Tannic Acid, A Hydrolysable Tannin, Prevents Transforming Growth Factor-β-Induced Epithelial–Mesenchymal Transition to Counteract Colorectal Tumor Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Drugs, Antibodies, and Chemical Reagents
2.3. Crystal Violet Staining Assay
2.4. Cell Adhesion Assay
2.5. Migration Using Wound-Healing Assay and Incucyte Device
2.6. Migration and Invasion Using Transwell Assay
2.7. Western Blotting
2.8. Animal Studies
2.9. Biochemical Assay
2.10. Satistical Analyses
3. Results
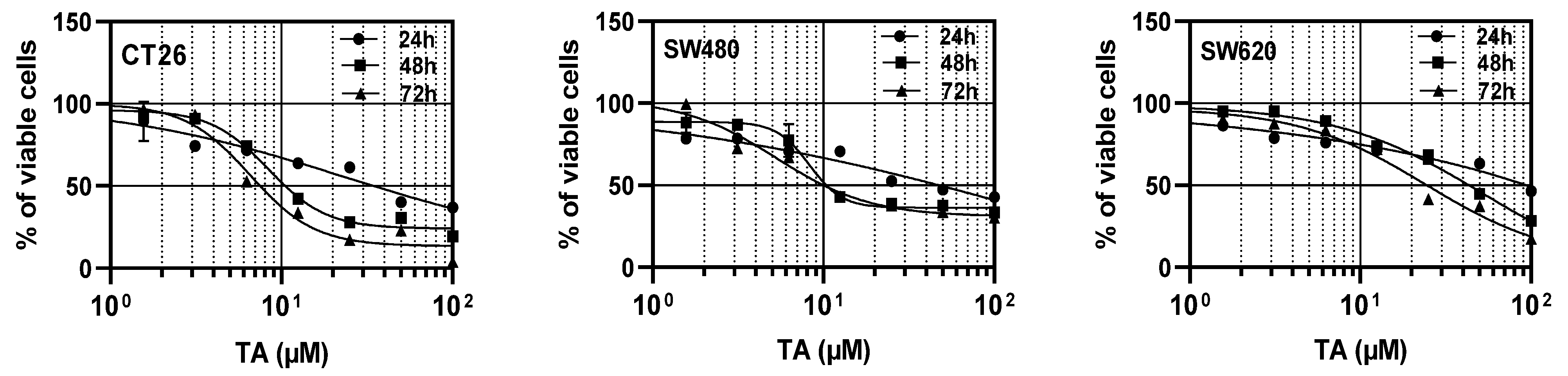
3.1. TA Inhibits the Proliferation of Colorectal Carcinoma Cells
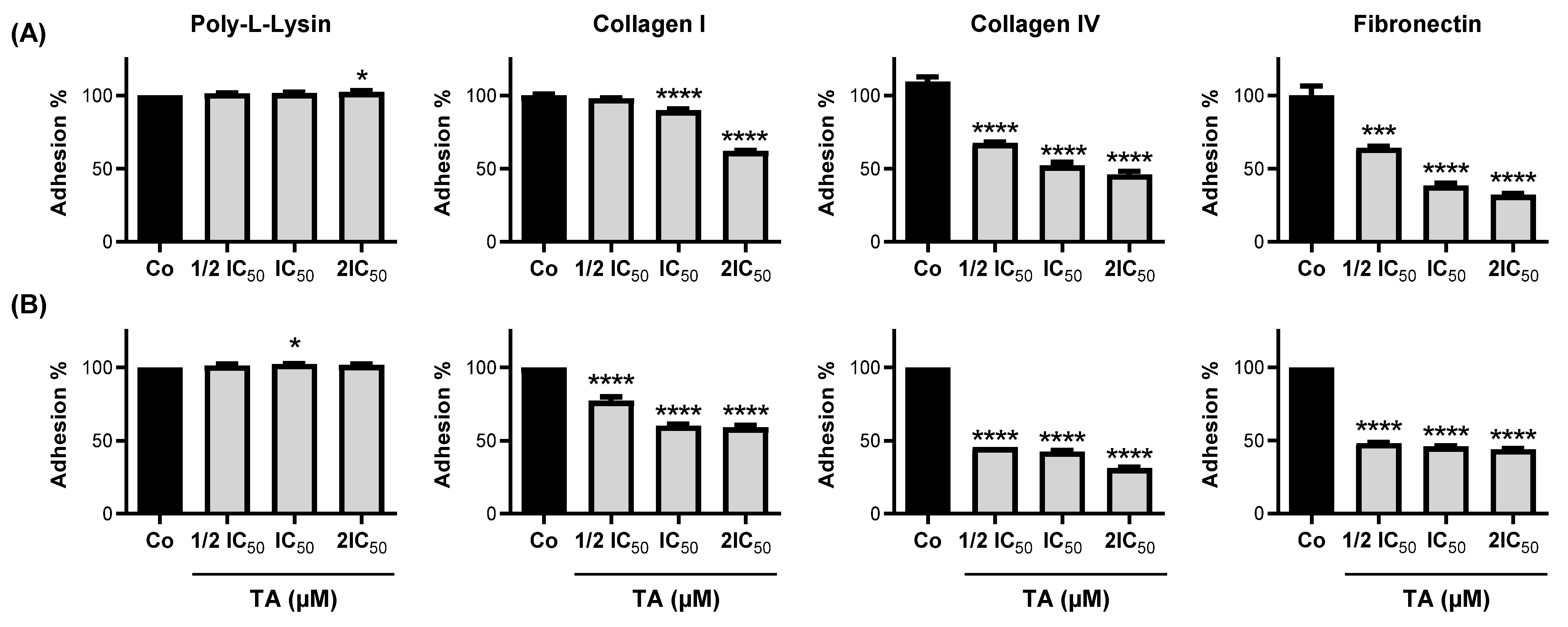
3.2. TA Inhibits Colorectal Carcinoma Cell Adhesion
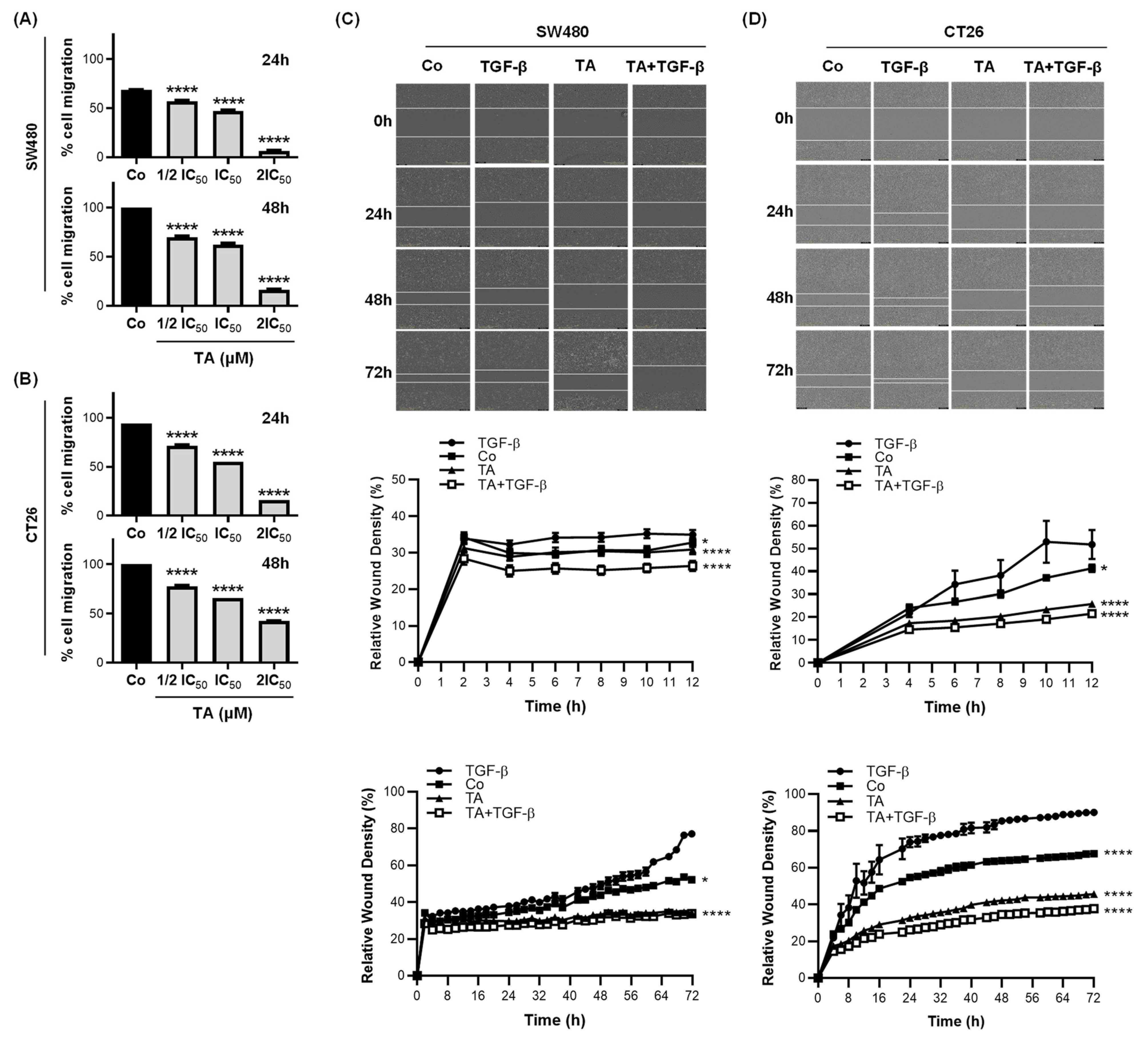
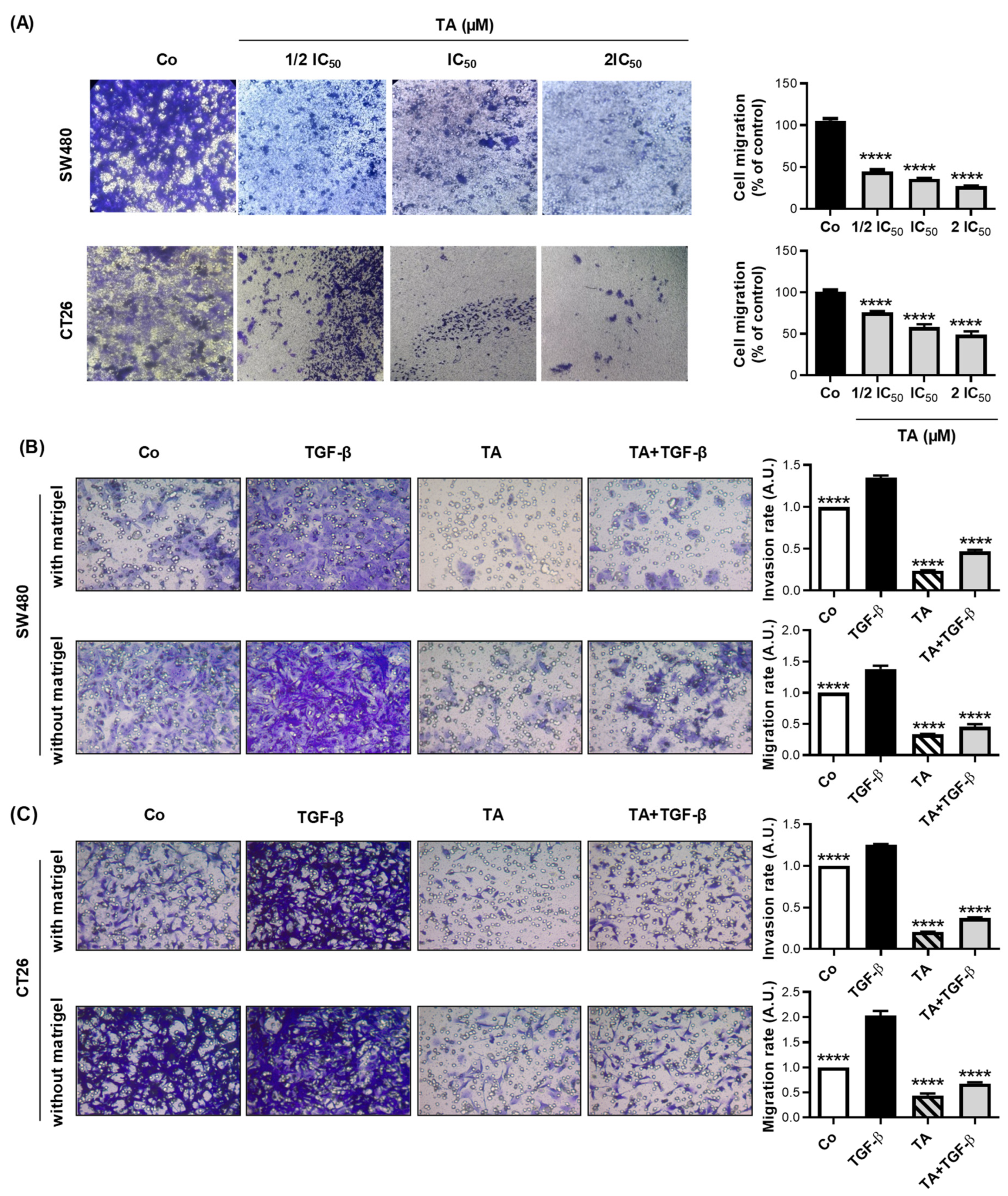
3.3. TA Inhibits Cell Migration
3.4. TA Inhibits TGF-β1-Induced Motility of Metastatic Colon Cancer Cells In Vitro
3.5. TA Restores Epithelial Markers Expressionin CRC Cells
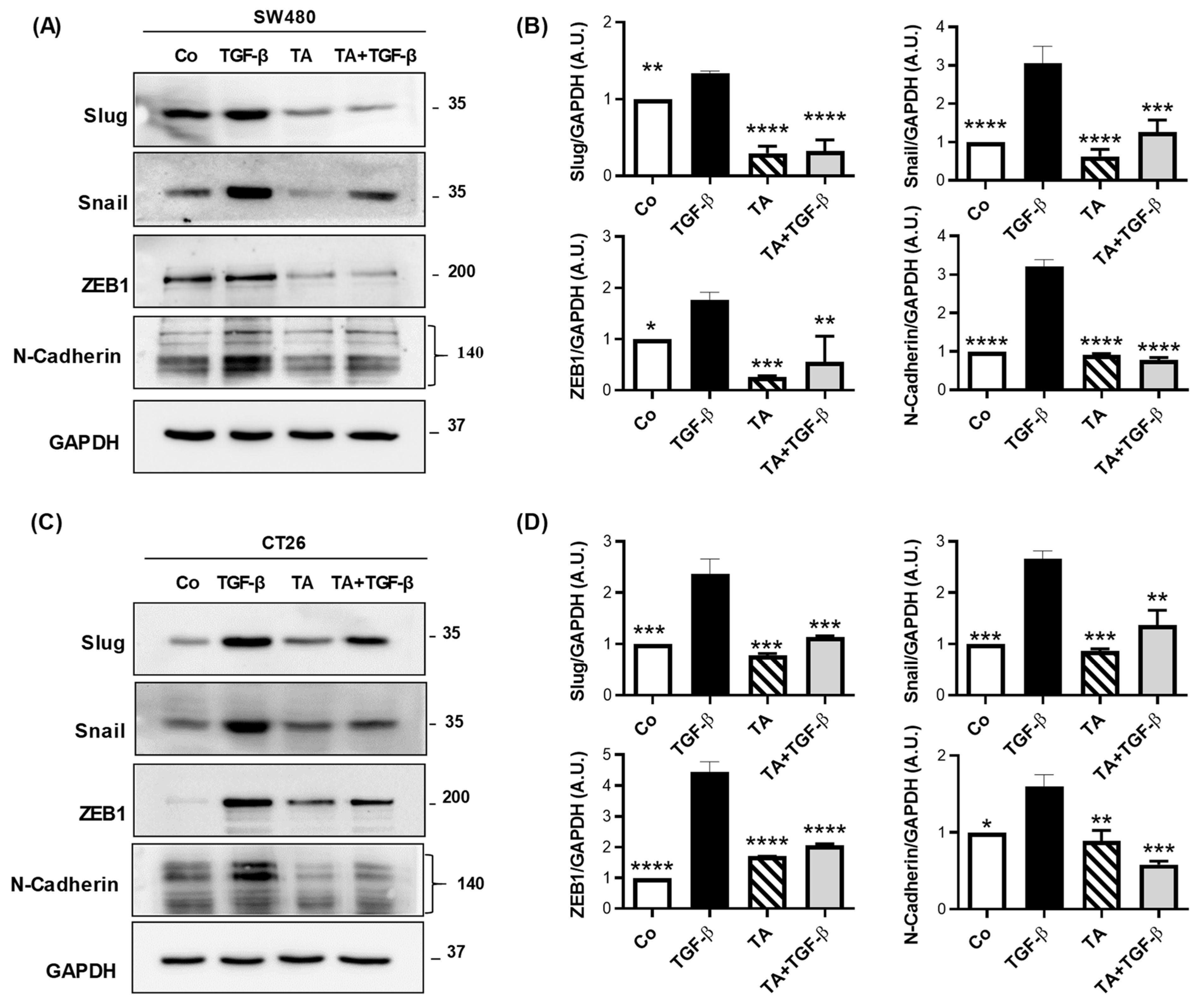
3.6. TA Inhibits Mesenchymal Marker Expression in Colorectal Cancer Cells
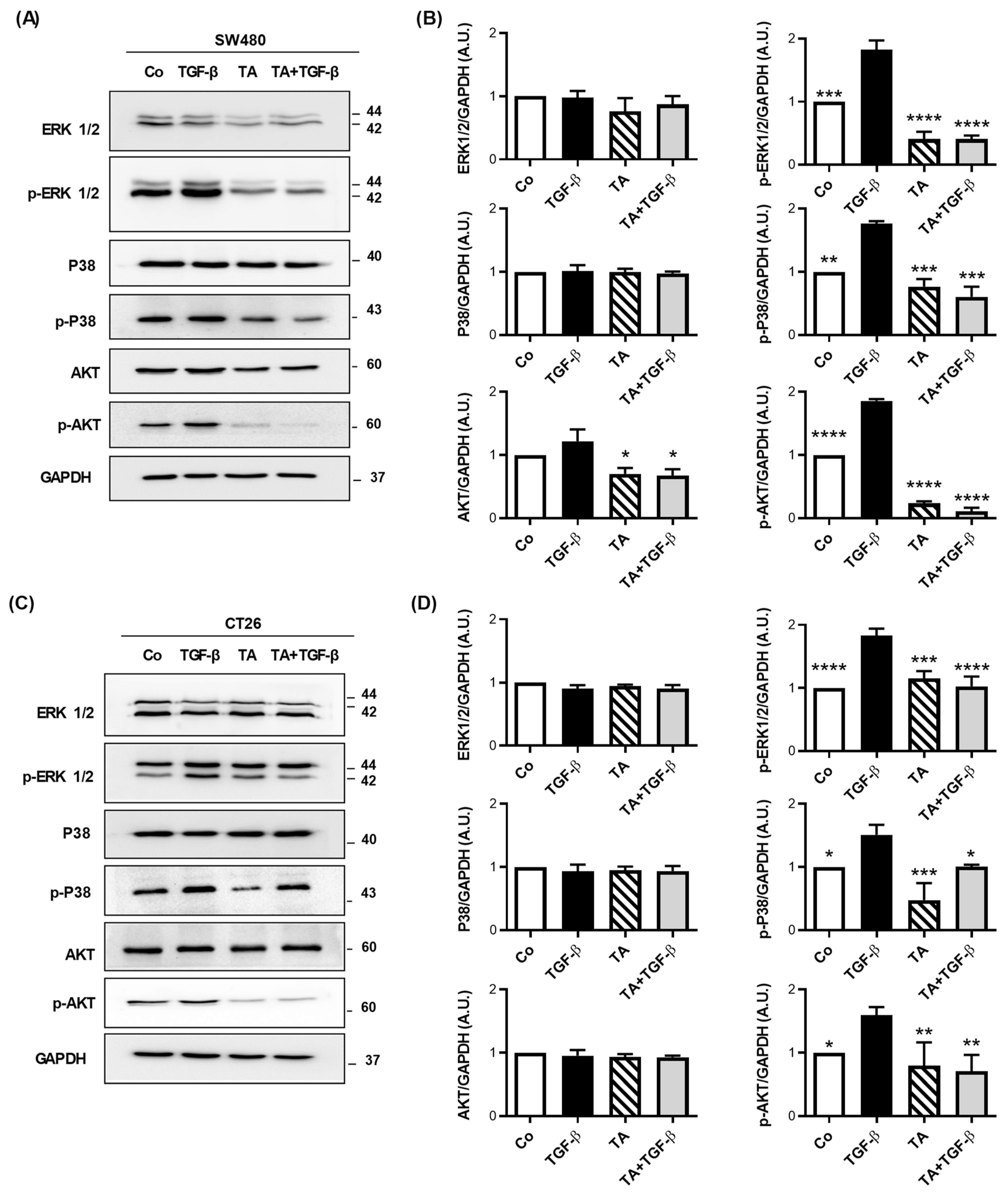
3.7. TA Antagonizes TGF-β1-Mediated Smad-Independent Signaling Pathways
3.8. TA Prevents Murine Colon Carcinoma Growth
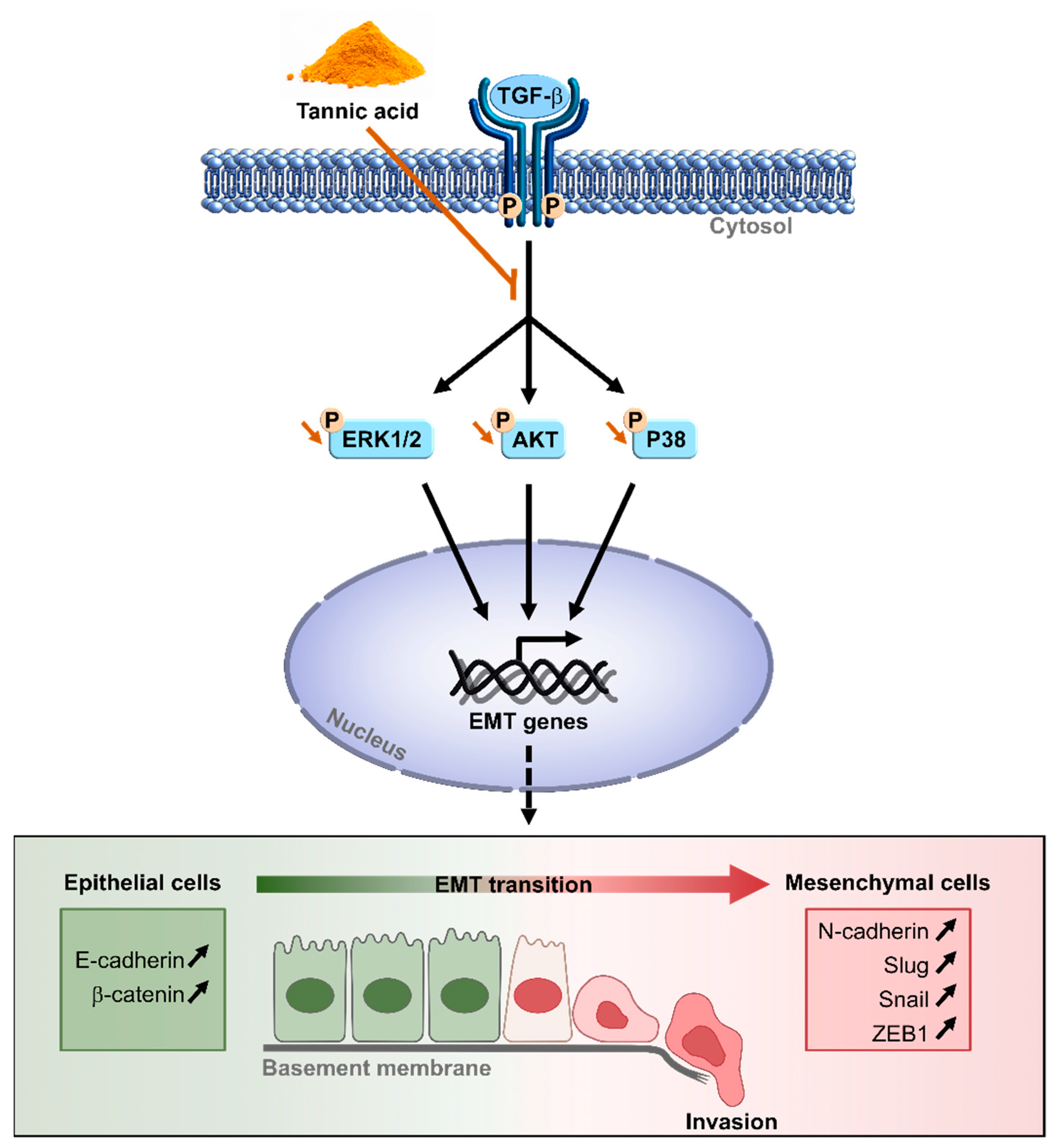
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Favoriti, P.; Carbone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.M.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Abdel-Samad, R.; Aouad, P.; Darwiche, N. Natural and synthetic retinoids in preclinical colorectal cancer models. Anti-Cancer Drugs 2019, 30, 655–669. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Diepenbruck, M.; Christofori, G. Epithelial–mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr. Opin. Cell Biol. 2016, 43, 7–13. [Google Scholar] [CrossRef]
- Vences-Catalán, F.; Levy, S. Immune targeting of tetraspanins involved in cell invasion and metastasis. Front. Immunol. 2018, 9, 1277. [Google Scholar] [CrossRef]
