Abstract
Sirtuins (SIRT1–7 in mammals) are evolutionarily conserved nicotinamide adenine dinucleotide-dependent lysine deacetylases/deacylases that regulate fundamental biological processes including aging. In this study, we reveal that male Sirt7 knockout (KO) mice exhibited an extension of mean and maximum lifespan and a delay in the age-associated mortality rate. In addition, aged male Sirt7 KO mice displayed better glucose tolerance with improved insulin sensitivity compared with wild-type (WT) mice. Fibroblast growth factor 21 (FGF21) enhances insulin sensitivity and extends lifespan when it is overexpressed. Serum levels of FGF21 were markedly decreased with aging in WT mice. In contrast, this decrease was suppressed in Sirt7 KO mice, and the serum FGF21 levels of aged male Sirt7 KO mice were higher than those of WT mice. Activating transcription factor 4 (ATF4) stimulates Fgf21 transcription, and the hepatic levels of Atf4 mRNA were increased in aged male Sirt7 KO mice compared with WT mice. Our findings indicate that the loss of SIRT7 extends lifespan and improves glucose metabolism in male mice. High serum FGF21 levels might be involved in the beneficial effect of SIRT7 deficiency.
1. Introduction
Sirtuins (SIRT1–7 in mammals) are evolutionarily conserved nicotinamide adenine dinucleotide-dependent lysine deacetylases/deacylases that regulate diverse biological processes, including metabolism, stress responses, genomic stability, and aging [1]. Genetic overexpression of sirtuins increases longevity in a variety of lower organisms such as yeast, worms, and flies [2,3,4]. Intriguingly, transgenic mice with brain-specific Sirt1 overexpression and whole-body Sirt6 transgenic mice also show an extended lifespan [5,6,7].
Metabolic dysfunction, including increased body fat and reduced glucose tolerance, is a hallmark of aging [8]. SIRT1, SIRT6, and SIRT7 are nuclear proteins, and SIRT1 and SIRT6 exert beneficial effects against metabolic diseases [9,10]. In sharp contrast, we demonstrated that Sirt7 knockout (KO) mice are resistant to high-fat diet-induced obesity, glucose intolerance, and fatty liver [11], suggesting that the loss of SIRT7 induces a metabolically healthy condition. Aging is also a major risk factor for cancer. With regard to cancer, SIRT7 is responsible for tumor phenotype maintenance by deacetylation of histone H3 lysine 18 (H3K18) [12], and SIRT7 expression is upregulated in the majority of human cancers, including hepatic, gastric, colorectal, and breast cancers [13,14]. In addition, SIRT7 may exert its oncogenic properties through the upregulation of ribosomal RNA synthesis to meet the increased demand for ribosomes in rapidly growing tumor cells [13,15,16]. Considering that SIRT1 and SIRT6 act as tumor suppressors [17,18], SIRT7 seems to have the opposite role in cancer. Of note, ribosomal protein gene deletion and inhibition of translation have been reported to extend lifespan in numerous model organisms, including mammals [12,19,20]. Together, these findings suggest the possibility that SIRT7 deficiency plays beneficial roles in aging-associated metabolic disorders, cancer, and even lifespan. However, a previous study reported that Sirt7 KO mice exhibit a shortened lifespan with severe cardiac disorders [21].
Because our Sirt7 KO mice [11] did not display such a shortened lifespan, in the present study, we reevaluated the impact of the loss of SIRT7 on lifespan in mice. We found that male, but not female, Sirt7 KO mice on a C57BL/6J background showed an extension of mean and maximum lifespan and a delay of the age-associated mortality rate. In addition, aged male Sirt7 KO mice displayed better glucose tolerance and higher serum levels of fibroblast growth factor 21 (FGF21) compared with wild-type (WT) mice. It has been reported that FGF21 improves glucose metabolism and extends lifespan in mice [22,23]; therefore, increased levels of FGF21 might be involved in the improved glucose tolerance and lifespan extension of male Sirt7 KO mice.
2. Materials and Methods
2.1. Mice
Sirt7 KO mice were obtained from Dr. Eva Bober [21]. These mice were backcrossed to C57BL/6J mice (Charles River Laboratory Japan, Inc., Kanagawa, Japan) for at least five generations. Male and female Sirt7 heterozygous mice were crossed to obtain WT and Sirt7 KO littermates. Genomic DNA was isolated from a 3-week-old mouse tail and PCR genotyping was performed as previously described [11]. All mouse experiments were performed in accordance with the guidelines of the Institutional Animal Committee of Kumamoto University. The mice were housed at a maximum of 5 mice/cage and maintained at 22 ± 2 °C with a 12-h light/dark cycle and free access to water and normal chow (CE-2; CLEA Japan, Inc., Tokyo, Japan).
2.2. Echocardiography and Cardiac Hypertrophy Measurements
Thirty-month-old male WT and Sirt7 KO mice were lightly anesthetized with 1% isoflurane for shaving and quickly subjected to echocardiography. Transthoracic echocardiography was performed using an Aprio 300 (Toshiba Corp., Tokyo, Japan) in awake and conscious conditions. For echocardiography, we held the mice gently with their back toward the palm and placed the transducer on the chest while avoiding the vagal reflex induced by the pressure of the transducer [24]. Left ventricular wall thickness, left ventricular end-diastolic dimension, left ventricular end-systolic dimension, and percentage fractional shortening were calculated in M-mode. To quantify cardiac hypertrophy, mouse hearts were dissected immediately, and heart weight and tibia length were measured to calculate the heart weight/tibia length ratio. Cross-sectional images of hematoxylin and eosin-stained cardiomyocytes were captured using a BZ-710 All-in-One Microscope (Keyence, Inc., Osaka, Japan), and the cross-sectional area of 50–100 cardiomyocytes in each section was measured with ImageJ software.
2.3. Lifespan Study
Male (WT, n = 50, Sirt7 KO, n = 42) and female (WT, n = 40, Sirt7 KO, n = 34) mice were inspected at least twice a day for health issues and their age was recorded when the mice were found dead. Animals showing signs of morbidity (immobility, lack of responsiveness to manual stimulation, and inability to eat or drink) were euthanized by manual cervical dislocation according to the institutional animal care guidelines of Kumamoto University. The time at euthanization was the endpoint. Lifespan was assessed using Kaplan–Meier survival curves. To estimate the hazard ratio, 2qx/(2 − qx), the age-related mortality rate (qx) was estimated as the number of mice at the end of an interval against the number of mice at the beginning of the interval [25]. The natural logarithm of the hazard ratio was plotted. Mice used in the longevity study were not used for any other experiment.
2.4. Histological Analyses
Mouse tissues (brain, thyroid, trachea, lung, stomach, pancreas, liver, spleen, intestine, kidney, urinary bladder, and skeletal muscle) were recovered from male WT (n = 22) and Sirt7 KO mice (n = 21) soon after death. The tissues were fixed in phosphate-buffered 4% paraformaldehyde and embedded in paraffin; 4-μm-thick sections were obtained and stained with hematoxylin and eosin. The sections were examined for neoplasms by a pathologist (T. I.).
The heart sections from each mouse group were stained with Masson’s trichrome stain to detect fibrosis.
2.5. Serum Parameters and Enzyme-Linked Immunosorbent Assay Measurements
After 16-h fasting, blood samples were collected from 24-month-old male WT and Sirt7 KO mice by cardiac puncture. Serum preparation was performed by centrifugation at 5000× g for 10 min at 4 °C using a blood collection tube (TS-801; SATO Chemical Industry Co., Ltd., Tochigi, Japan). Biochemical parameters were measured using an automatic biochemical analyzer (JCA-BM6070; JEOL Ltd., Tokyo, Japan). Serum hormone levels (except for adiponectin) were measured by using a Bio-Plex Pro Mouse Diabetes 8-Plex panel (Bio-Rad Laboratories, Inc., Hercules, CA, USA), a MILLIPLEX Mouse Myokine Magnetic Bead Panel (Millipore Co., Bedford, MA, USA), and a Bio-Plex200 system (Bio-Rad Laboratories, Inc.). Adiponectin was measured using a Bio-Plex Pro Mouse Adiponectin Assay Kit (171F7002M; Bio-Rad Laboratories, Inc.).
