Topical Application of Butyl Flufenamate Ointment Promotes Cranial Defect Healing in Mice by Inducing BMP2 Secretion in Skin Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation and Culture of mSMSCs
2.3. Osteogenic, Adipogenic, and Chondrogenic Induction of mSMSCs
2.4. Co-Culture of Mouse Bone Marrow Mesenchymal Stem Cells (mBMMSCs) with mSMSCs Using Transwell Membranes
2.5. Preparation of a Concentrated FFA Solution
2.6. ELISA
2.7. Alkaline Phosphatase (ALP) Staining and Quantification
2.8. Alizarin Red S (ARS) Staining and Quantification
2.9. Oil Red O Staining
2.10. Alcian Blue Staining
2.11. Quantitative Real-Time Reverse-Transcription PCR (qRT-PCR)
2.12. Cranial Defect Surgery in Mice
2.13. Application of Butyl Flufenamate Ointment to the Skin Surface of the Cranial Defect Area
2.14. Micro-Computed Tomography (CT) and Bone Morphometric Analysis
2.15. Statistical Analysis
3. Results
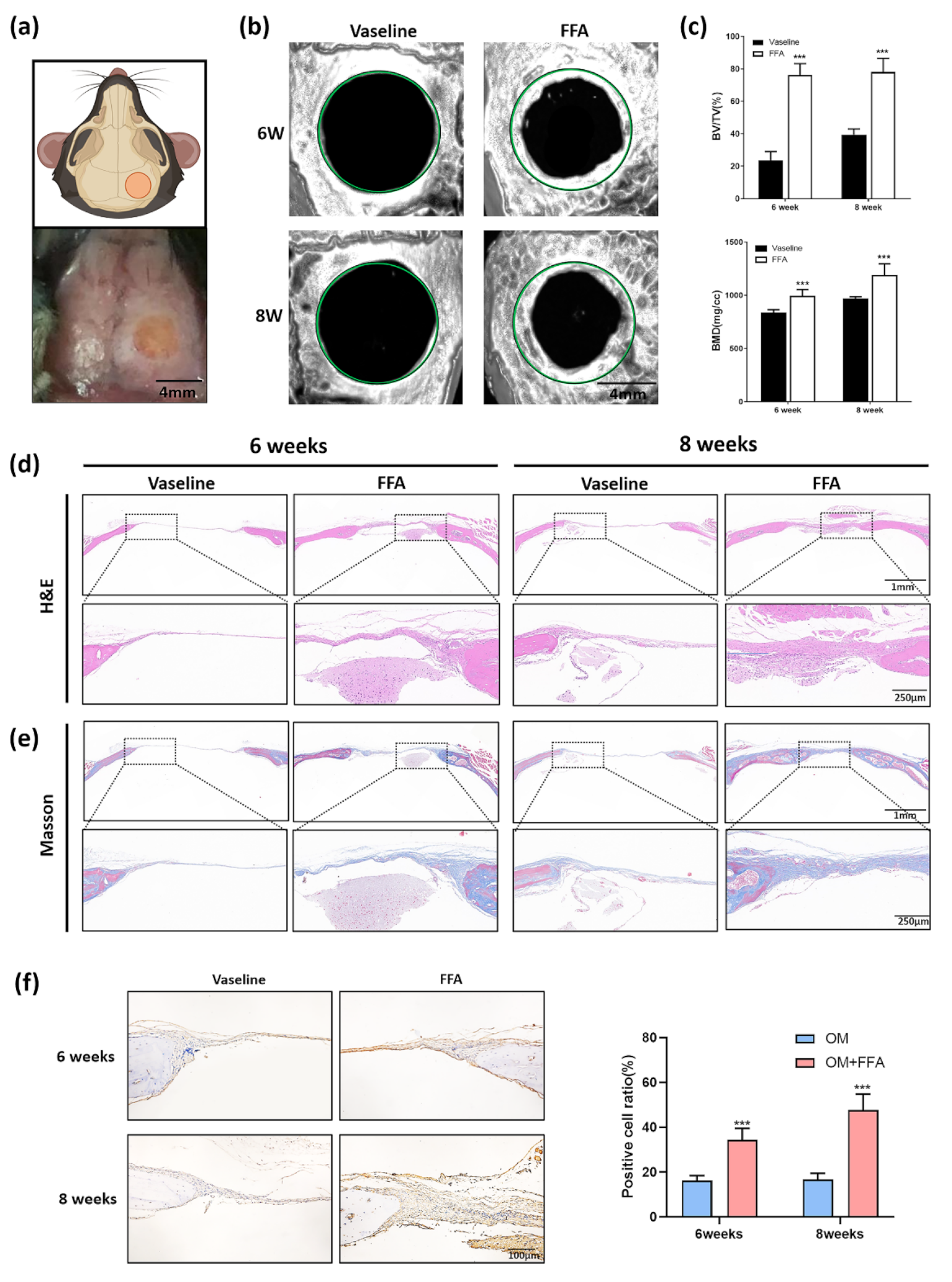
3.1. Application of Butyl Flufenamate Ointment to the Skin Surface Promoted Cranial Defect Healing in Mice
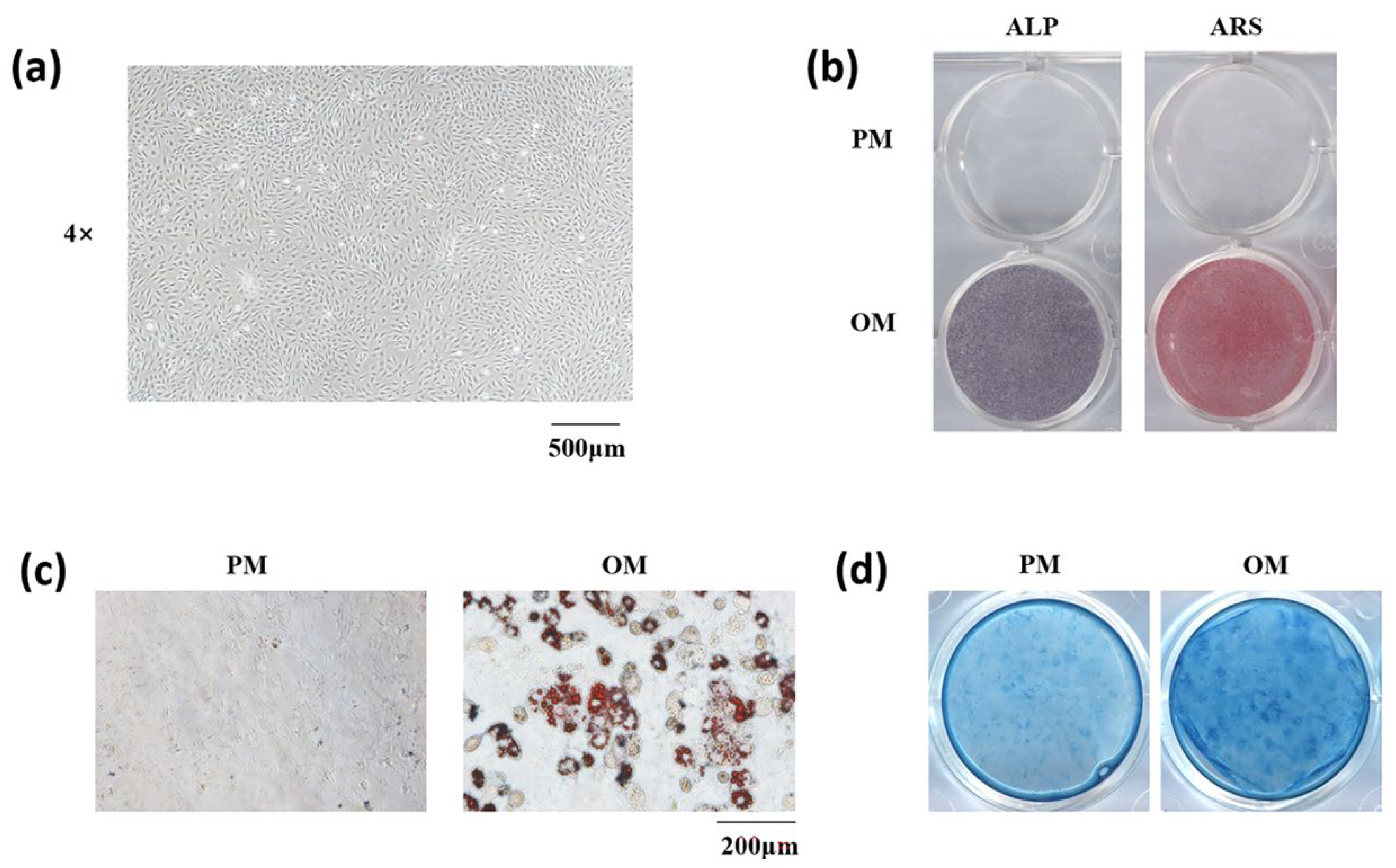
3.2. Isolation and Stemness Determination of mSMSCs
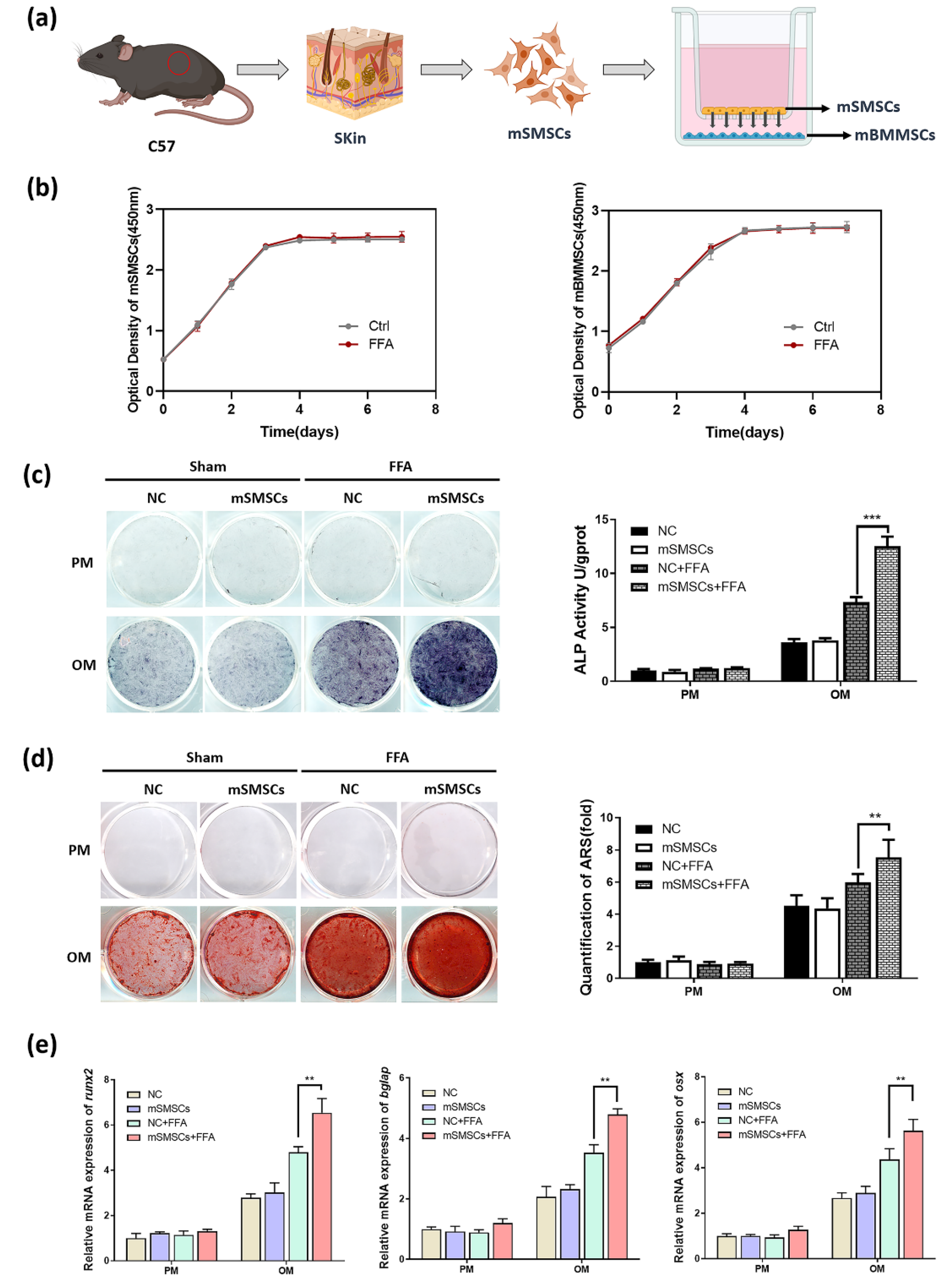
3.3. Co-Culture with mSMSCs Promoted Osteogenesis in mBMMSCs in the Presence of a Low Concentration of Flufenamic Acid
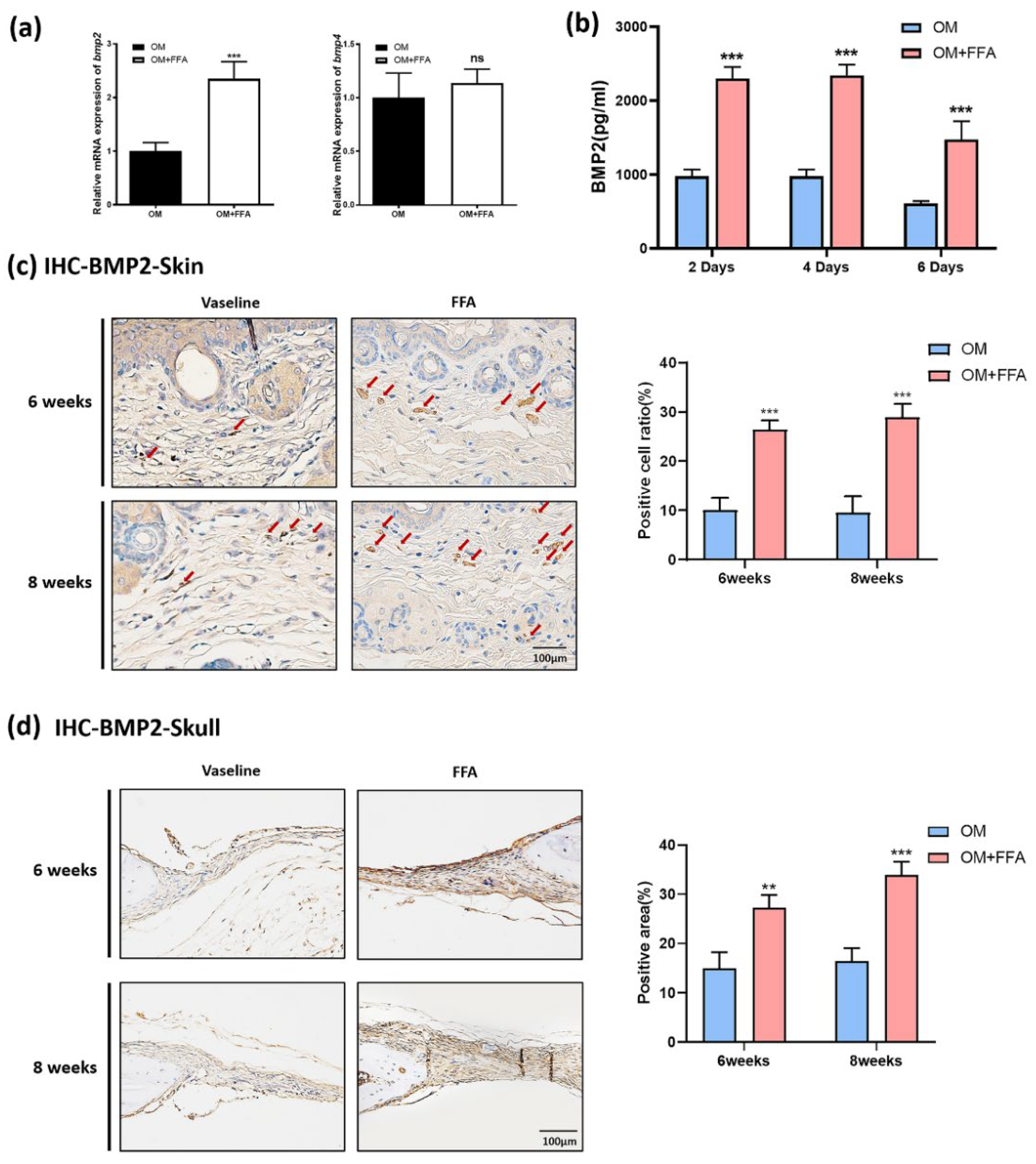
