The Complex Journey of the Calcium Regulation Downstream of TAS2R Activation
Abstract
:1. Introduction
2. Bitter Taste Receptor Ligands
3. Bitter Taste Signal Transduction Mechanisms
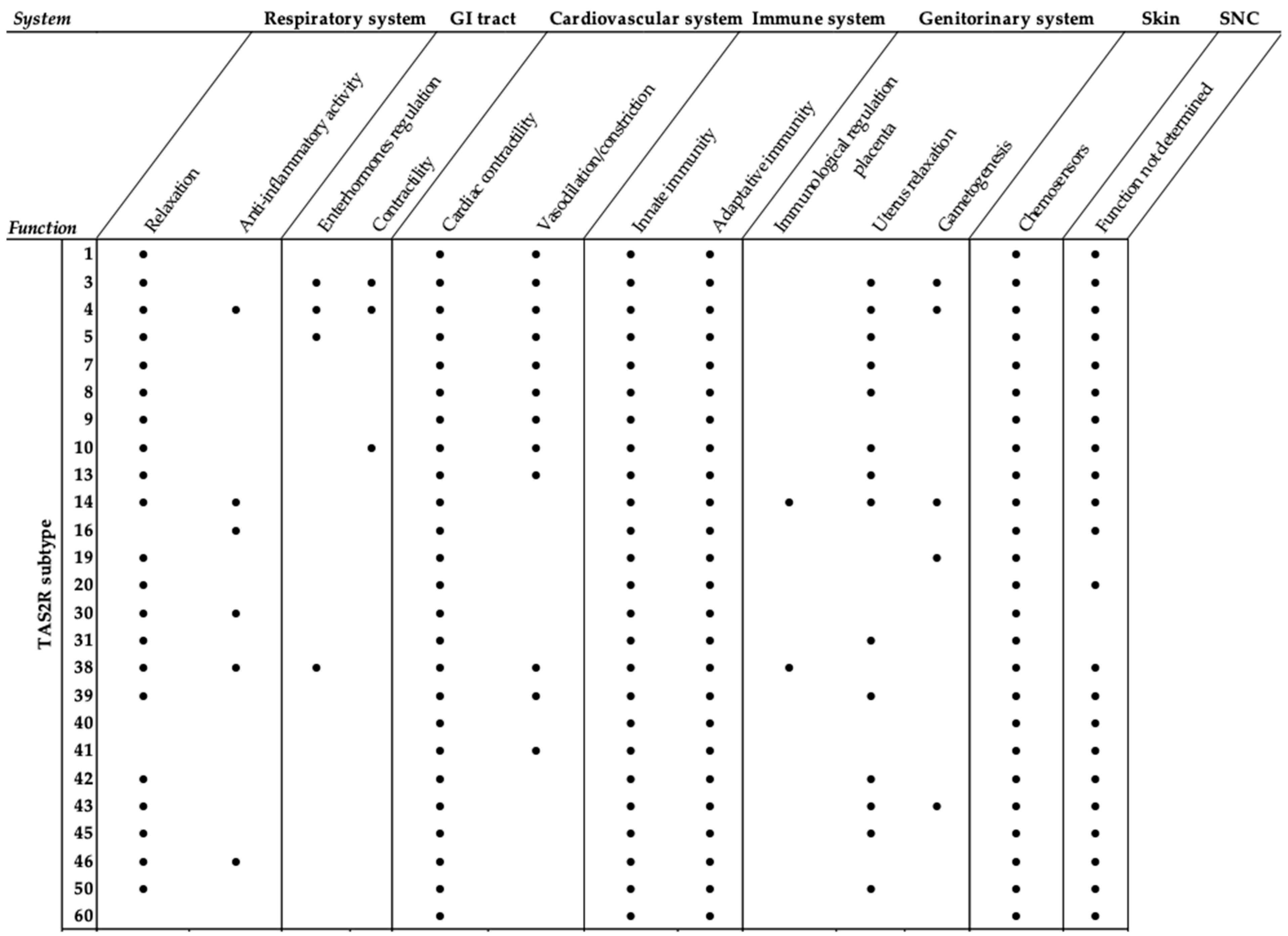
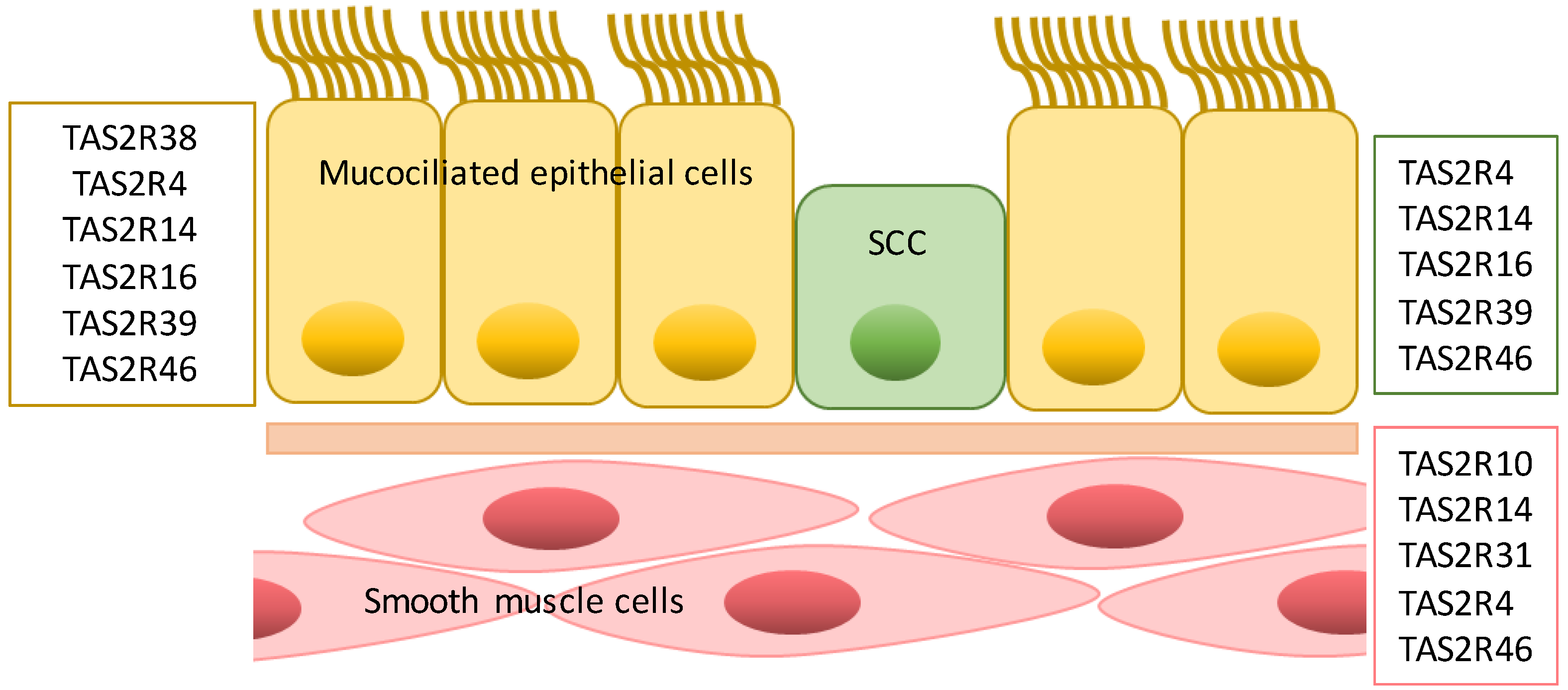
4. Type 2 Taste Receptors in the Respiratory System
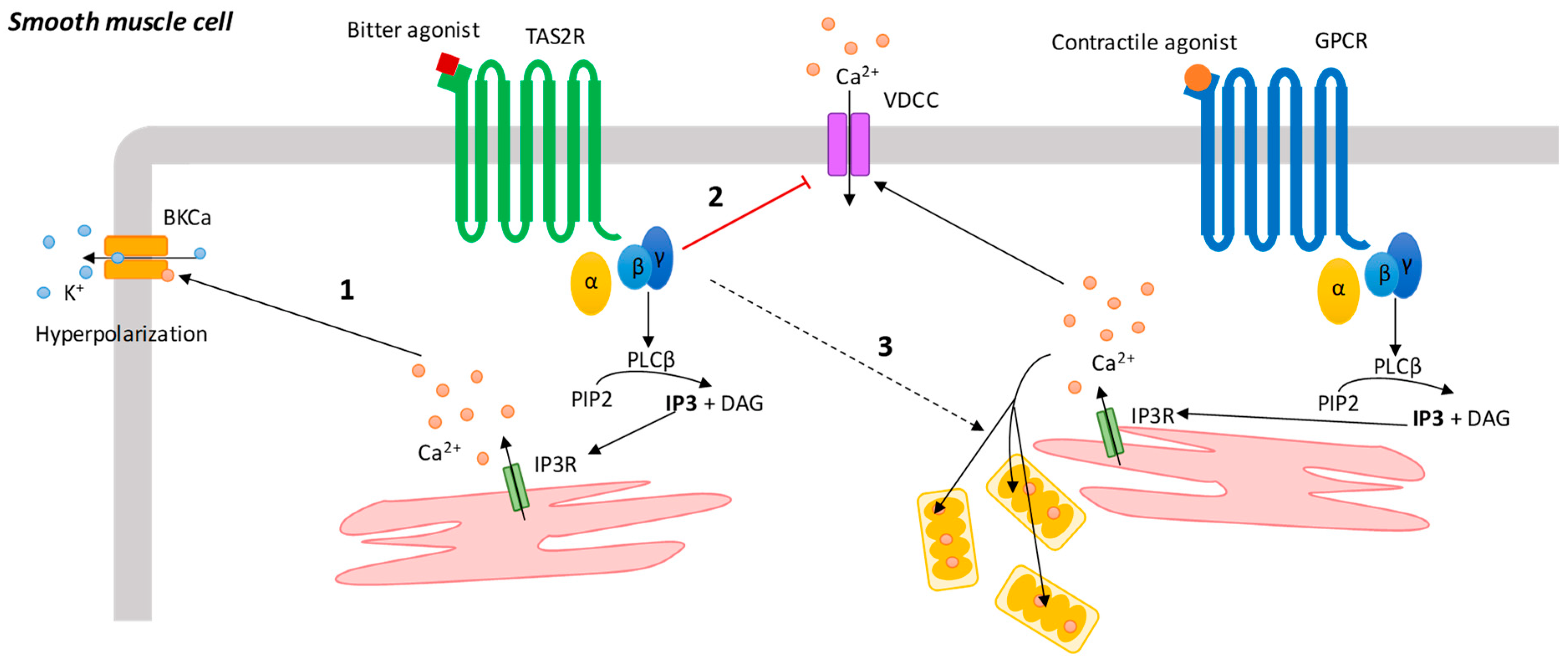
4.1. Airway Smooth Muscle Cells
4.2. Epithelial Cells
4.3. Immune Cells
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lush, I.E.; Hornigold, N.; King, P.; Stoye, J.P. The genetics of tasting in mice. VII. Glycine revisited, and the chromosom.al location of Sac and Soa. Genet. Res. 1995, 66, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bachmanov, A.A.; Beauchamp, G.K. Taste receptor genes. Annu. Rev. Nutr. 2007, 27, 389–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wooding, S.P.; Ramirez, V.A.; Behrens, M. Bitter taste receptors: Genes, evolution and health. Evol. Med. Public. Health 2021, 13, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Adler, E.; Hoon, M.A.; Mueller, K.L.; Chandrashekar, J.; Ryba, N.J.; Zuker, C.S. A novel family of mammalian taste receptors. Cell 2000, 100, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekar, J.; Mueller, K.L.; Hoon, M.A.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J. T2Rs function as bitter taste receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Meyerhof, W.; Behrens, M.; Brockhoff, A.; Bufe, B.; Kuhn, C. Human bitter taste perception. Chem. Senses 2005, 30, i14–i19. [Google Scholar] [CrossRef]
- Ahmad, R.; Dalziel, J.E. G Protein-Coupled Receptors in Taste Physiology and Pharmacology. Front. Pharmacol. 2020, 11, 587664. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Jaggupilli, A.; Howard, R.; Upadhyaya, J.D.; Bhullar, R.P.; Chelikani, P. Bitter taste receptors: Novel insights into the biochemistry and pharmacology. Int. J. Biochem. Cell. Biol. 2016, 77, 184–196. [Google Scholar] [CrossRef]
- Brockhoff, A.; Behrens, M.; Roudnitzky, N.; Appendino, G.; Avonto, C.; Meyerhof, W. Receptor agonism and antagonism of dietary bitter compounds. J. Neurosci. 2011, 31, 14775–14782. [Google Scholar] [CrossRef]
- Sanematsu, K.; Yoshida, R.; Shigemura, N.; Ninomiya, Y. Structure, function, and signaling of taste G-protein-coupled receptors. Curr. Pharm. Biotechnol. 2014, 15, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Brockhoff, A.; Behrens, M.; Niv, M.Y.; Meyerhof, W. Structural requirements of bitter taste receptor activation. Proc. Natl. Acad. Sci. USA 2010, 107, 11110–11115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pizio, A.; Levit, A.; Slutzki, M.; Behrens, M.; Karaman, R.; Niv, M.Y. Comparing Class A GPCRs to bitter taste receptors: Structural motifs, ligand interactions and agonist-to-antagonist ratios. Methods. Cell. Biol. 2016, 132, 401–427. [Google Scholar] [PubMed]