For serum FGF21 measurements (for Figure 5), blood was collected from the heart after 16-h fasting and centrifuged at 5000× g for 10 min at 4 °C to obtain serum. Serum samples from 4- and 30-month-old male mice were assayed for the quantification of FGF21 using an enzyme-linked immunosorbent assay kit (MF2100; R&D Systems, Inc., Minneapolis, MN, USA). An iMark™ Microplate Reader (Bio-Rad Laboratories, Inc.) was used to read samples at 450 nm and corrected at 540 nm.
2.6. Metabolic Tests
The body weight of 24-month-old male WT and Sirt7 KO mice was measured. For the glucose tolerance test, the mice were injected intraperitoneally with 2 g/kg glucose after 15-h fasting. Blood was collected from the tail vein and blood glucose was monitored by a Glutest Neo Super (Sanwa Kagaku Kenkyusyo Co., Ltd., Nagoya, Japan) at the indicated time points (0, 15, 30, 60, and 120 min). For the insulin tolerance test, 1 U/kg insulin was injected intraperitoneally after 4-h fasting. Blood insulin was monitored at the indicated time points (0, 30, 60, 90, and 120 min).
2.7. RNA-seq Analysis
Livers were collected from 24-month-old male WT and Sirt7 KO mice after 16-h fasting, and RNA isolation was performed using a ReliaPrep RNA Miniprep System (Z6012; Promega, Inc., Madison, WI, USA). RNA quality was determined with the RNA integrity number equivalent value using a High Sensitivity RNA ScreenTape Assay (5067-5579; Agilent Technologies, Inc., Santa Clara, CA, USA), and confirmed to be 6.9 or higher. Total RNA (1 ng) was used for the reverse transcription reaction with a SMART-seq HT (634455; Takara Bio, Inc., Shiga, Japan). An RNA-seq library was prepared using a Nextera XT Library Prep Kit (FC-131-1024; Illumina, Inc., San Diego, CA, USA) and sequenced on a NextSeq 500 Sequencer with 75 bp single-end reads (Illumina). The reads were trimmed for universal Illumina adaptors with Trim Galore (version 0.6.5) [26] and mapped to the mouse transcriptome (GRCm38) and quantified by Salmon (version 1.2.1) with default settings. Differential expression testing was performed with DESeq2 (version 1.28.0). Data were loaded into R using tximport (version 1.16.0) and aggregated to gene-level abundance in TPM. Differentially expressed genes were defined as p < 0.05, fold change > 1.5. Upregulated differentially expressed genes in Sirt7 KO mice were subjected to enrichment analysis with DAVID (version 6.8) [27]. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway analyses were used for annotation.
2.8. qRT-PCR
Frozen tissues were homogenized in Sepasol-RNA I Super G Solution (Nacalai Tesque, Inc., Kyoto, Japan). Total RNA was extracted using the phenol-chloroform extraction method, and cDNA was synthesized from total RNA using a Prime Script RT Kit (RR047A; Takara). qRT-PCR was performed on an ABI 7300 thermal cycler (Applied Biosystems, Foster City, CA, USA) using SYBR Premix Ex Taq II (RR820A; Takara). Relative gene expression was normalized by the mRNA expression level of mouse TATA-binding protein. The primer sequences are shown in Supplementary Table S1.
2.9. Statistical Analysis
Data are presented as the mean ± standard deviation (SD). The significance of differences was calculated with an unpaired two-tailed Student’s t-test or two-way analysis of variance with Tukey’s post hoc test. Kaplan–Meier survival curves were compared using the log-rank test. Changes in the age-associated mortality rate (slope and y-intercept) were measured using analysis of covariance. The frequency of cancer was compared using Fisher’s exact test. Statistical analysis was performed using GraphPad Prism 9 software version 9.4.0 (GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was assumed at p < 0.05.
3. Results
3.1. Lack of Cardiac Dysfunction in Aged Sirt7 KO Mice
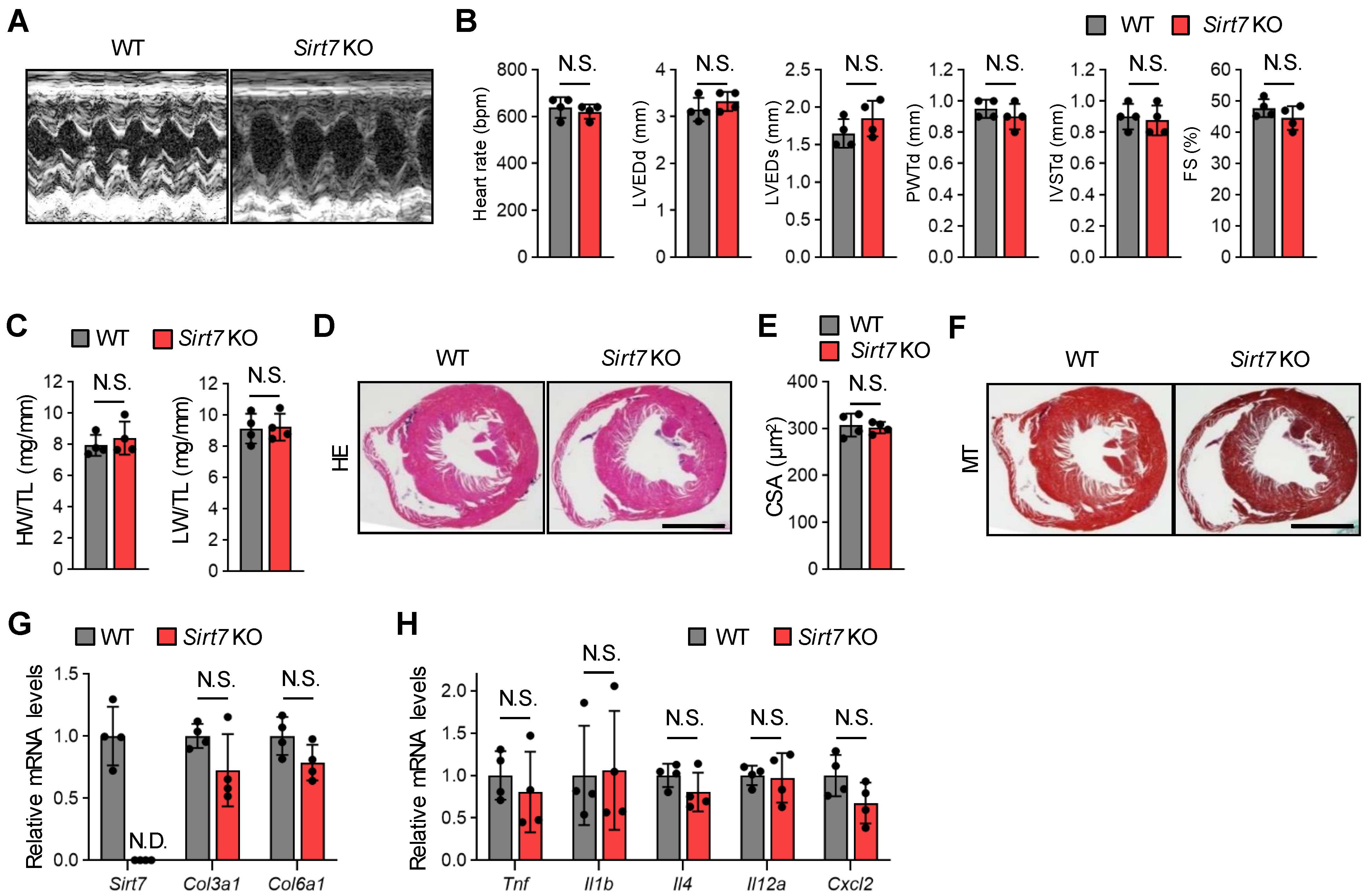
Vakhrusheva et al. [21] reported that Sirt7 KO mice (C57BL/6 × 129Sv mixed background mice were backcrossed to C57BL/6) exhibit inflammatory cardiomyopathy with a strong increase in fibrosis and shortened lifespan. Therefore, we investigated the cardiac function of our 30-month-old male Sirt7 KO mice on a C57BL/6J background by echocardiographic analysis. Interestingly, there were no significant differences in left ventricular dimension and contractile function between WT and Sirt7 KO mice (Figure 1A,B). The heart weight to tibial length ratio and lung weight to tibial length ratio of Sirt7 KO mice were both similar to those of WT mice (Figure 1C). Histological analysis revealed that the cross-sectional area, showing relative cardiomyocyte size, was similar between WT and Sirt7 KO mice (Figure 1D,E). Fibrosis was not detected in the heart of our Sirt7 KO mice by Masson’s trichrome staining (Figure 1F). Consistently, the cardiac mRNA expression levels of Col3a1 (encoding collagen type III) and Col6a1 (encoding collagen type VI) were similar between WT and Sirt7 KO mice (Figure 1G). In addition, increased expression of inflammatory genes was not detected in the heart of Sirt7 KO mice (Figure 1H). Taken together, these observations show that our aged Sirt7 KO mice do not exhibit cardiac dysfunction compared with WT mice.