3.4. Butyl Flufenamate Ointment Accelerated Cranial Defect Healing in Mice by Promoting BMP2 Secretion from mSMSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2018, 28, 351. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release Off. J. Control. Release Soc. 2018, 270, 203. [Google Scholar] [CrossRef]
- Bailey, S.C.; Wismer, G.A.; Parker, R.M.; Walton, S.M.; Wood, A.J.J.; Wallia, A.; Brokenshire, S.A.; Infanzon, A.C.; Curtis, L.M.; Kwasny, M.J.; et al. Development and rationale for a multifactorial, randomized controlled trial to test strategies to promote adherence to complex drug regimens among older adults. Contemp. Clin. Trials 2017, 62, 21. [Google Scholar] [CrossRef]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564. [Google Scholar] [CrossRef]
- Watkinson, A.C.; Kearney, M.C.; Quinn, H.L.; Courtenay, A.J.; Donnelly, R.F. Future of the transdermal drug delivery market—Have we barely touched the surface? Expert Opin. Drug Deliv. 2016, 13, 523. [Google Scholar] [CrossRef]
- Barnardo, D.E.; Currey, H.L.; Mason, R.M.; Fox, W.R.; Weatherall, M. Mefenamic acid and flufenamic acid compared with aspirin and phenylbutazone in rheumatoid arthritis. Br. Med. J. 1966, 2, 342. [Google Scholar] [CrossRef][Green Version]
- Simpson, M.R.; Simpson, N.R.W.; Masheter, H.C.; Hardy, S.M.J.R. Flufenamic Acid in Rheumatoid Arthritis. Rheumatology 1966, 8 (Suppl. S1), 208–213. [Google Scholar] [CrossRef]
