Development and In Vitro Differentiation of Schwann Cells
Abstract
:1. Introduction
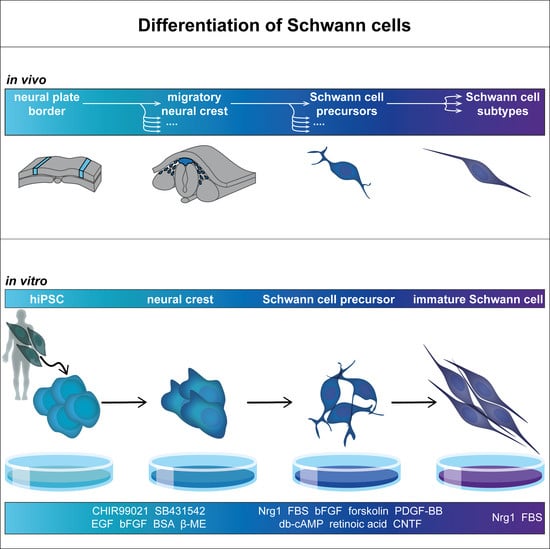
2. In Vivo Development of Schwann Cells
2.1. Development of Neural Crest and Schwann Cell Precursors
2.2. Commitment to Schwann Cell Lineage and Differentiation of Subtypes
3. In Vitro Differentiation of Schwann Cells
3.1. Molecular Mechanisms
3.1.1. Neural Crest Induction
3.1.2. Schwann Cell Specification
3.2. Cell Sources
3.3. Differentiation Protocols
3.3.1. Differentiation from hiPSC and hESC
3.3.2. Adult Tissue-Derived Multipotent Stem Cells
3.3.3. Other Cell Types
4. Characterization
5. Trends in Schwann Cell 3D Culture
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jessen, K.R.; Mirsky, R. Schwann Cell Precursors; Multipotent Glial Cells in Embryonic Nerves. Front. Mol. Neurosci. 2019, 12, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, C.B.; Feltri, M.L.; Wilson, E.R. Peripheral glia diversity. J. Anat. 2021, 241, 1219–1234. [Google Scholar] [CrossRef]
- Gerber, D.; Pereira, J.A.; Gerber, J.; Tan, G.; Dimitrieva, S.; Yángüez, E.; Suter, U. Transcriptional profiling of mouse peripheral nerves to the single-cell level to build a sciatic nerve ATlas (SNAT). eLife 2021, 10, e58591. [Google Scholar] [CrossRef] [PubMed]
- Sardella-Silva, G.; Mietto, B.S.; Ribeiro-Resende, V.T. Four Seasons for Schwann Cell Biology, Revisiting Key Periods: Development, Homeostasis, Repair, and Aging. Biomolecules 2021, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Abdo, H.; Calvo-Enrique, L.; Lopez, J.M.; Song, J.; Zhang, M.-D.; Usoskin, D.; El Manira, A.; Adameyko, I.; Hjerling-Leffler, J.; Ernfors, P. Specialized cutaneous Schwann cells initiate pain sensation. Science 2019, 365, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Rinwa, P.; Calvo-Enrique, L.; Zhang, M.-D.; Nyengaard, J.R.; Karlsson, P.; Ernfors, P. Demise of nociceptive Schwann cells causes nerve retraction and pain hyperalgesia. Pain 2021, 162, 1816–1827. [Google Scholar] [CrossRef]
- Ko, C.-P.; Robitaille, R. Perisynaptic Schwann Cells at the Neuromuscular Synapse: Adaptable, Multitasking Glial Cells. Cold Spring Harb. Perspect. Biol. 2015, 7, a020503. [Google Scholar] [CrossRef]
- Darabid, H.; St-Pierre-See, A.; Robitaille, R. Purinergic-Dependent Glial Regulation of Synaptic Plasticity of Competing Terminals and Synapse Elimination at the Neuromuscular Junction. Cell Rep. 2018, 25, 2070–2082. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.H.; Shurin, G.V.; Khosravi, H.; Kazi, R.; Kruglov, O.; Shurin, M.R.; Bunimovich, Y.L. Immunomodulation by Schwann cells in disease. Cancer Immunol. Immunother. 2020, 69, 245–253. [Google Scholar] [CrossRef]
- Meyer zu Hörste, G.; Hu, W.; Hartung, H.-P.; Lehmann, H.C.; Kieseier, B.C. The immunocompetence of Schwann cells. Muscle Nerve 2008, 37, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Sanchez, J.A.; Pilch, K.S.; van der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef] [Green Version]
- Negro, S.; Pirazzini, M.; Rigoni, M. Models and methods to study Schwann cells. J. Anat. 2022, 241, 1235–1258. [Google Scholar] [CrossRef] [PubMed]
- McGonigal, R.; Campbell, C.I.; Barrie, J.A.; Yao, D.; Cunningham, M.E.; Crawford, C.L.; Rinaldi, S.; Rowan, E.G.; Willison, H.J. Schwann cell nodal membrane disruption triggers bystander axonal degeneration in a Guillain-Barré syndrome mouse model. J. Clin. Investig. 2022, 132, 158524. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Sunada, Y. Schwann Cell and the Pathogenesis of Charcot-Marie-Tooth Disease. Adv. Exp. Med. Biol. 2019, 1190, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Rodella, U.; Negro, S.; Scorzeto, M.; Bergamin, E.; Jalink, K.; Montecucco, C.; Yuki, N.; Rigoni, M. Schwann cells are activated by ATP released from neurons in an in vitro cellular model of Miller Fisher syndrome. Dis. Model. Mech. 2017, 10, 597–603. [Google Scholar] [CrossRef] [Green Version]
- Monje, P.V.; Sant, D.; Wang, G. Phenotypic and Functional Characteristics of Human Schwann Cells as Revealed by Cell-Based Assays and RNA-SEQ. Mol. Neurobiol. 2018, 55, 6637–6660. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.V. The properties of human Schwann cells: Lessons from in vitro culture and transplantation studies. Glia 2020, 68, 797–810. [Google Scholar] [CrossRef]
- Monje, P.V. Schwann Cell Cultures: Biology, Technology and Therapeutics. Cells 2020, 9, 1848. [Google Scholar] [CrossRef]
- Morrissey, T.K.; Levi, A.D.; Nuijens, A.; Sliwkowski, M.X.; Bunge, R.P. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc. Natl. Acad. Sci. USA 1995, 92, 1431–1435. [Google Scholar] [CrossRef]
- Huang, Z.; Powell, R.; Phillips, J.B.; Haastert-Talini, K. Perspective on Schwann Cells Derived from Induced Pluripotent Stem Cells in Peripheral Nerve Tissue Engineering. Cells 2020, 9, 2497. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, H.C.; Höke, A. Use of engineered Schwann cells in peripheral neuropathy: Hopes and hazards. Brain Res. 2016, 1638, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.-S.; Boddeke, E.; Copray, S. Pluripotent stem cells for Schwann cell engineering. Stem Cell Rev. Rep. 2015, 11, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Betters, E.; Charney, R.M.; Garcia-Castro, M.I. Early specification and development of rabbit neural crest cells. Dev. Biol. 2018, 444 (Suppl. S1), 181–192. [Google Scholar] [CrossRef]
- Basch, M.L.; Bronner-Fraser, M.; García-Castro, M.I. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 2006, 441, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.M.; Teillet, M.-A.M. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev. Biol. 1974, 41, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Simões-Costa, M.; Bronner, M.E. Establishing neural crest identity: A gene regulatory recipe. Development 2015, 142, 242–257. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.S.; Charney, R.M.; García-Castro, M.I. Specification and formation of the neural crest: Perspectives on lineage segregation. Genesis 2019, 57, e23276. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Hao, H.; Reynolds, K.; McMahon, M.; Zhou, C.J. Wnt Signaling in Neural Crest Ontogenesis and Oncogenesis. Cells 2019, 8, 1173. [Google Scholar] [CrossRef]
- Lunn, J.S.; Fishwick, K.J.; Halley, P.A.; Storey, K.G. A spatial and temporal map of FGF/Erk1/2 activity and response repertoires in the early chick embryo. Dev. Biol. 2007, 302, 536–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steventon, B.; Araya, C.; Linker, C.; Kuriyama, S.; Mayor, R. Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development 2009, 136, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.M.; Stottmann, R.W.; Choi, M.; Klingensmith, J. Endogenous bone morphogenetic protein antagonists regulate mammalian neural crest generation and survival. Dev. Dyn. 2006, 235, 2507–2520. [Google Scholar] [CrossRef]
- Tribulo, C.; Aybar, M.J.; Nguyen, V.H.; Mullins, M.C.; Mayor, R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development 2003, 130, 6441–6452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchant, L.; Linker, C.; Ruiz, P.; Guerrero, N.; Mayor, R. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev. Biol. 1998, 198, 319–329. [Google Scholar] [CrossRef]
- Noisa, P.; Lund, C.; Kanduri, K.; Lund, R.; Lähdesmäki, H.; Lahesmaa, R.; Lundin, K.; Chokechuwattanalert, H.; Otonkoski, T.; Tuuri, T.; et al. Notch signaling regulates the differentiation of neural crest from human pluripotent stem cells. J. Cell Sci. 2014, 127, 2083–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bellard, M.E.; Ching, W.; Gossler, A.; Bronner-Fraser, M. Disruption of segmental neural crest migration and ephrin expression in delta-1 null mice. Dev. Biol. 2002, 249, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Endo, Y.; Osumi, N.; Wakamatsu, Y. Bimodal functions of Notch-mediated signaling are involved in neural crest formation during avian ectoderm development. Development 2002, 129, 863–873. [Google Scholar] [CrossRef]
- Morrison, S.J.; Perez, S.E.; Qiao, Z.; Verdi, J.M.; Hicks, C.; Weinmaster, G.; Anderson, D.J. Transient Notch Activation Initiates an Irreversible Switch from Neurogenesis to Gliogenesis by Neural Crest Stem Cells. Cell 2000, 101, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Rekler, D.; Kalcheim, C. Completion of neural crest cell production and emigration is regulated by retinoic-acid-dependent inhibition of BMP signaling. eLife 2022, 11, e72723. [Google Scholar] [CrossRef]
- Martínez-Morales, P.L.; Del Diez Corral, R.; Olivera-Martínez, I.; Quiroga, A.C.; Das, R.M.; Barbas, J.A.; Storey, K.G.; Morales, A.V. FGF and retinoic acid activity gradients control the timing of neural crest cell emigration in the trunk. J. Cell Biol. 2011, 194, 489–503. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Le, T.P.; Erhardt, S.; Findley, T.O.; Wang, J. Hippo-Yap Pathway Orchestrates Neural Crest Ontogenesis. Front. Cell Dev. Biol. 2021, 9, 706623. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Nitzan, E.; Kalcheim, C. YAP promotes neural crest emigration through interactions with BMP and Wnt activities. Cell Commun. Signal. 2019, 17, 69. [Google Scholar] [CrossRef] [Green Version]
- Hindley, C.J.; Condurat, A.L.; Menon, V.; Thomas, R.; Azmitia, L.M.; Davis, J.A.; Pruszak, J. The Hippo pathway member YAP enhances human neural crest cell fate and migration. Sci. Rep. 2016, 6, 23208. [Google Scholar] [CrossRef] [Green Version]
- Manderfield, L.J.; Aghajanian, H.; Engleka, K.A.; Lim, L.Y.; Liu, F.; Jain, R.; Li, L.; Olson, E.N.; Epstein, J.A. Hippo signaling is required for Notch-dependent smooth muscle differentiation of neural crest. Development 2015, 142, 2962–2971. [Google Scholar] [CrossRef] [Green Version]
- Dupin, E.; Calloni, G.W.; Coelho-Aguiar, J.M.; Le Douarin, N.M. The issue of the multipotency of the neural crest cells. Dev. Biol. 2018, 444, S47–S59. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, M.; Bhattacharya, D.; Simoes-Costa, M. The molecular basis of neural crest axial identity. Dev. Biol. 2018, 444 (Suppl. S1), 170–180. [Google Scholar] [CrossRef]
- Abzhanov, A.; Tzahor, E.; Lassar, A.B.; Tabin, C.J. Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development 2003, 130, 4567–4579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solovieva, T.; Bronner, M. Schwann cell precursors: Where they come from and where they go. Cells Dev. 2021, 166, 203686. [Google Scholar] [CrossRef]
- Rocha, M.; Beiriger, A.; Kushkowski, E.E.; Miyashita, T.; Singh, N.; Venkataraman, V.; Prince, V.E. From head to tail: Regionalization of the neural crest. Development 2020, 147, dev193888. [Google Scholar] [CrossRef]
- Mehrotra, P.; Tseropoulos, G.; Bronner, M.E.; Andreadis, S.T. Adult tissue-derived neural crest-like stem cells: Sources, regulatory networks, and translational potential. Stem Cells Transl. Med. 2020, 9, 328–341. [Google Scholar] [CrossRef] [Green Version]
- Monk, K.R.; Feltri, M.L.; Taveggia, C. New insights on Schwann cell development. Glia 2015, 63, 1376–1393. [Google Scholar] [CrossRef] [Green Version]
- Le Douarin, N.; Kalcheim, C. The Neural Crest, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999; ISBN 9780511897948. [Google Scholar]
