Minor Kinases with Major Roles in Cytokinesis Regulation
Abstract
1. Introduction
1.1. Importance of Cytokinesis in Living Cells
1.2. Overview of Cytokinesis in Animal Cells
1.3. Spatio-Temporal Control of Cytokinesis through Phosphorylation
2. Casein Kinase 2 (CK2)
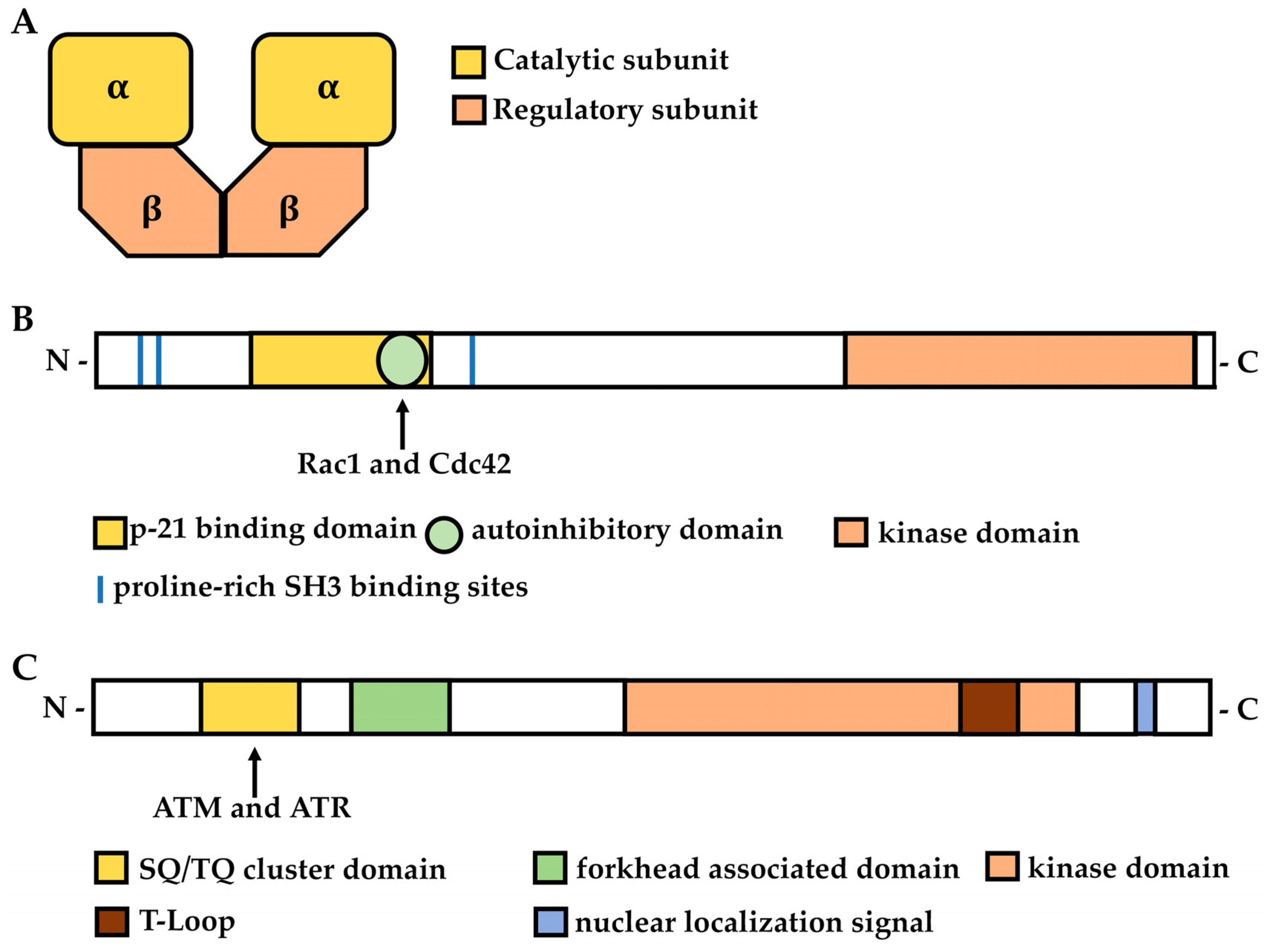
2.1. Structure and Function of CK2
2.2. Role of CK2 in Tumorigenesis
2.3. Role of CK2 in Cytokinesis
3. P21-Activated Kinase (PAK)
3.1. Structure and Function of PAKs
3.2. Role of PAKs in Tumorigenesis
3.3. Role of PAKs in Cytokinesis
4. Checkpoint Kinase 2 (Chk2)
4.1. Structure and Function of Chk2
4.2. Role of Chk2 in Tumorigenesis
4.3. Role of Chk2 in Cytokinesis
5. On the Crosstalk between Minor Kinases—Novel Insight into Cell-Cycle Control?
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Acronyms | Full name |
| ALIX | ALG-2-interacting protein X |
| AKT | Protein kinase B |
| ANCHR | Abscission/NoCut checkpoint regulator |
| ARF1 | ADP-ribosylation factor-1 |
| ATM | Ataxia telangiectasia-mutated |
| ATR | Ataxia telangiectasia and Rad3-related |
| Bni1 | Diaphanous-related formin |
| ßPIX | PAK-interacting exchange factor beta |
| BRCA1 | Breast cancer type 1 susceptibility protein |
| BRCA2 | Breast cancer type 2 susceptibility protein |
| ßTrCP | Beta-transducin repeat containing E3 ubiquitin protein ligase |
| Bud6 | Bud site selection protein 6 |
| Cdc3 | Cell division control protein 3 |
| Cdc10 | Cell division control protein 10 |
| Cdc11 | Cell division control protein 11 |
| Cdc12 | Cell division control protein 12 |
| Cdc15 | Cell division control protein 15 |
| Cdc25 | Cell division cycle 25 |
| Cdc25B | Cell division cycle 25B |
| Cdc42 | Cell division control protein 42 homolog |
| CDK1 | Cyclin-dependent kinase 1 |
| Cep55 | Centrosomal protein 55 |
| Chk2 | Checkpoint kinase 2 |
| CHMP4C | Charged multivesicular body protein 4C |
| CIN | Chromosome instability |
| Cit-K | Citron kinase |
| CK2 | Casein kinase 2 |
| CK2α | Casein kinase 2 subunit alpha |
| CK2β | Casein kinase 2 subunit beta |
| Ckb1 | Casein kinase 2 subunit beta |
| CKIP-1 | Casein kinase 2 interacting protein 1 |
| Cla4 | Serine/threonine protein kinase CLA4 |
| CPC | Chromosomal passenger complex |
| CR | Contractile ring |
| Cyk-4 | Cytokinesis defect-4 |
| DNA-PK | DNA-dependent protein kinase |
| DDR | DNA damage response |
| E2F1 | Transcription factor |
| ECT2 | Epithelial cell-transforming sequence 2 oncogene |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| ER | Endoplasmic reticulum |
| ESCRT-III | Endosomal sorting complexes required for transport-III |
| FHA | Forkhead-associated domain |
| GBF1 | Golgi brefeldin A-resistant guanine nucleotide exchange factor 1 |
| GOLPH3 | Golgi phosphoprotein 3 |
| GRB2 | Growth factor receptor-bound protein 2 |
| HDAC1 | Histone deacetylase 1 |
| HDAC2 | Histone deacetylase 2 |
| INCENP | Inner centromere protein |
| JAK | Janus tyrosine kinase |
| KD | Serine/threonine kinase domain |
| KIF23 | Kinesin-like protein |
| MAPK | Mitogen-activated protein Kinases |
| MB | Midbody |
| mbt | Mushroom bodies tiny |
| MgcRacGAP | Rac GTPase-activating protein 1 |
| Mid1 | Anillin-related medial ring protein mid1 |
| mhcA | Myosin heavy chain A |
| MKLP1 | Mitotic kinesin-like protein1 |
| MLCK | Myosin light chain kinase |
| MORC2 | Microrchidia family CW-type zinc finger protein 2 |
| MPS1 | MonoPolar spindle 1 |
| MRE11 | Double-strand break repair protein MRE11 |
| MRK | MLK-related kinase |
| MRLC | Myosin II regulatory light chain |
| Myo II | Myosin II |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NBS1 | Nijmegen breakage syndrome 1 |
| Nck | Non-catalytic region of tyrosine kinase |
| NM IIA | Non-muscle myosin II isoform A |
| Orb2p | Translational regulator orb2 |
| PAK | P21-activated kinases |
| PI3K | Phosphatidylnositol 3-kinase |
| PIN1 | Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
| PIX | PAK-interacting exchange |
| Plk1 | Polo-like kinase 1 |
| PML | Promyelocytic leukemia |
| PP2A | Phosphoprotein phosphatase 2A |
| PP6 | Protein phosphatase 6 |
| PXXP | Proline-Xaa-Xaa-Proline |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| Rlc1 | Myosin regulatory light chain |
| RhoA | Ras homolog family member A |
| SCD | SQ/TQ cluster domain |
| SCF | Skp1/Cul-1/F-box protein complex |
| Sep7 | Septation protein 7 |
| SH3 | SRC homology 3 domain |
| Shs1 | Seventh homolog of septin 1 |
| Shk1 | Serine/threonine protein kinase shk1/pak1 |
| Shk2 | Serine/threonine protein kinase shk2 |
| Skm1 | Serine/threonine protein kinase shk1/pak1 |
| Ste20 | Serine/threonine protein kinase STE20 |
| TSG101 | Tumor susceptibility gene 101 protein |
| zip | Zipper |
References
- D’Avino, P.P.; Giansanti, M.G.; Petronczki, M. Cytokinesis in animal cells. Cold Spring Harb. Perspect. Biol. 2015, 7, a015834. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; O’Shaughnessy, B. Molecular Mechanism of Cytokinesis. Annu. Rev. Biochem. 2020, 88, 661–689. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar-Jog, Y.P.; Bi, E. Mechanics and regulation of cytokinesis in budding yeast. Semin. Cell Dev. Biol. 2017, 66, 107–118. [Google Scholar] [CrossRef]
- Wangsa, D.; Quintanilla, I.; Torabi, K.; Vila-Casadesús, M.; Ercilla, A.; Klus, G.; Yuce, Z.; Galofré, C.; Cuatrecasas, M.; Lozano, J.J.; et al. Near-tetraploid cancer cells show chromosome instability triggered by replication stress and exhibit enhanced invasiveness. FASEB J. 2018, 32, 3502–3517. [Google Scholar] [CrossRef]
- Bach, D.H.; Zhang, W.; Sood, A.K. Chromosomal Instability in Tumor Initiation and Development. Cancer Res. 2019, 79, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Telentschak, S.; Soliwoda, M.; Nohroudi, K.; Addicks, K.; Klinz, F.J. Cytokinesis failure and successful multipolar mitoses drive aneuploidy in glioblastoma cells. Oncol. Rep. 2015, 33, 2001–2008. [Google Scholar] [CrossRef]
- Heng, H.H.; Bremer, S.W.; Stevens, J.B.; Horne, S.D.; Liu, G.; Abdallah, B.Y.; Ye, K.J.; Ye, C.Y. Chromosomal instability (CIN): What it is and why it is crucial to cancer evolution. Cancer Metastasis Rev. 2013, 32, 325–340. [Google Scholar] [CrossRef]
- Mishima, M. centralsplindin in Rappaport’s cleavage signaling. Semin. Cell Dev. Biol. 2016, 53, 45–56. [Google Scholar] [CrossRef]
- Murthy, K.; Wadsworth, P. Dual role for microtubules in regulating cortical contractility during cytokinesis. J. Cell Sci. 2008, 121, 2350–2359. [Google Scholar] [CrossRef]
- Mishima, M.; Kaitna, S.; Glotzer, M. Central spindle assembly and cytokinesis require a kinesin-like protein/ RhoGAP complex with microtubule bundling activity. Dev. Cell 2002, 2, 41–54. [Google Scholar] [CrossRef]
- White-Cooper, H.; Caporilli, S. Transcriptional and post-transcriptional regulation of Drosophila germline stem cells and their differentiating progeny. Adv. Exp. Med. Biol. 2013, 786, 47–61. [Google Scholar] [CrossRef]
- Somers, W.G.; Saint, R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 2003, 4, 29–39. [Google Scholar] [CrossRef]
- Yuce, O.; Piekny, A.; Glotzer, M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 2005, 170, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.M.; Fang, G. MgcRacGAP controls the assembly of the contractile ring and the initiation of cytokinesis. Proc. Natl. Acad. Sci. USA 2005, 102, 13158–13163. [Google Scholar] [CrossRef]