- Spaderna, S.; Schmalhofer, O.; Hlubek, F.; Berx, G.; Eger, A.; Merkel, S.; Jung, A.; Kirchner, T.; Brabletz, T. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology 2006, 131, 830–840. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Savagner, P. The epithelial–mesenchymal transition (EMT) phenomenon. Ann. Oncol. 2010, 21, vii89–vii92. [Google Scholar] [CrossRef]
- Batlle, E.; Sancho, E.; Francí, C.; Domínguez, D.; Monfar, M.; Baulida, J.; García de Herreros, A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000, 2, 84–89. [Google Scholar] [CrossRef]
- Kurrey, N.; Amit, K.; Bapat, S. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol. Oncol. 2005, 97, 155–165. [Google Scholar] [CrossRef]
- Tsuji, T.; Ibaragi, S.; Hu, G.-f. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009, 69, 7135–7139. [Google Scholar] [CrossRef]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-beta signaling in tumor suppression and cancer progression. Nat. Genet. 2001, 29, 117–129. [Google Scholar] [CrossRef]
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. [Google Scholar] [CrossRef]
- Colin, D.; Gimazane, A.; Lizard, G.; Izard, J.C.; Solary, E.; Latruffe, N.; Delmas, D. Effects of resveratrol analogs on cell cycle progression, cell cycle associated proteins and 5fluoro-uracil sensitivity in human derived colon cancer cells. Int. J. Cancer 2009, 124, 2780–2788. [Google Scholar] [CrossRef]
- Delmas, D. Silymarin and Derivatives: From Biosynthesis to Health Benefits. Molecules 2020, 25, 2415. [Google Scholar] [CrossRef]
- Delmas, D.; Xiao, J.; Vejux, A.; Aires, V. Silymarin and Cancer: A Dual Strategy in Both in Chemoprevention and Chemosensitivity. Molecules 2020, 25, 2009. [Google Scholar] [CrossRef]
- Sioud, F.; Amor, S.; Toumia, I.B.; Lahmar, A.; Aires, V.; Chekir-Ghedira, L.; Delmas, D. A New Highlight of Ephedra alata Decne Properties as Potential Adjuvant in Combination with Cisplatin to Induce Cell Death of 4T1 Breast Cancer Cells In Vitro and In Vivo. Cells 2020, 9, 362. [Google Scholar] [CrossRef]