- Fledrich, R.; Kungl, T.; Nave, K.-A.; Stassart, R.M. Axo-glial interdependence in peripheral nerve development. Development 2019, 146, dev151704. [Google Scholar] [CrossRef]
- Furlan, A.; Adameyko, I. Schwann cell precursor: A neural crest cell in disguise? Dev. Biol. 2018, 444 (Suppl. S1), 25–35. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef]
- Leimeroth, R.; Lobsiger, C.; Lüssi, A.; Taylor, V.; Suter, U.; Sommer, L. Membrane-bound neuregulin1 type III actively promotes Schwann cell differentiation of multipotent Progenitor cells. Dev. Biol. 2002, 246, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Meyer, D.; Yamaai, T.; Garratt, A.; Riethmacher-Sonnenberg, E.; Kane, D.; Theill, L.E.; Birchmeier, C. Isoform-specific expression and function of neuregulin. Development 1997, 124, 3575–3586. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Brennan, A.; Liu, N.; Yarden, Y.; Lefkowitz, G.; Mirsky, R.; Jessen, K.R. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat schwann cell precursors. Neuron 1995, 15, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Grinspan, J.B.; Marchionni, M.A.; Reeves, M.; Coulaloglou, M.; Scherer, S.S. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: Neuregulin receptors and the role of neuregulins. J. Neurosci. J. Soc. Neurosci. 1996, 16, 6107–6118. [Google Scholar] [CrossRef] [Green Version]
- Syroid, D.E.; Maycox, P.R.; Burrola, P.G.; Liu, N.; Wen, D.; Lee, K.F.; Lemke, G.; Kilpatrick, T.J. Cell death in the Schwann cell lineage and its regulation by neuregulin. Proc. Natl. Acad. Sci. USA 1996, 93, 9229–9234. [Google Scholar] [CrossRef]
- Kastriti, M.E.; Faure, L.; Von Ahsen, D.; Bouderlique, T.G.; Boström, J.; Solovieva, T.; Jackson, C.; Bronner, M.; Meijer, D.; Hadjab, S.; et al. Schwann cell precursors represent a neural crest-like state with biased multipotency. EMBO J. 2022, 41, e108780. [Google Scholar] [CrossRef]
- Kalcheim, C.; Kumar, D. Cell fate decisions during neural crest ontogeny. Int. J. Dev. Biol. 2017, 61, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Adameyko, I.; Lallemend, F.; Aquino, J.B.; Pereira, J.A.; Topilko, P.; Müller, T.; Fritz, N.; Beljajeva, A.; Mochii, M.; Liste, I.; et al. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 2009, 139, 366–379. [Google Scholar] [CrossRef] [Green Version]
- Muppirala, A.N.; Limbach, L.E.; Bradford, E.F.; Petersen, S.C. Schwann cell development: From neural crest to myelin sheath. Wiley Interdiscip. Rev. Dev. Biol. 2021, 10, e398. [Google Scholar] [CrossRef]
- Joseph, N.M.; Mukouyama, Y.; Mosher, J.T.; Jaegle, M.; Crone, S.A.; Dormand, E.-L.; Lee, K.-F.; Meijer, D.; Anderson, D.J.; Morrison, S.J. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 2004, 131, 5599–5612. [Google Scholar] [CrossRef] [Green Version]
- Dyachuk, V.; Furlan, A.; Shahidi, M.K.; Giovenco, M.; Kaukua, N.; Konstantinidou, C.; Pachnis, V.; Memic, F.; Marklund, U.; Müller, T.; et al. Neurodevelopment. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science 2014, 345, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Medina, I.; Outin, E.; Picard, C.A.; Chettouh, Z.; Dymecki, S.; Consalez, G.G.; Coppola, E.; Brunet, J.-F. Neurodevelopment. Parasympathetic ganglia derive from Schwann cell precursors. Science 2014, 345, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, T.; Nagashimada, M.; Enomoto, H. Neuronal Differentiation in Schwann Cell Lineage Underlies Postnatal Neurogenesis in the Enteric Nervous System. J. Neurosci. 2015, 35, 9879–9888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlan, A.; Dyachuk, V.; Kastriti, M.E.; Calvo-Enrique, L.; Abdo, H.; Hadjab, S.; Chontorotzea, T.; Akkuratova, N.; Usoskin, D.; Kamenev, D.; et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 2017, 357, eaal3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastriti, M.E.; Kameneva, P.; Kamenev, D.; Dyachuk, V.; Furlan, A.; Hampl, M.; Memic, F.; Marklund, U.; Lallemend, F.; Hadjab, S.; et al. Schwann Cell Precursors Generate the Majority of Chromaffin Cells in Zuckerkandl Organ and Some Sympathetic Neurons in Paraganglia. Front. Mol. Neurosci. 2019, 12, 6. [Google Scholar] [CrossRef]
- Kaukua, N.; Shahidi, M.K.; Konstantinidou, C.; Dyachuk, V.; Kaucka, M.; Furlan, A.; An, Z.; Wang, L.; Hultman, I.; Ahrlund-Richter, L.; et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature 2014, 513, 551–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Kamenev, D.; Kaucka, M.; Kastriti, M.E.; Zhou, B.; Artemov, A.V.; Storer, M.; Fried, K.; Adameyko, I.; Dyachuk, V.; et al. Schwann cell precursors contribute to skeletal formation during embryonic development in mice and zebrafish. Proc. Natl. Acad. Sci. USA 2019, 116, 15068–15073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, A.G.; Kameneva, P.; Adameyko, I. The transcriptional portraits of the neural crest at the individual cell level. Semin. Cell Dev. Biol. 2022, in press. [CrossRef]
- Lucas, T.A.; Zhu, L.; Buckwalter, M.S. Spleen glia are a transcriptionally unique glial subtype interposed between immune cells and sympathetic axons. Glia 2021, 69, 1799–1815. [Google Scholar] [CrossRef]
- Barlow-Anacker, A.J.; Fu, M.; Erickson, C.S.; Bertocchini, F.; Gosain, A. Neural Crest Cells Contribute an Astrocyte-like Glial Population to the Spleen. Sci. Rep. 2017, 7, 45645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belle, M.; Godefroy, D.; Couly, G.; Malone, S.A.; Collier, F.; Giacobini, P.; Chédotal, A. Tridimensional Visualization and Analysis of Early Human Development. Cell 2017, 169, 161–173.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessen, K.R.; Brennan, A.; Morgan, L.; Mirsky, R.; Kent, A.; Hashimoto, Y.; Gavrilovic, J. The schwann cell precursor and its fate: A study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron 1994, 12, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Kastriti, M.E.; Adameyko, I. Specification, plasticity and evolutionary origin of peripheral glial cells. Curr. Opin. Neurobiol. 2017, 47, 196–202. [Google Scholar] [CrossRef]
- D’Antonio, M.; Michalovich, D.; Paterson, M.; Droggiti, A.; Woodhoo, A.; Mirsky, R.; Jessen, K.R. Gene profiling and bioinformatic analysis of Schwann cell embryonic development and myelination. Glia 2006, 53, 501–515. [Google Scholar] [CrossRef]
- Buchstaller, J.; Sommer, L.; Bodmer, M.; Hoffmann, R.; Suter, U.; Mantei, N. Efficient isolation and gene expression profiling of small numbers of neural crest stem cells and developing Schwann cells. J. Neurosci. 2004, 24, 2357–2365. [Google Scholar] [CrossRef] [Green Version]
- Wanner, I.B.; Guerra, N.K.; Mahoney, J.; Kumar, A.; Wood, P.M.; Mirsky, R.; Jessen, K.R. Role of N-cadherin in Schwann cell precursors of growing nerves. Glia 2006, 54, 439–459. [Google Scholar] [CrossRef]
- Meier, C.; Parmantier, E.; Brennan, A.; Mirsky, R.; Jessen, K.R. Developing Schwann Cells Acquire the Ability to Survive without Axons by Establishing an Autocrine Circuit Involving Insulin-Like Growth Factor, Neurotrophin-3, and Platelet-Derived Growth Factor-BB. J. Neurosci. 1999, 19, 3847–3859. [Google Scholar] [CrossRef] [Green Version]
- Feltri, M.L.; Poitelon, Y.; Previtali, S.C. How Schwann Cells Sort Axons: New Concepts. Neuroscientist 2016, 22, 252–265. [Google Scholar] [CrossRef]
- Woodhoo, A.; Alonso, M.B.D.; Droggiti, A.; Turmaine, M.; D’Antonio, M.; Parkinson, D.B.; Wilton, D.K.; Al-Shawi, R.; Simons, P.; Shen, J.; et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat. Neurosci. 2009, 12, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Stewart, H.J.; Morgan, L.; Jessen, K.R.; Mirsky, R. Changes in DNA synthesis rate in the Schwann cell lineage in vivo are correlated with the precursor—Schwann cell transition and myelination. Eur. J. Neurosci. 1993, 5, 1136–1144. [Google Scholar] [CrossRef]
- Brown, M. Schwann cell proliferation in the postnatal mouse: Timing and topography. Exp. Neurol. 1981, 74, 170–186. [Google Scholar] [CrossRef]
- Barik, A.; Li, L.; Sathyamurthy, A.; Xiong, W.-C.; Mei, L. Schwann Cells in Neuromuscular Junction Formation and Maintenance. J. Neurosci. 2016, 36, 9770–9781. [Google Scholar] [CrossRef] [Green Version]
- Georgiou, J.; Charlton, M.P. Non-myelin-forming perisynaptic Schwann cells express protein zero and myelin-associated glycoprotein. Glia 1999, 27, 101–109. [Google Scholar] [CrossRef]
- Suazo, I.; Vega, J.A.; García-Mesa, Y.; García-Piqueras, J.; García-Suárez, O.; Cobo, T. The Lamellar Cells of Vertebrate Meissner and Pacinian Corpuscles: Development, Characterization, and Functions. Front. Neurosci. 2022, 16, 790130. [Google Scholar] [CrossRef]
- Etxaniz, U.; Pérez-San Vicente, A.; Gago-López, N.; García-Dominguez, M.; Iribar, H.; Aduriz, A.; Pérez-López, V.; Burgoa, I.; Irizar, H.; Muñoz-Culla, M.; et al. Neural-competent cells of adult human dermis belong to the Schwann lineage. Stem Cell Rep. 2014, 3, 774–788. [Google Scholar] [CrossRef]
- Taveggia, C.; Zanazzi, G.; Petrylak, A.; Yano, H.; Rosenbluth, J.; Einheber, S.; Xu, X.; Esper, R.M.; Loeb, J.A.; Shrager, P.; et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 2005, 47, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Petersen, S.C.; Luo, R.; Liebscher, I.; Giera, S.; Jeong, S.-J.; Mogha, A.; Ghidinelli, M.; Feltri, M.L.; Schöneberg, T.; Piao, X.; et al. The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron 2015, 85, 755–769. [Google Scholar] [CrossRef] [Green Version]
- Mogha, A.; Benesh, A.E.; Patra, C.; Engel, F.B.; Schöneberg, T.; Liebscher, I.; Monk, K.R. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J. Neurosci. 2013, 33, 17976–17985. [Google Scholar] [CrossRef] [Green Version]
- Monk, K.R.; Oshima, K.; Jörs, S.; Heller, S.; Talbot, W.S. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development 2011, 138, 2673–2680. [Google Scholar] [CrossRef] [Green Version]
- Monk, K.R.; Naylor, S.G.; Glenn, T.D.; Mercurio, S.; Perlin, J.R.; Dominguez, C.; Moens, C.B.; Talbot, W.S. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science 2009, 325, 1402–1405. [Google Scholar] [CrossRef] [Green Version]
- Topilko, P.; Schneider-Maunoury, S.; Levi, G.; Baron-Van Evercooren, A.; Chennoufi, A.B.; Seitanidou, T.; Babinet, C.; Charnay, P. Krox-20 controls myelination in the peripheral nervous system. Nature 1994, 371, 796–799. [Google Scholar] [CrossRef]
- Glenn, T.D.; Talbot, W.S. Analysis of Gpr126 function defines distinct mechanisms controlling the initiation and maturation of myelin. Development 2013, 140, 3167–3175. [Google Scholar] [CrossRef] [Green Version]
- Michailov, G.V.; Sereda, M.W.; Brinkmann, B.G.; Fischer, T.M.; Haug, B.; Birchmeier, C.; Role, L.; Lai, C.; Schwab, M.H.; Nave, K.-A. Axonal neuregulin-1 regulates myelin sheath thickness. Science 2004, 304, 700–703. [Google Scholar] [CrossRef] [Green Version]
- Faroni, A.; Castelnovo, L.F.; Procacci, P.; Caffino, L.; Fumagalli, F.; Melfi, S.; Gambarotta, G.; Bettler, B.; Wrabetz, L.; Magnaghi, V. Deletion of GABA-B receptor in Schwann cells regulates remak bundles and small nociceptive C-fibers. Glia 2014, 62, 548–565. [Google Scholar] [CrossRef]
- Procacci, P.; Ballabio, M.; Castelnovo, L.F.; Mantovani, C.; Magnaghi, V. GABA-B receptors in the PNS have a role in Schwann cells differentiation? Front. Cell. Neurosci. 2012, 6, 68. [Google Scholar] [CrossRef]