- Kamijo, K.; Ohara, N.; Abe, M.; Uchimura, T.; Hosoya, H.; Lee, J.S.; Miki, T. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol. Biol. Cell 2006, 17, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Yonemura, S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J. Cell Sci. 2006, 119, 104–114. [Google Scholar] [CrossRef]
- Piekny, A.; Werner, M.; Glotzer, M. Cytokinesis: Welcome to the Rho zone. Trends Cell Biol. 2005, 15, 651–658. [Google Scholar] [CrossRef]
- Kim, O.V.; Litvinov, R.I.; Mordakhanova, E.R.; Bi, E.; Vagin, O.; Weisel, J.W. Contribution of septins to human platelet structure and function. iScience 2022, 25, 104654. [Google Scholar] [CrossRef]
- Garno, C.; Irons, Z.H.; Gamache, C.M.; McKim, Q.; Reyes, G.; Wu, X.; Shuster, C.B.; Henson, J.H. Building the cytokinetic contractile ring in an early embryo: Initiation as clusters of myosin II, anillin and septin, and visualization of a septin filament network. PLoS ONE 2021, 16, e0252845. [Google Scholar] [CrossRef]
- Kučera, O.; Siahaan, V.; Janda, D.; Dijkstra, S.H.; Pilátová, E.; Zatecka, E.; Diez, S.; Braun, M.; Lansky, Z. Anillin propels myosin-independent constriction of actin rings. Nat. Commun. 2021, 12, 4595. [Google Scholar] [CrossRef]
- Carim, S.C.; Kechad, A.; Hickson, G.R.X. Animal Cell Cytokinesis: The Rho-dependent Actomyosin-Anillo septin Contractile Ring as a Membrane Microdomain Gathering, Compressing, and Sorting Machine. Front. Cell Dev. Biol. 2020, 8, 575226. [Google Scholar] [CrossRef] [PubMed]
- Gai, M.; Camera, P.; Dema, A.; Bianchi, F.; Berto, G.; Scarpa, E.; Germena, G.; Di Cunto, F. Citron kinase controls abscission through RhoA and anillin. Mol. Biol. Cell 2011, 22, 3768–3778. [Google Scholar] [CrossRef] [PubMed]
- El-Amine, N.; Carim, S.C.; Wernike, D.; Hickson, G.R.X. Rho-dependent control of the Citron kinase. Sticky, drives midbody ring maturation. Mol. Biol. Cell 2019, 30, 2185–2204. [Google Scholar] [CrossRef] [PubMed]
- Sirajuddin, M.; Farkasovsky, M.; Hauer, F.; Kühlmann, D.; Macara, I.G.; Weyand, M.; Stark, H.; Wittinghofer, A. Structural insight into filament formation by mammalian septins. Nature 2007, 449, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Piatti, S. Cytokinesis: An Anillin-RhoGEF Module Sets the Stage for Septin Double Ring Assembly. Curr. Biol. 2020, 30, R347–R349. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Field, C.M.; Coughlin, M.L.; Straight, A.F.; Mitchison, T.J. Self- and Actin-Templated Assembly of Mammalian Septins. Dev. Cell 2002, 3, 791–802. [Google Scholar] [CrossRef]
- Joo, E.; Surka, M.C.; Trimble, W.S. Mammalian SEPT2 Is Required for Scaffolding Nonmuscle Myosin II and its Kinases. Dev. Cell 2007, 13, 677–690. [Google Scholar] [CrossRef]
- Fremont, S.; Echard, A. Membrane traffic in the late steps of cytokinesis. Curr. Biol. 2018, 28, R458–R470. [Google Scholar] [CrossRef]
- Hu, C.K.; Coughlin, M.; Mitchison, T.J. Midbody assembly and its regulation during cytokinesis. Mol. Biol. Cell 2012, 23, 1024–1034. [Google Scholar] [CrossRef]
- Petsalaki, E.; Zachos, G. The Abscission Ceckpoint: A guardian of chromosomal stability. Cells 2021, 10, 3350. [Google Scholar] [CrossRef]
- Carlton, J.Z.; Martin-Serrano, J. Parallels between cytokinesis and retroviral budding: A role for the ESCRT machinery. Science 2007, 316, 1908–1912. [Google Scholar] [CrossRef] [PubMed]
- Horváth, P.; Müller-Reichert, T. A Structural View on ESCRT-Mediated Abscission. Front. Cell Dev. Biol. 2020, 8, 586880. [Google Scholar] [CrossRef] [PubMed]
- Morita, E.; Sandrin, V.; Chung, H.Y.; Morham, S.G.; Gygi, S.P.; Rodesch, C.K.; Sundquist, W.I. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007, 26, 4215–4227. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Elia, N.; Ghirlando, R.; Lippincott-Schwartz, J.; Hurley, J.H. Midbody targeting of the ESCRT machinery by a noncanonical coiled coil in CEP55. Science 2008, 322, 576–580. [Google Scholar] [CrossRef]
- Nasa, I.; Kettenbach, A.N. Coordination of Protein Kinase and Phosphoprotein Phosphatase Activities in Mitosis. Front. Cell Dev. Biol. 2018, 6, 30. [Google Scholar] [CrossRef]
- Smoly, I.; Shemesh, N.; Ziv-Ukelson, M.; Ben-Zvi, A.; Yeger-Lotem, E. An Asymmetrically Balanced Organization of Kinases versus Phosphatases across Eukaryotes Determines Their Distinct Impacts. PLoS Comput. Biol. 2017, 13, e1005221. [Google Scholar] [CrossRef]
- Manning, G.; Plowman, G.D.; Hunter, T.; Sudarsanam, S. Evolution of protein kinase signaling from yeast to man. Trends Biochem. Sci. 2002, 27, 514–520. [Google Scholar] [CrossRef]
- Bononi, A.; Agnoletto, C.; De Marchi, E.; Marchi, S.; Patergnani, S.; Bonora, M.; Giorgi, C.; Missiroli, S.; Poletti, F.; Rimessi, A.; et al. Protein kinases and phosphatases in the control of cell fate. Enzym. Res. 2011, 2011, 329098. [Google Scholar] [CrossRef]
- Audagnotto, M.; Dal Peraro, M. Protein post-translational modifications: In silico prediction tools and molecular modeling. Comput. Struct. Biotechnol. J. 2017, 15, 307–319. [Google Scholar] [CrossRef]
- Pinna, L.A.; Ruzzene, M. How do protein kinases recognize their substrates? Biochim. Et. Biophys. Acta 1996, 1314, 191–225. [Google Scholar] [CrossRef]
- Brennan, I.M.; Peters, U.; Kapoor, T.M.; Straight, A.F. Polo-like kinase controls vertebrate spindle elongation and cytokinesis. PLoS ONE 2007, 2, e409. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.; Neef, R.; Eberspächer, U.; Eis, K.; Husemann, M.; Mumberg, D.; Prechtl, S.; Schulze, V.; Siemeister, G.; Wortmann, L.; et al. Use of the novel Plk1 inhibitor ZK-thiazolidinone to elucidate functions of Plk1 in early and late stages of mitosis. Mol. Biol. Cell 2007, 18, 4024–4036. [Google Scholar] [CrossRef] [PubMed]
- Burkard, M.E.; Maciejowski, J.; Rodriguez-Bravo, V.; Repka, M.; Lowery, D.M.; Clauser, K.R.; Zhang, C.; Shokat, K.M.; Carr, S.A.; Yaffe, M.B.; et al. Plk1 self-organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells. PLoS Biol. 2009, 7, e1000111. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.A.; Takaki, T.; Petronczki, M.; Glotzer, M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 2009, 7, e1000110. [Google Scholar] [CrossRef]
- Barr, F.A.; Silljé, H.H.; Nigg, E.A. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 2004, 5, 429–440. [Google Scholar] [CrossRef]
- Van der Waal, M.S.; Hengeveld, R.C.; Van der Horst, A.; Lens, S.M. Cell division control by the Chromosomal Passenger Complex. Exp. Cell Res. 2012, 318, 1407–1420. [Google Scholar] [CrossRef]
- Carmena, M.; Wheelock, M.; Funabiki, H.; Earnshaw, W.C. The chromosomal passenger complex (CPC): From easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell Biol. 2012, 13, 789–803. [Google Scholar] [CrossRef]
- Bastos, R.N.; Barr, F.A. Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission. J. Cell Biol. 2010, 191, 751–760. [Google Scholar] [CrossRef]
- Capalbo, L.; Montembault, E.; Takeda, T.; Bassi, Z.I.; Glover, D.M.; D’Avino, P.P. The chromosomal passenger complex controls the function of endosomal sorting complex required for transport-III Snf7 proteins during cytokinesis. Open Biol. 2012, 2, 120070. [Google Scholar] [CrossRef]
- Thoresen, S.B.; Campsteijn, C.; Vietri, M.; Schink, K.O.; Liestøl, K.; Andersen, J.S.; Raiborg, C.; Stenmark, H. ANCHR mediates Aurora-B-dependent abscission checkpoint control through retention of VPS4. Nat. Cell Biol. 2014, 16, 550–560. [Google Scholar] [CrossRef]