- Al Hanbali, O.A.; Khan, H.M.S.; Sarfraz, M.; Arafat, M.; Ijaz, S.; Hameed, A. Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharm. 2019, 69, 197. [Google Scholar] [CrossRef]
- Ghica, M.V.; Albu Kaya, M.G.; Dinu-Pîrvu, C.E.; Lupuleasa, D.; Udeanu, D.I. Development, Optimization and In Vitro/In Vivo Characterization of Collagen-Dextran Spongious Wound Dressings Loaded with Flufenamic Acid. Molecules 2017, 22, 1552. [Google Scholar] [CrossRef] [PubMed]
- Lentjes, E.G.; van Ginneken, C.A. Pharmacokinetics of flufenamic acid in man. Int. J. Clin. Pharmacol. Ther. Toxicol. 1987, 25, 185. [Google Scholar] [PubMed]
- Malinovskaja-Gomez, K.; Labouta, H.I.; Schneider, M.; Hirvonen, J.; Laaksonen, T. Transdermal iontophoresis of flufenamic acid loaded PLGA nanoparticles. Eur. J. Pharm. Sci. 2016, 89, 154. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Z.; Liu, H.; Zhu, Y.; Xia, D.; Wang, S.; Gu, R.; Wu, W.; Zhang, P.; Liu, Y.; et al. Low concentration flufenamic acid enhances osteogenic differentiation of mesenchymal stem cells and suppresses bone loss by inhibition of the NF-κB signaling pathway. Stem Cell Res. Ther. 2019, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Z.; Liu, H.; Zhu, Y.; Xia, D.; Wang, S.; Gu, R.; Zhang, P.; Liu, Y.; Zhou, Y. Flufenamic Acid Inhibits Adipogenic Differentiation of Mesenchymal Stem Cells by Antagonizing the PI3K/AKT Signaling Pathway. Stem Cells Int. 2020, 2020, 1540905. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, M.; Juric, S.; Ettl, H.; Valenta, C. Effect of monoacyl phosphatidylcholine content on the formation of microemulsions and the dermal delivery of flufenamic acid. Int. J. Pharm. 2015, 479, 70. [Google Scholar] [CrossRef] [PubMed]
- Luengo, J.; Weiss, B.; Schneider, M.; Ehlers, A.; Stracke, F.; König, K.; Kostka, K.H.; Lehr, C.M.; Schaefer, U.F. Influence of nanoencapsulation on human skin transport of flufenamic acid. Ski. Pharmacol. Physiol. 2006, 19, 190. [Google Scholar] [CrossRef]
- Ciuffreda, M.C.; Malpasso, G.; Musarò, P.; Turco, V.; Gnecchi, M. Protocols for in vitro Differentiation of Human Mesenchymal Stem Cells into Osteogenic, Chondrogenic and Adipogenic Lineages. Methods Mol. Biol. 2016, 1416, 149. [Google Scholar]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Liddle, D.M.; Hutchinson, A.L.; Robinson, L.E. Studying Adipocyte and Immune Cell Cross Talk Using a Co-culture System. Methods Mol. Biol. 2020, 2184, 111. [Google Scholar]
- Arias-Betancur, A.; Badilla-Wenzel, N.; Astete-Sanhueza, Á.; Farfán-Beltrán, N.; Dias, F.J. Carrier systems for bone morphogenetic proteins: An overview of biomaterials used for dentoalveolar and maxillofacial bone regeneration. Jpn. Dent. Sci. Rev. 2022, 58, 316. [Google Scholar] [CrossRef] [PubMed]