- Magnaghi, V.; Ballabio, M.; Cavarretta, I.T.R.; Froestl, W.; Lambert, J.J.; Zucchi, I.; Melcangi, R.C. GABAB receptors in Schwann cells influence proliferation and myelin protein expression. Eur. J. Neurosci. 2004, 19, 2641–2649. [Google Scholar] [CrossRef]
- Milichko, V.; Dyachuk, V. Novel Glial Cell Functions: Extensive Potency, Stem Cell-Like Properties, and Participation in Regeneration and Transdifferentiation. Front. Cell Dev. Biol. 2020, 8, 809. [Google Scholar] [CrossRef] [PubMed]
- Boerboom, A.; Dion, V.; Chariot, A.; Franzen, R. Molecular Mechanisms Involved in Schwann Cell Plasticity. Front. Mol. Neurosci. 2017, 10, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stierli, S.; Napoli, I.; White, I.J.; Cattin, A.-L.; Monteza Cabrejos, A.; Garcia Calavia, N.; Malong, L.; Ribeiro, S.; Nihouarn, J.; Williams, R.; et al. The regulation of the homeostasis and regeneration of peripheral nerve is distinct from the CNS and independent of a stem cell population. Development 2018, 145, dev170316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguayo, A.J.; Epps, J.; Charron, L.; Bray, G.M. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: Quantitative microscopy and radioautography. Brain Res. 1976, 104, 1–20. [Google Scholar] [CrossRef] [PubMed]
- El-Nachef, W.N.; Bronner, M.E. De novo enteric neurogenesis in post-embryonic zebrafish from Schwann cell precursors rather than resident cell types. Development 2020, 147, dev186619. [Google Scholar] [CrossRef]
- Fröb, F.; Wegner, M. The role of chromatin remodeling complexes in Schwann cell development. Glia 2020, 68, 1596–1603. [Google Scholar] [CrossRef] [Green Version]
- Jacob, C. Chromatin-remodeling enzymes in control of Schwann cell development, maintenance and plasticity. Curr. Opin. Neurobiol. 2017, 47, 24–30. [Google Scholar] [CrossRef]
- Ma, K.H.; Hung, H.A.; Svaren, J. Epigenomic Regulation of Schwann Cell Reprogramming in Peripheral Nerve Injury. J. Neurosci. 2016, 36, 9135–9147. [Google Scholar] [CrossRef]
- Liu, Q.; Spusta, S.C.; Mi, R.; Lassiter, R.N.T.; Stark, M.R.; Höke, A.; Rao, M.S.; Zeng, X. Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: Induction, maintenance, and differentiation into functional schwann cells. Stem Cells Transl. Med. 2012, 1, 266–278. [Google Scholar] [CrossRef]
- Ziegler, L.; Grigoryan, S.; Yang, I.H.; Thakor, N.V.; Goldstein, R.S. Efficient generation of schwann cells from human embryonic stem cell-derived neurospheres. Stem Cell Rev. Rep. 2011, 7, 394–403. [Google Scholar] [CrossRef]
- Jiang, X.; Gwye, Y.; McKeown, S.J.; Bronner-Fraser, M.; Lutzko, C.; Lawlor, E.R. Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2009, 18, 1059–1070. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Kim, H.; Elkabetz, Y.; Al Shamy, G.; Panagiotakos, G.; Barberi, T.; Tabar, V.; Studer, L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat. Biotechnol. 2007, 25, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Pomp, O.; Brokhman, I.; Ben-Dor, I.; Reubinoff, B.; Goldstein, R.S. Generation of peripheral sensory and sympathetic neurons and neural crest cells from human embryonic stem cells. Stem Cells 2005, 23, 923–930. [Google Scholar] [CrossRef]
- Shi, L.; Huang, L.; He, R.; Huang, W.; Wang, H.; Lai, X.; Zou, Z.; Sun, J.; Ke, Q.; Zheng, M.; et al. Modeling the Pathogenesis of Charcot-Marie-Tooth Disease Type 1A Using Patient-Specific iPSCs. Stem Cell Rep. 2018, 10, 120–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-W.; Huang, W.-C.; Qiu, X.; Da Fernandes Ferreira Silva, F.; Wang, A.; Patel, S.; Nesti, L.J.; Poo, M.-M.; Li, S. The Differentiation Stage of Transplanted Stem Cells Modulates Nerve Regeneration. Sci. Rep. 2017, 7, 17401. [Google Scholar] [CrossRef] [Green Version]
- Hörner, S.J.; Couturier, N.; Bruch, R.; Koch, P.; Hafner, M.; Rudolf, R. hiPSC-Derived Schwann Cells Influence Myogenic Differentiation in Neuromuscular Cocultures. Cells 2021, 10, 3292. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee-Clavin, B.; Mi, R.; Kern, B.; Choi, I.Y.; Lim, H.; Oh, Y.; Lannon, B.; Kim, K.J.; Bell, S.; Hur, J.K.; et al. Comparison of three congruent patient-specific cell types for the modelling of a human genetic Schwann-cell disorder. Nat. Biomed. Eng. 2019, 3, 571–582. [Google Scholar] [CrossRef]
- Muller, Q.; Beaudet, M.-J.; De Serres-Bérard, T.; Bellenfant, S.; Flacher, V.; Berthod, F. Development of an innervated tissue-engineered skin with human sensory neurons and Schwann cells differentiated from iPS cells. Acta Biomater. 2018, 82, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Lee, J.; Da Lee, Y.; Kim, Y.-D.; Kim, J.Y.; Lim, H.J.; Lim, S.; Cho, Y.S. Schwann Cell Precursors from Human Pluripotent Stem Cells as a Potential Therapeutic Target for Myelin Repair. Stem Cell Rep. 2017, 8, 1714–1726. [Google Scholar] [CrossRef]
- Elkouby, Y.M.; Frank, D. Wnt/β-Catenin Signaling in Vertebrate Posterior Neural Development; Biota Publishing: San Rafael, CA, USA, 2010; ISBN 9781615040544. [Google Scholar]
- Kiecker, C.; Niehrs, C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 2001, 128, 4189–4201. [Google Scholar] [CrossRef] [PubMed]
- Gomez, G.A.; Prasad, M.S.; Sandhu, N.; Shelar, P.B.; Leung, A.W.; García-Castro, M.I. Human neural crest induction by temporal modulation of WNT activation. Dev. Biol. 2019, 449, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Leung, A.W.; Murdoch, B.; Salem, A.F.; Prasad, M.S.; Gomez, G.A.; García-Castro, M.I. WNT/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate. Development 2016, 143, 398–410. [Google Scholar] [CrossRef] [Green Version]
- Mica, Y.; Lee, G.; Chambers, S.M.; Tomishima, M.J.; Studer, L. Modeling neural crest induction, melanocyte specification, and disease-related pigmentation defects in hESCs and patient-specific iPSCs. Cell Rep. 2013, 3, 1140–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menendez, L.; Kulik, M.J.; Page, A.T.; Park, S.S.; Lauderdale, J.D.; Cunningham, M.L.; Dalton, S. Directed differentiation of human pluripotent cells to neural crest stem cells. Nat. Protoc. 2013, 8, 203–212. [Google Scholar] [CrossRef]
- Chambers, S.M.; Qi, Y.; Mica, Y.; Lee, G.; Zhang, X.-J.; Niu, L.; Bilsland, J.; Cao, L.; Stevens, E.; Whiting, P.; et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat. Biotechnol. 2012, 30, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Menendez, L.; Yatskievych, T.A.; Antin, P.B.; Dalton, S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc. Natl. Acad. Sci. USA 2011, 108, 19240–19245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrió, M.; Mazuelas, H.; Richaud-Patin, Y.; Gel, B.; Terribas, E.; Rosas, I.; Jimenez-Delgado, S.; Biayna, J.; Vendredy, L.; Blanco, I.; et al. Reprogramming Captures the Genetic and Tumorigenic Properties of Neurofibromatosis Type 1 Plexiform Neurofibromas. Stem Cell Rep. 2019, 12, 411–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pla, P.; Monsoro-Burq, A.H. The neural border: Induction, specification and maturation of the territory that generates neural crest cells. Dev. Biol. 2018, 444 (Suppl. S1), 36–46. [Google Scholar] [CrossRef]
- Groves, A.K.; LaBonne, C. Setting appropriate boundaries: Fate, patterning and competence at the neural plate border. Dev. Biol. 2014, 389, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Kreitzer, F.R.; Salomonis, N.; Sheehan, A.; Huang, M.; Park, J.S.; Spindler, M.J.; Lizarraga, P.; Weiss, W.A.; So, P.-L.; Conklin, B.R. A robust method to derive functional neural crest cells from human pluripotent stem cells. Am. J. Stem Cells 2013, 2, 119–131. [Google Scholar]
- Huang, M.; Miller, M.L.; McHenry, L.K.; Zheng, T.; Zhen, Q.; Ilkhanizadeh, S.; Conklin, B.R.; Bronner, M.E.; Weiss, W.A. Generating trunk neural crest from human pluripotent stem cells. Sci. Rep. 2016, 6, 19727. [Google Scholar] [CrossRef] [Green Version]
- Avery, J.; Dalton, S. Methods for Derivation of Multipotent Neural Crest Cells Derived from Human Pluripotent Stem Cells. Methods Mol. Biol. 2016, 1341, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Fukuta, M.; Nakai, Y.; Kirino, K.; Nakagawa, M.; Sekiguchi, K.; Nagata, S.; Matsumoto, Y.; Yamamoto, T.; Umeda, K.; Heike, T.; et al. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS ONE 2014, 9, e112291. [Google Scholar] [CrossRef] [Green Version]
- Denham, M.; Hasegawa, K.; Menheniott, T.; Rollo, B.; Zhang, D.; Hough, S.; Alshawaf, A.; Febbraro, F.; Ighaniyan, S.; Leung, J.; et al. Multipotent caudal neural progenitors derived from human pluripotent stem cells that give rise to lineages of the central and peripheral nervous system. Stem Cells 2015, 33, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Cimadamore, F.; Fishwick, K.; Giusto, E.; Gnedeva, K.; Cattarossi, G.; Miller, A.; Pluchino, S.; Brill, L.M.; Bronner-Fraser, M.; Terskikh, A.V. Human ESC-derived neural crest model reveals a key role for SOX2 in sensory neurogenesis. Cell Stem Cell 2011, 8, 538–551. [Google Scholar] [CrossRef] [Green Version]
- Hackland, J.O.S.; Frith, T.J.R.; Thompson, O.; Marin Navarro, A.; Garcia-Castro, M.I.; Unger, C.; Andrews, P.W. Top-Down Inhibition of BMP Signaling Enables Robust Induction of hPSCs Into Neural Crest in Fully Defined, Xeno-free Conditions. Stem Cell Rep. 2017, 9, 1043–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackland, J. Top-Down Inhibition (TDi) and Baseline Activation (BLa): Controlling Signal Transduction When Endogenous Cytokines are Ruining Your Differentiation. Curr. Protoc. Stem Cell Biol. 2019, 51, e98. [Google Scholar] [CrossRef]
- Wang, A.; Tang, Z.; Park, I.-H.; Zhu, Y.; Patel, S.; Daley, G.Q.; Li, S. Induced pluripotent stem cells for neural tissue engineering. Biomaterials 2011, 32, 5023–5032. [Google Scholar] [CrossRef] [Green Version]
- Cooper, F.; Gentsch, G.E.; Mitter, R.; Bouissou, C.; Healy, L.E.; Rodriguez, A.H.; Smith, J.C.; Bernardo, A.S. Rostrocaudal patterning and neural crest differentiation of human pre-neural spinal cord progenitors in vitro. Stem Cell Rep. 2022, 17, 894–910. [Google Scholar] [CrossRef]
- Gomez, G.A.; Prasad, M.S.; Wong, M.; Charney, R.M.; Shelar, P.B.; Sandhu, N.; Hackland, J.O.S.; Hernandez, J.C.; Leung, A.W.; García-Castro, M.I. WNT/β-catenin modulates the axial identity of embryonic stem cell-derived human neural crest. Development 2019, 146, dev175604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackland, J.O.S.; Shelar, P.B.; Sandhu, N.; Prasad, M.S.; Charney, R.M.; Gomez, G.A.; Frith, T.J.R.; García-Castro, M.I. FGF Modulates the Axial Identity of Trunk hPSC-Derived Neural Crest but Not the Cranial-Trunk Decision. Stem Cell Rep. 2019, 12, 920–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frith, T., Jr.; Granata, I.; Wind, M.; Stout, E.; Thompson, O.; Neumann, K.; Stavish, D.; Heath, P.R.; Ortmann, D.; Hackland, J.O.; et al. Human axial progenitors generate trunk neural crest cells in vitro. eLife 2018, 7, e35786. [Google Scholar] [CrossRef]
- Cooper, F.; Tsakiridis, A. Shaping axial identity during human pluripotent stem cell differentiation to neural crest cells. Biochem. Soc. Trans. 2022, 50, 499–511. [Google Scholar] [CrossRef]
- Wilson, E.R.; Della-Flora Nunes, G.; Weaver, M.R.; Frick, L.R.; Feltri, M.L. Schwann cell interactions during the development of the peripheral nervous system. Dev. Neurobiol. 2020, 81, 464–489. [Google Scholar] [CrossRef]