- Aires, V.; Delmas, D. Common pathways in health benefit properties of RSV in cardiovascular diseases, cancers and degenerative pathologies. Curr. Pharm. Biotechnol. 2015, 16, 219–244. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Madana, R.M.; V, A.K.; Gogoi, R.; Barua, C.C. Chemopreventive and therapeutic potential of chrysin in cancer: Mechanistic perspectives. Toxicol. Lett. 2015, 233, 214–225. [Google Scholar] [CrossRef]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Stevens, J.F.; Revel, J.S.; Maier, C.S. Mitochondria-Centric Review of Polyphenol Bioactivity in Cancer Models. Antioxid Redox Signal 2018, 29, 1589–1611. [Google Scholar] [CrossRef]
- Avila-Carrasco, L.; Majano, P.; Sanchez-Tomero, J.A.; Selgas, R.; Lopez-Cabrera, M.; Aguilera, A.; Gonzalez Mateo, G. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front Pharm. 2019, 10, 715. [Google Scholar] [CrossRef]
- Kuo, M.-L.; Lee, K.-C.; Lin, J.-K. Genotoxicities of nitropyrenes and their modulation by apigenin, tannic acid, ellagic acid and indole-3-carbinol in the Salmonella and CHO systems. Mutat. Res./Fundam. Mol. Mech. Mutagenes. 1992, 270, 87–95. [Google Scholar] [CrossRef]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Youness, R.A.; Kamel, R.; Elkasabgy, N.A.; Shao, P.; Farag, M.A. Recent advances in tannic acid (gallotannin) anticancer activities and drug delivery systems for efficacy improvement; a comprehensive review. Molecules 2021, 26, 1486. [Google Scholar] [CrossRef]
- Pattarayan, D.; Sivanantham, A.; Krishnaswami, V.; Loganathan, L.; Palanichamy, R.; Natesan, S.; Muthusamy, K.; Rajasekaran, S. Tannic acid attenuates TGF-beta1-induced epithelial-to-mesenchymal transition by effectively intervening TGF-beta signaling in lung epithelial cells. J. Cell. Physiol. 2018, 233, 2513–2525. [Google Scholar] [CrossRef]