- Rosen, V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009, 20, 475. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006, 38, 1424. [Google Scholar] [CrossRef] [PubMed]
- Riley, E.H.; Lane, J.M.; Urist, M.R.; Lyons, K.M.; Lieberman, J.R. Bone morphogenetic protein-2: Biology and applications. Clin. Orthop. Relat. Res. 1996, 324, 39. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature 1971, 231, 232. [Google Scholar] [CrossRef]
- Vane, J.R. The mode of action of aspirin and similar compounds. J. Allergy Clin. Immunol. 1976, 58, 691. [Google Scholar] [CrossRef]
- Moncada, S.; Ferreira, S.H.; Vane, J.R. Prostaglandins, aspirin-like drugs and the oedema of inflammation. Nature 1973, 246, 217. [Google Scholar] [CrossRef]
- Hasenbein, I.; Sachse, A.; Hortschansky, P.; Schmuck, K.D.; Horbert, V.; Anders, C.; Lehmann, T.; Huber, R.; Maslaris, A.; Layher, F.; et al. Single Application of Low-Dose, Hydroxyapatite-Bound BMP-2 or GDF-5 Induces Long-Term Bone Formation and Biomechanical Stabilization of a Bone Defect in a Senile Sheep Lumbar Osteopenia Model. Biomedicines 2022, 10, 513. [Google Scholar] [CrossRef]
- Rickard, D.J.; Sullivan, T.A.; Shenker, B.J.; Leboy, P.S.; Kazhdan, I. Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev. Biol. 1994, 161, 218. [Google Scholar] [CrossRef]