Figure 1.
Cardiac function and morphology in aged male Sirt7 KO mice. (A) Representative M-mode echocardiogram images of 30-month-old male WT and Sirt7 KO mice. (B) Quantitative analysis of heart rate, left ventricular end-diastolic diameter (LVEDd), left ventricular end-systolic diameter (LVED), posterior wall thickness in diastole (PWTd), interventricular septum thickness in diastole (IVSTd), and fractional shortening (FS) in 30-month-old male WT and Sirt7 KO mice (n = 4). (C) Quantification of the heart weight/tibia length (HW/TL) ratio and lung weight/tibia length (LW/TL) ratio in 30-month-old male WT and Sirt7 KO mice (n = 4). (D) Representative images of hematoxylin and eosin (HE)-stained heart tissue from 30-month-old male WT and Sirt7 KO mice. Scale bar, 2 mm. (E) Quantitative analysis of cardiomyocyte cross-sectional area in 30-month-old male WT and Sirt7 KO mice (n = 4). (F) Representative images of Masson’s trichrome (MT)-stained heart tissue from 30-month-old male WT and Sirt7 KO mice. Scale bar, 2 mm. (G,H) qRT-PCR analysis of Sirt7 and fibrosis-related genes (Col3a1 and Col6a1) (G) and inflammation-related genes (Tnf, Il1b, Il4, Il12a, and Cxcl2) (H) in the heart of 30-month-old male WT and Sirt7 KO mice (n = 4). The data are expressed as the mean ± SD; N.D., not detected; N.S., not significant by unpaired Student’s t-test.
3.2. Male Sirt7 KO Mice Exhibit an Extension of Lifespan
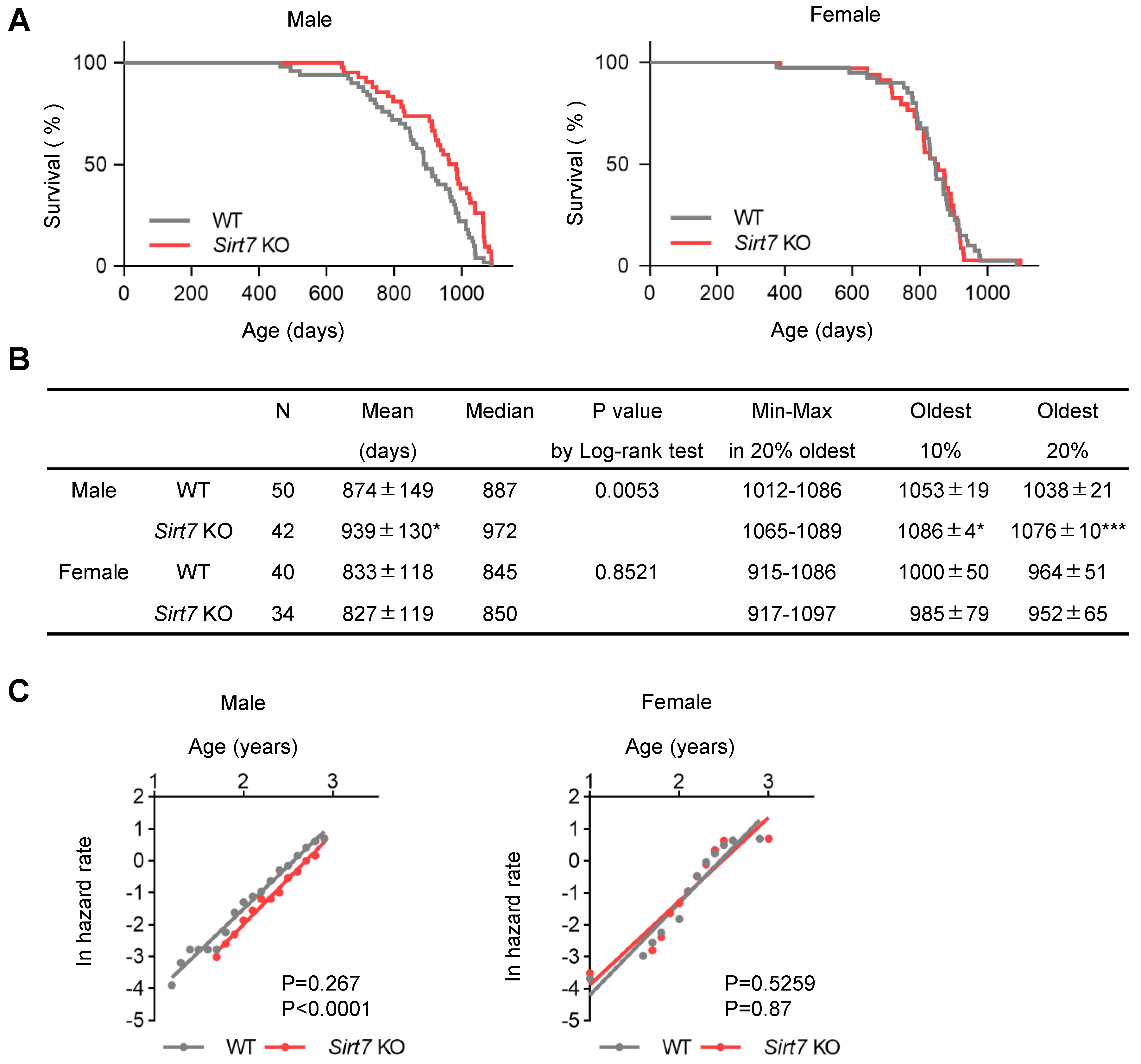
These findings encouraged us to reevaluate the lifespan of Sirt7 KO mice. When fed a standard chow diet, neither male nor female Sirt7 KO mice on a C57BL/6J background exhibited a shortened lifespan (Figure 2A). Interestingly, the survival curves of WT and Sirt7 KO male, but not female, mice were significantly different by log-rank testing (p = 0.0053), and male Sirt7 KO mice showed an 11.2% extension of median lifespan (the day at which the probability of survival equals 50%: WT 887 days vs. Sirt7 KO 972 days) (Figure 2A,B). They also exhibited a significant extension of mean lifespan (WT 874 ± 149 days vs. Sirt7 KO 939 ± 130 days, p = 0.031). Furthermore, the maximum lifespan (the average of the mean lifespan of the longest-lived 10% or 20% of mice) [6,28] of male Sirt7 KO mice was also significantly increased (10% oldest WT 1053 ± 19 days vs. Sirt7 KO 1086 ± 4 days, p = 0.021; 20% oldest WT 1038 ± 21 days vs. Sirt7 KO 1076 ± 10 days, p = 0.0005) (Figure 2B). We next assessed age-associated mortality in Sirt7 KO mice. Male Sirt7 KO mice exhibited a significant delay in age-associated mortality compared with WT mice, whereas the slope of age-associated mortality change, which defines the rate of aging [25], did not differ between WT and Sirt7 KO mice (Figure 2C). In contrast to males, this delay in age-associated mortality was not detected in female Sirt7 KO mice. Taken together, these results suggest that SIRT7 deficiency extends lifespan in male mice by delaying the onset of age-associated physiological decline.