- Deshpande, D.A.; Wang, W.C.; McIlmoyle, E.L.; Robinett, K.S.; Schillinger, R.M.; An, S.S.; Sham, J.S.; Liggett, S.B. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 2010, 16, 1299–1304. [Google Scholar] [CrossRef] [Green Version]
- Talmon, M.; Rossi, S.; Lim, D.; Pollastro, F.; Palattella, G.; Ruffinatti, F.A.; Marotta, P.; Boldorini, R.; Genazzani, A.A.; Fresu, L.G. Absinthin, an agonist of the bitter taste receptor hTAS2R46, uncovers an ER-to-mitochondria Ca2+-shuttling event. J. Biol. Chem. 2019, 294, 12472–12482. [Google Scholar] [CrossRef]
- Morris, J.L.; Puttick, M.N.; Clark, J.W.; Edwards, D.; Kenrick, P.; Pressel, S.; Wellman, C.H.; Yang, Z.; Schneider, H.; Donoghue, P.C.J. The timescale of early land plant evolution. Proc. Natl. Acad. Sci. USA 2018, 115, E2274–E2283. [Google Scholar] [CrossRef] [Green Version]
- Tuzim, K.; Korolczuk, A. An update on extra-oral bitter taste receptors. J. Transl. Med. 2021, 19, 440. [Google Scholar] [CrossRef]
- Behrens, M.; Lang, T. Extra-Oral Taste Receptors-Function, Disease, and Perspectives. Front. Nutr. 2022, 9, 881177. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Abrial, C.; Fayad-Kobeissi, S.; Brollo, M.; Faisy, C.; Alvarez, J.C.; Naline, E.; Devillier, P. The expression and relaxant effect of bitter taste receptors in human bronchi. Respir. Res. 2013, 14, 134. [Google Scholar] [CrossRef] [Green Version]
- Rozengurt, E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G171–G178. [Google Scholar]
- Bloxham, C.J.; Foster, S.R.; Thomas, W.G. A Bitter Taste in Your Heart. Front. Physiol. 2020, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Ping, N.N.; Liang, D.; Li, M.Y.; Mi, Y.N.; Li, S.; Cao, L.; Cai, Y.; Cao, Y.X. The expression of bitter taste receptors in mesenteric, cerebral and omental arteries. Life. Sci. 2017, 170, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Welcome, M.O. The bitterness of genitourinary infections: Properties, ligands of genitourinary bitter taste receptors and mechanisms linking taste sensing to inflammatory processes in the genitourinary tract. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, J.D.; Singh, N.; Sikarwar, A.S.; Chakraborty, R.; Pydi, S.P.; Bhullar, R.P.; Dakshinamurti, S.; Chelikani, P. Dextromethorphan mediated bitter taste receptor activation in the pulmonary circuit causes vasoconstriction. PLoS ONE 2014, 9, e110373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef] [Green Version]
- Gopallawa, I.; Freund, J.R.; Lee, R.J. Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling. Cell. Mol. Life Sci. 2021, 78, 271–286. [Google Scholar] [CrossRef]