- Yang, Y.I.; Jung, D.W.; Bai, D.G.; Yoo, G.S.; Choi, J.K. Counterion-dye staining method for DNA in agarose gels using crystal violet and methyl orange. Electrophoresis 2001, 22, 855–859. [Google Scholar] [CrossRef]
- Reid, K.J.; Lang, K.; Froscio, S.; Humpage, A.J.; Young, F.M. Undifferentiated murine embryonic stem cells used to model the effects of the blue–green algal toxin cylindrospermopsin on preimplantation embryonic cell proliferation. Toxicon 2015, 106, 79–88. [Google Scholar] [CrossRef]
- Kwak, Y.; Ju, J. Inhibitory activities of Perilla frutescens britton leaf extract against the growth, migration, and adhesion of human cancer cells. Nutr. Res. Pract. 2015, 9, 11–16. [Google Scholar] [CrossRef]
- Li, S.; Chen, M.; Wu, H.; Li, Y.; Tollefsbol, T.O. Maternal epigenetic regulation contributes to prevention of estrogen receptor–negative mammary cancer with broccoli sprout consumption. Cancer Prev. Res. 2020, 13, 449–462. [Google Scholar] [CrossRef]
- Ottaviano, A.J.; Sun, L.; Ananthanarayanan, V.; Munshi, H.G. Extracellular matrix-mediated membrane-type 1 matrix metalloproteinase expression in pancreatic ductal cells is regulated by transforming growth factor-beta1. Cancer Res. 2006, 66, 7032–7040. [Google Scholar] [CrossRef]
- Ou, J.; Peng, Y.; Deng, J.; Miao, H.; Zhou, J.; Zha, L.; Zhou, R.; Yu, L.; Shi, H.; Liang, H. Endothelial cell-derived fibronectin extra domain A promotes colorectal cancer metastasis via inducing epithelial-mesenchymal transition. Carcinogenesis 2014, 35, 1661–1670. [Google Scholar] [CrossRef]
- Karlsson, S.; Nystrom, H. The extracellular matrix in colorectal cancer and its metastatic settling—Alterations and biological implications. Crit. Rev. Oncol. Hematol. 2022, 175, 103712. [Google Scholar] [CrossRef]
