Clinical Value of Ultrasonography and Serum Markers in Preoperative N Staging of Thyroid Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
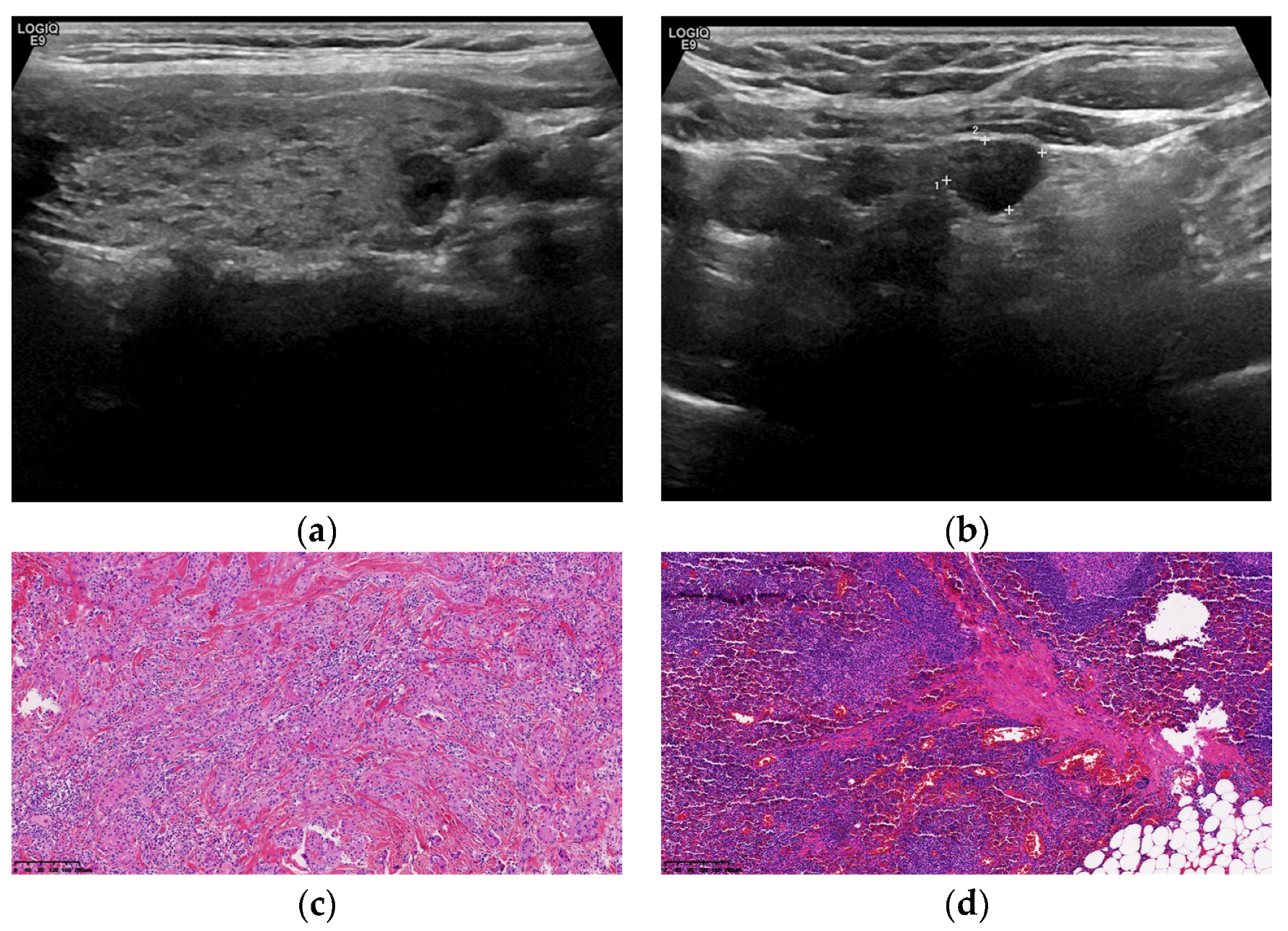
2.2. Ultrasonic Instruments and Inspection Methods
2.3. Type of Surgery
2.4. TNM Staging Criteria for TC
2.5. Detection of Serum Markers
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Pathological and Ultrasonic Diagnostic Results of LNM
3.3. Comparison of Serum Markers between the pN0 and pN1 Groups
3.4. Analysis of the Correlation of fT3, Tg, and TPOAb with the pN0 and pN1 Groups
3.5. Comparison of the Diagnostic Value of Tg, Age, US Examination Alone, and the Combined Method in LNM
3.6. Verification of the Diagnostic Value of Tg, Age, US Examination Alone, and Combined Method in LNM
3.7. Comparison of TPOAb and Tg between the pN1a and pN1b Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solis-Pazmino, P.; Salazar-Vega, J.; Lincango-Naranjo, E.; Garcia, C.; Koupermann, G.J.; Ortiz-Prado, E.; Ledesma, T.; Rojas, T.; Alvarado-Mafla, B.; Carcamo, C.; et al. Thyroid Cancer Overdiagnosis and Overtreatment: A Cross- Sectional Study at a Thyroid Cancer Referral Center in Ecuador. BMC Cancer 2021, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Zhou, W.; Zhan, W.; Li, W.; Wang, Y.; Fan, J. Preoperative Prediction of Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma via Conventional and Contrast-Enhanced Ultrasound. J. Ultrasound. Med. 2020, 39, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.H.; Lee, Y.H.; Lim, H.K.; Moon, W.J.; Na, D.G.; Park, J.S.; et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Kim, W.G.; Choi, Y.M.; Kwon, H.; Song, D.E.; Lee, Y.M.; Sung, T.Y.; Yoon, J.H.; Hong, S.J.; Baek, J.H.; et al. Recent Changes in the Clinical Outcome of Papillary Thyroid Carcinoma with Cervical Lymph Node Metastasis. J. Clin. Endocrinol. Metab. 2015, 100, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Meng, Y.; Guo, R.; Lu, G.; Jin, L.; Wang, Y.; Yang, D. Value of Ultrasound Combined with Immunohistochemistry Evaluation of Central Lymph Node Metastasis for the Prognosis of Papillary Thyroid Carcinoma. Cancer Manag. Res. 2020, 12, 8787–8799. [Google Scholar] [CrossRef]
- Yang, Z.; Heng, Y.; Lin, J.; Lu, C.; Yu, D.; Tao, L.; Cai, W. Nomogram for Predicting Central Lymph Node Metastasis in Papillary Thyroid Cancer: A Retrospective Cohort Study of Two Clinical Centers. Cancer Res Treat. 2020, 52, 1010–1018. [Google Scholar] [CrossRef]
- Feng, J.W.; Ye, J.; Wu, W.X.; Qu, Z.; Qin, A.C.; Jiang, Y. Management of cN0 Papillary Thyroid Microcarcinoma Patients According to Risk-Scoring Model for Central Lymph Node Metastasis and Predictors of Recurrence. J. Endocrinol. Investig. 2020, 43, 1807–1817. [Google Scholar] [CrossRef]
- Detweiler, K.; Elfenbein, D.M.; Mayers, D. Evaluation of Thyroid Nodules. Surg. Clin. N. Am. 2019, 99, 571–586. [Google Scholar] [CrossRef]