- Lee, R.J.; Cohen, N.A. Role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Curr. Opin. Allergy. Clin. Immunol. 2015, 15, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Grassin-Delyle, S.; Salvator, H.; Mantov, N.; Abrial, C.; Brollo, M.; Faisy, C.; Naline, E.; Couderc, L.J.; Devillier, P. Bitter Taste Receptors (TAS2Rs) in Human Lung Macrophages: Receptor Expression and Inhibitory Effects of TAS2R Agonists. Front. Physiol. 2019, 10, 1267. [Google Scholar] [CrossRef]
- Wölfle, U.; Elsholz, F.A.; Kersten, A.; Haarhaus, B.; Schumacher, U.; Schempp, C.M. Expression and Functional Activity of the Human Bitter Taste Receptor TAS2R38 in Human Placental Tissues and JEG-3 Cells. Molecules 2016, 21, 306. [Google Scholar] [CrossRef] [Green Version]
- Taher, S.; Borja, Y.; Cabanela, L.; Costers, V.J.; Carson-Marino, M.; Bailes, J.C.; Dhar, B.; Beckworth, M.T.; Rabaglino, M.B.; Post Uiterweer, E.D.; et al. Cholecystokinin, gastrin, cholecystokinin/gastrin receptors, and bitter taste receptor TAS2R14: Trophoblast expression and signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R628–R639, Erratum in Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R355. [Google Scholar] [CrossRef]
- Liszt, K.I.; Wang, Q.; Farhadipour, M.; Segers, A.; Thijs, T.; Nys, L.; Deleus, E.; Van der Schueren, B.; Gerner, C.; Neuditschko, B.; et al. Human intestinal bitter taste receptors regulate innate immune responses and metabolic regulators in obesity. J. Clin. Invest. 2022, 132, e144828. [Google Scholar] [CrossRef] [PubMed]
- Avau, B.; Rotondo, A.; Thijs, T.; Andrews, C.N.; Janssen, P.; Tack, J.; Depoortere, I. Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci. Rep. 2015, 5, 15985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, S.R.; Porrello, E.R.; Purdue, B.; Chan, H.W.; Voigt, A.; Frenzel, S.; Hannan, R.D.; Moritz, K.M.; Simmons, D.G.; Molenaar, P.; et al. Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS ONE 2013, 8, e64579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, S.R.; Porrello, E.R.; Stefani, M.; Smith, N.J.; Molenaar, P.; dos Remedios, C.G.; Thomas, W.G.; Ramialison, M. Cardiac gene expression data and in silico analysis provide novel insights into human and mouse taste receptor gene regulation. Naunyn. Schmiedebergs. Arch. Pharmacol. 2015, 388, 1009–1027. [Google Scholar] [CrossRef]
- Carey, R.M.; Hariri, B.M.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. HSP90 Modulates T2R Bitter Taste Receptor Nitric Oxide Production and Innate Immune Responses in Human Airway Epithelial Cells and Macrophages. Cells 2022, 11, 1478. [Google Scholar] [CrossRef]
- Gaida, M.M.; Dapunt, U.; Hänsch, G.M. Sensing developing biofilms: The bitter receptor T2R38 on myeloid cells. Pathog. Dis. 2016, 74, ftw004. [Google Scholar] [CrossRef] [Green Version]