- Smith-Thomas, L.C.; Fawcett, J.W. Expression of Schwann cell markers by mammalian neural crest cells in vitro. Development 1989, 105, 251–262. [Google Scholar] [CrossRef]
- Lyons, S.A.; Morell, P.; McCarthy, K.D. Schwann cell ATP-mediated calcium increases in vitro and in situ are dependent on contact with neurons. Glia 1995, 13, 27–38. [Google Scholar] [CrossRef]
- Mirsky, R.; Dubois, C.; Morgan, L.; Jessen, K.R. 04 and A007-sulfatide antibodies bind to embryonic Schwann cells prior to the appearance of galactocerebroside; regulation of the antigen by axon-Schwann cell signals and cyclic AMP. Development 1990, 109, 105–116. [Google Scholar] [CrossRef]
- Lemke, G.; Chao, M. Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development 1988, 102, 499–504. [Google Scholar] [CrossRef]
- Stemple, D.L.; Anderson, D.J. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell 1992, 71, 973–985. [Google Scholar] [CrossRef]
- Aquino, J.B.; Hjerling-Leffler, J.; Koltzenburg, M.; Edlund, T.; Villar, M.J.; Ernfors, P. In vitro and in vivo differentiation of boundary cap neural crest stem cells into mature Schwann cells. Exp. Neurol. 2006, 198, 438–449. [Google Scholar] [CrossRef]
- Dong, Z.; Sinanan, A.; Parkinson, D.; Parmantier, E.; Mirsky, R.; Jessen, K.R. Schwann cell development in embryonic mouse nerves. J. Neurosci. Res. 1999, 56, 334–348. [Google Scholar] [CrossRef]
- Shah, N.M.; Marchionni, M.A.; Isaacs, I.; Stroobant, P.; Anderson, P.J. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell 1994, 77, 349–360. [Google Scholar] [CrossRef]
- Lemke, G.E.; Brockes, J.P. Identification and purification of glial growth factor. J. Neurosci. 1984, 4, 75–83. [Google Scholar] [CrossRef]
- Birchmeier, C.; Nave, K.-A. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 2008, 56, 1491–1497. [Google Scholar] [CrossRef]
- Meintanis, S.; Thomaidou, D.; Jessen, K.R.; Mirsky, R.; Matsas, R. The neuron-glia signal beta-neuregulin promotes Schwann cell motility via the MAPK pathway. Glia 2001, 34, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Garratt, A.N.; Voiculescu, O.; Topilko, P.; Charnay, P.; Birchmeier, C. A dual role of erbB2 in myelination and in expansion of the schwann cell precursor pool. J. Cell Biol. 2000, 148, 1035–1046. [Google Scholar] [CrossRef] [Green Version]
- Maurel, P.; Salzer, J.L. Axonal Regulation of Schwann Cell Proliferation and Survival and the Initial Events of Myelination Requires PI 3-Kinase Activity. J. Neurosci. 2000, 20, 4635–4645. [Google Scholar] [CrossRef] [Green Version]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef]
- Dezawa, M.; Takahashi, I.; Esaki, M.; Takano, M.; Sawada, H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur. J. Neurosci. 2001, 14, 1771–1776. [Google Scholar] [CrossRef]
- Monje, P.V.; Rendon, S.; Athauda, G.; Bates, M.; Wood, P.M.; Bunge, M.B. Non-antagonistic relationship between mitogenic factors and cAMP in adult Schwann cell re-differentiation. Glia 2009, 57, 947–961. [Google Scholar] [CrossRef] [Green Version]
- Fregien, N.L.; White, L.A.; Bunge, M.B.; Wood, P.M. Forskolin increases neuregulin receptors in human Schwann cells without increasing receptor mRNA. Glia 2005, 49, 24–35. [Google Scholar] [CrossRef]
- Meyer-Franke, A.; Wilkinson, G.A.; Kruttgen, A.; Hu, M.; Munro, E.; Hanson, M.G.; Reichardt, L.F.; Barres, B.A. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron 1998, 21, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Bacallao, K.; Monje, P.V. Requirement of cAMP signaling for Schwann cell differentiation restricts the onset of myelination. PLoS ONE 2015, 10, e0116948. [Google Scholar] [CrossRef] [Green Version]
- Jessen, K.R.; Mirsky, R.; Morgan, L. Role of cyclic AMP and proliferation controls in Schwann cell differentiation. Ann. N. Y. Acad. Sci. 1991, 633, 78–89. [Google Scholar] [CrossRef]
- Arthur-Farraj, P.; Wanek, K.; Hantke, J.; Davis, C.M.; Jayakar, A.; Parkinson, D.B.; Mirsky, R.; Jessen, K.R. Mouse schwann cells need both NRG1 and cyclic AMP to myelinate. Glia 2011, 59, 720–733. [Google Scholar] [CrossRef] [Green Version]
- Huff, T.C.; Sant, D.W.; Camarena, V.; van Booven, D.; Andrade, N.S.; Mustafi, S.; Monje, P.V.; Wang, G. Vitamin C regulates Schwann cell myelination by promoting DNA demethylation of pro-myelinating genes. J. Neurochem. 2021, 157, 1759–1773. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, C.F.; Bunge, M.B.; Bunge, R.P.; Wood, P.M. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J. Cell Biol. 1987, 105, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- Wakao, S.; Matsuse, D.; Dezawa, M. Mesenchymal stem cells as a source of Schwann cells: Their anticipated use in peripheral nerve regeneration. Cells Tissues Organs 2014, 200, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Matsuse, D.; Kitada, M.; Kohama, M.; Nishikawa, K.; Makinoshima, H.; Wakao, S.; Fujiyoshi, Y.; Heike, T.; Nakahata, T.; Akutsu, H.; et al. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J. Neuropathol. Exp. Neurol. 2010, 69, 973–985. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Cheng, H.-Y.; Zhang, L.; Fan, J.; Chen, Y.-Z.; Jiang, X.-D.; Xu, R.-X. Umbilical cord blood cell-derived neurospheres differentiate into Schwann-like cells. Neuroreport 2009, 20, 354–359. [Google Scholar] [CrossRef]
- Latasa, M.-J.; Jiménez-Lara, A.M.; Cosgaya, J.M. Retinoic acid regulates Schwann cell migration via NEDD9 induction by transcriptional and post-translational mechanisms. Biochim. Biophys. Acta 2016, 1863, 1510–1518. [Google Scholar] [CrossRef]
- Latasa, M.-J.; Ituero, M.; Moran-Gonzalez, A.; Aranda, A.; Cosgaya, J.M. Retinoic acid regulates myelin formation in the peripheral nervous system. Glia 2010, 58, 1451–1464. [Google Scholar] [CrossRef]
- Mazzara, P.G.; Massimino, L.; Pellegatta, M.; Ronchi, G.; Ricca, A.; Iannielli, A.; Giannelli, S.G.; Cursi, M.; Cancellieri, C.; Sessa, A.; et al. Two factor-based reprogramming of rodent and human fibroblasts into Schwann cells. Nat. Commun. 2017, 8, 14088. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.B.; Stroobant, P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J. Cell Biol. 1990, 110, 1353–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodhoo, A.; Dean, C.H.; Droggiti, A.; Mirsky, R.; Jessen, K.R. The trunk neural crest and its early glial derivatives: A study of survival responses, developmental schedules and autocrine mechanisms. Mol. Cell. Neurosci. 2004, 25, 30–41. [Google Scholar] [CrossRef]
- Lobsiger, C.S.; Schweitzer, B.; Taylor, V.; Suter, U. Platelet-derived growth factor-BB supports the survival of cultured rat schwann cell precursors in synergy with neurotrophin-3. Glia 2000, 30, 290–300. [Google Scholar] [CrossRef]
- Sendtner, M.; Arakawa, Y.; Stöckli, K.A.; Kreutzberg, G.W.; Thoenen, H. Effect of ciliary neurotrophic factor (CNTF) on motoneuron survival. J. Cell Sci. Suppl. 1991, 15, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Stankoff, B.; Aigrot, M.-S.; Noël, F.; Wattilliaux, A.; Zalc, B.; Lubetzki, C. Ciliary Neurotrophic Factor (CNTF) Enhances Myelin Formation: A Novel Role for CNTF and CNTF-Related Molecules. J. Neurosci. 2002, 22, 9221–9227. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A.; Burne, J.F.; Holtmann, B.; Thoenen, H.; Sendtner, M.; Raff, M.C. Ciliary Neurotrophic Factor Enhances the Rate of Oligodendrocyte Generation. Mol. Cell. Neurosci. 1996, 8, 146–156. [Google Scholar] [CrossRef]
- Mayer, M.; Bhakoo, K.; Noble, M. Ciliary neurotrophic factor and leukemia inhibitory factor promote the generation, maturation and survival of oligodendrocytes in vitro. Development 1994, 120, 143–153. [Google Scholar] [CrossRef]
- Murphy, P.; Topilko, P.; Schneider-Maunoury, S.; Seitanidou, T.; Baron-Van Evercooren, A.; Charnay, P. The regulation of Krox-20 expression reveals important steps in the control of peripheral glial cell development. Development 1996, 122, 2847–2857. [Google Scholar] [CrossRef]
- Najafabadi, M.M.; Bayati, V.; Orazizadeh, M.; Hashemitabar, M.; Absalan, F. Impact of Cell Density on Differentiation Efficiency of Rat Adipose-derived Stem Cells into Schwann-like Cells. Int. J. Stem Cells 2016, 9, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Piovesana, R.; Pisano, A.; Loreti, S.; Ricordy, R.; Talora, C.; Tata, A.M. Notch Signal Mediates the Cross-Interaction between M2 Muscarinic Acetylcholine Receptor and Neuregulin/ErbB Pathway: Effects on Schwann Cell Proliferation. Biomolecules 2022, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Jeanette, H.; Marziali, L.N.; Bhatia, U.; Hellman, A.; Herron, J.; Kopec, A.M.; Feltri, M.L.; Poitelon, Y.; Belin, S. YAP and TAZ regulate Schwann cell proliferation and differentiation during peripheral nerve regeneration. Glia 2021, 69, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Castelnovo, L.F.; Bonalume, V.; Melfi, S.; Ballabio, M.; Colleoni, D.; Magnaghi, V. Schwann cell development, maturation and regeneration: A focus on classic and emerging intracellular signaling pathways. Neural Regen. Res. 2017, 12, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Grove, M.; Kim, H.; Santerre, M.; Krupka, A.J.; Han, S.B.; Zhai, J.; Cho, J.Y.; Park, R.; Harris, M.; Kim, S.; et al. YAP/TAZ initiate and maintain Schwann cell myelination. eLife 2017, 6, e20982. [Google Scholar] [CrossRef] [PubMed]
- Poitelon, Y.; Lopez-Anido, C.; Catignas, K.; Berti, C.; Palmisano, M.; Williamson, C.; Ameroso, D.; Abiko, K.; Hwang, Y.; Gregorieff, A.; et al. YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat. Neurosci. 2016, 19, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Colciago, A.; Melfi, S.; Giannotti, G.; Bonalume, V.; Ballabio, M.; Caffino, L.; Fumagalli, F.; Magnaghi, V. Tumor suppressor Nf2/merlin drives Schwann cell changes following electromagnetic field exposure through Hippo-dependent mechanisms. Cell Death Discov. 2015, 1, 15021. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Orkwis, J.A.; Harris, G.M. Cell Shape and Matrix Stiffness Impact Schwann Cell Plasticity via YAP/TAZ and Rho GTPases. Int. J. Mol. Sci. 2021, 22, 4821. [Google Scholar] [CrossRef]
- Belin, S.; Zuloaga, K.L.; Poitelon, Y. Influence of Mechanical Stimuli on Schwann Cell Biology. Front. Cell. Neurosci. 2017, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-M.; Chen, Z.-L.; North, A.J.; Strickland, S. Laminin is required for Schwann cell morphogenesis. J. Cell Sci. 2009, 122, 929–936. [Google Scholar] [CrossRef] [Green Version]
- McKee, K.K.; Yang, D.-H.; Patel, R.; Chen, Z.-L.; Strickland, S.; Takagi, J.; Sekiguchi, K.; Yurchenco, P.D. Schwann cell myelination requires integration of laminin activities. J. Cell Sci. 2012, 125, 4609–4619. [Google Scholar] [CrossRef] [Green Version]
- Ghidinelli, M.; Poitelon, Y.; Shin, Y.K.; Ameroso, D.; Williamson, C.; Ferri, C.; Pellegatta, M.; Espino, K.; Mogha, A.; Monk, K.; et al. Laminin 211 inhibits protein kinase A in Schwann cells to modulate neuregulin 1 type III-driven myelination. PLoS Biol. 2017, 15, e2001408. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.; Muguruma, K.; et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef]