- O’Connell, K.; Yen, T.W.; Quiroz, F.; Evans, D.B.; Wang, T.S. The Utility of Routine Preoperative Cervical Ultrasonography in Patients Undergoing Thyroidectomy for Differentiated Thyroid Cancer. Surgery 2013, 154, 697–701. [Google Scholar] [CrossRef]
- Popoveniuc, G.; Jonklaas, J. Thyroid Nodules. Med. Clin. N. Am. 2012, 96, 329–349. [Google Scholar] [CrossRef]
- Jo, K.; Lim, D.J. Clinical Implications of Anti-Thyroglobulin Antibody Measurement Before Surgery in Thyroid Cancer. Korean J. Intern. Med. 2018, 33, 1050–1057. [Google Scholar] [CrossRef]
- Medenica, S.; Radojevic, N.; Stojkovic, M.; Nedeljkovic-Beleslin, B.; Savic, S.; Ciric, J.; Trbojevic, B.; Zarkovic, M. Autoimmunity and Thyrotropin Level in Developing Thyroid Malignancy. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2824–2829. [Google Scholar]
- Wong, S.L.; Grodski, S.; Yeung, M.J.; Serpell, J.W. Anti-Thyroid Antibodies as a Predictor of Thyroid Cancer. ANZ J. Surg. 2015, 85, 849–853. [Google Scholar] [CrossRef]
- Hu, M.J.; Zhang, C.; Liang, L.; Wang, S.Y.; Zheng, X.C.; Zhang, Q.; Jiang, C.X.; Zhong, Q.; Huang, F. Fasting Serum Glucose, Thyroid-Stimulating Hormone, and Thyroid Hormones and Risk of Papillary Thyroid Cancer: A Case-Control Study. Head Neck 2019, 41, 2277–2284. [Google Scholar] [CrossRef]
- Cho, Y.A.; Kong, S.Y.; Shin, A.; Lee, J.; Lee, E.K.; Lee, Y.J.; Kim, J. Biomarkers of Thyroid Function and Autoimmunity for Predicting High-Risk Groups of Thyroid Cancer: A Nested Case-Control Study. BMC Cancer 2014, 14, 873. [Google Scholar] [CrossRef]
- Davis, P.J.; Lin, H.Y.; Hercbergs, A.; Mousa, S.A. Actions of L-thyroxine (T4) and Tetraiodothyroacetic Acid (Tetrac) on Gene Expression in Thyroid Cancer Cells. Genes 2020, 11, 755. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Zhou, Y.; Cheng, R. Risk Factors for Central Lymph Node Metastasis in the Cervical Region in Papillary Thyroid Carcinoma: A Retrospective Study. World J. Surg. Oncol. 2021, 19, 138. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Sherma, S.I. Thyroid Carcinoma. Lancet 2003, 361, 501–511. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.Y.; Choe, J.H.; Kim, J.S.; Hahn, S.Y.; Kim, S.W.; Chung, J.H.; Jung, J.; Kim, T.H. Preoperative Serum Thyroglobulin and its Correlation with the Burden and Extent of Differentiated Thyroid Cancer. Cancers 2020, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Nambron, R.; Rosenthal, R.; Bahl, D. Diagnosis and Evaluation of Thyroid Nodules-The Clinician’s Perspective. Radiol. Clin. N. Am. 2020, 58, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.N.; Song, L.H.; Yu, P.Y.; Yu, S.Y.; Deng, S.H.; Mao, X.H.; Xiu, C.; Sun, J. Ultrasound Elastic Parameters Predict Central Lymph Node Metastasis of Papillary Thyroid Carcinoma. J. Surg. Res. 2020, 253, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.P.; Zhang, L. Using Ultrasonography to Evaluate the Relationship Between Capsular Invasion or Extracapsular Extension and Lymph Node Metastasis in Papillary Thyroid Carcinomas. Chin. Med. J. (Engl.) 2017, 130, 1309–1313. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, F.; Wang, G.; Liu, T.; Dong, T.; Sun, Y. Value of Combining Clinical Factors, Conventional Ultrasound, and Contrast-Enhanced Ultrasound Features in Preoperative Prediction of Central Lymph Node Metastases of Different Sized Papillary Thyroid Carcinomas. Cancer Manag. Res. 2021, 13, 3403–3415. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H. Meta-Analysis of Ultrasound for Cervical Lymph Nodes in Papillary Thyroid Cancer: Diagnosis of Central and Lateral Compartment Nodal Metastases. Eur. J. Radiol. 2019, 112, 14–21. [Google Scholar] [CrossRef]
- Leboulleux, S.; Rubino, C.; Baudin, E.; Caillou, B.; Hartl, D.M.; Bidart, J.M.; Travagli, J.P.; Schlumberger, M. Prognostic Factors for Persistent or Recurrent Disease of Papillary Thyroid Carcinoma with Neck Lymph Node Metastases and/or Tumor Extension Beyond the Thyroid Capsule at Initial Diagnosis. J. Clin. Endocrinol. Metab. 2005, 90, 5723–5729. [Google Scholar] [CrossRef]
- Leboulleux, S.; Girard, E.; Rose, M.; Travagli, J.P.; Sabbah, N.; Caillou, B.; Hartl, D.M.; Lassau, N.; Baudin, E.; Schlumberger, M. Ultrasound Criteria of Malignancy for Cervical Lymph Nodes in Patients Followed up for Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2007, 92, 3590–3594. [Google Scholar] [CrossRef]
- Jo, Y.J.; Choi, H.R.; Park, S.H.; Jeong, Y.J. Extent of Thyroid Surgery for Clinically Node-Negative Papillary Thyroid Carcinoma with Confirmed Nodal Metastases After Prophylactic Central Neck Dissection: A 15-Year Experience in a Single Center. Ann. Surg. Treat. Res. 2020, 99, 197–204. [Google Scholar] [CrossRef]
- Agcaoglu, O.; Sengun, B.; Ozoran, E.; Bilgic, C.; Karabay, O.; Taskin, O.C.; Yazici, D.; Tezelman, S. Should we Perform Routine Prophylactic Central Neck Dissection in Patients with Thyroid Papillary Microcarcinoma? Ann. Ital. Chir. 2018, 89, 485–488. [Google Scholar]
- Li, W.; Chen, T. An Insight into the Clinical Application of Gut Microbiota during Anticancer Therapy. Adv. Gut Microbiome Res. 2022, 2022, 8183993. [Google Scholar] [CrossRef]
- Zhao, W.J.; Luo, H.; Zhou, Y.M.; Dai, W.Y.; Zhu, J.Q. Evaluating the Effectiveness of Prophylactic Central Neck Dissection with Total Thyroidectomy for cN0 Papillary Thyroid Carcinoma: An Updated Meta-Analysis. Eur. J. Surg. Oncol. 2017, 43, 1989–2000. [Google Scholar] [CrossRef]
- Moreno, M.A.; Edeiken-Monroe, B.S.; Siegel, E.R.; Sherman, S.I.; Clayman, G.L. In Papillary Thyroid Cancer, Preoperative Central Neck Ultrasound Detects Only Macroscopic Surgical Disease, but Negative Findings Predict Excellent Long-Term Regional Control and Survival. Thyroid 2012, 22, 347–355. [Google Scholar] [CrossRef]