- Glover, C.V.C.; Shelton, E.R.; Brutlag, D.L. Purification and characterization of a type II casein kinase from Drosophila melanogaster. J. Biol. Chem. 1983, 258, 3258–3265. [Google Scholar] [CrossRef]
- Dahmus, M.E. Purification and properties of calf thymus casein kinase I and II. J. Biol. Chem. 1981, 256, 3319–3325. [Google Scholar] [CrossRef]
- Litchfield, D.W.; Lozeman, F.J.; Piening, C.; Sommercorn, J.; Takio, K.; Walsh, K.A.; Krebs, E.G. Subunit structure of casein kinase II from bovine testis. Demonstration that the α and α′ subunits are distinct polypeptides. J. Biol. Chem. 1990, 265, 7638–7644. [Google Scholar] [CrossRef]
- Hathaway, G.M.; Zoller, M.J.; Traugh, J.A. Identification of the catalytic subunit of casein kinase II by affinity labeling with 5′-p-fluorosulfonylbenzoyl adenosine. J. Biol. Chem. 1981, 256, 11442–11446. [Google Scholar] [CrossRef]
- Meggio, F.; Brunati, A.M.; Pinna, L.A. Autophosphorylation of type 2 casein kinase TS at both its α- and β- subunits. FEBS Lett. 1983, 160, 203–208. [Google Scholar] [CrossRef]
- Gietz, R.D.; Graham, K.C.; Litchfield, D.W. Interactions between the subunits of casein kinase II. J. Biol. Chem. 1995, 270, 13017–13021. [Google Scholar] [CrossRef]
- Kuenzel, E.A.; Mulligan, J.A.; Sommercorn, J.; Krebs, E.G. Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J. Biol. Chem. 1987, 262, 9136–9140. [Google Scholar] [CrossRef]
- Meggio, F.; Marin, O.; Pinna, L.A. Substrate specificity of protein kinase CK2. Cell. Mol. Biol. Res. 1994, 40, 401–409. [Google Scholar]
- Magliozzi, R.; Carrero, Z.I.; Low, T.Y.; Yuniati, L.; Valdes-Quezada, C.; Kruiswijk, F.; Van Wijk, K.; Heck, A.J.R.; Jackson, C.L.; Guardavaccaro, D. Inheritance of the Golgi Apparatus and Cytokinesis Are Controlled by Degradation of GBF1. Cell Rep. 2018, 23, 3381–3391. [Google Scholar] [CrossRef]
- Dulyaninova, N.G.; Malashkevich, V.N.; Almo, S.C.; Bresnick, A.R. Regulation of Myosin-IIA Assembly and Mts1 Binding by Heavy Chain Phosphorylation. Biochemistry 2005, 44, 6867–6876. [Google Scholar] [CrossRef]
- Goehring, A.S.; Mitchell, D.A.; Tong, A.H.; Keniry, M.E.; Boone, C.; Sprague, G.F., Jr. Synthetic lethal analysis implicates Ste20p, a p21-activated protein kinase, in polarisome activation. Mol. Biol. Cell 2003, 14, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Versele, M.; Thorner, J. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J. Cell Biol. 2004, 164, 701–715. [Google Scholar] [CrossRef]
- Weiss, E.L.; Bishop, A.C.; Shokat, K.M.; Drubin, D.G. Chemical genetic analysis of the budding-yeast p21-activated kinase Cla4p. Nat. Cell Biol. 2000, 2, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Frazier, J.A.; Wong, M.L.; Longtine, M.S.; Pringle, J.R.; Mann, M.; Mitchison, T.J.; Field, C. Polymerization of purified yeast septins: Evidence that organized filament arrays may not be required for septin function. J. Cell Biol. 1998, 143, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Field, C.M.; Kellogg, D. Septins: Cytoskeletal polymers or signalling GTPases? Trends Cell Biol. 1999, 9, 387–394. [Google Scholar] [CrossRef]
- Kadota, J.; Yamamoto, T.; Yoshiuchi, S.; Bi, E.; Tanaka, K. Septin ring assembly requires concerted action of polarisome components, a PAK kinase Cla4p, and the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 2004, 15, 5329–5345. [Google Scholar] [CrossRef]
- Magliozzi, J.O.; Sears, J.; Cressey, L.; Brady, M.; Opalko, H.E.; Kettenbach, A.N.; Moseley, J.B. Fission yeast Pak1 phosphorylates anillin-like Mid1 for spatial control of cytokinesis. J. Cell Biol. 2020, 219, e201908017. [Google Scholar] [CrossRef]
- Loo, T.H.; Balasubramanian, M. Schizosaccharomyces pombe Pak-related protein, Pak1p/Orb2p, phosphorylates myosin regulatory light chain to inhibit cytokinesis. J. Cell Biol. 2008, 183, 785–793. [Google Scholar] [CrossRef]
- Goeckeler, Z.M.; Masaracchia, R.A.; Zeng, Q.; Chew, T.L.; Gallagher, P.; Wysolmerski, R.B. Phosphorylation of Myosin Light Chain Kinase by p21-activated Kinase PAK2. J. Biol. Chem. 2000, 275, 18366–18374. [Google Scholar] [CrossRef]
- Chew, T.L.; Masaracchia, R.A.; Goeckeler, Z.M.; Wysolmerski, R.B. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J. Muscle Res. Cell Motil. 1998, 19, 839–854. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Liu, X.L.; Zhu, Y.Y.; Huang, H. Revealing PAK2′s Function in the Cell Division through MKLP1′s Interactome. Biomed Res. Int. 2020, 2020, 8854245. [Google Scholar] [CrossRef] [PubMed]
- Petsalaki, E.; Zachos, G. An ATM–Chk2–INCENP pathway activates the abscission checkpoint. J. Cell Biol. 2020, 220, e202008029. [Google Scholar] [CrossRef]
- Litchfield, D.W. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem. J. 2003, 369, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Faust, M.; Montenarh, M. Subcellular localization of protein kinase CK2. A key to its function? Cell Tissue Res. 2000, 301, 329–340. [Google Scholar] [CrossRef]
- Sarrouilhe, D.; Filhol, O.; Leroy, D.; Bonello, G.; Baudry, M.; Chambaz, E.; Cochet, C. The tight association of protein kinase CK2 with plasma membrane is mediated by a specific domain of its regulatory β-subunit. Biochim. Biophys. Acta 1998, 1403, 199–210. [Google Scholar] [CrossRef][Green Version]
- Dittie, A.S.; Thomas, L.; Thomas, G.; Tooze, S.A. Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II phosphorylation. EMBO J. 1997, 16, 4859–4870. [Google Scholar] [CrossRef]
- Walter, J.; Schnolzer, M.; Pyerin, W.; Kinzel, V.; Kubler, D. Induced release of cell surface protein kinase yields CK1- and CK2-like enzymes in tandem. J. Biol. Chem. 1996, 271, 111–119. [Google Scholar] [CrossRef][Green Version]
- Meggio, F.; Pinna, L.A. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003, 17, 349–368. [Google Scholar] [CrossRef]
- Montenarh, M. Protein kinase CK2 in DNA damage and repair. Transl. Cancer Res. 2016, 5, 49–63. [Google Scholar] [CrossRef]
- Pinna, L.; Meggio, F. Protein kinase CK2 (‘‘casein kinase-2′’) and its implication in cell division and proliferation. Prog. Cell Cycle Res. 1997, 3, 77–97. [Google Scholar] [CrossRef]
- Ahmed, K. Nuclear matrix and protein kinase CK2 signaling. Crit. Rev. Eukaryot. Gene Expr. 1999, 9, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Issinger, O.G. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis 1999, 20, 391–408. [Google Scholar] [CrossRef]
- Ahmed, K.; Gerber, D.A.; Cochet, C. Joining the cell survival squad: An emerging role for protein kinase CK2. Trends Cell Biol. 2002, 12, 226–230. [Google Scholar] [CrossRef]
- Fragoso, R.; Barata, J.T. Kinases, tails and more: Regulation of PTEN function by phosphorylation. Methods 2015, 77–78, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ruzzene, M.; Bertacchini, J.; Toker, A.; Marmiroli, S. Cross-talk between the CK2 and AKT signaling pathways in cancer. Adv. Biol. Regul. 2017, 64, 1–8. [Google Scholar] [CrossRef]
- Dominguez, I.; Sonenshein, G.E.; Seldin, D.C. Protein kinase CK2 in health and disease: CK2 and its role in Wnt and NF-kappaB signaling: Linking development and cancer. Cell. Mol. Life Sci. 2009, 66, 1850–1857. [Google Scholar] [CrossRef]
- Manni, S.; Brancalion, A.; Mandato, E.; Quotti Tubi, L.; Colpo, A.; Pizzi, M.; Cappellesso, R.; Zaffino, F.; Di Maggio, S.A.; Cabrelle, A.; et al. Protein kinase CK2 inhibition down modulates the NF-κB and STAT3 survival pathways, enhances the cellular proteotoxic stress and synergistically boosts the cytotoxic effect of bortezomib on multiple myeloma and mantle cell lymphoma cells. PLoS ONE 2013, 8, e75280. [Google Scholar] [CrossRef]