- Maldonado, M.; Luu, R.J.; Ramos, M.E.P.; Nam, J. ROCK inhibitor primes human induced pluripotent stem cells to selectively differentiate towards mesendodermal lineage via epithelial-mesenchymal transition-like modulation. Stem Cell Res. 2016, 17, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.; Wen, J.; Li, L.; Wang, X.; Qian, C.; Pan, M.; Lai, M.; Deng, J.; Hu, X.; Zhang, H.; et al. Inhibition of RhoA-Subfamily GTPases Suppresses Schwann Cell Proliferation Through Regulating AKT Pathway Rather Than ROCK Pathway. Front. Cell. Neurosci. 2018, 12, 437. [Google Scholar] [CrossRef] [Green Version]
- Saulite, L.; Vavers, E.; Zvejniece, L.; Dambrova, M.; Riekstina, U. The Differentiation of Skin Mesenchymal Stem Cells Towards a Schwann Cell Phenotype: Impact of Sigma-1 Receptor Activation. Mol. Neurobiol. 2018, 55, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Tsui, Y.-P.; Tam, K.-W.; Shea, G.K.-H.; Chang, R.S.-K.; Ao, Q.; Shum, D.K.-Y.; Chan, Y.-S. Directed Differentiation of Human Bone Marrow Stromal Cells to Fate-Committed Schwann Cells. Stem Cell Rep. 2017, 9, 1097–1108. [Google Scholar] [CrossRef] [Green Version]
- Jung, N.; Park, S.; Choi, Y.; Park, J.-W.; Hong, Y.B.; Park, H.H.C.; Yu, Y.; Kwak, G.; Kim, H.S.; Ryu, K.-H.; et al. Tonsil-Derived Mesenchymal Stem Cells Differentiate into a Schwann Cell Phenotype and Promote Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2016, 17, 1867. [Google Scholar] [CrossRef]
- Tomita, K.; Madura, T.; Sakai, Y.; Yano, K.; Terenghi, G.; Hosokawa, K. Glial differentiation of human adipose-derived stem cells: Implications for cell-based transplantation therapy. Neuroscience 2013, 236, 55–65. [Google Scholar] [CrossRef]
- Razavi, S.; Ahmadi, N.; Kazemi, M.; Mardani, M.; Esfandiari, E. Efficient transdifferentiation of human adipose-derived stem cells into Schwann-like cells: A promise for treatment of demyelinating diseases. Adv. Biomed. Res. 2012, 1, 12. [Google Scholar] [CrossRef]
- Shimizu, S.; Kitada, M.; Ishikawa, H.; Itokazu, Y.; Wakao, S.; Dezawa, M. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem. Biophys. Res. Commun. 2007, 359, 915–920. [Google Scholar] [CrossRef]
- Xie, S.; Lu, F.; Han, J.; Tao, K.; Wang, H.; Simental, A.; Hu, D.; Yang, H. Efficient generation of functional Schwann cells from adipose-derived stem cells in defined conditions. Cell Cycle 2017, 16, 841–851. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, M.B.; Sharma, A.D.; Mallapragada, S.K.; Sakaguchi, D.S. Transdifferentiation of brain-derived neurotrophic factor (BDNF)-secreting mesenchymal stem cells significantly enhance BDNF secretion and Schwann cell marker proteins. J. Biosci. Bioeng. 2017, 124, 572–582. [Google Scholar] [CrossRef]
- Di Summa, P.G.; Kingham, P.J.; Raffoul, W.; Wiberg, M.; Terenghi, G.; Kalbermatten, D.F. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1544–1552. [Google Scholar] [CrossRef]
- Shea, G.K.H.; Tsui, A.Y.P.; Chan, Y.S.; Shum, D.K.Y. Bone marrow-derived Schwann cells achieve fate commitment—A prerequisite for remyelination therapy. Exp. Neurol. 2010, 224, 448–458. [Google Scholar] [CrossRef]
- Jiang, L.; Zhu, J.-K.; Liu, X.-L.; Xiang, P.; Hu, J.; Yu, W.-H. Differentiation of rat adipose tissue-derived stem cells into Schwann-like cells in vitro. Neuroreport 2008, 19, 1015–1019. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Liu, L.; Zhao, C.; Xiong, F.; Zhou, C.; Li, Y.; Shan, Y.; Peng, F.; Zhang, C. Neurospheres from rat adipose-derived stem cells could be induced into functional Schwann cell-like cells in vitro. BMC Neurosci. 2008, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höving, A.L.; Windmöller, B.A.; Knabbe, C.; Kaltschmidt, B.; Kaltschmidt, C.; Greiner, J.F.W. Between Fate Choice and Self-Renewal-Heterogeneity of Adult Neural Crest-Derived Stem Cells. Front. Cell Dev. Biol. 2021, 9, 662754. [Google Scholar] [CrossRef] [PubMed]
- Al-Zer, H.; Apel, C.; Heiland, M.; Friedrich, R.E.; Jung, O.; Kroeger, A.; Eichhorn, W.; Smeets, R. Enrichment and Schwann cell differentiation of neural crest-derived dental pulp stem cells. In Vivo 2015, 29, 319–326. [Google Scholar] [PubMed]
- Sakaue, M.; Sieber-Blum, M. Human epidermal neural crest stem cells as a source of Schwann cells. Development 2015, 142, 3188–3197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, Y.; Zhang, K.; Liu, X.; Yang, T.; Wang, B.; Fu, L.; Lan, A.; Zhou, Y. miR-21 promotes the differentiation of hair follicle-derived neural crest stem cells into Schwann cells. Neural Regen. Res. 2014, 9, 828–836. [Google Scholar] [CrossRef]
- Martens, W.; Sanen, K.; Georgiou, M.; Struys, T.; Bronckaers, A.; Ameloot, M.; Phillips, J.; Lambrichts, I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014, 28, 1634–1643. [Google Scholar] [CrossRef] [Green Version]
- Sieber-Blum, M.; Grim, M.; Hu, Y.F.; Szeder, V. Pluripotent neural crest stem cells in the adult hair follicle. Dev. Dyn. 2004, 231, 258–269. [Google Scholar] [CrossRef]
- Krause, M.P.; Dworski, S.; Feinberg, K.; Jones, K.; Johnston, A.P.W.; Paul, S.; Paris, M.; Peles, E.; Bagli, D.; Forrest, C.R.; et al. Direct genesis of functional rodent and human schwann cells from skin mesenchymal precursors. Stem Cell Rep. 2014, 3, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Min, S.-K.; Jung, S.Y.; Jung, K.; Da Jang, H.; Kim, O.B.; Chun, G.-S.; Lee, Z.H.; Min, B.-M. The potential of mouse skin-derived precursors to differentiate into mesenchymal and neural lineages and their application to osteogenic induction in vivo. Int. J. Mol. Med. 2011, 28, 1001–1011. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Biernaskie, J.; Toma, J.G.; Midha, R.; Miller, F.D. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. J. Soc. Neurosci. 2006, 26, 6651–6660. [Google Scholar] [CrossRef]
- Toma, J.G.; Akhavan, M.; Fernandes, K.J.; Barnabé-Heider, F.; Sadikot, A.; Kaplan, D.R.; Miller, F.D. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 2001, 3, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-H.; Zhao, N.; Feng, X.; Jie, Y.; Jin, Z.-B. Conversion of mouse embryonic fibroblasts into neural crest cells and functional corneal endothelia by defined small molecules. Sci. Adv. 2021, 7, eabg5749. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, J.Y.; Song, C.L.; Jeong, J.E.; Cho, Y.S. Directly induced human Schwann cell precursors as a valuable source of Schwann cells. Stem Cell Res. Ther. 2020, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Murakami, T.; Wakao, S.; Li, G.; Dezawa, M. Direct conversion of adult human skin fibroblasts into functional Schwann cells that achieve robust recovery of the severed peripheral nerve in rats. Glia 2019, 67, 950–966. [Google Scholar] [CrossRef]
- Sowa, Y.; Kishida, T.; Tomita, K.; Yamamoto, K.; Numajiri, T.; Mazda, O. Direct Conversion of Human Fibroblasts into Schwann Cells that Facilitate Regeneration of Injured Peripheral Nerve In Vivo. Stem Cells Transl. Med. 2017, 6, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lim, H.; Li, Z.; Oh, Y.; Kovlyagina, I.; Choi, I.Y.; Dong, X.; Lee, G. Generation of multipotent induced neural crest by direct reprogramming of human postnatal fibroblasts with a single transcription factor. Cell Stem Cell 2014, 15, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoma, E.C.; Merkl, C.; Heckel, T.; Haab, R.; Knoflach, F.; Nowaczyk, C.; Flint, N.; Jagasia, R.; Jensen Zoffmann, S.; Truong, H.H.; et al. Chemical conversion of human fibroblasts into functional Schwann cells. Stem Cell Rep. 2014, 3, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Moghadasi Boroujeni, S.; Koontz, A.; Tseropoulos, G.; Kerosuo, L.; Mehrotra, P.; Bajpai, V.K.; Selvam, S.R.; Lei, P.; Bronner, M.E.; Andreadis, S.T. Neural crest stem cells from human epidermis of aged donors maintain their multipotency in vitro and in vivo. Sci. Rep. 2019, 9, 9750. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, V.K.; Kerosuo, L.; Tseropoulos, G.; Cummings, K.A.; Wang, X.; Lei, P.; Liu, B.; Liu, S.; Popescu, G.K.; Bronner, M.E.; et al. Reprogramming Postnatal Human Epidermal Keratinocytes Toward Functional Neural Crest Fates. Stem Cells 2017, 35, 1402–1415. [Google Scholar] [CrossRef] [Green Version]
- Mizuseki, K.; Sakamoto, T.; Watanabe, K.; Muguruma, K.; Ikeya, M.; Nishiyama, A.; Arakawa, A.; Suemori, H.; Nakatsuji, N.; Kawasaki, H.; et al. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc. Natl. Acad. Sci. USA 2003, 100, 5828–5833. [Google Scholar] [CrossRef] [Green Version]
- Pomp, O.; Brokhman, I.; Ziegler, L.; Almog, M.; Korngreen, A.; Tavian, M.; Goldstein, R.S. PA6-induced human embryonic stem cell-derived neurospheres: A new source of human peripheral sensory neurons and neural crest cells. Brain Res. 2008, 1230, 50–60. [Google Scholar] [CrossRef]
- Zhou, Y.; Snead, M.L. Derivation of cranial neural crest-like cells from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2008, 376, 542–547. [Google Scholar] [CrossRef]
- Curchoe, C.L.; Maurer, J.; McKeown, S.J.; Cattarossi, G.; Cimadamore, F.; Nilbratt, M.; Snyder, E.Y.; Bronner-Fraser, M.; Terskikh, A.V. Early acquisition of neural crest competence during hESCs neuralization. PLoS ONE 2010, 5, e13890. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Swistowski, A.; Zeng, X. Human neural crest stem cells derived from human pluripotent stem cells. Methods Mol. Biol. 2014, 1210, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Toh, Y.-C. Human Pluripotent Stem Cell-Derived Neural Crest Cells for Tissue Regeneration and Disease Modeling. Front. Mol. Neurosci. 2019, 12, 39. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama-Nakagiri, Y.; Fujimura, T.; Moriwaki, S. Induction of Skin-Derived Precursor Cells from Human Induced Pluripotent Stem Cells. PLoS ONE 2016, 11, e0168451. [Google Scholar] [CrossRef]
- Mazuelas, H.; Magallón-Lorenz, M.; Fernández-Rodríguez, J.; Uriarte-Arrazola, I.; Richaud-Patin, Y.; Terribas, E.; Villanueva, A.; Castellanos, E.; Blanco, I.; Raya, Á.; et al. Modeling iPSC-derived human neurofibroma-like tumors in mice uncovers the heterogeneity of Schwann cells within plexiform neurofibromas. Cell Rep. 2022, 38, 110385. [Google Scholar] [CrossRef]
- Li, W.; Huang, L.; Lin, W.; Ke, Q.; Chen, R.; Lai, X.; Wang, X.; Zhang, J.; Jiang, M.; Huang, W.; et al. Engraftable neural crest stem cells derived from cynomolgus monkey embryonic stem cells. Biomaterials 2015, 39, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.M.; Ramamurthy, P.; Ebisu, F.; Lisak, R.P.; Bealmear, B.M.; Barald, K.F. A mouse embryonic stem cell model of Schwann cell differentiation for studies of the role of neurofibromatosis type 1 in Schwann cell development and tumor formation. Glia 2007, 55, 1123–1133. [Google Scholar] [CrossRef] [Green Version]
- Okawa, T.; Kamiya, H.; Himeno, T.; Kato, J.; Seino, Y.; Fujiya, A.; Kondo, M.; Tsunekawa, S.; Naruse, K.; Hamada, Y.; et al. Transplantation of neural crest-like cells derived from induced pluripotent stem cells improves diabetic polyneuropathy in mice. Cell Transplant. 2013, 22, 1767–1783. [Google Scholar] [CrossRef] [Green Version]
- Uemura, T.; Takamatsu, K.; Ikeda, M.; Okada, M.; Kazuki, K.; Ikada, Y.; Nakamura, H. Transplantation of induced pluripotent stem cell-derived neurospheres for peripheral nerve repair. Biochem. Biophys. Res. Commun. 2012, 419, 130–135. [Google Scholar] [CrossRef]