- Raffaelli, M.; De Crea, C.; Sessa, L.; Giustacchini, P.; Revelli, L.; Bellantone, C.; Lombardi, C.P. Prospective Evaluation of Total Thyroidectomy Versus Ipsilateral Versus Bilateral Central Neck Dissection in Patients with Clinically Node-Negative Papillary Thyroid Carcinoma. Surgery 2012, 152, 957–964. [Google Scholar] [CrossRef]
- Viola, D.; Materazzi, G.; Valerio, L.; Molinaro, E.; Agate, L.; Faviana, P.; Seccia, V.; Sensi, E.; Romei, C.; Piaggi, P.; et al. Prophylactic Central Compartment Lymph Node Dissection in Papillary Thyroid Carcinoma: Clinical Implications Derived from the First Prospective Randomized Controlled Single Institution Study. J. Clin. Endocrinol. Metab. 2015, 100, 1316–1324. [Google Scholar] [CrossRef]
- Zhao, W.; You, L.; Hou, X.; Chen, S.; Ren, X.; Chen, G.; Zhao, Y. The Effect of Prophylactic Central Neck Dissection on Locoregional Recurrence in Papillary Thyroid Cancer After Total Thyroidectomy: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2017, 24, 2189–2198. [Google Scholar] [CrossRef]
- Suh, I.; Vriens, M.R.; Guerrero, M.A.; Griffin, A.; Shen, W.T.; Duh, Q.Y.; Clark, O.H.; Kebebew, E. Serum Thyroglobulin is a Poor Diagnostic Biomarker of Malignancy in Follicular and Hurthle-Cell Neoplasms of the Thyroid. Am. J. Surg. 2010, 200, 41–46. [Google Scholar] [CrossRef]
- Lee, E.K.; Chung, K.W.; Min, H.S.; Kim, T.S.; Kim, T.H.; Ryu, J.S.; Jung, Y.S.; Kim, S.K.; Lee, Y.J. Preoperative Serum Thyroglobulin as a Useful Predictive Marker to Differentiate Follicular Thyroid Cancer from Benign Nodules in Indeterminate Nodules. J. Korean Med. Sci. 2012, 27, 1014–1018. [Google Scholar] [CrossRef]
- Sands, N.B.; Karls, S.; Rivera, J.; Tamilia, M.; Hier, M.P.; Black, M.J.; Gologan, O.; Payne, R.J. Preoperative Serum Thyroglobulin as an Adjunct to Fine-Needle Aspiration in Predicting Well-Differentiated Thyroid Cancer. J. Otolaryngol. Head Neck Surg. 2010, 39, 669–673. [Google Scholar]
- Algeciras-Schimnich, A. Thyroglobulin Measurement in the Management of Patients with Differentiated Thyroid Cancer. Crit Rev. Clin. Lab. Sci. 2018, 55, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Qiu, Y.; Li, Z.; Zhang, L.; Fei, Y.; Zhu, J.; Su, A. Predictors of Thyroglobulin in the Lymph Nodes Recurrence of Papillary Thyroid Carcinoma Undergoing Total Thyroidectomy. BMC Surg. 2021, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Mizrachi, A.; Bachar, G.; Vainer, I.; Shimon, I.; Hirsch, D.; Diker-Cohen, T.; Duskin-Bitan, H.; Robenshtok, E. Detecting Recurrence Following Lobectomy for Thyroid Cancer: Role of Thyroglobulin and Thyroglobulin Antibodies. J. Clin. Endocrinol. Metab. 2020, 105, e2145–e2151. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, W.Y.; Cui, L.L. Clinical Significance of STAT3 and MAPK Phosphorylation, and the Protein Expression of Cyclin D1 in Skin Squamous Cell Carcinoma Tissues. Mol. Med. Rep. 2015, 12, 8129–8134. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.F.; Skeaff, S.A. Thyroglobulin as a Biomarker of Iodine Deficiency: A Review. Thyroid 2014, 24, 1195–1209. [Google Scholar] [CrossRef]