Figure 2.
Lifespan analysis of male and female Sirt7 KO mice. (A) Kaplan–Meier survival curves for male (left: WT [n = 50], Sirt7 KO [n = 42]) and female (right: WT [n = 40], Sirt7 KO [n = 34]) mice. (B) Parameters of lifespan analysis. The mean (average lifespan) and oldest 10% and 20% (mean lifespan of the longest-lived 10% and 20% mice) of each group are shown as the mean ± SD; * p < 0.05, *** p < 0.001 by unpaired Student’s t-test. P-values of (A) were calculated by the log-rank test. (C) The age-associated mortality rate of male (left) and female (right) WT and Sirt7 KO mice. P-values for the differences between the slopes (age-associated mortality rate) and y-intercepts (initial mortality rate) were calculated by analysis of covariance (top = p-value of slopes, bottom = p-value of y-intercepts).
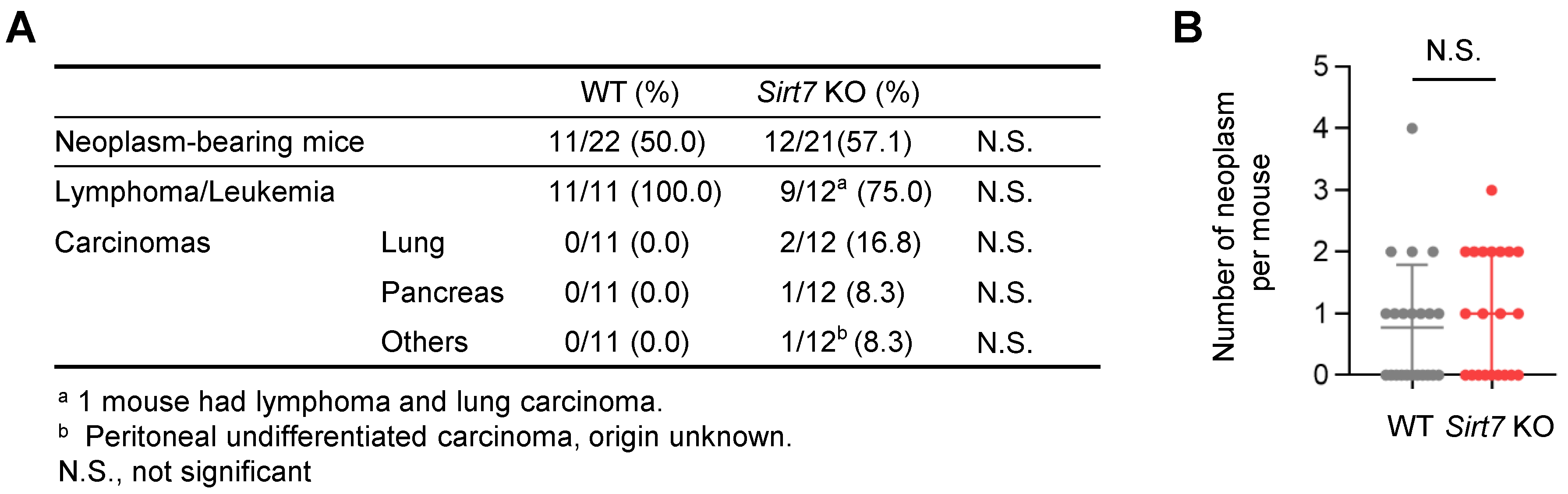
Malignant neoplasm is a major cause of death in laboratory mice. Because SIRT7 has oncogenic properties [13,14], we investigated whether the extension of lifespan in male Sirt7 KO mice was due to the reduced incidence of neoplasms. However, post-mortem gross and microscopic examinations revealed that the incidence of malignant neoplasms was similar between male WT (11 out of 22 mice, 50%) and Sirt7 KO mice (12 out of 21 mice, 57.1%) (Figure 3A). In addition, the average number of tumors per mouse did not differ between WT and Sirt7 KO mice (Figure 3B). These results indicate that the pro-longevity effect of SIRT7 deficiency cannot be reasoned by the decreased incidence of neoplasms.

Figure 3.
Tumor spectrum and incidence in aged male Sirt7 KO mice. (A) Microscopic pathological findings at death in male WT (n = 22) and Sirt7 KO (n = 21) mice. Statistically significant differences were calculated using Fisher’s exact test. (B) Number of neoplasms in male WT (n = 22) and Sirt7 KO (n = 21) mice. Data are expressed as the mean ± SD; N.S., not significant by unpaired Student’s t-test.
3.3. Sirt7 KO Mice are Protected from Aging-Associated Metabolic Dysfunction
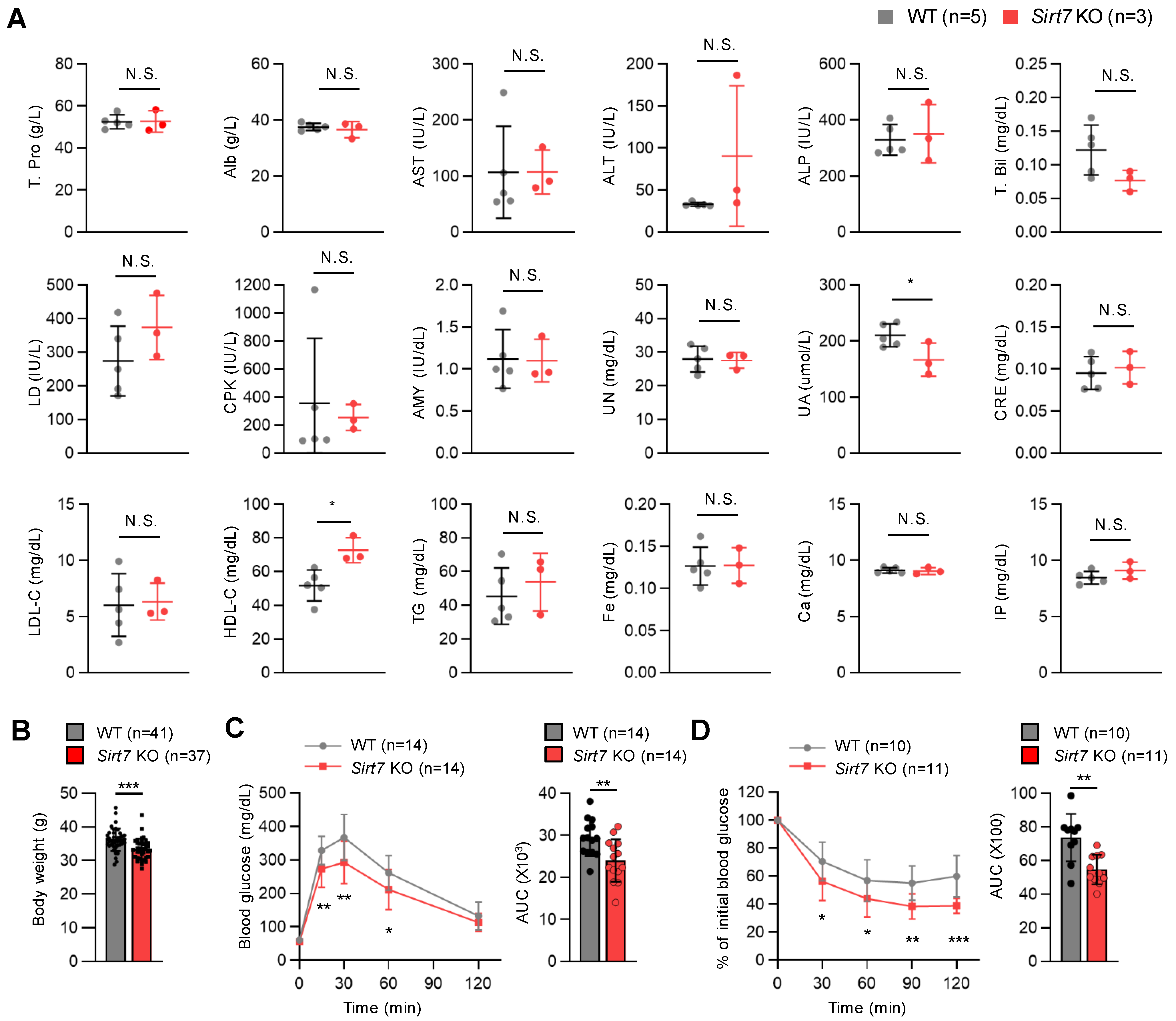
Next, we examined serum biochemical parameters in fasted 24-month-old male mice. Serum uric acid levels and high-density lipoprotein (HDL) levels were significantly lower and higher, respectively, in aged Sirt7 KO mice than in WT mice (Figure 4A). High HDL levels are associated with a reduced risk of atherosclerosis and cardiovascular diseases in humans [29], but the significance of increased HDL levels on lifespan extension in mice is unclear. There was a trend for altered levels of alanine aminotransferase, total bilirubin, and lactate dehydrogenase in aged Sirt7 KO mice, but the difference did not reach significance probably due to small sample sizes. Metabolic dysfunction is a hallmark of aging. As glucose tolerance and insulin sensitivity were improved in Sirt7 KO mice fed a high-fat diet [11], we next investigated the metabolic parameters of aged male mice. Sirt7 KO animals weighed significantly less than WT mice (Figure 4B). A glucose tolerance test demonstrated better glucose tolerance in aged Sirt7 KO mice (Figure 4C), and an insulin tolerance test revealed significantly better insulin tolerance in aged Sirt7 KO mice (Figure 4D). These results indicate that the loss of SIRT7 confers protection against aging-associated dysfunction in glucose metabolism.