- Yokoi, T.; Uemura, T.; Takamatsu, K.; Shintani, K.; Onode, E.; Hama, S.; Miyashima, Y.; Okada, M.; Nakamura, H. Fate and contribution of induced pluripotent stem cell-derived neurospheres transplanted with nerve conduits to promote peripheral nerve regeneration in mice. Biomed. Mater. Eng. 2021, 32, 171–181. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Y.; Yin, H.-Y.; Guo, Z.-Y.; Xu, F.; Xiao, B.; Jiang, W.-L.; Guo, W.-M.; Meng, H.-Y.; Lu, S.-B.; et al. Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: Potential advantage of cellular transient memory function. Stem Cell Res. Ther. 2018, 9, 133. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.D.; Wiederin, J.; Uz, M.; Ciborowski, P.; Mallapragada, S.K.; Gendelman, H.E.; Sakaguchi, D.S. Proteomic analysis of mesenchymal to Schwann cell transdifferentiation. J. Proteom. 2017, 165, 93–101. [Google Scholar] [CrossRef]
- Uz, M.; Büyüköz, M.; Sharma, A.D.; Sakaguchi, D.S.; Altinkaya, S.A.; Mallapragada, S.K. Gelatin-based 3D conduits for transdifferentiation of mesenchymal stem cells into Schwann cell-like phenotypes. Acta Biomater. 2017, 53, 293–306. [Google Scholar] [CrossRef]
- Ladak, A.; Olson, J.; Tredget, E.E.; Gordon, T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp. Neurol. 2011, 228, 242–252. [Google Scholar] [CrossRef]
- Lin, W.; Chen, X.; Wang, X.; Liu, J.; Gu, X. Adult rat bone marrow stromal cells differentiate into Schwann cell-like cells in vitro. Vitr. Cell. Dev. Biol. Anim. 2008, 44, 31–40. [Google Scholar] [CrossRef]
- Keilhoff, G.; Goihl, A.; Langnäse, K.; Fansa, H.; Wolf, G. Transdifferentiation of mesenchymal stem cells into Schwann cell-like myelinating cells. Eur. J. Cell Biol. 2006, 85, 11–24. [Google Scholar] [CrossRef]
- Caddick, J.; Kingham, P.J.; Gardiner, N.J.; Wiberg, M.; Terenghi, G. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia 2006, 54, 840–849. [Google Scholar] [CrossRef]
- Tohill, M.; Mantovani, C.; Wiberg, M.; Terenghi, G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci. Lett. 2004, 362, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Ching, R.C.; Wiberg, M.; Kingham, P.J. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res. Ther. 2018, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Tong, Z.; Li, Q.; Niu, Q.; Zhang, Z.; Tong, X.; Tong, L.; Zhang, X. Induction of adipose-derived stem cells into Schwann-like cells and observation of Schwann-like cell proliferation. Mol. Med. Rep. 2016, 14, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zheng, Y.; Cai, Q.; Wu, X.; Yao, W.; Wang, J. Different methods for inducing adipose-derived stem cells to differentiate into Schwann-like cells. Arch. Med. Sci. 2015, 11, 886–892. [Google Scholar] [CrossRef]
- Faroni, A.; Mantovani, C.; Shawcross, S.G.; Motta, M.; Terenghi, G.; Magnaghi, V. Schwann-like adult stem cells derived from bone marrow and adipose tissue express γ-aminobutyric acid type B receptors. J. Neurosci. Res. 2011, 89, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Sun, M.; Wiberg, M.; Downes, S.; Terenghi, G.; Kingham, P.J. Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience 2011, 199, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Kaewkhaw, R.; Scutt, A.M.; Haycock, J.W. Anatomical site influences the differentiation of adipose-derived stem cells for Schwann-cell phenotype and function. Glia 2011, 59, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhou, K.; Mi, W.; Qiu, J. Combined use of decellularized allogeneic artery conduits with autologous transdifferentiated adipose-derived stem cells for facial nerve regeneration in rats. Biomaterials 2011, 32, 8118–8128. [Google Scholar] [CrossRef]
- Brohlin, M.; Mahay, D.; Novikov, L.N.; Terenghi, G.; Wiberg, M.; Shawcross, S.G.; Novikova, L.N. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci. Res. 2009, 64, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Park, S.Y.; Shin, Y.H.; Heo, S.-H.; Kim, K.-H.; Lee, H.I.; Kim, J.K. Mesenchymal Stem Cells Derived from Wharton’s Jelly Can Differentiate into Schwann Cell-Like Cells and Promote Peripheral Nerve Regeneration in Acellular Nerve Grafts. Tissue Eng. Regen. Med. 2021, 18, 467–478. [Google Scholar] [CrossRef]
- Xiao, Y.-Z.; Wang, S. Differentiation of Schwann-like cells from human umbilical cord blood mesenchymal stem cells in vitro. Mol. Med. Rep. 2015, 11, 1146–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Wang, Y.; Zhang, L.; Zhao, B.; Zhao, Z.; Chen, J.; Guo, Q.; Liu, S.; Sui, X.; Xu, W.; et al. Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res. Bull. 2011, 84, 235–243. [Google Scholar] [CrossRef]
- Liu, B.; Kong, Y.; Shi, W.; Kuss, M.; Liao, K.; Hu, G.; Xiao, P.; Sankarasubramanian, J.; Guda, C.; Wang, X.; et al. Exosomes derived from differentiated human ADMSC with the Schwann cell phenotype modulate peripheral nerve-related cellular functions. Bioact. Mater. 2022, 14, 61–75. [Google Scholar] [CrossRef]
- Wu, S.; Qi, Y.; Shi, W.; Kuss, M.; Chen, S.; Duan, B. Electrospun conductive nanofiber yarns for accelerating mesenchymal stem cells differentiation and maturation into Schwann cell-like cells under a combination of electrical stimulation and chemical induction. Acta Biomater. 2020, 139, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Wakao, S.; Hayashi, T.; Kitada, M.; Kohama, M.; Matsue, D.; Teramoto, N.; Ose, T.; Itokazu, Y.; Koshino, K.; Watabe, H.; et al. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp. Neurol. 2010, 223, 537–547. [Google Scholar] [CrossRef]
- Radtke, C.; Schmitz, B.; Spies, M.; Kocsis, J.D.; Vogt, P.M. Peripheral glial cell differentiation from neurospheres derived from adipose mesenchymal stem cells. Int. J. Dev. Neurosci. 2009, 27, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Gong, Y.; Qiang, L.; Li, X.; Zhang, S.; Gao, J.; Li, K.; Ji, X.; Tian, L.; Gu, X.; et al. Derivation of Schwann cell precursors from neural crest cells resident in bone marrow for cell therapy to improve peripheral nerve regeneration. Biomaterials 2016, 89, 25–37. [Google Scholar] [CrossRef]
- Park, S.; Jung, N.; Myung, S.; Choi, Y.; Chung, K.W.; Choi, B.-O.; Jung, S.-C. Differentiation of Human Tonsil-Derived Mesenchymal Stem Cells into Schwann-Like Cells Improves Neuromuscular Function in a Mouse Model of Charcot-Marie-Tooth Disease Type 1A. Int. J. Mol. Sci. 2018, 19, 2393. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Shum, D.K.Y.; Chan, Y.-S. Human Induced Pluripotent Stem Cell-Derived Sensory Neurons for Fate Commitment of Bone Marrow Stromal Cell-Derived Schwann Cells. Methods Mol. Biol. 2018, 1739, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Das, S.R.; Uz, M.; Ding, S.; Lentner, M.T.; Hondred, J.A.; Cargill, A.A.; Sakaguchi, D.S.; Mallapragada, S.; Claussen, J.C. Electrical Differentiation of Mesenchymal Stem Cells into Schwann-Cell-Like Phenotypes Using Inkjet-Printed Graphene Circuits. Adv. Healthc. Mater. 2017, 6, 1601087. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.D.; Zbarska, S.; Petersen, E.M.; Marti, M.E.; Mallapragada, S.K.; Sakaguchi, D.S. Oriented growth and transdifferentiation of mesenchymal stem cells towards a Schwann cell fate on micropatterned substrates. J. Biosci. Bioeng. 2016, 121, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.-J.; Zhang, S.; Mi, R.; Liu, Q.; Zeng, X.; Rao, M.; Hoke, A.; Mao, H.-Q. Enhanced differentiation of human neural crest stem cells towards the Schwann cell lineage by aligned electrospun fiber matrix. Acta Biomater. 2013, 9, 7727–7736. [Google Scholar] [CrossRef]
- Coste, C.; Neirinckx, V.; Sharma, A.; Agirman, G.; Rogister, B.; Foguenne, J.; Lallemend, F.; Gothot, A.; Wislet, S. Human bone marrow harbors cells with neural crest-associated characteristics like human adipose and dermis tissues. PLoS ONE 2017, 12, e0177962. [Google Scholar] [CrossRef]
- Toma, J.G.; McKenzie, I.A.; Bagli, D.; Miller, F.D. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells 2005, 23, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Jinno, H.; Morozova, O.; Jones, K.L.; Biernaskie, J.A.; Paris, M.; Hosokawa, R.; Rudnicki, M.A.; Chai, Y.; Rossi, F.; Marra, M.A.; et al. Convergent genesis of an adult neural crest-like dermal stem cell from distinct developmental origins. Stem Cells 2010, 28, 2027–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergeron, L.; Busuttil, V.; Botto, J.-M. Multipotentiality of skin-derived precursors: Application to the regeneration of skin and other tissues. Int. J. Cosmet. Sci. 2020, 42, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biernaskie, J.A.; McKenzie, I.A.; Toma, J.G.; Miller, F.D. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat. Protoc. 2006, 1, 2803–2812. [Google Scholar] [CrossRef]
- Shakhbazau, A.; Mirfeizi, L.; Walsh, T.; Wobma, H.M.; Kumar, R.; Singh, B.; Kallos, M.S.; Midha, R. Inter-microcarrier transfer and phenotypic stability of stem cell-derived Schwann cells in stirred suspension bioreactor culture. Biotechnol. Bioeng. 2016, 113, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Khuong, H.T.; Kumar, R.; Senjaya, F.; Grochmal, J.; Ivanovic, A.; Shakhbazau, A.; Forden, J.; Webb, A.; Biernaskie, J.; Midha, R. Skin derived precursor Schwann cells improve behavioral recovery for acute and delayed nerve repair. Exp. Neurol. 2014, 254, 168–179. [Google Scholar] [CrossRef]
- Walsh, S.; Biernaskie, J.; Kemp, S.W.P.; Midha, R. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience 2009, 164, 1097–1107. [Google Scholar] [CrossRef]
- Hunt, D.P.J.; Morris, P.N.; Sterling, J.; Anderson, J.A.; Joannides, A.; Jahoda, C.; Compston, A.; Chandran, S. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells 2008, 26, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Biernaskie, J.; Sparling, J.S.; Liu, J.; Shannon, C.P.; Plemel, J.R.; Xie, Y.; Miller, F.D.; Tetzlaff, W. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J. Neurosci. J. Soc. Neurosci. 2007, 27, 9545–9559. [Google Scholar] [CrossRef] [Green Version]
- Yun, W.; Kim, Y.J.; Lee, G. Direct Conversion to Achieve Glial Cell Fates: Oligodendrocytes and Schwann Cells. Int. J. Stem Cells 2022, 15, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Dezawa, M. Mesenchymal stem cells and their subpopulation, pluripotent muse cells, in basic research and regenerative medicine. Anat. Rec. 2014, 297, 98–110. [Google Scholar] [CrossRef]
- Windmöller, B.A.; Höving, A.L.; Knabbe, C.; Greiner, J.F.W. Inter- and Intrapopulational Heterogeneity of Characteristic Markers in Adult Human Neural Crest-derived Stem Cells. Stem Cell Rev. Rep. 2021, 18, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Chambers, S.M.; Tomishima, M.J.; Studer, L. Derivation of neural crest cells from human pluripotent stem cells. Nat. Protoc. 2010, 5, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jin, Y.-Q.; Chen, L.; Wang, Y.; Yang, X.; Cheng, J.; Wu, W.; Qi, Z.; Shen, Z. Specific marker expression and cell state of Schwann cells during culture in vitro. PLoS ONE 2015, 10, e0123278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, A.K.Y.; Wang, P.L.; Bermingham, J.R.; Hackett, A.; Strickland, A.; Miller, T.M.; Ly, C.; Mitra, R.D.; Milbrandt, J. Disentangling glial diversity in peripheral nerves at single-nuclei resolution. Nat. Neurosci. 2022, 25, 238–251. [Google Scholar] [CrossRef]