- Trimboli, P.; Treglia, G.; Giovanella, L. Preoperative Measurement of Serum Thyroglobulin to Predict Malignancy in Thyroid Nodules: A Systematic Review. Horm. Metab. Res. 2015, 47, 247–252. [Google Scholar] [CrossRef]
- Rinaldi, S.; Plummer, M.; Biessy, C.; Tsilidis, K.K.; Østergaard, J.N.; Overvad, K.; Tjønneland, A.; Halkjaer, J.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; et al. Thyroid-Stimulating Hormone, Thyroglobulin, and Thyroid Hormones and Risk of Differentiated Thyroid Carcinoma: The EPIC Study. J. Natl. Cancer Inst. 2014, 106, dju097. [Google Scholar] [CrossRef]
- Scheffler, P.; Forest, V.I.; Leboeuf, R.; Florea, A.V.; Tamilia, M.; Sands, N.B.; Hier, M.P.; Mlynarek, A.M.; Payne, R.J. Serum Thyroglobulin Improves the Sensitivity of the McGill Thyroid Nodule Score for Well-Differentiated Thyroid Cancer. Thyroid 2014, 24, 852–857. [Google Scholar] [CrossRef]
- Hulikal, N.; Re, A.; Banoth, M.; Chowhan, A.K.; Yutla, M.; Sachan, A. Can Preoperative Serum Thyroglobulin Levels Predict the Risk of Malignancy? Results From Prospective Analysis of Biochemical Predictors of Malignancy in Thyroid Nodules. Acta Otorhinolaryngol. Ital. 2020, 40, 33–37. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, W.J.; Liang, L.; Li, L.L.; Yin, W.; Su, Q.L.; Lin, F.F. Establishing a Predictive Nomogram for Cervical Lymph Node Metastasis in Patients with Papillary Thyroid Carcinoma. Front. Oncol. 2021, 11, 766650. [Google Scholar] [CrossRef]
- Rui, Z.Y.; Liu, Y.; Zheng, W.; Wang, X.; Meng, Z.W.; Tan, J.; Li, N.; Jia, Q. A Retrospective Study of the Risk Factors and the Prognosis in Patients with Papillary Thyroid Carcinoma Depending on the Number of Lymph Node Metastasis. Clin. Exp. Med. 2021, 21, 277–286. [Google Scholar] [CrossRef]
- Jiang, H.J.; Hsiao, P.J. Clinical Application of the Ultrasound-Guided Fine Needle Aspiration for Thyroglobulin Measurement to Diagnose Lymph Node Metastasis from Differentiated Thyroid Carcinoma-Literature Review. Kaohsiung J. Med. Sci. 2020, 36, 236–243. [Google Scholar] [CrossRef]
- Prpić, M.; Franceschi, M.; Romić, M.; Jukić, T.; Kusić, Z. Thyroglobulin as a Tumor Marker in Differentiated Thyroid Cancer—Clinical Considerations. Acta Clin. Croat. 2018, 57, 518–527. [Google Scholar] [CrossRef]