- Cano, A.; Perez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Onder, T.T.; Gupta, P.B.; Mani, S.A.; Yang, J.; Lander, E.S.; Weinberg, R.A. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008, 68, 3645–3654. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Provenzale, D.; Gupta, S.; Ahnen, D.J.; Markowitz, A.J.; Chung, D.C.; Mayer, R.J.; Regenbogen, S.E.; Blanco, A.M.; Bray, T.; Cooper, G.; et al. NCCN Guidelines Insights: Colorectal Cancer Screening, Version 1.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 939–949. [Google Scholar] [CrossRef]
- Escalante, P.I.; Quinones, L.A.; Contreras, H.R. Epithelial-Mesenchymal Transition and MicroRNAs in Colorectal Cancer Chemoresistance to FOLFOX. Pharmaceutics 2021, 13, 75. [Google Scholar] [CrossRef]
- Cheng, M.; Jiang, Y.; Yang, H.; Zhao, D.; Li, L.; Liu, X. FLNA promotes chemoresistance of colorectal cancer through inducing epithelial-mesenchymal transition and smad2 signaling pathway. Am. J. Cancer Res. 2020, 10, 403–423. [Google Scholar]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef]
- Zhou, B.P.; Deng, J.; Xia, W.; Xu, J.; Li, Y.M.; Gunduz, M.; Hung, M.C. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 2004, 6, 931–940. [Google Scholar] [CrossRef]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef]
- Sanchez-Tillo, E.; Lazaro, A.; Torrent, R.; Cuatrecasas, M.; Vaquero, E.C.; Castells, A.; Engel, P.; Postigo, A. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene 2010, 29, 3490–3500. [Google Scholar] [CrossRef]
- Hatami, E.; PK, B.N.; Sikander, M.; Dhasmana, A.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Tannic Acid Exhibits Antiangiogenesis Activity in Nonsmall-Cell Lung Cancer Cells. ACS Omega 2022, 7, 23939–23949. [Google Scholar] [CrossRef]
- Karakurt, S.; Adali, O. Tannic Acid Inhibits Proliferation, Migration, Invasion of Prostate Cancer and Modulates Drug Metabolizing and Antioxidant Enzymes. Anti-Cancer Agents Med. Chem. 2016, 16, 781–789. [Google Scholar] [CrossRef]
- Savagner, P.; Yamada, K.M.; Thiery, J.P. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J. Cell Biol. 1997, 137, 1403–1419. [Google Scholar] [CrossRef]