- Betters, E.; Murdoch, B.; Leung, A.W.; García-Castro, M.I. Human Neural Crest Cells and Stem Cell-Based Models. In Neural Crest Cells: Evolution, Development and Disease; Trainor, P.A., Ed.; Academic Press: London, UK, 2014; pp. 395–412. ISBN 9780124017306. [Google Scholar]
- Betters, E.; Liu, Y.; Kjaeldgaard, A.; Sundström, E.; García-Castro, M.I. Analysis of early human neural crest development. Dev. Biol. 2010, 344, 578–592. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Stykel, M.G.; Touahri, Y.; Stratton, J.A.; Biernaskie, J.; Schuurmans, C. Temporal Analysis of Gene Expression in the Murine Schwann Cell Lineage and the Acutely Injured Postnatal Nerve. PLoS ONE 2016, 11, e0153256. [Google Scholar] [CrossRef] [Green Version]
- Jacob, C.; Lötscher, P.; Engler, S.; Baggiolini, A.; Varum Tavares, S.; Brügger, V.; John, N.; Büchmann-Møller, S.; Snider, P.L.; Conway, S.J.; et al. HDAC1 and HDAC2 control the specification of neural crest cells into peripheral glia. J. Neurosci. 2014, 34, 6112–6122. [Google Scholar] [CrossRef] [Green Version]
- Doddrell, R.D.S.; Dun, X.-P.; Moate, R.M.; Jessen, K.R.; Mirsky, R.; Parkinson, D.B. Regulation of Schwann cell differentiation and proliferation by the Pax-3 transcription factor. Glia 2012, 60, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Kioussi, C.; Gross, M.K.; Gruss, P. Pax3: A paired domain gene as a regulator in PNS myelination. Neuron 1995, 15, 553–562. [Google Scholar] [CrossRef]
- Blake, J.A.; Ziman, M.R. The characterisation of Pax3 expressant cells in adult peripheral nerve. PLoS ONE 2013, 8, e59184. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Sorci, G.; Riuzzi, F.; Arcuri, C.; Bianchi, R.; Brozzi, F.; Tubaro, C.; Giambanco, I. S100B’s double life: Intracellular regulator and extracellular signal. Biochim. Biophys. Acta 2009, 1793, 1008–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef] [Green Version]
- Aquino, J.B.; Sierra, R. Schwann cell precursors in health and disease. Glia 2018, 66, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir-Yilmaz, O.E.; Druckenbrod, N.R.; Olukoya, O.O.; Dong, W.; Yung, A.R.; Bastille, I.; Pazyra-Murphy, M.F.; Sitko, A.A.; Hale, E.B.; Vigneau, S.; et al. Diversity of developing peripheral glia revealed by single-cell RNA sequencing. Dev. Cell 2021, 56, 2516–2535.e8. [Google Scholar] [CrossRef]
- Jablonka-Shariff, A.; Broberg, C.; Rios, R.; Snyder-Warwick, A.K. T-box transcription factor 21 is expressed in terminal Schwann cells at the neuromuscular junction. Muscle Nerve 2021, 64, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Taetzsch, T.; Vaughan, S.K.; Godbe, K.; Chappell, J.; Settlage, R.E.; Valdez, G. Specific labeling of synaptic schwann cells reveals unique cellular and molecular features. eLife 2020, 9, e56935. [Google Scholar] [CrossRef] [PubMed]
- Gorlewicz, A.; Wlodarczyk, J.; Wilczek, E.; Gawlak, M.; Cabaj, A.; Majczynski, H.; Nestorowicz, K.; Herbik, M.A.; Grieb, P.; Slawinska, U.; et al. CD44 is expressed in non-myelinating Schwann cells of the adult rat, and may play a role in neurodegeneration-induced glial plasticity at the neuromuscular junction. Neurobiol. Dis. 2009, 34, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Kraniak, J.M.; Chalasani, A.; Wallace, M.R.; Mattingly, R.R. Development of 3D culture models of plexiform neurofibroma and initial application for phenotypic characterization and drug screening. Exp. Neurol. 2018, 299, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.A.; Diaz, A.; Errante, E.L.; Smartz, T.; Khan, A.; Silvera, R.; Brooks, A.E.; Lee, Y.-S.; Burks, S.S.; Levi, A.D. Systematic review of the therapeutic use of Schwann cells in the repair of peripheral nerve injuries: Advancements from animal studies to clinical trials. Front. Cell. Neurosci. 2022, 16, 929593. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, C.A.; Grochmal, J.; Kung, T.A.; Cederna, P.S.; Midha, R.; Kemp, S.W.P. Stem-cell-based therapies to enhance peripheral nerve regeneration. Muscle Nerve 2020, 61, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Hopf, A.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System. Cells 2020, 9, 1990. [Google Scholar] [CrossRef]
- Han, G.-H.; Peng, J.; Liu, P.; Ding, X.; Wei, S.; Lu, S.; Wang, Y. Therapeutic strategies for peripheral nerve injury: Decellularized nerve conduits and Schwann cell transplantation. Neural Regen. Res. 2019, 14, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Strategic Design and Fabrication of Nerve Guidance Conduits for Peripheral Nerve Regeneration. Biotechnol. J. 2018, 13, e1700635. [Google Scholar] [CrossRef] [PubMed]
- Gregory, H.; Phillips, J.B. Materials for peripheral nerve repair constructs: Natural proteins or synthetic polymers? Neurochem. Int. 2021, 143, 104953. [Google Scholar] [CrossRef] [PubMed]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. SDF-1α gene-activated collagen scaffold drives functional differentiation of human Schwann cells for wound healing applications. Biotechnol. Bioeng. 2021, 118, 725–736. [Google Scholar] [CrossRef]
- Panzer, K.V.; Burrell, J.C.; Helm, K.V.T.; Purvis, E.M.; Zhang, Q.; Le, A.D.; O’Donnell, J.C.; Cullen, D.K. Tissue Engineered Bands of Büngner for Accelerated Motor and Sensory Axonal Outgrowth. Front. Bioeng. Biotechnol. 2020, 8, 580654. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhao, Y.; Wu, Y.; Li, Z.; Lv, H.; Li, S.; Jiang, Y.; Song, C.; Wang, F.; Huang, Y. Fabrication, characterization and biocompatibility of collagen/oxidized regenerated cellulose-Ca composite scaffold for carrying Schwann cells. Int. J. Biol. Macromol. 2018, 119, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Golding, J.P.; Loughlin, A.J.; Kingham, P.J.; Phillips, J.B. Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials 2015, 37, 242–251. [Google Scholar] [CrossRef] [Green Version]
- van Neerven, S.G.A.; Krings, L.; Haastert-Talini, K.; Vogt, M.; Tolba, R.H.; Brook, G.; Pallua, N.; Bozkurt, A. Human Schwann cells seeded on a novel collagen-based microstructured nerve guide survive, proliferate, and modify neurite outgrowth. Biomed Res. Int. 2014, 2014, 493823. [Google Scholar] [CrossRef] [PubMed]
- Zaminy, A.; Shokrgozar, M.A.; Sadeghi, Y.; Noroozian, M.; Heidari, M.H.; Piryaei, A. Mesenchymal stem cells as an alternative for Schwann cells in rat spinal cord injury. Iran. Biomed. J. 2013, 17, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B.I.; Hang, T.; Tranquillo, R.T. Schwann cell behavior in three-dimensional collagen gels: Evidence for differential mechano-transduction and the influence of TGF-beta 1 in morphological polarization and differentiation. Exp. Neurol. 2005, 195, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, D.; Wang, L.; Hou, J.; Zhang, H.; Li, Y.; Zhong, S.; Wang, Y.; Wu, Y.; Huang, W. 3D bioprinted multiscale composite scaffolds based on gelatin methacryloyl (GelMA)/chitosan microspheres as a modular bioink for enhancing 3D neurite outgrowth and elongation. J. Colloid Interface Sci. 2020, 574, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Wang, K.; Ho, C.-C.; Kao, C.-T.; Ng, H.Y.; Shie, M.-Y. Cyclic tensile stimulation enrichment of Schwann cell-laden auxetic hydrogel scaffolds towards peripheral nerve tissue engineering. Mater. Des. 2020, 195, 108982. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Liang, R.; Chen, Y.; Li, S.; Li, S.; Sun, Z.; Wang, Y.; Li, G.; Ming, A.; et al. Fabrication of alignment polycaprolactone scaffolds by combining use of electrospinning and micromolding for regulating Schwann cells behavior. J. Biomed. Mater. Res. A 2018, 106, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Ciardelli, G.; Zanetti, M.; Geuna, S.; Perroteau, I. The influence of electrospun fibre size on Schwann cell behaviour and axonal outgrowth. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 620–631. [Google Scholar] [CrossRef]
- Jain, S.; Webster, T.J.; Sharma, A.; Basu, B. Intracellular reactive oxidative stress, cell proliferation and apoptosis of Schwann cells on carbon nanofibrous substrates. Biomaterials 2013, 34, 4891–4901. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xiao, Q.; McNaughton, R.; Han, L.; Zhang, L.; Wang, Y.; Yang, Y. Nanoengineered porous chitosan/CaTiO3 hybrid scaffolds for accelerating Schwann cells growth in peripheral nerve regeneration. Colloids Surf. B Biointerfaces 2017, 158, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, X.; Zhao, W.; Zhang, L.; Wang, C.; Jiang, M.; Gu, X.; Yang, Y. Porous chitosan scaffolds with surface micropatterning and inner porosity and their effects on Schwann cells. Biomaterials 2014, 35, 8503–8513. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Li, L.-T.; Su, W.-T. Three dimensional chitosan scaffolds influence the extra cellular matrix expression in Schwann cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 474–478. [Google Scholar] [CrossRef] [PubMed]
- England, S.; Rajaram, A.; Schreyer, D.J.; Chen, X. Bioprinted fibrin-factor XIII-hyaluronate hydrogel scaffolds with encapsulated Schwann cells and their in vitro characterization for use in nerve regeneration. Bioprinting 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Bayat, N.; Ebrahimi-Barough, S.; Ardakan, M.M.M.; Ai, A.; Kamyab, A.; Babaloo, H.; Ai, J. Differentiation of Human Endometrial Stem Cells into Schwann Cells in Fibrin Hydrogel as 3D Culture. Mol. Neurobiol. 2016, 53, 7170–7176. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Sarker, M.D.; Abelseth, E.; Chen, X. Indirect 3D bioprinting and characterization of alginate scaffolds for potential nerve tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 93, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, L.; Betancourt, N.; Schreyer, D.J.; Chen, X. Characterization of Cell Damage and Proliferative Ability during and after Bioprinting. ACS Biomater. Sci. Eng. 2018, 4, 3906–3918. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Q.; Xie, S.; Shan, X.; Cai, Z. In vitro and in vivo biocompatibility evaluation of a 3D bioprinted gelatin-sodium alginate/rat Schwann-cell scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110530. [Google Scholar] [CrossRef]
- Hyung, S.; Lee, S.-R.; Kim, J.; Kim, Y.; Kim, S.; Kim, H.N.; Jeon, N.L. A 3D disease and regeneration model of peripheral nervous system-on-a-chip. Sci. Adv. 2021, 7, eabd9749. [Google Scholar] [CrossRef]
- Sharma, A.D.; McCoy, L.; Jacobs, E.; Willey, H.; Behn, J.Q.; Nguyen, H.; Bolon, B.; Curley, J.L.; Moore, M.J. Engineering a 3D functional human peripheral nerve in vitro using the Nerve-on-a-Chip platform. Sci. Rep. 2019, 9, 8921. [Google Scholar] [CrossRef] [Green Version]
- Fangmann, L.; Teller, S.; Stupakov, P.; Friess, H.; Ceyhan, G.O.; Demir, I.E. 3D Cancer Migration Assay with Schwann Cells. Methods Mol. Biol. 2018, 1739, 317–325. [Google Scholar] [CrossRef]
- Demir, I.E.; Boldis, A.; Pfitzinger, P.L.; Teller, S.; Brunner, E.; Klose, N.; Kehl, T.; Maak, M.; Lesina, M.; Laschinger, M.; et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. J. Natl. Cancer Inst. 2014, 106, dju184. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Li, S.; Zhang, L.; Chen, S.; Sun, Z.; Li, S.; Zhang, L.; Yang, Y. Construction of Biofunctionalized Anisotropic Hydrogel Micropatterns and Their Effect on Schwann Cell Behavior in Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37397–37410. [Google Scholar] [CrossRef] [PubMed]