Figure 4.
Serum biochemical parameters and glucose metabolism in aged male Sirt7 KO mice. (A) Biochemical analysis of 24-month-old male WT (n = 5) and Sirt7 KO (n = 3) mice after 16-h fasting. (B) Body weight of 24-month-old male WT (n = 41) and Sirt7 KO (n = 37) mice. (C) Glucose tolerance test in 24-month-old male WT (n = 14) and Sirt7 KO (n = 14) mice after intraperitoneal injection of glucose (2 g/kg body weight). (D) Insulin tolerance test in 24-month-old male WT (n = 10) and Sirt7 KO (n = 11) mice after intraperitoneal injection of insulin (1 U/kg body weight). The area under the curve (AUC) for each tolerance test (C,D) is calculated and shown. Data are expressed as the mean ± SD; * p < 0.05, ** p < 0.01, *** p < 0.001; N.S., not significant by unpaired Student’s t-test. Alb, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMY, amylase; AST, aspartate aminotransferase; Ca, calcium; CPK, creatine phosphokinase; CRE, creatinine; Fe, iron; HDL-C, high-density lipoprotein cholesterol; IP, inorganic phosphorus; LD, lactate dehydrogenase; LDL-C, low-density lipoprotein cholesterol; T. Bil, total bilirubin; TG, triglyceride; T. Pro, total protein; UA, uric acid; UN, urea nitrogen.
3.4. Hepatic FGF21 Expression is Maintained in Aged Sirt7 KO Mice
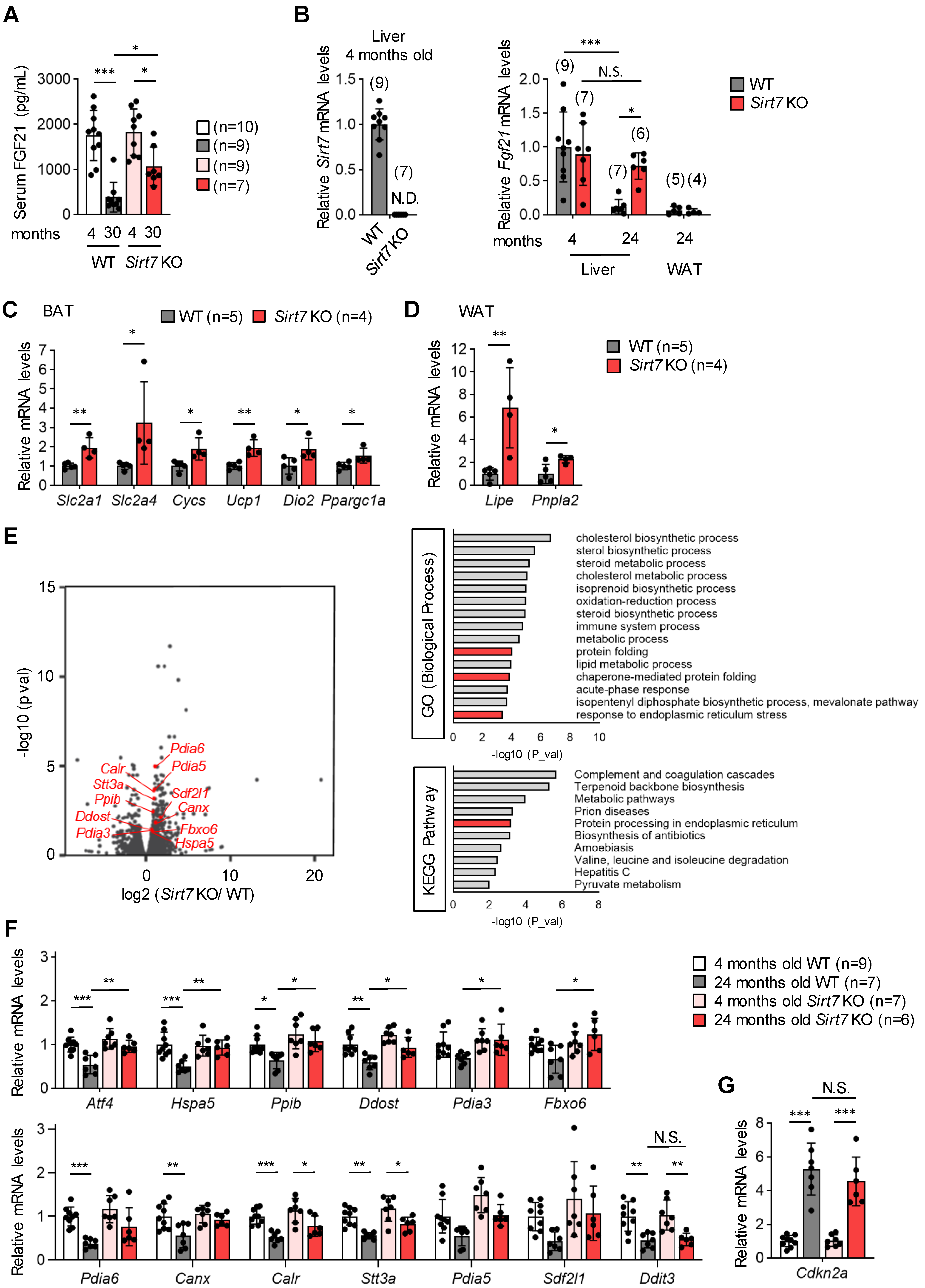
We next measured the serum levels of secreted polypeptides and proteins that are involved in glucose metabolism. Among the secreted factors, the serum levels of FGF21 were significantly increased in 24-month-old male Sirt7 KO mice compared with WT mice (Table 1). FGF21 has a fundamental role in the regulation of energy expenditure, and FGF21 administration promotes weight loss and improves insulin sensitivity and glucose homeostasis [22]. Moreover, transgenic mice overexpressing FGF21 show an extended lifespan [22,30,31,32]. We also measured serum FGF21 levels in male mice in an independent cohort (Figure 5A). The serum FGF21 levels of young mice did not differ between genotypes. Serum FGF21 levels were markedly decreased with aging in WT mice. In contrast, this decrease was suppressed in Sirt7 KO mice, and the increased serum FGF21 levels of aged male Sirt7 KO mice were confirmed in this cohort.

Table 1.
Serum hormone levels in aged male WT and Sirt7 KO mice.

Figure 5.