- Wels, C.; Joshi, S.; Koefinger, P.; Bergler, H.; Schaider, H. Transcriptional activation of ZEB1 by Slug leads to cooperative regulation of the epithelial-mesenchymal transition-like phenotype in melanoma. J. Investig. Dermatol. 2011, 131, 1877–1885. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metast. Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef]
- Comoglio, P.M.; Boccaccio, C.; Trusolino, L. Interactions between growth factor receptors and adhesion molecules: Breaking the rules. Curr. Opin. Cell Biol. 2003, 15, 565–571. [Google Scholar] [CrossRef]
- Booth, B.W.; Inskeep, B.D.; Shah, H.; Park, J.P.; Hay, E.J.; Burg, K.J. Tannic Acid preferentially targets estrogen receptor-positive breast cancer. Int. J. Breast Cancer 2013, 2013, 369609. [Google Scholar] [CrossRef]
- Nie, F.; Liang, Y.; Jiang, B.; Li, X.; Xun, H.; He, W.; Lau, H.T.; Ma, X. Apoptotic effect of tannic acid on fatty acid synthase over-expressed human breast cancer cells. Tumour Biol. 2016, 37, 2137–2143. [Google Scholar] [CrossRef]
- Nagesh, P.K.B.; Hatami, E.; Chowdhury, P.; Kashyap, V.K.; Khan, S.; Hafeez, B.B.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Tannic Acid Induces Endoplasmic Reticulum Stress-Mediated Apoptosis in Prostate Cancer. Cancers 2018, 10, 68. [Google Scholar] [CrossRef]
- Ridley, A.J. Life at the leading edge. Cell 2011, 145, 1012–1022. [Google Scholar] [CrossRef]
- Moustakas, A.; Heldin, C.H. Non-Smad TGF-beta signals. J. Cell Sci. 2005, 118, 3573–3584. [Google Scholar] [CrossRef]
- Makrodouli, E.; Oikonomou, E.; Koc, M.; Andera, L.; Sasazuki, T.; Shirasawa, S.; Pintzas, A. BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: A comparative study. Mol. Cancer 2011, 10, 118. [Google Scholar] [CrossRef]

 ), 5-FU (15 mg/kg,
), 5-FU (15 mg/kg,  ), or TA (15 mg/kg,