- Zheng, Y.; Qin, H.; Frank, S.J.; Deng, L.; Litchfield, D.W.; Tefferi, A.; Pardanani, A.; Lin, F.T.; Li, J.; Sha, B.; et al. A CK2-dependent mechanism for activation of the JAK-STAT signaling pathway. Blood 2011, 118, 156–166. [Google Scholar] [CrossRef]
- Jia, H.; Liu, Y.; Xia, R.; Tong, C.; Yue, T.; Jiang, J.; Jia, J. Casein kinase 2 promotes Hedgehog signaling by regulating both smoothened and Cubitus interruptus. J. Biol. Chem. 2010, 285, 37218–37226. [Google Scholar] [CrossRef]
- Litchfield, D.W.; Lüscher, B. Casein kinase II in signal transduction and cell cycle regulation. Mol. Cell. Biochem. 1993, 127–128, 187–199. [Google Scholar] [CrossRef]
- Zhang, S.; Long, H.; Yang, Y.L.; Wang, Y.; Hsieh, D.; Li, W.; Au, A.; Stoppler, H.J.; Xu, Z.; Jablons, D.M.; et al. Inhibition of CK2α down-regulates Notch1 signalling in lung cancer cells. J. Cell. Mol. Med. 2013, 17, 854–862. [Google Scholar] [CrossRef] [PubMed]
- De Gooijer, M.C.; Guillén, N.M.; Bernards, R.; Wurdinger, T.; Van Tellingen, O. An experimenter’s guide to glioblastoma invasion pathways. Trends Mol. Med. 2018, 24, 763–780. [Google Scholar] [CrossRef] [PubMed]
- Salaun, P.; Rannou, Y.; Prigent, C. Cdk1, Plks, Auroras, and Neks: The mitotic bodyguards. Adv. Exp. Med. Biol. 2008, 617, 41–56. [Google Scholar] [CrossRef]
- Takemoto, A.; Kimura, K.; Yanagisawa, J.; Yokoyama, S.; Hanaoka, F. Negative regulation of condensin I by CK2-mediated phosphorylation. EMBO J. 2006, 25, 5339–5348. [Google Scholar] [CrossRef] [PubMed]
- Yde, C.W.; Olsen, B.B.; Meek, D.; Watanabe, N.; Guerra, B. The regulatory beta-subunit of protein kinase CK2 regulates cell-cycle progression at the onset of mitosis. Oncogene 2008, 27, 4986–4997. [Google Scholar] [CrossRef]
- St-Denis, N.A.; Derksen, D.R.; Litchfield, D.W. Evidence for regulation of mitotic progression through temporal phosphorylation and dephosphorylation of CK2α. Mol. Cell. Biol. 2009, 29, 2068–2081. [Google Scholar] [CrossRef]
- St-Denis, N.; Gabriel, M.; Turowec, J.P.; Gloor, G.B.; Li, S.S.; Gingras, A.C.; Litchfield, D.W. Systematic investigation of hierarchical phosphorylation by protein kinase CK2. J. Proteom. 2015, 118, 49–62. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.S.; Yang, X.; Wang, Y.; Wang, Y.; Turner, J.R.; Liu, X. Phosphorylation of CLIP-170 by Plk1 and CK2 promotes timely formation of kinetochore-microtubule attachments. EMBO J. 2010, 29, 2953–2965. [Google Scholar] [CrossRef]
- Barrett, R.M.; Colnaghi, R.; Wheatley, S.P. Threonine 48 in the BIR domain of survivin is critical to its mitotic and anti-apoptotic activities and can be phosphorylated by CK2 in vitro. Cell Cycle 2011, 10, 538–548. [Google Scholar] [CrossRef]
- Peng, Y.; Wong, C.C.; Nakajima, Y.; Tyers, R.G.; Sarkeshik, A.S.; Yates, J., III; Drubina, D.G.; Barnes, G. Overlapping kinetochore targets of CK2 and Aurora B kinases in mitotic regulation. Mol. Biol. Cell 2011, 22, 2680–2689. [Google Scholar] [CrossRef]
- Rusin, S.F.; Schlosser, K.A.; Adamo, M.E.; Kettenbach, A.N. Quantitative phosphoproteomics reveals new roles for the protein phosphatase PP6 in mitotic cells. Sci. Signal. 2015, 8, rs12. [Google Scholar] [CrossRef] [PubMed]
- Pepperkok, R.; Lorenz, P.; Jakobi, R.; Ansorge, W.; Pyerin, W. Cell growth stimulation by EGF: Inhibition through antisense- oligodeoxynucleotides demonstrates important role of casein kinase II. Exp. Cell Res. 1991, 197, 245–253. [Google Scholar] [CrossRef]
- Pepperkok, R.; Lorenz, P.; Ansorge, W.; Pyerin, W. Casein kinase II is required for transition of G0/G1, early G1, and G1/S phases of the cell cycle. J. Biol. Chem. 1994, 269, 6986–6991. [Google Scholar] [CrossRef]
- Lorenz, P.; Pepperkok, R.; Ansorge, W.; Pyerin, W. Cell biological studies with monoclonal and polyclonal antibodies against human casein kinase II subunit beta demonstrate participation of the kinase in mitogenic signaling. J. Biol. Chem. 1993, 268, 2733–2739. [Google Scholar] [CrossRef]
- Glover, C.V., III. On the physiological role of casein kinase II in Saccharomyces cerevisiae. Prog. Nucleic Acid Res. Mol. Biol. 1998, 59, 95–133. [Google Scholar] [CrossRef]
- Hériché, J.K.; Lebrin, F.; Rabilloud, T.; Leroy, D.; Chambaz, E.M.; Goldberg, Y. Regulation of protein phosphatase 2A by direct interaction with casein kinase 2α. Science 1997, 276, 952–955. [Google Scholar] [CrossRef]
- Daum, J.R.; Gorbsky, G.J. Casein kinase II catalyzes a mitotic phosphorylation on threonine 1342 of human DNA topoisomerase IIalpha, which is recognized by the 3F3/2 phosphoepitope antibody. J. Biol. Chem. 1998, 273, 30622–30629. [Google Scholar] [CrossRef]
- Escargueil, A.E.; Plisov, S.Y.; Filhol, O.; Cochet, C.; Larsen, A.K. Mitotic phosphorylation of DNA topoisomerase II alpha by protein kinase CK2 creates the MPM-2 phosphoepitope on Ser-1469. J. Biol. Chem. 2000, 275, 34710–34718. [Google Scholar] [CrossRef]
- Theis-Febvre, N.; Filhol, O.; Froment, C.; Cazales, M.; Cochet, C.; Monsarrat, B.; Ducommun, B.; Baldin, B. Protein kinase CK2 regulates CDC25B phosphatase activity. Oncogene 2003, 22, 220–232. [Google Scholar] [CrossRef]
- Khan, D.H.; He, S.; Yu, J.; Winter, S.; Cao, W.; Seiser, C.; Davie, J.R. Protein kinase CK2 regulates the dimerization of histone deacetylase 1 (HDAC1) and HDAC2 during mitosis. J. Biol. Chem. 2013, 288, 16518–16528. [Google Scholar] [CrossRef]
- Roussou, I.; Draetta, G. The Schizosaccharomyces pombe casein kinase II alpha and beta subunits: Evolutionary conservation and positive role of the beta subunit. Mol. Cell. Biol. 1994, 14, 576–586. [Google Scholar] [CrossRef]
- Singh, N.N.; Ramji, D.P. Protein kinase CK2, an important regulator of the inflammatory response? J. Mol. Med. 2008, 86, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.E.; Seidner, Y.; Dominguez, I. Mining CK2 in cancer. PLoS ONE 2014, 9, e115609. [Google Scholar] [CrossRef] [PubMed]
- Iegre, J.; Atkinson, E.L.; Brear, P.D.; Cooper, B.M.; Hyvönen, M.; Spring, D.R. Chemical probes targeting the kinase CK2: A journey outside the catalytic box. Org. Biomol. Chem. 2021, 19, 4380–4396. [Google Scholar] [CrossRef] [PubMed]
- Pucko, E.B.; Ostrowski, R.P. Inhibiting CK2 among Promising Therapeutic Strategies for Gliomas and Several Other Neoplasms. Pharmaceutics 2022, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Pinna, L.A. Protein kinase CK2: A challenge to canons. J. Cell Sci. 2002, 115, 3873–3878. [Google Scholar] [CrossRef]
- Rusin, S.F.; Adamo, M.E.; Kettenbach, A.N. Identification of Candidate Casein Kinase 2 Substrates in Mitosis by Quantitative Phosphoproteomics. Front. Cell Dev. Biol. 2017, 22, 5–97. [Google Scholar] [CrossRef]
- Trembley, J.H.; Kren, B.T.; Afzal, M.; Scaria, G.A.; Klein, M.A.; Ahmed, K. Protein kinase CK2-diverse roles in cancer cell biology and therapeutic promise. Mol. Cell. Biochem. 2022, 17, 1–28. [Google Scholar] [CrossRef]
- Faust, R.A.; Niehans, G.; Gapany, M.; Hoistad, D.; Knapp, D.; Cherwitz, D.; Davis, A.; Adams, G.L.; Ahmed, K. Subcellular immunolocalization of protein kinase CK2 in normal and carcinoma cells. Int. J. Biochem. Cell Biol. 1999, 31, 941–949. [Google Scholar] [CrossRef]
- Nozawa, R.S.; Gilbert, N. RNA: Nuclear Glue for Folding the Genome. Trends Cell Biol. 2019, 29, 201–211. [Google Scholar] [CrossRef]