- Shukla, N.; Osazuwa-Peters, N.; Megwalu, U.C. Association Between Age and Nodal Metastasis in Papillary Thyroid Carcinoma. Otolaryngol. Head Neck Surg. 2021, 165, 43–49. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Q.; Zhang, H.; Zheng, K.; Wang, R.; Wang, G. Risk Factors for Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front. Endocrinol. (Lausanne) 2020, 11, 265. [Google Scholar] [CrossRef]
- Feng, Y.; Min, Y.; Chen, H.; Xiang, K.; Wang, X.; Yin, G. Construction and validation of a nomogram for predicting cervical lymph node metastasis in classic papillary thyroid carcinoma. J. Endocrinol. Investig. 2021, 44, 2203–2211. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Shu, X.; Yu, P.; Wang, H.; Su, X.; Tan, J. Risk Factors and a Prediction Model of Lateral Lymph Node Metastasis in CN0 Papillary Thyroid Carcinoma Patients With 1-2 Central Lymph Node Metastases. Front. Endocrinol. (Lausanne) 2021, 12, 716728. [Google Scholar] [CrossRef]
- Spinelli, C.; Ghionzoli, M.; Oreglio, C.; Sanna, B.; De Napoli, L.; Morganti, R.; Antonelli, A.; Morabito, A.; Miccoli, P. Increased trend of thyroid cancer in childhood over the last 30 years in EU countries: A call for the pediatric surgeon. Eur. J. Pediatr. 2022, 181, 3907–3913. [Google Scholar] [CrossRef]
- Spinelli, C.; Tognetti, F.; Strambi, S.; Morganti, R.; Massimino, M.; Collini, P. Cervical Lymph Node Metastases of Papillary Thyroid Carcinoma, in the Central and Lateral Compartments, in Children and Adolescents: Predictive Factors. World J. Surg. 2018, 42, 2444–2453. [Google Scholar] [CrossRef]
- Howard, S.R.; Freeston, S.; Harrison, B.; Izatt, L.; Natu, S.; Newbold, K.; Pomplun, S.; Spoudeas, H.A.; Wilne, S.; Kurzawinski, T.R.; et al. Paediatric differentiated thyroid carcinoma: A UK National Clinical Practice Consensus Guideline. Endocr. Relat. Cancer 2022, 29, G1–G33. [Google Scholar] [CrossRef]



| Characteristics | Model Establishing Group | External Validation Group | ||||
|---|---|---|---|---|---|---|
| N0 (%) | N1a (%) | N1b (%) | N0 (%) | N1a (%) | N1b (%) | |
| Sex | ||||||
| Male | 261 (23.5) | 173 (30.3) | 105 (34.8) | 17 (19.3) | 18 (38.3) | 6 (50.0) |
| Female | 849 (76.5) | 398 (69.7) | 197 (65.2) | 71 (80.7) | 29 (61.7) | 6 (50.0) |
| Age, median (IQR), years | 48 (19) | 43 (18) | 41 (21) | 48 (16) | 42 (18) | 34 (30) |
| Pathology 1 | ||||||
| Papillary | 1075 (96.8) | 566 (99.1) | 293 (97.0) | 85 (96.6) | 47 (100) | 12 (100) |
| Follicular | 18 (1.6) | 1 (0.2) | 1 (0.3) | 1 (1.1) | 0 (0) | 0 (0) |
| Medullary | 15 (1.4) | 4 (0.7) | 8 (2.6) | 2 (2.3) | 0 (0) | 1 (8.3) |
| Undifferentiated | 2 (0.2) | 0 (0) | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) |
| Serum markers | ||||||
| TT3, median (IQR), ng/mL | 1.07 (0.23) | 1.07 (0.23) | 1.09 (0.22) | 0.995 (0.21) | 1.03 (0.27) | 0.98 (0.22) |
| TT4, median (IQR), μg/dL | 7.80 (2.00) | 7.70 (1.80) | 7.90 (2.00) | 8.10 (2.12) | 8.40 (2.34) | 7.85 (1.95) |
| fT3, median (IQR), pg/mL | 3.20 (0.42) | 3.23 (0.48) | 3.21 (0.47) | 3.15 (0.50) | 3.24 (0.66) | 3.275 (0.46) |