- Sakakibara, M.; Sumida, H.; Yanagida, K.; Miyasato, S.; Nakamura, M.; Sato, S. Bitter taste receptor T2R38 is expressed on skin-infiltrating lymphocytes and regulates lymphocyte migration. Sci. Rep. 2022, 12, 11790. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Herz, C.; Ruf, P.; Stetter, R.; Lamy, E. Human T2R38 Bitter Taste Receptor Expression in Resting and Activated Lymphocytes. Front. Immunol. 2018, 9, 2949. [Google Scholar] [CrossRef]
- Malki, A.; Fiedler, J.; Fricke, K.; Ballweg, I.; Pfaffl, M.W.; Krautwurst, D. Class I odorant receptors, TAS1R and TAS2R taste receptors, are markers for subpopulations of circulating leukocytes. J. Leukoc. Biol. 2015, 97, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Warning, J.C.; McCracken, S.A.; Morris, J.M. A balancing act: Mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 2011, 141, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Lu, P.; Delpapa, E.; Bellve, K.; Deng, R.; Condon, J.C.; Fogarty, K.; Lifshitz, L.M.; Simas, T.A.M.; Shi, F.; et al. Bitter taste receptors as targets for tocolytics in preterm labor therapy. FASEB J. 2017, 31, 4037–4052. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cao, J.; Iguchi, N.; Riethmacher, D.; Huang, L. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol. Hum. Reprod. 2013, 19, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Gentiluomo, M.; Crifasi, L.; Luddi, A.; Locci, D.; Barale, R.; Piomboni, P.; Campa, D. Taste receptor polymorphisms and male infertility. Hum. Reprod. 2017, 32, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Governini, L.; Semplici, B.; Pavone, V.; Crifasi, L.; Marrocco, C.; De Leo, V.; Arlt, E.; Gudermann, T.; Boekhoff, I.; Luddi, A.; et al. Expression of Taste Receptor 2 Subtypes in Human Testis and Sperm. J. Clin. Med. 2020, 9, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, M.G.; Kim, Y.; Cha, Y.K.; Park, T.H.; Kim, Y. Bitter taste receptors protect against skin aging by inhibiting cellular senescence and enhancing wound healing. Nutr. Res. Pract. 2022, 16, 1–13. [Google Scholar] [CrossRef]
- Shaw, L.; Mansfield, C.; Colquitt, L.; Lin, C.; Ferreira, J.; Emmetsberger, J.; Reed, D.R. Personalized expression of bitter ‘taste’ receptors in human skin. PLoS ONE 2018, 13, e0205322. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Vrontakis, M.; Parkinson, F.; Chelikani, P. Functional bitter taste receptors are expressed in brain cells. Biochem. Biophys. Res. Commun. 2011, 406, 146–151. [Google Scholar] [CrossRef]
- Duarte, A.C.; Santos, J.; Costa, A.R.; Ferreira, C.L.; Tomás, J.; Quintela, T.; Ishikawa, H.; Schwerk, C.; Schroten, H.; Ferrer, I.; et al. Bitter taste receptors profiling in the human blood-cerebrospinal fluid-barrier. Biochem. Pharmacol. 2020, 177, 113954. [Google Scholar] [CrossRef]