- Trembley, J.H.; Hu, D.; Slaughter, C.A.; Lahti, J.M.; Kidd, V.J. Casein kinase 2 interacts with cyclin-dependent kinase 11 (CDK11) in vivo and phosphorylates both the RNA polymerase II carboxyl-terminal domain and CDK11 in vitro. J. Biol. Chem. 2003, 278, 2265–2270. [Google Scholar] [CrossRef] [PubMed]
- Cabrejos, M.E.; Allende, C.C.; Maldonado, E. Effects of phosphorylation by protein kinase CK2 on the human basal components of the RNA polymerase II transcription machinery. J. Cell. Biochem. 2004, 93, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Palancade, B.; Dubois, M.F.; Bensaude, O. FCP1 phosphorylation by casein kinase 2 enhances binding to TFIIF and RNA polymerase II carboxyl-terminal domain phosphatase activity. J. Biol. Chem. 2002, 277, 36061–36067. [Google Scholar] [CrossRef] [PubMed]
- Újvári, A.; Pal, M.; Luse, D.S. The functions of TFIIF during initiation and transcript elongation are differentially affected by phosphorylation by casein kinase 2. J. Biol. Chem. 2011, 286, 23160–23167. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Wada, T.; Suzuki, F.; Takagi, T.; Hasegawa, J.; Handa, H. Casein kinase II interacts with the bZIP domains of several transcription factors. Nucleic Acids Res. 1998, 26, 3854–3861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bird, T.A.; Schooley, K.; Dower, S.K.; Hagen, H.; Virca, G.D. Activation of Nuclear Transcription Factor NF-κB by Interleukin-1 Is Accompanied by Casein Kinase II-mediated Phosphorylation of the p65 Subunit. J. Biol. Chem. 1997, 272, 32606–32612. [Google Scholar] [CrossRef]
- Voit, R.; Schnapp, A.; Kuhn, A.; Rosenbauer, H.; Hirschmann, P.; Stunnenberg, H.G.; Grummt, I. The nucleolar transcription factor mUBF is phosphorylated by casein kinase II in the C-terminal hyperacidic tail which is essential for transactivation. EMBO J. 1992, 11, 2211–2218. [Google Scholar] [CrossRef]
- Bian, Y.; Ye, M.; Wang, C.; Cheng, K.; Song, C.; Dong, M.; Pan, Y.; Qin, H.; Zou, H. Global screening of CK2 kinase substrates by an integrated phosphoproteomics workflow. Sci. Rep. 2013, 3, 3460. [Google Scholar] [CrossRef]
- Trembley, J.H.; Tatsumi, S.; Sakashita, E.; Loyer, P.; Slaughter, C.A.; Suzuki, H.; Endo, H.; Kidd, V.J.; Mayeda, A. Activation of pre-mRNA splicing by human RNPS1 is regulated by CK2 phosphorylation. Mol. Cell. Biol. 2005, 25, 1446–1457. [Google Scholar] [CrossRef]
- Lehnert, S.; Gotz, C.; Kartarius, S.; Schafer, B.; Montenarh, M. Protein kinase CK2 interacts with the splicing factor hPrp3p. Oncogene 2008, 27, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Mylonis, I.; Giannakouros, T. Protein kinase CK2 phosphorylates and activates the SR protein-specific kinase 1. Biochem. Biophys. Res. Commun. 2003, 301, 650–656. [Google Scholar] [CrossRef]
- Borgo, C.; D’Amore, C.; Sarno, S.; Salvi, M.; Ruzzene, M. Protein kinase CK2: A potential therapeutic target for diverse human diseases. Signal Transduct. Target. Ther. 2021, 6, 183. [Google Scholar] [CrossRef]
- Salvi, M.; Borgo, C.; Pinna, L.A.; Ruzzene, M. Targeting CK2 in cancer: A valuable strategy or a waste of time? Cell Death Discov. 2021, 7, 325. [Google Scholar] [CrossRef]
- Medley, J.C.; Kabara, M.M.; Stubenvoll, M.D.; DeMeyer, L.E.; Song, M.H. Casein kinase II is required for proper cell division and acts as a negative regulator of centrosome duplication in Caenorhabditis elegans embryos. Biol. Open. 2017, 6, 17–28. [Google Scholar] [CrossRef]
- Skop, A.R.; Liu, H.; Yates, J., 3rd; Meyer, B.J.; Heald, R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 2004, 305, 61–66. [Google Scholar] [CrossRef]
- Salvi, M.; Raiborg, C.; Hanson, P.I.; Campsteijn, C.; Stenmark, H.; Pinna, L.A. CK2 involvement in ESCRT-III complex phosphorylation. Arch. Biochem. Biophys. 2014, 545, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.G.; Jackson, C.L. Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol. 2000, 12, 475–482. [Google Scholar] [CrossRef]
- Donaldson, J.G.; Jackson, C.L. ARF family G proteins and their regulators: Roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 2011, 12, 362–375. [Google Scholar] [CrossRef]
- Fath, K.R. Characterization of myosin-II binding to Golgi stacks in vitro. Cell Motil. Cytoskelet. 2005, 60, 222–235. [Google Scholar] [CrossRef]
- Manstein, D.J.; Titus, M.A.; De Lozanne, A.; Spudich, J.A. Gene replacement in Dictyostelium: Generation of myosin null mutants. EMBO J. 1989, 8, 923–932. [Google Scholar] [CrossRef]
- Young, P.E.; Richman, A.M.; Ketchum, A.S.; Kiehart, D.P. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 1993, 7, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Sechi, S.; Frappaolo, A.; Karimpour-Ghahnavieh, A.; Fraschini, R.; Giansanti, M.G. A novel coordinated function of Myosin II with GOLPH3 controls centralspindlin localization during cytokinesis in Drosophila. J. Cell Sci. 2020, 133, jcs252965. [Google Scholar] [CrossRef] [PubMed]
- Babkoff, A.; Cohen-Kfir, E.; Aharon, H.; Ravid, S. Aurora-B phosphorylates the myosin II heavy chain to promote cytokinesis. J. Biol. Chem. 2021, 297, 101024. [Google Scholar] [CrossRef] [PubMed]
- Betapudi, V.; Gokulrangan, G.; Chance, M.R.; Egelhoff, T.T. A proteomic study of myosin II motor proteins during tumor cell migration. J. Mol. Biol. 2011, 407, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Sechi, S.; Karimpour-Ghahnavieh, A.; Frappaolo, A.; Di Francesco, L.; Piergentili, R.; Schininà, E.; D’Avino, P.P.; Giansanti, M.G. Identification of GOLPH3 Partners in Drosophila Unveils Potential Novel Roles in Tumorigenesis and Neural Disorders. Cells 2021, 10, 2336. [Google Scholar] [CrossRef]
- St-Denis, N.A.; Bailey, M.L.; Parker, E.L.; Vilk, G.; Litchfield, D.W. Localization of phosphorylated CK2alpha to the mitotic spindle requires the peptidyl-prolyl isomerase Pin1. J. Cell Sci. 2011, 124, 2341–2348. [Google Scholar] [CrossRef]
- Van der Horst, A.; Khanna, K.K. The peptidyl-prolyl isomerase Pin1 regulates cytokinesis through Cep55. Cancer Res. 2009, 69, 6651–6659. [Google Scholar] [CrossRef]
- Manser, E.; Leung, T.; Salihuddin, H.; Zhao, Z.S.; Lim, L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 1994, 367, 40–46. [Google Scholar] [CrossRef]
- Knaus, U.G.; Morris, S.; Dong, H.J.; Chernoff, J.; Bokoch, G.M. Regulation of human leukocyte p21-activated kinases through G protein–coupled receptors. Science 1995, 269, 221–223. [Google Scholar] [CrossRef]
- Rane, C.K.; Minden, A. P21 activated kinase signaling in cancer. Semin. Cancer Biol. 2019, 54, 40–49. [Google Scholar] [CrossRef]
- Hofmann, C.; Shepelev, M.; Chernoff, J. The genetics of Pak. J. Cell Sci. 2004, 117, 4343–4354. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, B.; Raabe, T. Phylogenetic and structural analysis of the Drosophila melanogaster p21-activated kinase DmPAK3. Gene 2005, 349, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Unden, H.; Jacquier, N.; Schneiter, R.; Just, U.; Höfken, T. The Cdc42 effectors Ste20, Cla4, and Skm1 down-regulate the expression of genes involved in sterol uptake by a mitogen-activated protein kinase-independent pathway. Mol. Biol. Cell 2009, 20, 4826–4837. [Google Scholar] [CrossRef]