Gene expression profiles in the liver of aged male Sirt7 KO mice. (A) Serum FGF21 levels in 4- or 30-month-old male WT and Sirt7 KO mice after 16-h fasting. (B) qRT-PCR analysis of Sirt7 and Fgf21 in the liver and epididymal white adipose tissue (WAT) from male WT and Sirt7 KO mice. N.D., not detected. (C,D) qRT-PCR analysis of Fgf21 target genes in brown adipose tissue (BAT) (Slc2a1, Slc2a4, Cycs, Ucp1, Dio2, and Ppargc1a) (C) and epididymal WAT (Lipe and Pnpla2) (D) of 24-month-old male WT and Sirt7 KO mice. (E) Volcano plot (left) and enrichment analysis (right) of RNA-seq data in liver samples from 24-month-old male WT and Sirt7 KO mice (n = 3). UPRER-related genes are shown in red (left). GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes. (F,G) qRT-PCR analysis of UPRER-related genes (F) and a marker of senescence (G) in the liver of 4- and 24-month-old male WT and Sirt7 KO mice. Data are expressed as the mean ± SD; * p < 0.05, ** p < 0.01, *** p < 0.001; N.S., not significant. Statistical significance was determined by either two-way analysis of variance with Tukey’s post hoc test (A,B,F,G) or unpaired Student’s t-test (C,D).
The liver is a major source of the expression and secretion of FGF21 [33]. Hepatic Fgf21 mRNA expression levels were similar between young male Sirt7 KO and WT mice (Figure 5B). In accordance with a previous report [34], hepatic Fgf21 mRNA expression decreased markedly with aging in male WT mice, but such a decrease was not detected in Sirt7 KO mice, and Fgf21 mRNA expression was significantly increased in aged male Sirt7 KO mice compared with WT mice (Figure 5B). FGF21 induces the expression of genes involved in glucose transport (Slc2a1 and Slc2a4), mitochondrial oxidation (Cycs), and thermogenesis (Ucp1, Dio2, and Ppargc1a) in brown adipose tissue and lipid metabolism (Lipe and Pnpla2) in white adipose tissue [35,36]. The expression of these genes was significantly increased in aged male Sirt7 KO mice compared with WT mice (Figure 5C,D). These results indicate that the increased serum levels of FGF21 in aged male Sirt7 KO mice are not due to FGF21 resistance, a state of impaired FGF21 signaling [37].
To further investigate the mechanism underlying the increase in hepatic FGF21 expression in aged male Sirt7 KO mice, we performed RNA-seq analysis of the liver. We identified 243 differentially expressed genes (173 upregulated and 70 downregulated; adjusted p < 0.05) in the liver of aged male Sirt7 KO mice compared with WT mice (Figure 5E). Intriguingly, Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses revealed that genes involved in the unfolded protein response of the endoplasmic reticulum (UPRER), including Hspa5 (encoding GRP78) and Pdia3 (encoding protein disulfide isomerase, family A, member 3), were increased in the liver of aged male Sirt7 KO mice (Figure 5E). Altered ER homeostasis leads to the accumulation of unfolded proteins in the ER, called ER stress, which activates the UPRER signaling pathway to mitigate the stress [38,39]. Activating transcription factor 4 (ATF4) is one of the main effectors of the UPRER and controls the expression of stress-resistance genes including Fgf21 [22]. Therefore, we analyzed the hepatic expression of genes involved in the UPRER in male WT and Sirt7 KO mice by qRT-PCR (Figure 5F). The hepatic expression of several UPRER-related genes such as Atf4 and Hspa5 decreased with aging in male WT mice, whereas this decrease was diminished in Sirt7 KO mice. As a result, Atf4 mRNA expression was significantly increased in aged Sirt7 KO mice compared with WT mice. ATF4 can induce apoptosis via the induction of Ddit3 (encoding C/EBP-homologous protein); however, Ddit3 expression was similar between male Sirt7 KO and WT mice (Figure 5F). Cdkn2a (encoding p16, a marker of senescence) expression did not differ between aged male Sirt7 KO and WT mice (Figure 5G), indicating that cellular senescence occurs similarly in the hepatic cells of these mice. These results suggest that the loss of SIRT7 helps to maintain serum FGF21 levels at high levels in aged male Sirt7 KO mice by maintaining hepatic ATF4 expression.
4. Discussion
The phenotypes of Sirt7 KO mice are controversial. Vazquez et al. reported that their Sirt7 KO mice exhibit increased perinatal lethality [40], but such an increase was not observed in our Sirt7 KO mice [41] or in another independent line of Sirt7 KO mice [42]. We have no adequate explanation for these contrasting results, but differences in the construct used (absence of LacZ in [11] and [42]) may have contributed to these discrepancies. A reduction in SIRT7 levels with aging has been reported in tissues of humans and animal models [43], while SIRT7 levels increase during calorie restriction [44,45], suggesting that SIRT7 might exhibit a protective effect on longevity. Accordingly, Vakhrusheva et al. [21] reported that their Sirt7 KO mice exhibit a shortened lifespan due to cardiac dysfunction with a strong increase in fibrosis [21]. Although we and Vakhrusheva et al. [21] used the same Sirt7 KO line, such phenotypes were not detected in this study. The reason is again unclear, but the lack of cardiac dysfunction may have contributed to the extended lifespan of our Sirt7 KO mice. We also would like to emphasize that the phenotype of shortened lifespan was not observed in another line of Sirt7 KO mice [42] (Professor Johan Auwerx, personal communication). We backcrossed Vakhrusheva’s Sirt7 KO mice (C57BL/6 × 129Sv mixed background was backcrossed onto C57BL/6 background) with C57BL/6J mice for at least five generations before use in this study. Ryu et al. backcrossed their Sirt7 KO mice (129Sv background) for ten generations onto the C57BL/6J background [42]. Since genetic background affects lifespan in mice [28], the different genetic backgrounds of these mice might have contributed to their altered cardiac phenotypes and lifespans. Furthermore, differences in environmental factors (e.g., diet and housing conditions) may have affected their phenotypes. Further studies are necessary to clarify the reasons for these discrepancies.
FGF21 is an endocrine hormone that exerts a fundamental role in the regulation of energy metabolism, and FGF21 administration promotes weight loss and improves glucose homeostasis by enhancing insulin sensitivity [22]. In addition, FGF21 is a potent longevity factor, and transgenic mice overexpressing FGF21 show an extended lifespan [30]. We found that serum FGF21 levels were significantly higher in our aged male Sirt7 KO mice than in aged male WT mice. Therefore, there is a possibility that the increase in serum FGF21 levels might contribute to the extended lifespan of male Sirt7 KO mice. However, female Sirt7 KO mice did not show an extension of lifespan, but we did not examine their serum FGF21 levels. Thus, we cannot conclude that the prolonged lifespan of our male Sirt7 KO mice was due to increased FGF21 levels. It has been reported that female mice exhibit higher serum concentrations of FGF21 than males [46]. Investigation of serum FGF21 levels in female mice is a major future undertaking to address the contribution of FGF21 to the prolonged lifespan of male Sirt7 KO mice.
We also revealed that aged male Sirt7 KO mice showed less body weight gain and improved glucose homeostasis compared with WT mice of the same age. FGF21 promotes weight loss and enhances insulin sensitivity by increasing energy expenditure [22]. Hence, it is plausible that the increased levels of FGF21 contribute to the improved glucose metabolism in aged male Sirt7 KO mice. Additionally, FGF21 is reported to increase serum HDL-cholesterol levels [47]. Thus, the increased serum HDL-cholesterol concentrations in aged male Sirt7 KO mice might also be attributable to the upregulation of FGF21. However, we do not claim that the improved glucose and lipid metabolism in male Sirt7 KO mice can be explained by the increase in FGF21 only. Serum FGF21 levels were similar between young Sirt7 KO and control mice, but we demonstrated previously that young Sirt7 KO mice show resistance to high-fat diet-induced obesity, glucose intolerance, and fatty liver [11].