- Bufe, B.; Hofmann, T.; Krautwurst, D.; Raguse, J.D.; Meyerhof, W. The human TAS2R16 receptor mediates bitter taste in response to beta-glucopyranosides. Nat. Genet. 2002, 32, 397–401. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W. Bitter taste receptor research comes of age: From characterization to modulation of TAS2Rs. Semin. Cell. Dev. Biol. 2013, 24, 215–221. [Google Scholar] [CrossRef]
- Brockhoff, A.; Behrens, M.; Massarotti, A.; Appendino, G.; Meyerhof, W. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J. Agric. Food. Chem. 2007, 55, 6236–6243. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Ugawa, S.; Yamamura, H.; Imaizumi, Y.; Shimada, S. Functional interaction between T2R taste receptors and G-protein alpha subunits expressed in taste receptors cells. J. Neurosci. 2003, 23, 7376–7380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Kraker, J.W.; Franssen, M.C.; de Groot, A.; Shibata, T.; Bouwmeester, H.J. Germacrenes from fresh costus roots. Phytochemistry 2001, 58, 481–487. [Google Scholar] [CrossRef]
- Liggett, S.B. Bitter taste receptors on airway smooth muscle as targets for novel bronchodilators. Expert. Opin. Ther. Targets 2013, 17, 721–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, A.P.; Shah, S.D.; Michael, J.V.; Deshpande, D.A. Bitter Taste Receptors for Asthma Therapeutics. Front. Physiol. 2019, 10, 884. [Google Scholar] [CrossRef] [Green Version]
- Dalesio, N.M.; Barreto Ortiz, S.F.; Pluznick, J.L.; Berkowitz, D.E. Olfactory, Taste, and Photo Sensory Receptors in Non-sensory Organs: It Just Makes Sense. Front. Physiol. 2018, 9, 1673. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; An, S.S.; Lam, H.; Leahy, J.W.; Liggett, S.B. Identification and Characterization of Novel Bronchodilator Agonists Acting at Human Airway Smooth Muscle Cell TAS2R5. ACS. Pharmacol. Transl. Sci. 2020, 3, 1069–1075. [Google Scholar] [CrossRef]
- Guo, N.; Xu, Y.; Cao, Z. Absinthin attenuates LPS-induced ALI through MIP-1α-mediated inflammatory cell infiltration. Exp. Lung. Res. 2015, 41, 514–524. [Google Scholar] [CrossRef]
- Talmon, M.; Bosso, L.; Quaregna, M.; Lopatriello, A.; Rossi, S.; Gavioli, D.; Marotta, P.; Caprioglio, D.; Boldorini, R.; Miggiano, R.; et al. Anti-inflammatory Activity of Absinthin and Derivatives in Human Bronchoepithelial Cells. J. Nat. Prod. 2020, 83, 1740–1750, Erratum in J. Nat. Prod. 2020, 83, 2815–2816. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, S.; Fang, F.; Chen, Q.; Hu, H.; Jia, X.; Zhai, H. Total synthesis of absinthin. J. Am. Chem. Soc. 2005, 127, 18–27. [Google Scholar] [CrossRef]