- Qyang, Y.; Yang, P.; Du, H.; Lai, H.; Kim, H.; Marcus, S. The p21-activated kinase, Shk1, is required for proper regulation of microtubule dynamics in the fission yeast, Schizosaccharomyces pombe. Mol. Microbiol. 2002, 44, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.A.; Bollag, G.; McCormick, F.; Abo, A. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 1995, 14, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Bagrodia, S.; Taylor, S.J.; Creasy, C.L.; Chernoff, J.; Cerione, R.A. Identification of a mouse p21Cdc42/Rac activated kinase. J. Biol. Chem. 1995, 270, 22731–22737. [Google Scholar] [CrossRef]
- Sells, M.A.; Barratt, J.T.; Caviston, J.; Ottilie, S.; Leberer, E.; Chernoff, J. Characterization of Pak2p, a pleckstrin homology domain-containing, p21-activated protein kinase from fission yeast. J. Biol. Chem. 1998, 273, 18490–18498. [Google Scholar] [CrossRef]
- Iden, S.; Collard, J.G. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat. Rev. Mol. Cell Biol. 2008, 9, 846–859. [Google Scholar] [CrossRef]
- Rane, C.K.; Minden, A. P21 activated kinases: Structure, regulation, and functions. Small GTPases 2014, 5, e28003. [Google Scholar] [CrossRef]
- Jha, R.K.; Strauss, C.E.M. 3D structure analysis of PAKs: A clue to the rational design for affinity reagents and blockers. Cell. Logist. 2012, 2, 69–77. [Google Scholar] [CrossRef]
- Baker, N.M.; Chow, H.Y.; Chernoff, J.; Der, C.J. Molecular pathways: Targeting RAC-p21-activated serine-threonine kinase signaling in RAS-driven cancers. Clin. Cancer Res. 2014, 20, 4740–4746. [Google Scholar] [CrossRef] [PubMed]
- Pirruccello, M.; Sondermann, H.; Pelton, J.G.; Pellicena, P.; Hoelz, A.; Chernoff, J.; Wemmer, D.E.; Kuriyan, J. A dimeric kinase assembly underlying autophosphorylation in the p21 activated kinases. J. Mol. Biol. 2006, 361, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, G.; Hostinova, E.; Rudolph, M.G.; Kraemer, A.; Sickmann, A.; Meyer, H.E.; Scheffzek, K.; Wittinghofer, A. Conformational switch and role of phosphorylation in PAK activation. Mol. Cell. Biol. 2001, 21, 5179–5189. [Google Scholar] [CrossRef] [PubMed]
- Parrini, M.C. Untangling the complexity of PAK1 dynamics: The future challenge. Cell. Logist. 2012, 2, 78–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shin, Y.J.; Kim, Y.B.; Kim, J.H. Protein kinase CK2 phosphorylates and activates p21-activated kinase 1. Mol. Biol. Cell 2013, 24, 2990–2999. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.; Tan, L.; Lim, L.; Manser, E. The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J. Biol. Chem. 2001, 276, 17347–17353. [Google Scholar] [CrossRef]
- Puto, L.A.; Pestonjamasp, K.; King, C.C.; Bokoch, G.M. p21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. J. Biol. Chem. 2003, 278, 9388–9393. [Google Scholar] [CrossRef]
- Zhao, Z.S.; Manser, E.; Lim, L. Interaction between PAK and Nck: A Template for Nck Targets and Role of PAK Autophosphorylation. Mol. Cell. Biol. 2000, 20, 3906–3917. [Google Scholar] [CrossRef]
- Zhou, G.L.; Zhuo, Y.; King, C.C.; Fryer, B.H.; Bokoch, G.M.; Field, J. Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol. Cell. Biol. 2003, 23, 8058–8069. [Google Scholar] [CrossRef]
- Fryer, B.H.; Wang, C.; Vedantam, S.; Zhou, G.L.; Jin, S.; Fletcher, L.; Simon, M.C.; Fiel, J. cGMP-dependent protein kinase phosphorylates p21-activated kinase (Pak) 1, inhibiting Pak/Nck binding and stimulating Pak/vasodilator-stimulated phosphoprotein association. J. Biol. Chem. 2006, 281, 11487–11495. [Google Scholar] [CrossRef]
- Morrice, N.A.; Gabrielli, B.; Kemp, B.E.; Wettenhall, R.E.A. Cardiolipin-activated protein kinase from rat liver structurally distinct from the protein kinases C. J. Biol. Chem. 1994, 269, 20040–20046. [Google Scholar] [CrossRef]
- Kumar, R.; Li, D.Q. PAKs in human cancer progression: From inception to cancer therapeutic to future oncobiology. Adv. Cancer Res. 2016, 130, 137–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, K.; Dong, Z. The Role of p21-Activated Kinases in Cancer and Beyond: Where Are We Heading? Front. Cell Dev. Biol. 2021, 16, 9–641381. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.; Liu, Y.; Xu, L.; Zhang, W.; Zhu, Y.; Xu, J.; Gu, J. Prognostic significance of p21-activated kinase 6 expression in patients with clear cell renal cell carcinoma. Ann. Surg. Oncol. 2014, 21, S575–S583. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Liu, H.; Zhang, W.; Fu, Q.; Xu, J. Tumor suppressive function of p21-activated Kinase 6 in hepatocellular carcinoma. J. Biol. Chem. 2015, 290, 28489–28501. [Google Scholar] [CrossRef]
- Chen, J.; Lu, H.; Yan, D.; Cui, F.; Wang, X.; Yu, F.; Xue, Y.; Feng, X.; Wang, J.; Wang, X.; et al. PAK6 increase chemoresistance and is a prognostic marker for stage II and III colon cancer patients undergoing 5-FU based chemotherapy. Oncotarget 2015, 6, 355–367. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, W.; Li, T.; Hu, Y.; Chen, S.; Xi, S.; Wen, Y.; Huang, L.; Zhao, L.; Xiao, C.; et al. Prognostic and predictive value of p21-activated Kinase 6 associated support vector machine classifier in gastric cancer treated by 5-fluorouracil/oxaliplatin chemotherapy. EBioMedicine 2017, 22, 78–88. [Google Scholar] [CrossRef]
- Zhao, Z.S.; Lim, J.P.; Ng, Y.W.; Lim, L.; Manser, E. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol. Cell. 2005, 20, 237–249. [Google Scholar] [CrossRef]
- Vadlamudi, R.K.; Barnes, C.J.; Rayala, S.; Li, F.; Balasenthil, S.; Marcus, S. p21-activated kinase 1 regulates microtubule dynamics by phosphorylating tubulin cofactor B. Mol. Cell. Biol. 2005, 25, 3726–3736. [Google Scholar] [CrossRef]
- Pakala, S.B.; Nair, V.S.; Reddy, S.D.; Kumar, R. Signaling-dependent phosphorylation of mitotic centromere-associated kinesin regulates microtubule depolymerization and its centrosomal localization. J. Biol. Chem. 2012, 287, 40560–40569. [Google Scholar] [CrossRef]
- Maroto, B.; Ye, M.B.; von Lohneysen, K.; Schnelzer, A.; Knaus, U.G. P21-activated kinase is required for mitotic progression and regulates Plk1. Oncogene 2008, 27, 4900–4908. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Adam, L.; Vadlamudi, R.K.; Zhou, H.; Sen, S.; Chernoff, J.; Mandal, M.; Kumar, R. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep. 2002, 3, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Nair, S.S.; Ohshiro, K.; Kumar, A.; Nair, V.S.; Pakala, S.B.; Reddy, S.D.N.; Gajula, R.P.; Eswaran, J.; Aravind, L.; et al. MORC2 signaling integrates phosphorylation-dependent, ATPase-coupled chromatin remodeling during the DNA damage response. Cell Rep. 2012, 2, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.; Crawford, J.J.; Hoeflich, K.P.; Wang, W. Inhibitors of p21-Activated Kinases (PAKs). J. Med. Chem. 2015, 58, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Juanes, M.A.; Piatti, S. The final cut: Cell polarity meets cytokinesis at the bud neck in S. cerevisiae. Cell Mol. Life Sci. 2016, 73, 3115–3136. [Google Scholar] [CrossRef]
- Cvrckova, F.; De Virgilio, C.; Manser, E.; Pringle, J.R.; Nasmyth, K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 1995, 9, 1817–1830. [Google Scholar] [CrossRef]
- Annan, R.B.; Lee, A.Y.; Reid, I.D.; Sayad, A.; Whiteway, M.; Hallett, M.; Thomas, D.Y. A biochemical genomics screen for substrates of Ste20p kinase enables the in silico prediction of novel substrates. PLoS ONE 2009, 4, e8279. [Google Scholar] [CrossRef]
- Atkins, B.D.; Yoshida, S.; Saito, K.; Wu, C.F.; Lew, D.J.; Pellman, D. Inhibition of Cdc42 during mitotic exit is required for cytokinesis. J. Cell Biol. 2013, 202, 231–240. [Google Scholar] [CrossRef]
- Dobbelaere, J.; Gentry, M.S.; Hallberg, R.L.; Barral, Y. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev. Cell 2003, 4, 345–357. [Google Scholar] [CrossRef]