| fT4, median (IQR), ng/dL | 1.24 (0.22) | 1.24 (0.24) | 1.27 (0.24) | 1.095 (0.22) | 1.15 (0.28) | 1.165 (0.19) |
| TSH, median (IQR), μIU/mL | 1.55 (1.18) | 1.54 (1.07) | 1.78 (1.36) | 1.46965 (0.7965) | 1.368 (1.225) | 1.522 (0.6345) |
| TgAb, median (IQR), IU/mL | 15.60 (15.62) | 15.90 (15.65) | 15.00 (18.35) | 14.00 (34.82) | 14.00 (46.28) | 17.60 (133.03) |
| TPOAb, median (IQR), IU/mL | 38.55 (29.80) | 33.70 (22.70) | 31.30 (24.65) | 26.20 (48.24) | 26.20 (40.09) | 26.20 (3.63) |
| Tg, median (IQR), ng/mL | 10.43 (14.43) | 13.89 (19.57) | 31.55 (72.26) | 8.105 (13.59) | 10.24 (18.80) | 28.09 (79.67) |
| Clinical N Stage | Pathological N Stage | Sum | Kappa | p-Value | ||
|---|---|---|---|---|---|---|
| pN0 | pN1a | pN1b | ||||
| cN0 | 946 | 405 | 9 | 1360 | 0.437 | <0.001 |
| cN1a | 125 | 133 | 3 | 261 | ||
| cN1b | 39 | 33 | 290 | 362 | ||
| Sum | 1110 | 571 | 302 | 1983 | ||
| Serum Markers | Median | Z | p-Value | |
|---|---|---|---|---|
| pN0 | pN1 | |||
| TT3 (ng/mL) | 1.070 | 1.070 | −0.342 | 0.733 |
| TT4 (μg/dL) | 7.800 | 7.700 | −1.249 | 0.212 |
| fT3 (pg/mL) | 3.200 | 3.220 | −2.789 | 0.005 |
| fT4 (ng/dL) | 1.240 | 1.250 | −1.212 | 0.226 |
| TSH (μIU/mL) | 1.547 | 1.611 | −1.039 | 0.299 |
| TgAb (IU/mL) | 15.600 | 15.600 | −0.454 | 0.650 |
| TPOAb (IU/mL) | 38.550 | 32.800 | −5.212 | <0.001 |
| Tg (ng/mL) | 10.430 | 16.580 | −8.399 | <0.001 |
| Variables | β | SE | Wald | p-Value | OR | 95% CI for OR |
|---|---|---|---|---|---|---|
| Constant | −0.058 | 0.493 | 0.014 | 0.906 | 0.944 | |
| TPOAb (IU/mL) | −0.001 | 0.000 | 25.651 | <0.001 | 0.999 | 0.999–1.000 |
| Tg (ng/mL) | 0.003 | 0.001 | 17.276 | <0.001 | 1.003 | 1.001–1.004 |
| fT3 (pg/mL) | 0.147 | 0.130 | 1.288 | 0.256 | 1.158 | 0.899–1.494 |
| Age | −0.027 | 0.004 | 39.817 | <0.001 | 0.973 | 0.965–0.981 |
| cN (0 or 1) | 1.849 | 0.115 | 257.547 | <0.001 | 6.357 | 5.071–7.967 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhao, S.; Xu, C.; Yao, J.; Yu, X.; Xu, D. Clinical Value of Ultrasonography and Serum Markers in Preoperative N Staging of Thyroid Cancer. Cells 2022, 11, 3621. https://doi.org/10.3390/cells11223621
Wang H, Zhao S, Xu C, Yao J, Yu X, Xu D. Clinical Value of Ultrasonography and Serum Markers in Preoperative N Staging of Thyroid Cancer. Cells. 2022; 11(22):3621. https://doi.org/10.3390/cells11223621
Chicago/Turabian StyleWang, Hui, Shanshan Zhao, Chunyang Xu, Jincao Yao, Xiuhua Yu, and Dong Xu. 2022. "Clinical Value of Ultrasonography and Serum Markers in Preoperative N Staging of Thyroid Cancer" Cells 11, no. 22: 3621. https://doi.org/10.3390/cells11223621
APA StyleWang, H., Zhao, S., Xu, C., Yao, J., Yu, X., & Xu, D. (2022). Clinical Value of Ultrasonography and Serum Markers in Preoperative N Staging of Thyroid Cancer. Cells, 11(22), 3621. https://doi.org/10.3390/cells11223621