- Pulkkinen, V.; Manson, M.L.; Säfholm, J.; Adner, M.; Dahlén, S.E. The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L956–L966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Lifshitz, L.M.; Uy, K.F.; Ikebe, M.; Fogarty, K.E.; ZhuGe, R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol. 2013, 11, e1001501, Erratum in PLoS Biol. 2013, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Camoretti-Mercado, B.; Pauer, S.H.; Yong, H.M.; Smith, D.C.; Deshpande, D.A.; An, S.S.; Liggett, S.B. Pleiotropic Effects of Bitter Taste Receptors on [Ca2+]i Mobilization, Hyperpolarization, and Relaxation of Human Airway Smooth Muscle Cells. PLoS ONE 2015, 10, e0131582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Conaway, S., Jr.; Deshpande, D. Bitter Taste Receptors in the Airway Cells Functions. Handb. Exp. Pharmacol. 2022, 275, 203–227. [Google Scholar] [PubMed]
- Billington, C.K.; Penn, R.B. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir. Res. 2003, 4, 2. [Google Scholar] [CrossRef]
- An, S.S.; Wang, W.C.; Koziol-White, C.J.; Ahn, K.; Lee, D.Y.; Kurten, R.C.; Panettieri, R.A., Jr.; Liggett, S.B. TAS2R activation promotes airway smooth muscle relaxation despite β(2)-adrenergic receptor tachyphylaxis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L304–L311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, A.; Zhang, M.; Zeng, H.; Lu, Y.; Liu, L.; Li, J.; Deng, L. Artesunate attenuates airway resistance in vivo and relaxes airway smooth muscle cells in vitro via bitter taste receptor-dependent calcium signalling. Exp. Physiol. 2019, 104, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Sanderson, M.J. Bitter tasting compounds dilate airways by inhibiting airway smooth muscle calcium oscillations and calcium sensitivity. Br. J. Pharmacol. 2014, 171, 646–662. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.W.; Sun, J.; Wang, Y.; Chen, C.P.; Tao, T.; Ma, M.; Chen, X.; Zhang, X.N.; Yang, L.Y.; Zhang, Z.L.; et al. Tas2R activation relaxes airway smooth muscle by release of Gαt targeting on AChR signaling. Proc. Natl. Acad. Sci. USA 2022, 119, e2121513119. [Google Scholar] [CrossRef]
- Lee, R.J.; Cohen, N.A. The emerging role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Am. J. Rhinol. Allergy 2013, 27, 283–289. [Google Scholar] [CrossRef]
- Barham, H.P.; Cooper, S.E.; Anderson, C.B.; Tizzano, M.; Kingdom, T.T.; Finger, T.E.; Kinnamon, S.C.; Ramakrishnan, V.R. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int. Forum. Allergy Rhinol. 2013, 3, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Larson, E.D.; Anderson, C.B.; Agarwal, P.; Frank, D.N.; Kinnamon, S.C.; Ramakrishnan, V.R. Expression of Bitter Taste Receptors and Solitary Chemosensory Cell Markers in the Human Sinonasal Cavity. Chem. Senses 2019, 44, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Freund, J.R.; Mansfield, C.J.; Doghramji, L.J.