- Schmidt, M.; Varma, A.; Drgon, T.; Bowers, B.; Cabib, E. Septins, under Cla4p regulation, and the chitin ring are required for neck integrity in budding yeast. Mol. Biol. Cell 2003, 14, 2128–2141. [Google Scholar] [CrossRef]
- Sanders, L.C.; Matsumura, F.; Bokoch, G.M.; de Lanerolle, P. Inhibition of myosin light chain kinase by p21-activated kinase. Science 1999, 283, 2083–2085. [Google Scholar] [CrossRef] [PubMed]
- Tuazon, P.T.; Spanos, W.C.; Gump, E.L.; Monnig, C.A.; Traugh, J.A. Determinants for substrate phosphorylation by p21-activated protein kinase (gamma-PAK). Biochemistry 1997, 36, 16059–16064. [Google Scholar] [CrossRef] [PubMed]
- Sells, M.A.; Knaus, U.G.; Bagrodia, S.; Ambrose, D.M.; Bokoch, G.M.; Chernoff, J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 1997, 7, 202–210. [Google Scholar] [CrossRef]
- Frost, J.A.; Khokhlatchev, A.; Stippec, S.; White, M.A.; Cobb, M.H. Differential effects of Pak1-activating mutations reveal activity-dependent and -independent effects on cytoskeletal regulation. J. Biol. Chem. 1998, 273, 28191–28198. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Huang, M.; Elledge, S.J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 1998, 282, 1893–1897. [Google Scholar] [CrossRef]
- Cai, Z.; Chehab, N.H.; Pavletich, N.P. Structure and Activation Mechanism of the CHK2 DNA Damage Checkpoint Kinase. Mol. Cell 2009, 35, 818–829. [Google Scholar] [CrossRef]
- Buscemi, G.; Carlessi, L.; Zannini, L.; Lisanti, S.; Fontanella, E.; Canevari, S.; Delia, D. DNA damage-induced cell cycle regulation and function of novel Chk2 phosphoresidues. Mol. Cell. Biol. 2006, 26, 7832–7845. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Williams, B.L.; Haire, L.F.; Goldberg, M.; Wilker, E.; Durocher, D.; Yaffe, M.B.; Jackson, S.P.; Smerdon, S.J. Structural and functional versatility of the FHA domain in DNA-damage signaling by the tumor suppressor kinase Chk2. Mol. Cell 2002, 9, 1045–1054. [Google Scholar] [CrossRef]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef]
- Schwarz, J.K.; Lovly, C.M.; Piwnica-Worms, H. Regulation of the Chk2 protein kinase by oligomerization-mediated cis- and trans-phosphorylation. Mol. Cancer Res. 2003, 1, 598–609. [Google Scholar]
- Zannini, L.; Lecis, D.; Lisanti, S.; Benetti, R.; Buscemi, G.; Schneider, C.; Delia, D. Karyopherin-alpha2 protein interacts with Chk2 and contributes to its nuclear import. J. Biol. Chem. 2003, 278, 42346–42351. [Google Scholar] [CrossRef]
- Xu, X.; Tsvetkov, L.M.; Stern, D.F. Chk2 activation and phosphorylation-dependent oligomerization. Mol. Cell. Biol. 2002, 22, 4419–4432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tosti, E.; Waldbaum, L.; Warshaw, G.; Gross, E.A.; Ruggieri, R. The stress kinase MRK contributes to regulation of DNA damage checkpoints through a p38g-independent pathway. J. Biol. Chem. 2004, 279, 47652–47660. [Google Scholar] [CrossRef]
- Wei, J.H.; Chou, Y.F.; Ou, Y.H.; Yeh, Y.H.; Tyan, S.W.; Sun, T.P.; Shen, C.Y.; Shieh, S.Y. TTK/hMps1 Participates in the Regulation of DNA Damage Checkpoint Response by Phosphorylating CHK2 on Threonine 68. J. Biol. Chem. 2005, 280, 7748–7757. [Google Scholar] [CrossRef]
- Tsvetkov, L.; Xu, X.; Li, J.; Stern, D.F. Polo-like kinase 1 and Chk2 interact and co-localize to centrosomes and the midbody. J. Biol. Chem. 2003, 278, 8468–8475. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Schwarz, J.K.; Piwnica-Worms, H.; Canman, C.E. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000, 60, 5934–5936. [Google Scholar]
- Matsuoka, S.; Rotman, G.; Ogawa, A.; Shiloh, Y.; Tamai, K.; Elledge, S.J. Ataxia telangiectasia- mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2000, 97, 10389–10394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.B.; Elledge, S.J. The DNA damage response: Putting checkpoints in perspective. Nature 2000, 408, 433–439. [Google Scholar] [CrossRef]
- Abraham, R.T. Cell cycle checkpoints: Preventing an identity crisis. Genes Dev. 2001, 15, 2177–2196. [Google Scholar] [CrossRef]
- Falck, J.; Mailand, N.; Syljuasen, R.G.; Bartek, J.; Lukas, J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 2001, 410, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Urist, M.; Prives, C. The Chk2 protein kinase. DNA Repair (Amst.) 2004, 3, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Ballif, B.A.; Smogorzewska, A.; McDonald, E.R., 3rd; Hurov, K.E.; Luo, J.; Bakalarski, C.E.; Zhao, Z.; Solimini, N.; Lerenthal, Y.; et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007, 316, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Gatei, M.; Scott, S.P.; Filippovitch, I.; Soronika, N.; Lavin, M.F.; Weber, B.; Khannaet, K.K. Role for ATM in DNA damage-induced phosphorylation of BRCA1. Cancer Res. 2000, 60, 3299–3304. [Google Scholar]
- Banin, S.; Moyal, L.; Shieh, S.; Taya, Y.; Anderson, C.W.; Chessa, L.; Smorodinsky, N.I.; Prives, C.; Reiss, Y.; Shiloh, Y.; et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 1998, 281, 1674–1677. [Google Scholar] [CrossRef]
- Stevens, C.; Smith, L.; La Thangue, N.B. Chk2 activates E2F-1 in response to DNA damage. Nat. Cell Biol. 2003, 5, 401–409. [Google Scholar] [CrossRef]
- Chehab, N.H.; Malikzay, A.; Appel, M.; Halazonetis, T.D. Chk2/hCds1 functions as a DNA damage checkpoint in G-1 by stabilizing p53. Genes Dev. 2000, 14, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Lee, C.H.; Schwarz, J.K.; Mitiku, N.; Piwnica-Worms, H.; Chung, J.H. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc. Natl. Acad. Sci. USA 1999, 96, 3745–3750. [Google Scholar] [CrossRef]
- Lee, J.S.; Collins, K.M.; Brown, A.L.; Lee, C.H.; Chung, J.H. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature 2000, 404, 201–204. [Google Scholar] [CrossRef]
- Falck, J.; Petrini, J.H.J.; Williams, B.R.; Lukas, J.; Bartek, J. The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nat. Genet. 2002, 30, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kuo, C.; Bisi, J.E.; Kim, M.K. PML-dependent apoptosis after DNA damage is regulated by the checkpoint kinase hCds1/Chk2. Nat. Cell Biol. 2002, 4, 865–870. [Google Scholar] [CrossRef]
- Bartkova, J.; Horejsi, Z.; Koed, K.; Kramer, A.; Tort, F.; Zieger, K.; Guldberg, P.; Sehested, M.; Nesland, J.M.; Lukas, C.; et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005, 434, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.W.; Varley, J.M.; Szydlo, T.E.; Kang, D.H.; Wahrer, D.C.R.; Shannon, K.E.; Lubratovich, M.; Verselis, S.J.; Isselbacher, K.J.; Fraumeni, J.F.; et al. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 1999, 286, 2528–2531. [Google Scholar] [CrossRef]
- Vahteristo, P.; Tamminen, A.; Karvinen, P.; Eerola, H.; Eklund, C.; Aaltonen, L.A.; Blomqvist, C.; Aittomaki, K.; Nevanlinna, H. p53, CHK2, and CHK1 genes in Finnish families with Li-Fraumeni syndrome: Further evidence of CHK2 in inherited cancer predisposition. Cancer Res. 2001, 61, 5718–5722. [Google Scholar]
- Miller, C.W.; Ikezoe, T.; Krug, U.; Hofmann, W.K.; Tavor, S.; Vegesna, V.; Tsukasaki, K.; Takeuchi, S.; Koeffler, H.P. Mutations of the CHK2 gene are found in some osteosarcomas, but are rare in breast, lung, and ovarian tumors. Genes Chromosomes Cancer 2002, 33, 17–21. [Google Scholar] [CrossRef]