The diminished ability to maintain protein homeostasis is a hallmark of aging, and aged cells are unable to properly trigger UPRER-related transcriptional responses [48,49,50]. Consistently, we demonstrated that the expression of several hepatic UPRER-related genes, including Atf4, was decreased in aged male WT mice. In sharp contrast, this decrease was suppressed in aged male Sirt7 KO mice, and Atf4 mRNA expression was significantly increased in aged male Sirt7 KO mice compared with WT mice. Given that ATF4 stimulates Fgf21 transcription, the increased hepatic Fgf21 expression and serum FGF21 levels in aged male Sirt7 KO mice might be, at least in part, a consequence of higher ATF4 expression. However, it is unlikely that Atf4 expression is regulated directly by SIRT7 in the liver since the hepatic Atf4 mRNA levels of young mice did not differ between genotypes. What could be the underlying mechanism for the altered expression of UPRER-related genes in aged male Sirt7 KO mice? Previous studies have demonstrated that the chromatin landscape of aged cells is largely different from that of young cells, and the reduction of activating histone marks and the induction of repressive marks at the promoter regions of stress response genes are features of aged chromatin [48,49]. H3K18 acetylation, H3K36 acetylation, and H4K91 glutarylation are histone marks for active gene expression, and SIRT7 functions as an eraser of these modifications [12,51,52]. Loss of SIRT7 may help to induce gene expression by preserving active histone marks. Future studies are necessary to define whether histone marks at UPRER-related gene loci are regulated by SIRT7.
Enhancing the function of SIRT1 and SIRT6 improves the healthspan in mice. Although the phenotypes of Sirt7 KO mice are controversial, we revealed that the loss of SIRT7 extends lifespan and confers protection against aging-associated metabolic dysfunction in male mice. Thus, SIRT7 and SIRT1/SIRT6 may play opposite roles in aging. Further studies are necessary to improve our understanding of the roles of SIRT7 in aging.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11223609/s1, Table S1: List of the primer sequences used for qRT-PCR.
Author Contributions
T.M., Y.S. and K.Y. conceived the project and designed the experiments; T.M., Y.S., T.I., T.Y., T.T., A.S., S.A., K.T., M.T. and Y.O. carried out experiments, analyzed the data, and provided useful suggestions. T.M., Y.S. and K.Y. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from the Japan Agency for Medical Research and Development under Grant Number (JP21gm5010002: K.Y. and JP20gm5010001s0604: A.S.); by Grants-in-Aid for Scientific Research (B) (19H03711 and 22H03129; K.Y. and 20H04107; T.Y.); by a Grant-in-Aid for Challenging Research (Exploratory) (19K22639; K.Y.); by a Grant-in-Aid for Scientific Research (C) (19K09008; Y.S.); and by grants from the Naito Foundation (K.Y.) and Takeda Science Foundation (K.Y.)
Institutional Review Board Statement
The animal study protocol was approved by the Kumamoto University Ethics Review Committee for Animal Experimentation (Approval ID: A29-001, A 2019-048, A 2021-001).
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw and processed RNA sequencing were deposited in the Gene Expression Omnibus (GEO) under accession number GSE207272. Any other relevant data are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Johan Auwerx (École Polytechnique Fédérale de Lausanne) and Shin-ichiro Imai (Washington University School of Medicine) for their helpful discussions. We also thank Shingo Usuki (Kumamoto University) and Masaya Yamazaki (Kumamoto University) for technical support with RNA-seq analysis and data mining.
Conflicts of Interest
The authors declare no competing interest.
References
- Imai, S.; Guarente, L. NAD+ and Sirtuins in Aging and Disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A.; Guarente, L. Increased Dosage of a Sir-2 Gene Extends Lifespan in Caenorhabditis elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small Molecule Activators of Sirtuins Extend Saccharomyces cerevisiae Lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Rogina, B.; Helfand, S.L. Sir2 Mediates Longevity in the Fly through a Pathway Related to Calorie Restriction. Proc. Natl. Acad. Sci. USA 2004, 101, 15998–16003. [Google Scholar] [CrossRef] [PubMed]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The Sirtuin SIRT6 Regulates Lifespan in Male Mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S.I. Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef]
- Roichman, A.; Elhanati, S.; Aon, M.A.; Abramovich, I.; di Francesco, A.; Shahar, Y.; Avivi, M.Y.; Shurgi, M.; Rubinstein, A.; Wiesner, Y.; et al. Restoration of Energy Homeostasis by SIRT6 Extends Healthy Lifespan. Nat. Commun. 2021, 12, 3208. [Google Scholar] [CrossRef]
- López-Otín, C.; Galluzzi, L.; Freije, J.M.P.; Madeo, F.; Kroemer, G. Metabolic Control of Longevity. Cell 2016, 166, 802–821. [Google Scholar] [CrossRef]
- Herranz, D.; Muñoz-Martin, M.; Cañamero, M.; Mulero, F.; Martinez-Pastor, B.; Fernandez-Capetillo, O.; Serrano, M. Sirt1 Improves Healthy Ageing and Protects from Metabolic Syndrome-Associated Cancer. Nat. Commun. 2010, 1, 3. [Google Scholar] [CrossRef]
- Kanfi, Y.; Peshti, V.; Gil, R.; Naiman, S.; Nahum, L.; Levin, E.; Kronfeld-Schor, N.; Cohen, H.Y. SIRT6 Protects against Pathological Damage Caused by Diet-Induced Obesity. Aging Cell 2010, 9, 162–173. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Karim, M.F.; Sato, Y.; Senokuchi, T.; Miyata, K.; Fukuda, T.; Go, C.; Tasaki, M.; Uchimura, K.; Kadomatsu, T.; et al. SIRT7 Controls Hepatic Lipid Metabolism by Regulating the Ubiquitin-Proteasome Pathway. Cell Metab. 2014, 19, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.F.; Michishita-Kioi, E.; Xi, Y.; Tasselli, L.; Kioi, M.; Moqtaderi, Z.; Tennen, R.I.; Paredes, S.; Young, N.L.; Chen, K.; et al. SIRT7 Links H3K18 Deacetylation to Maintenance of Oncogenic Transformation. Nature 2012, 487, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.F.; Grummt, I. The Seven Faces of SIRT7. Transcription 2017, 8, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, Y.; Zhu, K.S.; Wang, H.; Zhu, W.G. Advances in Cellular Characterization of the Sirtuin Isoform, SIRT7. Front. Endocrinol. 2018, 9, 652. [Google Scholar] [CrossRef]
- Ford, E.; Voit, R.; Liszt, G.; Magin, C.; Grummt, I.; Guarente, L. Mammalian Sir2 Homolog SIRT7 Is an Activator of RNA Polymerase I Transcription. Genes Dev. 2006, 20, 1075–1080. [Google Scholar] [CrossRef]
- Chen, S.; Seiler, J.; Santiago-Reichelt, M.; Felbel, K.; Grummt, I.; Voit, R. Repression of RNA Polymerase I upon Stress Is Caused by Inhibition of RNA-Dependent Deacetylation of PAF53 by SIRT7. Mol. Cell 2013, 52, 303–313. [Google Scholar] [CrossRef]
- Wang, R.H.; Sengupta, K.; Li, C.; Kim, H.S.; Cao, L.; Xiao, C.; Kim, S.; Xu, X.; Zheng, Y.; Chilton, B.; et al. Impaired DNA Damage Response, Genome Instability, and Tumorigenesis in SIRT1 Mutant Mice. Cancer Cell 2008, 14, 312–323. [Google Scholar] [CrossRef]
- Tasselli, L.; Zheng, W.; Chua, K.F. SIRT6: Novel Mechanisms and Links to Aging and Disease. Trends Endocrinol. Metab. 2017, 28, 168–185. [Google Scholar] [CrossRef]
- Hansen, M.; Taubert, S.; Crawford, D.; Libina, N.; Lee, S.J.; Kenyon, C. Lifespan Extension by Conditions That Inhibit Translation in Caenorhabditis elegans. Aging Cell 2007, 6, 95–110. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin Fed Late in Life Extends Lifespan in Genetically Heterogeneous Mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef]
- Vakhrusheva, O.; Smolka, C.; Gajawada, P.; Kostin, S.; Boettger, T.; Kubin, T.; Braun, T.; Bober, E. Sirt7 Increases Stress Resistance of Cardiomyocytes and Prevents Apoptosis and Inflammatory Cardiomyopathy in Mice. Circ. Res. 2008, 102, 703–710. [Google Scholar] [CrossRef]
- BonDurant, L.D.; Potthoff, M.J. Fibroblast Growth Factor 21: A Versatile Regulator of Metabolic Homeostasis. Annu. Rev. Nutr. 2018, 38, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Regulation of Longevity by FGF21: Interaction between Energy Metabolism and Stress Responses. Ageing Res. Rev. 2017, 37, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ho, D.; Vatner, D.E.; Vatner, S.F. Echocardiography in Mice. Curr. Protoc. Mouse. Biol. 2011, 1, 71–83. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Cabral, J.A.S.; Magalhães, D. The Influence of Genes on the Aging Process of Mice: A Statistical Assessment of the Genetics of Aging. Genetics 2005, 169, 265–274. [Google Scholar] [CrossRef]
- Trim Galore. Available online: http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 24 June 2020).