- Chen, C.N.; Chang, H.I.; Yen, C.K.; Liu, W.L.; Huang, K.Y. Mechanical Stretch Induced Osteogenesis on Human Annulus Fibrosus Cells through Upregulation of BMP-2/6 Heterodimer and Activation of P38 and SMAD1/5/8 Signaling Pathways. Cells 2022, 11, 2600. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Liu, X.; Wei, D.; Zhu, Y.; Wang, F.; Liu, X.; Yan, F.; Zhang, X.; Liu, Y. Topical Application of Butyl Flufenamate Ointment Promotes Cranial Defect Healing in Mice by Inducing BMP2 Secretion in Skin Mesenchymal Stem Cells. Cells 2022, 11, 3620. https://doi.org/10.3390/cells11223620
Yang F, Liu X, Wei D, Zhu Y, Wang F, Liu X, Yan F, Zhang X, Liu Y. Topical Application of Butyl Flufenamate Ointment Promotes Cranial Defect Healing in Mice by Inducing BMP2 Secretion in Skin Mesenchymal Stem Cells. Cells. 2022; 11(22):3620. https://doi.org/10.3390/cells11223620
Chicago/Turabian StyleYang, Fan, Xuenan Liu, Donghao Wei, Yuan Zhu, Feilong Wang, Xuejiao Liu, Fanyu Yan, Xiao Zhang, and Yunsong Liu. 2022. "Topical Application of Butyl Flufenamate Ointment Promotes Cranial Defect Healing in Mice by Inducing BMP2 Secretion in Skin Mesenchymal Stem Cells" Cells 11, no. 22: 3620. https://doi.org/10.3390/cells11223620
APA StyleYang, F., Liu, X., Wei, D., Zhu, Y., Wang, F., Liu, X., Yan, F., Zhang, X., & Liu, Y. (2022). Topical Application of Butyl Flufenamate Ointment Promotes Cranial Defect Healing in Mice by Inducing BMP2 Secretion in Skin Mesenchymal Stem Cells. Cells, 11(22), 3620. https://doi.org/10.3390/cells11223620