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Reed, D.R.; Jiang, P.; Lee, R.J. Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic-AMP, and nitric oxide signaling. J. Biol. Chem. 2018, 293, 9824–9840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalševac, F.; Terra, X.; Rodríguez-Gallego, E.; Beltran-Debón, R.; Blay, M.T.; Pinent, M.; Ardévol, A. The Hidden One: What We Know About Bitter Taste Receptor 39. Front. Endocrinol. 2022, 13, 854718. [Google Scholar] [CrossRef]
- Lee, R.J.; Cohen, N.A. Sinonasal solitary chemosensory cells “taste” the upper respiratory environment to regulate innate immunity. Am. J. Rhinol. Allergy 2014, 28, 366–373. [Google Scholar] [CrossRef]
- Medapati, M.R.; Bhagirath, A.Y.; Singh, N.; Chelikani, P. Pharmacology of T2R Mediated Host-Microbe Interactions. Handb. Exp. Pharmacol. 2022, 275, 177–202. [Google Scholar]
- Liu, L.P.; Huang, L.H.; Ding, X.T.; Yan, L.; Jia, S.R.; Dai, Y.J.; Xie, Y.Y.; Zhong, C. Identification of Quorum-Sensing Molecules of N-Acyl-Homoserine Lactone in Gluconacetobacter Strains by Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2019, 24, 2694. [Google Scholar] [CrossRef] [Green Version]
- Perniss, A.; Liu, S.; Boonen, B.; Keshavarz, M.; Ruppert, A.L.; Timm, T.; Pfeil, U.; Soultanova, A.; Kusumakshi, S.; Delventhal, L.; et al. Chemosensory Cell-Derived Acetylcholine Drives Tracheal Mucociliary Clearance in Response to Virulence-Associated Formyl Peptides. Immunity 2020, 52, 683–699.e11. [Google Scholar] [CrossRef]
- Cohen, N.A. The genetics of the bitter taste receptor T2R38 in upper airway innate immunity and implications for chronic rhinosinusitis. Laryngoscope 2017, 127, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Freund, J.R.; Lee, R.J. Taste receptors in the upper airway. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 67–76. [Google Scholar] [CrossRef]
- Hariri, B.M.; McMahon, D.B.; Chen, B.; Freund, J.R.; Mansfield, C.J.; Doghramji, L.J.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Reed, D.R.; et al. Flavones modulate respiratory epithelial innate immunity: Anti-inflammatory effects and activation of the T2R14 receptor. J. Biol. Chem. 2017, 292, 8484–8497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile cilia of human airway epithelia are chemosensory. Science 2009, 325, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Medapati, M.R.; Singh, N.; Bhagirath, A.Y.; Duan, K.; Triggs-Raine, B.; Batista, E.L., Jr.; Chelikani, P. Bitter taste receptor T2R14 detects quorum sensing molecules from cariogenic Streptococcus mutans and mediates innate immune responses in gingival epithelial cells. FASEB J. 2021, 35, e21375. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.B.; Kuek, L.E.; Johnson, M.E.; Johnson, P.O.; Horn, R.L.J.; Carey, R.M.