), or TA (15 mg/kg,  ), (n = 5 mice per group)), and the treatment was continuing at a rate of one injection every 2 days. The evolution of tumor volumes (mm3) in time was determined. (B) Images of tumors harvested from the mice on day 14 (scale bar = 1 cm). (C) Measurement of serum aspartate transaminase (ASAT), alanine transaminase (ALT), and creatinine levels in Co, 5-FU-, or TA-treated mice. The data are (A) medians ± SEM or (C) means ± SD of 3 independent experiments (n = 3). Statistical significance was determined by one-way ANOVA, followed by Tukey’s test for multiple comparison tests, with ** p < 0.01, *** p < 0.001, and **** p < 0.0001, vs. the corresponding control group.
), (n = 5 mice per group)), and the treatment was continuing at a rate of one injection every 2 days. The evolution of tumor volumes (mm3) in time was determined. (B) Images of tumors harvested from the mice on day 14 (scale bar = 1 cm). (C) Measurement of serum aspartate transaminase (ASAT), alanine transaminase (ALT), and creatinine levels in Co, 5-FU-, or TA-treated mice. The data are (A) medians ± SEM or (C) means ± SD of 3 independent experiments (n = 3). Statistical significance was determined by one-way ANOVA, followed by Tukey’s test for multiple comparison tests, with ** p < 0.01, *** p < 0.001, and **** p < 0.0001, vs. the corresponding control group.
 ), 5-FU (15 mg/kg,
), 5-FU (15 mg/kg,  ), or TA (15 mg/kg,
), or TA (15 mg/kg,  ), (n = 5 mice per group)), and the treatment was continuing at a rate of one injection every 2 days. The evolution of tumor volumes (mm3) in time was determined. (B) Images of tumors harvested from the mice on day 14 (scale bar = 1 cm). (C) Measurement of serum aspartate transaminase (ASAT), alanine transaminase (ALT), and creatinine levels in Co, 5-FU-, or TA-treated mice. The data are (A) medians ± SEM or (C) means ± SD of 3 independent experiments (n = 3). Statistical significance was determined by one-way ANOVA, followed by Tukey’s test for multiple comparison tests, with ** p < 0.01, *** p < 0.001, and **** p < 0.0001, vs. the corresponding control group.