- Nevanlinna, H.; Bartek, J. The CHEK2 gene and inherited breast cancer susceptibility. Oncogene 2006, 25, 5912–5919. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Kim, S.H.; Bell, D.W.; Wahrer, D.C.R.; Schiripo, T.A.; Jorczak, M.M.; Sgroi, D.C.; Garber, J.E.; Li, F.P.; Nichols, K.E.; et al. Destabilization of CHK2 by a missense mutation associated with Li-Fraumeni syndrome. Cancer Res. 2001, 61, 8062–8067. [Google Scholar]
- Meijers-Heijboer, H.; Ouweland, A.V.D.; Klijn, J.; Wasielewski, M.; De Snoo, A.; Oldenburg, R.; Hollestelle, A.; Houben, M.; Crepin, E.; Van Veghel-Plandsoen, M.; et al. Low-penetrance susceptibility to breast cancer due to CHEK2*1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat. Genet. 2002, 31, 55–59. [Google Scholar] [CrossRef]
- Dong, X.; Wang, L.; Taniguchi, K.; Wang, X.; Cunningham, J.M.; McDonnell, S.K.; Qian, C.; Marks, A.F.; Slager, S.L.; Peterson, B.J.; et al. Mutations in CHEK2 associated with prostate cancer risk. Am. J. Hum. Genet. 2003, 72, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Dong, X.; Liu, W.; Chen, J. Characterization of CHEK2 mutations in prostate cancer. Hum. Mutat. 2006, 27, 742–747. [Google Scholar] [CrossRef]
- Cybulski, C.; Gorski, B.; Huzarski, T.; Masojc, B.; Mierzejewski, M.; Debniak, T.; Teodorczyk, U.; Byrski, T.; Gronwald, J.; Matyjasik, J.; et al. CHEK2 is a multiorgan cancer susceptibility gene. Am. J. Hum. Genet. 2004, 75, 1131–1135. [Google Scholar] [CrossRef]
- Chabalier-Taste, C.; Racca, C.; Dozier, C.; Larminat, F. BRCA1 is regulated by Chk2 in response to spindle damage. Biochim. Et. Biophys. Acta 2008, 1783, 2223–2233. [Google Scholar] [CrossRef]
- Choi, W.; Lee, E.S. Therapeutic Targeting of DNA Damage Response in Cancer. Int. J. Mol. Sci. 2022, 23, 1701. [Google Scholar] [CrossRef] [PubMed]
- Gisselsson, D. Classification of chromosome segregation errors in cancer. Chromosoma 2008, 117, 511–519. [Google Scholar] [CrossRef]
- Bai, J.; Wioland, H.; Advedissian, T.; Cuvelier, F.; Romet-Lemonne, G.; Echard, A. Actin reduction by MsrB2 is a key component of the cytokinetic abscission checkpoint and prevents tetraploidy. Proc. Natl. Acad. Sci. USA 2020, 117, 4169–4179. [Google Scholar] [CrossRef] [PubMed]
- Norden, C.; Mendoza, M.; Dobbelaere, J.; Kotwaliwale, C.V.; Biggins, S.; Barral, Y. The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell 2006, 125, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Petsalaki, E.; Zachos, G. Clks 1, 2 and 4 prevent chromatin breakage by regulating the Aurora B-dependent abscission checkpoint. Nat. Commun. 2016, 7, 11451. [Google Scholar] [CrossRef]
- Steigemann, P.; Wurzenberger, C.; Schmitz, M.H.A.; Held, M.; Guizetti, J.; Maar, S.; Gerlich, D.W. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 2009, 136, 473–484. [Google Scholar] [CrossRef]
- Honda, R.; Körner, R.; Nigg, E.A. Exploring the functional inter- actions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell 2003, 14, 3325–3341. [Google Scholar] [CrossRef]
- Adriaans, I.E.; Hooikaas, P.J.; Aher, A.; Vromans, M.J.M.; Van Es, R.M.; Grigoriev, I.; Akhmanova, A.; Lens, S.M.A. MKLP2 Is a Motile Kinesin that Transports the Chromosomal Passenger Complex during Anaphase. Curr. Biol. 2020, 30, 2628–2637.e9. [Google Scholar] [CrossRef]
- Van der Horst, A.; Vromans, M.J.M.; Bouwman, K.; van der Waal, M.S.; Hadders, M.A.; Lens, S.M.A. Inter-domain Cooperation in INCENP Promotes Aurora B Relocation from Centromeres to Microtubules. Cell Rep. 2015, 12, 380–387. [Google Scholar] [CrossRef]
- Kim, Y.B.; Shin, Y.J.; Roy, A.; Kim, J.H. The Role of the Pleckstrin Homology Domain-containing Protein CKIP-1 in Activation of p21-activated Kinase 1 (PAK1). J. Biol. Chem. 2015, 290, 21076–21085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.G.; Zhang, J.; Mao, L.L.; Wu, J.X.; Cao, W.J.; Zheng, J.N.; Pei, D.S. p21-Activated kinase 5 affects cisplatin-induced apoptosis and proliferation in hepatocellular carcinoma cells. Tumour Biol. 2015, 36, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T. Protein kinase CK2 interacts with Chk2 and phosphorylates Mre11 on serine 649. Biochem. Biophys. Res. Commun. 2005, 331, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Bjørling-Poulsen, M.; Siehler, S.; Wiesmüller, L.; Meek, D.; Niefind, K.; Issinger, O.G. The ‘regulatory’ beta-subunit of protein kinase CK2 negatively influences p53-mediated allosteric effects on Chk2 activation. Oncogene 2005, 24, 6194–6200. [Google Scholar] [CrossRef]
- Kroonen, J.; Artesi, M.; Capraro, V.; Nguyen-Khac, M.T.; Willems, M.; Chakravarti, A.; Bours, V.; Robe, P.A. Casein kinase 2 inhibition modulates the DNA damage response but fails to radiosensitize malignant glioma cells. Int. J. Oncol. 2012, 41, 776–782. [Google Scholar] [CrossRef]
- Levine, M.S.; Holland, A.J. The impact of mitotic errors on cell proliferation and tumorigenesis. Genes Dev. 2018, 32, 620–638. [Google Scholar] [CrossRef]
- McKenzie, C.; D’Avino, P.P. Investigating cytokinesis failure as a strategy in cancer therapy. Oncotarget 2016, 7, 87323–87341. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, H. Cytokinesis and the Hippo Pathway: New Molecular Links Between Intimate Partners. Gastroenterology 2018, 155, 976–978. [Google Scholar] [CrossRef]
- Lens, S.M.A.; Medema, R.H. Cytokinesis defects and cancer. Nat. Rev. Cancer 2019, 19, 32–45. [Google Scholar] [CrossRef]

| CK2 Kinase | Organism | Cytokinesis Functions | Phosphorylation Target | Phosphorylated Residues | Ref. |
|---|---|---|---|---|---|
| CK2 | Homo sapiens | Intercellular bridge stabilization | GBF1 | Ser292 Ser297 | [59] |
| CK2 | Homo sapiens | NM II filament disassembly | NMHC II | Ser1944 | [60] |
| PAK Kinase | Organism | Cytokinesis Functions | Phosphorylation Target | Phosphorylated Residues | Ref. |
| Ste20 | Saccharomyces cerevisiae | Formin activation, F-actin ring assembly and dynamics | Bni1 | ND | [61] |
| Cla4 | Saccharomyces cerevisiae | Formin activation, F-actin ring assembly and dynamics | Bni1 | ND | [61] |
| Cla4 | Saccharomyces cerevisiae | Septin ring assembly | Septins | ND | [62,63,64,65,66] |
| Pak1 | Schizosaccharomyces pombe | CR positioning | Mid1 | N-terminus phosphorylation | [67] |
| Pak1 | Schizosaccharomyces pombe | CR assembly | Cdc15 | ND | [67] |
| Pak1 | Schizosaccharomyces pombe | CR constriction | Rlc1 | Ser35 and Ser36 | [68] |
| Pak2 | Homo sapiens | MLCK inhibition | MLCK | Ser439 and Ser991 | [69] |
| Pak2 | Homo sapiens | MRLC activation | MRLC | Ser19 | [70] |
| Pak2 | Homo sapiens | Midbody dynamics | MKLP1 | tail domain * | [71] |
| Chk2 Kinase | Organism | Cytokinesis Functions | Phosphorylation Target | Phosphorylated Residues | Ref. |
| Chk2 | Homo sapiens | CPC localization | INCENP | Ser91 | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sechi, S.; Piergentili, R.; Giansanti, M.G. Minor Kinases with Major Roles in Cytokinesis Regulation. Cells 2022, 11, 3639. https://doi.org/10.3390/cells11223639
Sechi S, Piergentili R, Giansanti MG. Minor Kinases with Major Roles in Cytokinesis Regulation. Cells. 2022; 11(22):3639. https://doi.org/10.3390/cells11223639
Chicago/Turabian StyleSechi, Stefano, Roberto Piergentili, and Maria Grazia Giansanti. 2022. "Minor Kinases with Major Roles in Cytokinesis Regulation" Cells 11, no. 22: 3639. https://doi.org/10.3390/cells11223639
APA StyleSechi, S., Piergentili, R., & Giansanti, M. G. (2022). Minor Kinases with Major Roles in Cytokinesis Regulation. Cells, 11(22), 3639. https://doi.org/10.3390/cells11223639