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. The bitter end: T2R bitter receptor agonists elevate nuclear calcium and induce apoptosis in non-ciliated airway epithelial cells. Cell. Calcium 2022, 101, 102499. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Panebra, A.; Pera, T.; Tiegs, B.C.; Hershfeld, A.; Kenyon, L.C.; Deshpande, D.A. Antimitogenic effect of bitter taste receptor agonists on airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L365–L376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, D.; Watarai, T.; Ozawa, M.; Kanda, Y.; Saika, F.; Kiguchi, N.; Takeuchi, A.; Ikawa, M.; Matsuzaki, S.; Katakai, T. Tas2R signaling enhances mouse neutrophil migration via a ROCK-dependent pathway. Front. Immunol. 2022, 13, 973880. [Google Scholar] [CrossRef]
- Ekoff, M.; Choi, J.H.; James, A.; Dahlén, B.; Nilsson, G.; Dahlén, S.E. Bitter taste receptor (TAS2R) agonists inhibit IgE-dependent mast cell activation. J. Allergy Clin. Immunol. 2014, 134, 475–478. [Google Scholar] [CrossRef]
- Orsmark-Pietras, C.; James, A.; Konradsen, J.R.; Nordlund, B.; Söderhäll, C.; Pulkkinen, V.; Pedroletti, C.; Daham, K.; Kupczyk, M.; Dahlén, B.; et al. Transcriptome analysis reveals upregulation of bitter taste receptors in severe asthmatics. Eur. Respir. J. 2013, 42, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Mahn, K.; Ojo, O.O.; Chadwick, G.; Aaronson, P.I.; Ward, J.P.; Lee, T.H. Ca(2+) homeostasis and structural and functional remodelling of airway smooth muscle in asthma. Thorax 2010, 65, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. Smooth muscle cell calcium activation mechanisms. J. Physiol. 2008, 586, 5047–5061. [Google Scholar] [CrossRef]
- Ozier, A.; Allard, B.; Bara, I.; Girodet, P.O.; Trian, T.; Marthan, R.; Berger, P. The pivotal role of airway smooth muscle in asthma pathophysiology. J. Allergy 2011, 2011, 742710. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talmon, M.; Pollastro, F.; Fresu, L.G. The Complex Journey of the Calcium Regulation Downstream of TAS2R Activation. Cells 2022, 11, 3638. https://doi.org/10.3390/cells11223638
Talmon M, Pollastro F, Fresu LG. The Complex Journey of the Calcium Regulation Downstream of TAS2R Activation. Cells. 2022; 11(22):3638. https://doi.org/10.3390/cells11223638
Chicago/Turabian StyleTalmon, Maria, Federica Pollastro, and Luigia Grazia Fresu. 2022. "The Complex Journey of the Calcium Regulation Downstream of TAS2R Activation" Cells 11, no. 22: 3638. https://doi.org/10.3390/cells11223638
APA StyleTalmon, M., Pollastro, F., & Fresu, L. G. (2022). The Complex Journey of the Calcium Regulation Downstream of TAS2R Activation. Cells, 11(22), 3638. https://doi.org/10.3390/cells11223638





