; Annamalai, A.A.; Hendifar, A.E.; Nissen, N.N.; Tomlinson, J.S.; Lewis, M.; Palanisamy, N.; Tseng, H.R.; et al. Cultured circulating tumor cells and their derived xenografts for personalized oncology. Asian J. Urol. 2016, 3, 240–253. [Google Scholar] [CrossRef]
- Olea, N.; Villalobos, M.; Ruiz de Almodovar, J.M.; Pedraza, V. MCF-7 breast cancer cells grown as multicellular spheroids in vitro: Effect of 17 beta-estradiol. Int. J. Cancer 1992, 50, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Pourreau-Schneider, N.; Berthois, Y.; Mittre, H.; Charpin, C.; Jacquemier, J.; Martin, P.M. Estrogen response of MCF-7 cells grown on diverse substrates and in suspension culture: Promotion of morphological heterogeneity, modulation of progestin receptor induction; cell-substrate interactions on collagen gels. J. Steroid Biochem. 1984, 21, 763–771. [Google Scholar] [CrossRef]
- Boterberg, T.; Vennekens, K.M.; Thienpont, M.; Mareel, M.M.; Bracke, M.E. Internalization of the E-cadherin/catenin complex and scattering of human mammary carcinoma cells MCF-7/AZ after treatment with conditioned medium from human skin squamous carcinoma cells COLO 16. Cell Adhes. Commun. 2000, 7, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Parise, C.A.; Caggiano, V. Breast Cancer Survival Defined by the ER/PR/HER2 Subtypes and a Surrogate Classification according to Tumor Grade and Immunohistochemical Biomarkers. J. Cancer Epidemiol. 2014, 2014, 469251. [Google Scholar] [CrossRef] [Green Version]
- Parise, C.A.; Caggiano, V. Risk of mortality of node-negative, ER/PR/HER2 breast cancer subtypes in T1, T2, and T3 tumors. Breast Cancer Res. Treat. 2017, 165, 743–750. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal. Transduct. Targ. 2020, 5, 145. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Di Zazzo, E.; Bilancio, A.; Migliaccio, A. The Androgen Receptor in Breast Cancer. Front. Endocrinol. 2018, 9, 492. [Google Scholar] [CrossRef]





| Samples Co-Cultured with RMCF7-2 Cells | Suspension Growth Observed? | Cloned by Limiting Dilution? |
|---|---|---|
| Healthy donor PBMCs (3 donors) | Yes, with all 3 samples | No |
| Clinical breast cancer patient PBMCs (4 patients) | Yes, with all 4 samples | Yes, the first 12 MAF clones (including MAF-2 and MAF-12) from the second sample |
| HL-60, human promyelocytic leukemia cell line | Yes, in 3 co-cultures | Yes, the first 24 clones |
| HMC-1, human mast cell leukemia cell line | Yes, in 3 co-cultures | Yes, the first w clones |
| THP-1, human monocytic leukemia cell line | Yes, in 3 co-cultures | Yes, the first 6 clones |
| Jurkat, human T cell leukemia cell line | Yes, in 3 co-cultures | No |
| CA46, human Burkitt’s lymphoma cell line | Yes, in 3 co-cultures | No |
| Daudi, human Burkitt’s lymphoma cell line | Yes, in 3 co-cultures | No |
| Namalwa, human Burkitt’s lymphoma cell line | Yes, in 3 co-cultures | No |
| Raji, human Burkitt’s lymphoma cell line | Yes, in 3 co-cultures | No |
| Ramos, human Burkitt’s lymphoma cell line | Yes, in 3 co-cultures | No |
| Balb/c mouse PBMCs (3 mice) | Yes, with all 3 mice | No |
| Balb/c mouse spleen cells (2 mice) | Yes, with the 2 mice tested | No |
| Balb/c mBM-HCs (2 mice) | Yes, with the 2 mice tested | Yes, the first 2 clones from 1 mouse |
| A1.1, mouse T cell hybridoma cell line | Yes, in 6 co-cultures | Yes, the first 6 clones |
| EML, mouse multipotent hematopoietic cell line | Yes, in 3 co-cultures | Yes, the first 6 clones |
| >WEHI-231, mouse B cell lymphoma cell line | Yes, in 3 co-cultures | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, X.; Yin, L.; Yin, L.; Chu, G.C.-Y.; Hu, P.; Ou, Y.; Zhang, Y.; Lewis, M.S.; Pandol, S.J. Breast Cancer MCF-7 Cells Acquire Heterogeneity during Successive Co-Culture with Hematopoietic and Bone Marrow-Derived Mesenchymal Stem/Stromal Cells. Cells 2022, 11, 3553. https://doi.org/10.3390/cells11223553
Wang R, Wang X, Yin L, Yin L, Chu GC-Y, Hu P, Ou Y, Zhang Y, Lewis MS, Pandol SJ. Breast Cancer MCF-7 Cells Acquire Heterogeneity during Successive Co-Culture with Hematopoietic and Bone Marrow-Derived Mesenchymal Stem/Stromal Cells. Cells. 2022; 11(22):3553. https://doi.org/10.3390/cells11223553
Chicago/Turabian StyleWang, Ruoxiang, Xudong Wang, Liyuan Yin, Lijuan Yin, Gina Chia-Yi Chu, Peizhen Hu, Yan Ou, Yi Zhang, Michael S. Lewis, and Stephen J. Pandol. 2022. "Breast Cancer MCF-7 Cells Acquire Heterogeneity during Successive Co-Culture with Hematopoietic and Bone Marrow-Derived Mesenchymal Stem/Stromal Cells" Cells 11, no. 22: 3553. https://doi.org/10.3390/cells11223553