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics Enrichment Tools: Paths toward the Comprehensive Functional Analysis of Large Gene Lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ladiges, W.; van Remmen, H.; Strong, R.; Ikeno, Y.; Treuting, P.; Rabinovitch, P.; Richardson, A. Lifespan Extension in Genetically Modified Mice. Aging Cell 2009, 8, 346–352. [Google Scholar] [CrossRef]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High Density Lipoprotein as a Protective Factor against Coronary Heart Disease: The Framingham Study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Berglund, E.D.; Colbert Coate, K.; He, T.T.; Katafuchi, T.; Xiao, G.; Potthoff, M.J.; Wei, W.; Wan, Y.; et al. The Starvation Hormone, Fibroblast Growth Factor-21, Extends Lifespan in Mice. Elife 2012, 1, e00065. [Google Scholar] [CrossRef]
- Owen, B.M.; Mangelsdorf, D.J.; Kliewer, S.A. Tissue-Specific Actions of the Metabolic Hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 2015, 26, 22–29. [Google Scholar] [CrossRef]
- Youm, Y.H.; Horvath, T.L.; Mangelsdorf, D.J.; Kliewer, S.A.; Dixit, V.D. Prolongevity Hormone FGF21 Protects against Immune Senescence by Delaying Age-Related Thymic Involution. Proc. Natl. Acad. Sci. USA 2016, 113, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 Is Liver Derived and Enhances Glucose Uptake during Refeeding and Overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Makishima, M.; Bhawal, U.K. Differentiated Embryo Chondrocyte 1 (DEC1) Is a Novel Negative Regulator of Hepatic Fibroblast Growth Factor 21 (FGF21) in Aging Mice. Biochem. Biophys. Res. Commun. 2016, 469, 477–482. [Google Scholar] [CrossRef]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine Regulation of the Fasting Response by PPARα-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef]
- Hondares, E.; Rosell, M.; Gonzalez, F.J.; Giralt, M.; Iglesias, R.; Villarroya, F. Hepatic FGF21 Expression Is Induced at Birth via PPARα in Response to Milk Intake and Contributes to Thermogenic Activation of Neonatal Brown Fat. Cell Metab. 2010, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity Is a Fibroblast Growth Factor 21 (FGF21)-Resistant State. Diabetes 2010, 59, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kaufman, R.J. Protein Misfolding in the Endoplasmic Reticulum as a Conduit to Human Disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef]
- Metcalf, M.G.; Higuchi-Sanabria, R.; Garcia, G.; Kimberly Tsui, C.; Dillin, A. Beyond the Cell Factory: Homeostatic Regulation of and by the UPRER. Sci. Adv. 2020, 6, eabb9614. [Google Scholar] [CrossRef]
- Vazquez, B.N.; Thackray, J.K.; Simonet, N.G.; Kane-Goldsmith, N.; Martinez-Redondo, P.; Nguyen, T.; Bunting, S.; Vaquero, A.; Tischfield, J.A.; Serrano, L. SIRT7 Promotes Genome Integrity and Modulates Non-Homologous End Joining DNA Repair. EMBO J. 2016, 35, 1488–1503. [Google Scholar] [CrossRef]
- Fukuda, M.; Yoshizawa, T.; Karim, M.F.; Sobuz, S.U.; Korogi, W.; Kobayasi, D.; Okanishi, H.; Tasaki, M.; Ono, K.; Sawa, T.; et al. SIRT7 Has a Critical Role in Bone Formation by Regulating Lysine Acylation of SP7/Osterix. Nat. Commun. 2018, 9, 2833. [Google Scholar] [CrossRef]
- Ryu, D.; Jo, Y.S.; lo Sasso, G.; Stein, S.; Zhang, H.; Perino, A.; Lee, J.U.; Zeviani, M.; Romand, R.; Hottiger, M.O.; et al. A SIRT7-Dependent Acetylation Switch of GABPβ1 Controls Mitochondrial Function. Cell Metab. 2014, 20, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A. SIRT7 in the Aging Process. Cell Mol. Life Sci. 2022, 79, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wronska, A.; Lawniczak, A.; Wierzbicki, P.M.; Kmiec, Z. Age-Related Changes in Sirtuin 7 Expression in Calorie-Restricted and Refed Rats. Gerontology 2016, 62, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Simonet, N.G.; Thackray, J.K.; Vazquez, B.N.; Ianni, A.; Espinosa-Alcantud, M.; Morales-Sanfrutos, J.; Hurtado-Bagès, S.; Sabidó, E.; Buschbeck, M.; Tischfield, J.; et al. SirT7 Auto-ADP-Ribosylation Regulates Glucose Starvation Response through MH2A1. Sci. Adv. 2020, 6, eaaz2590. [Google Scholar] [CrossRef]
- Allard, C.; Bonnet, F.; Xu, B.; Coons, L.; Albarado, D.; Hill, C.; Fagherazzi, G.; Korach, K.S.; Levin, E.R.; Lefante, J.; et al. Activation of Hepatic Estrogen Receptor-α Increases Energy Expenditure by Stimulating the Production of Fibroblast Growth Factor 21 in Female Mice. Mol. Metab. 2019, 22, 62–70. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Wroblewski, V.J.; Koester, A.; Chen, Y.F.; Clutinger, C.K.; Tigno, X.T.; Hansen, B.C.; Shanafelt, A.B.; Etgen, G.J. The Metabolic State of Diabetic Monkeys Is Regulated by Fibroblast Growth Factor-21. Endocrinology 2007, 148, 774–781. [Google Scholar] [CrossRef]
- Sabath, N.; Levy-Adam, F.; Younis, A.; Rozales, K.; Meller, A.; Hadar, S.; Soueid-Baumgarten, S.; Shalgi, R. Cellular Proteostasis Decline in Human Senescence. Proc. Natl. Acad. Sci. USA 2020, 117, 31902–31913. [Google Scholar] [CrossRef]
- Meller, A.; Shalgi, R. The Aging Proteostasis Decline: From Nematode to Human. Exp. Cell Res. 2021, 399, 112474. [Google Scholar] [CrossRef]
- Wodrich, A.P.K.; Scott, A.W.; Shukla, A.K.; Harris, B.T.; Giniger, E. The Unfolded Protein Responses in Health, Aging, and Neurodegeneration: Recent Advances and Future Considerations. Front. Mol. Neurosci. 2022, 15, 831116. [Google Scholar] [CrossRef]
- Wang, W.W.; Angulo-Ibanez, M.; Lyu, J.; Kurra, Y.; Tong, Z.; Wu, B.; Zhang, L.; Sharma, V.; Zhou, J.; Lin, H.; et al. A Click Chemistry Approach Reveals the Chromatin-Dependent Histone H3K36 Deacylase Nature of SIRT7. J. Am. Chem. Soc. 2019, 141, 2462–2473. [Google Scholar] [CrossRef]
- Bao, X.; Liu, Z.; Zhang, W.; Gladysz, K.; Fung, Y.M.E.; Tian, G.; Xiong, Y.; Wong, J.W.H.; Yuen, K.W.Y.; Li, X.D. Glutarylation of Histone H4 Lysine 91 Regulates Chromatin Dynamics. Mol. Cell 2019, 76, 660–675. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).