), (n = 5 mice per group)), and the treatment was continuing at a rate of one injection every 2 days. The evolution of tumor volumes (mm3) in time was determined. (B) Images of tumors harvested from the mice on day 14 (scale bar = 1 cm). (C) Measurement of serum aspartate transaminase (ASAT), alanine transaminase (ALT), and creatinine levels in Co, 5-FU-, or TA-treated mice. The data are (A) medians ± SEM or (C) means ± SD of 3 independent experiments (n = 3). Statistical significance was determined by one-way ANOVA, followed by Tukey’s test for multiple comparison tests, with ** p < 0.01, *** p < 0.001, and **** p < 0.0001, vs. the corresponding control group.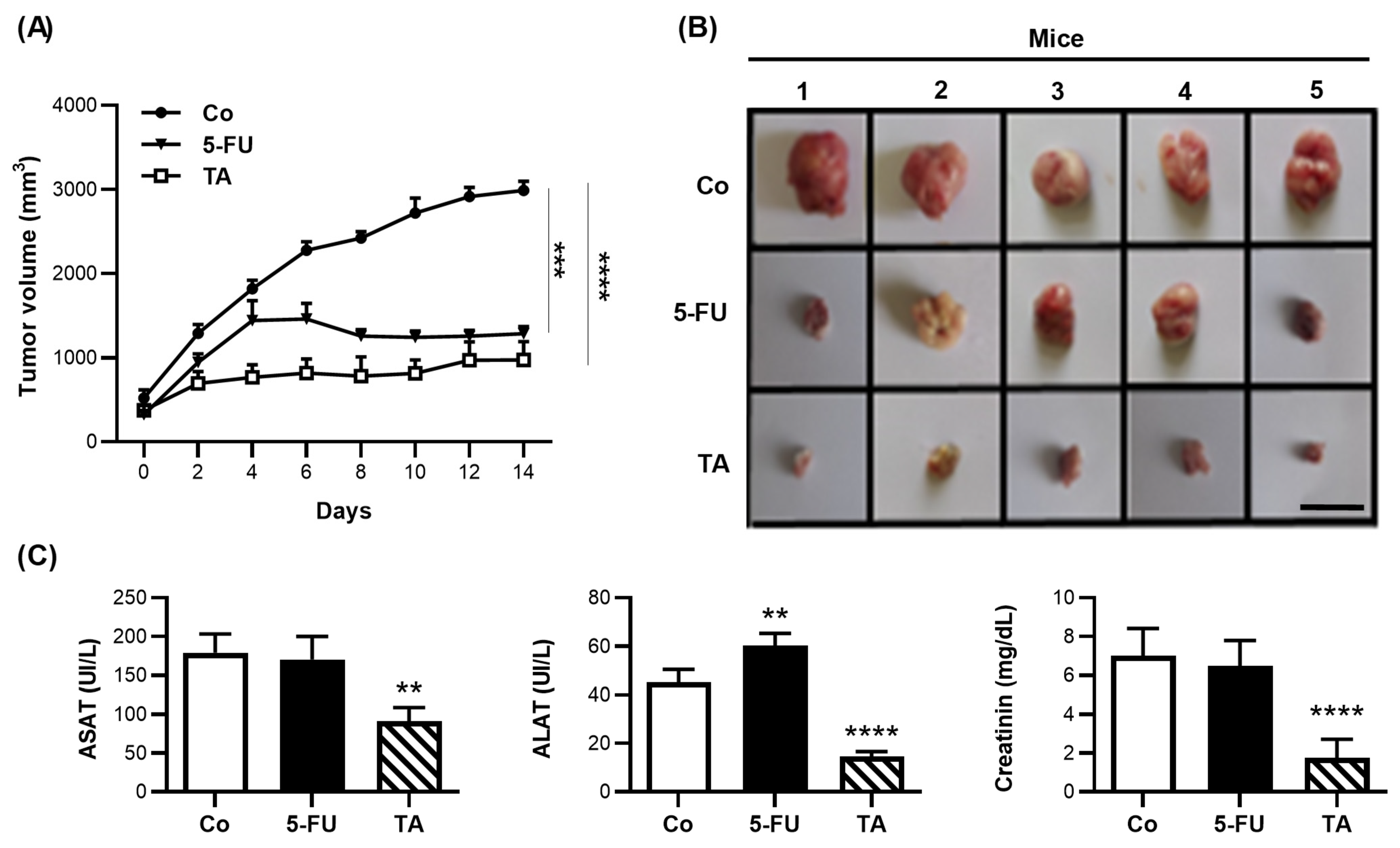
| Time of Treatment (h) | Cell Line | ||
|---|---|---|---|
| CT26 | SW480 | SW620 | |
| 24 | 34.48 ± 1.20 | 48.49 ± 1.68 *** | 88.17 ± 2.77 ****, #### |
| 48 | 11.16 ± 0.14 | 12.16 ± 0.11 $$$ | 44.20 ± 0.20 $$$$, ££££ |
| 72 | 7.61 ± 0.14 | 9.40 ± 0.08 ¥¥¥¥ | 25.75 ± 0.02 ¥¥¥¥, &&&& |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barboura, M.; Cornebise, C.; Hermetet, F.; Guerrache, A.; Selmi, M.; Salek, A.; Chekir-Ghedira, L.; Aires, V.; Delmas, D. Tannic Acid, A Hydrolysable Tannin, Prevents Transforming Growth Factor-β-Induced Epithelial–Mesenchymal Transition to Counteract Colorectal Tumor Growth. Cells 2022, 11, 3645. https://doi.org/10.3390/cells11223645
Barboura M, Cornebise C, Hermetet F, Guerrache A, Selmi M, Salek A, Chekir-Ghedira L, Aires V, Delmas D. Tannic Acid, A Hydrolysable Tannin, Prevents Transforming Growth Factor-β-Induced Epithelial–Mesenchymal Transition to Counteract Colorectal Tumor Growth. Cells. 2022; 11(22):3645. https://doi.org/10.3390/cells11223645
Chicago/Turabian StyleBarboura, Mahassen, Clarisse Cornebise, François Hermetet, Abderrahmane Guerrache, Mouna Selmi, Abir Salek, Leila Chekir-Ghedira, Virginie Aires, and Dominique Delmas. 2022. "Tannic Acid, A Hydrolysable Tannin, Prevents Transforming Growth Factor-β-Induced Epithelial–Mesenchymal Transition to Counteract Colorectal Tumor Growth" Cells 11, no. 22: 3645. https://doi.org/10.3390/cells11223645
APA StyleBarboura, M., Cornebise, C., Hermetet, F., Guerrache, A., Selmi, M., Salek, A., Chekir-Ghedira, L., Aires, V., & Delmas, D. (2022). Tannic Acid, A Hydrolysable Tannin, Prevents Transforming Growth Factor-β-Induced Epithelial–Mesenchymal Transition to Counteract Colorectal Tumor Growth. Cells, 11(22), 3645. https://doi.org/10.3390/cells11223645