- Rosso, G.; Liashkovich, I.; Young, P.; Röhr, D.; Shahin, V. Schwann cells and neurite outgrowth from embryonic dorsal root ganglions are highly mechanosensitive. Nanomedicine 2017, 13, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ji, Y.; Zhao, Y.; Liu, Y.; Ding, F.; Gu, X.; Yang, Y. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials 2012, 33, 6672–6681. [Google Scholar] [CrossRef] [PubMed]
- Millesi, F.; Weiss, T.; Mann, A.; Haertinger, M.; Semmler, L.; Supper, P.; Pils, D.; Naghilou, A.; Radtke, C. Defining the regenerative effects of native spider silk fibers on primary Schwann cells, sensory neurons, and nerve-associated fibroblasts. FASEB J. 2021, 35, e21196. [Google Scholar] [CrossRef] [PubMed]
- Naghilou, A.; Pöttschacher, L.; Millesi, F.; Mann, A.; Supper, P.; Semmler, L.; Weiss, T.; Backus, E.H.G.; Radtke, C. Correlating the secondary protein structure of natural spider silk with its guiding properties for Schwann cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111219. [Google Scholar] [CrossRef]
- Resch, A.; Wolf, S.; Mann, A.; Weiss, T.; Stetco, A.-L.; Radtke, C. Co-Culturing Human Adipose Derived Stem Cells and Schwann Cells on Spider Silk-A New Approach as Prerequisite for Enhanced Nerve Regeneration. Int. J. Mol. Sci. 2018, 20, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Gong, J.; Niu, C.; Wei, Z.; Shi, J.; Li, G.; Yang, Y.; Wang, H. A new electrospun graphene-silk fibroin composite scaffolds for guiding Schwann cells. J. Biomater. Sci. Polym. Ed. 2017, 28, 2171–2185. [Google Scholar] [CrossRef]
- Ning, L.; Zhu, N.; Mohabatpour, F.; Sarker, M.D.; Schreyer, D.J.; Chen, X. Bioprinting Schwann cell-laden scaffolds from low-viscosity hydrogel compositions. J. Mater. Chem. B 2019, 7, 4538–4551. [Google Scholar] [CrossRef]
- Ning, L.; Sun, H.; Lelong, T.; Guilloteau, R.; Zhu, N.; Schreyer, D.J.; Chen, X. 3D bioprinting of scaffolds with living Schwann cells for potential nerve tissue engineering applications. Biofabrication 2018, 10, 35014. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Niu, C.; Zhang, L.; Li, G.; Yang, Y. Construction of polyacrylamide/graphene oxide/gelatin/sodium alginate composite hydrogel with bioactivity for promoting Schwann cells growth. J. Biomed. Mater. Res. A 2018, 106, 1951–1964. [Google Scholar] [CrossRef]
- Hodde, D.; Gerardo-Nava, J.; Wöhlk, V.; Weinandy, S.; Jockenhövel, S.; Kriebel, A.; Altinova, H.; Steinbusch, H.W.M.; Möller, M.; Weis, J.; et al. Characterisation of cell-substrate interactions between Schwann cells and three-dimensional fibrin hydrogels containing orientated nanofibre topographical cues. Eur. J. Neurosci. 2016, 43, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, Y.; Zhang, L.; Gao, M.; Kong, Y.; Yang, Y. Preparation of graphene oxide/polyacrylamide composite hydrogel and its effect on Schwann cells attachment and proliferation. Colloids Surf. B Biointerfaces 2016, 143, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Cadena, M.; Ning, L.; King, A.; Hwang, B.; Jin, L.; Serpooshan, V.; Sloan, S.A. 3D Bioprinting of Neural Tissues. Adv. Healthc. Mater. 2021, 10, e2001600. [Google Scholar] [CrossRef] [PubMed]
- Soltan, N.; Ning, L.; Mohabatpour, F.; Papagerakis, P.; Chen, X. Printability and Cell Viability in Bioprinting Alginate Dialdehyde-Gelatin Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 2976–2987. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Wang, X.; Chen, H.; Zhang, X.; Zhou, L.; Xu, T. 3D bioprinted rat Schwann cell-laden structures with shape flexibility and enhanced nerve growth factor expression. 3 Biotech 2018, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Xu, Y.; Chen, X.; Schreyer, D.J. Influence of mechanical properties of alginate-based substrates on the performance of Schwann cells in culture. J. Biomater. Sci. Polym. Ed. 2016, 27, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, A.; Schreyer, D.; Chen, D. Bioplotting Alginate/Hyaluronic Acid Hydrogel Scaffolds with Structural Integrity and Preserved Schwann Cell Viability. 3D Print. Addit. Manuf. 2014, 1, 194–203. [Google Scholar] [CrossRef]
- Haring, A.P.; Thompson, E.G.; Tong, Y.; Laheri, S.; Cesewski, E.; Sontheimer, H.; Johnson, B.N. Process- and bio-inspired hydrogels for 3D bioprinting of soft free-standing neural and glial tissues. Biofabrication 2019, 11, 25009. [Google Scholar] [CrossRef]
- Podder, A.K.; Mohamed, M.A.; Tseropoulos, G.; Nasiri, B.; Andreadis, S.T. Engineering Nanofiber Scaffolds with Biomimetic Cues for Differentiation of Skin-Derived Neural Crest-like Stem Cells to Schwann Cells. Int. J. Mol. Sci. 2022, 23, 10834. [Google Scholar] [CrossRef] [PubMed]
- Babaloo, H.; Ebrahimi-Barough, S.; Derakhshan, M.A.; Yazdankhah, M.; Lotfibakhshaiesh, N.; Soleimani, M.; Joghataei, M.-T.; Ai, J. PCL/gelatin nanofibrous scaffolds with human endometrial stem cells/Schwann cells facilitate axon regeneration in spinal cord injury. J. Cell. Physiol. 2019, 234, 11060–11069. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Yu, J.; Ng, H.-Y.; Lee, A.K.-X.; Chen, C.-C.; Chen, Y.-S.; Shie, M.-Y. The Physicochemical Properties of Decellularized Extracellular Matrix-Coated 3D Printed Poly(ε-caprolactone) Nerve Conduits for Promoting Schwann Cells Proliferation and Differentiation. Materials 2018, 11, 1665. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, M.-S.; Maurel, P.; Lee, Y.-S.; Bunge, M.B.; Arinzeh, T.L. Aligned fibrous PVDF-TrFE scaffolds with Schwann cells support neurite extension and myelination in vitro. J. Neural Eng. 2018, 15, 56010. [Google Scholar] [CrossRef]
- Xue, J.; Yang, J.; O’Connor, D.M.; Zhu, C.; Da Huo; Boulis, N.M.; Xia, Y. Differentiation of Bone Marrow Stem Cells into Schwann Cells for the Promotion of Neurite Outgrowth on Electrospun Fibers. ACS Appl. Mater. Interfaces 2017, 9, 12299–12310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.; Sun, X.; Liu, D.; Zhou, Y.; Zhong, D. Oriented growth of rat Schwann cells on aligned electrospun poly(methyl methacrylate) nanofibers. J. Neurol. Sci. 2016, 369, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Daud, M.F.B.; Pawar, K.C.; Claeyssens, F.; Ryan, A.J.; Haycock, J.W. An aligned 3D neuronal-glial co-culture model for peripheral nerve studies. Biomaterials 2012, 33, 5901–5913. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Lin, Z.; Song, P.; Quan, D.; Bai, Y. Biomaterial-Based Schwann Cell Transplantation and Schwann Cell-Derived Biomaterials for Nerve Regeneration. Front. Cell. Neurosci. 2022, 16, 323. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.; Eleftheriadou, D.; Kellaway, S.; Phillips, J.B. Natural Biomaterials as Instructive Engineered Microenvironments That Direct Cellular Function in Peripheral Nerve Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 674473. [Google Scholar] [CrossRef]
- Lotfi, L.; Khakbiz, M.; Moosazadeh Moghaddam, M.; Bonakdar, S. A biomaterials approach to Schwann cell development in neural tissue engineering. J. Biomed. Mater. Res. A 2019, 107, 2425–2446. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, P.; Burrell, J.C.; Zeng, J.; Shi, S.; Shanti, R.M.; Kulischak, G.; Cullen, D.K.; Le, A.D. Harnessing 3D collagen hydrogel-directed conversion of human GMSCs into SCP-like cells to generate functionalized nerve conduits. NPJ Regen. Med. 2021, 6, 59. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.; Liu, J.; He, Q.; Zhou, Y.; Shao, G.; Sun, X.; Cao, X.; Gong, A.; Jiang, P. A fibrin matrix promotes the differentiation of EMSCs isolated from nasal respiratory mucosa to myelinating phenotypical Schwann-like cells. Mol. Cells 2015, 38, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.K.; Madigan, N.N.; Hakim, J.S.; Dadsetan, M.; McMahon, S.S.; Yaszemski, M.J.; Windebank, A.J. GDNF Schwann cells in hydrogel scaffolds promote regional axon regeneration, remyelination and functional improvement after spinal cord transection in rats. J. Tissue Eng. Regen. Med. 2018, 12, e398–e407. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, F.; Hernández, J.; Heimann, C.; Phillips, J.B.; Udina, E.; Navarro, X. Schwann cells and mesenchymal stem cells in laminin- or fibronectin-aligned matrices and regeneration across a critical size defect of 15 mm in the rat sciatic nerve. J. Neurosurg. Spine 2018, 28, 109–118. [Google Scholar] [CrossRef]
- Urbanski, M.M.; Kingsbury, L.; Moussouros, D.; Kassim, I.; Mehjabeen, S.; Paknejad, N.; Melendez-Vasquez, C.V. Myelinating glia differentiation is regulated by extracellular matrix elasticity. Sci. Rep. 2016, 6, 33751. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.; Prantl, L.; Vykoukal, J.; Loibl, M.; Felthaus, O. Differential Effects of Coating Materials on Viability and Migration of Schwann Cells. Materials 2016, 9, 150. [Google Scholar] [CrossRef]
- Masaeli, E.; Wieringa, P.A.; Morshed, M.; Nasr-Esfahani, M.H.; Sadri, S.; van Blitterswijk, C.A.; Moroni, L. Peptide functionalized polyhydroxyalkanoate nanofibrous scaffolds enhance Schwann cells activity. Nanomedicine 2014, 10, 1559–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kijeńska, E.; Prabhakaran, M.P.; Swieszkowski, W.; Kurzydlowski, K.J.; Ramakrishna, S. Interaction of Schwann cells with laminin encapsulated PLCL core–shell nanofibers for nerve tissue engineering. Eur. Polym. J. 2014, 50, 30–38. [Google Scholar] [CrossRef]
- Moosazadeh Moghaddam, M.; Bonakdar, S.; Shokrgozar, M.A.; Zaminy, A.; Vali, H.; Faghihi, S. Engineered substrates with imprinted cell-like topographies induce direct differentiation of adipose-derived mesenchymal stem cells into Schwann cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Pi, W.; Zhou, L.; Zhang, W.; Liu, S.; Li, C.; Zhang, M.; Wen, Y.; Zhang, P. Three-dimensional conductive polycaprolactone/carbon nanotubes scaffolds for peripheral nerve regeneration. J. Mater. Sci. 2022, 57, 11289–11299. [Google Scholar] [CrossRef]
- Wang, M.; Tremblay, P.-L.; Zhang, T. Optimizing the electrical conductivity of polyacrylonitrile/polyaniline with nickel nanoparticles for the enhanced electrostimulation of Schwann cells proliferation. Bioelectrochemistry 2021, 140, 107750. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, X.; Xu, Y.; Li, L.; Liu, J.; He, Y.; Zou, Y.; Yu, L.; Qiu, X.; Guo, J. Electric Conductivity on Aligned Nanofibers Facilitates the Transdifferentiation of Mesenchymal Stem Cells into Schwann Cells and Regeneration of Injured Peripheral Nerve. Adv. Healthc. Mater. 2020, 9, e1901570. [Google Scholar] [CrossRef]
- Entezari, M.; Mozafari, M.; Bakhtiyari, M.; Moradi, F.; Bagher, Z.; Soleimani, M. Three-dimensional-printed polycaprolactone/polypyrrole conducting scaffolds for differentiation of human olfactory ecto-mesenchymal stem cells into Schwann cell-like phenotypes and promotion of neurite outgrowth. J. Biomed. Mater. Res. A 2022, 110, 1134–1146. [Google Scholar] [CrossRef]
- Rosso, G.; Guck, J. Mechanical changes of peripheral nerve tissue microenvironment and their structural basis during development. APL Bioeng. 2019, 3, 36107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosso, G.; Young, P.; Shahin, V. Implications of Schwann Cells Biomechanics and Mechanosensitivity for Peripheral Nervous System Physiology and Pathophysiology. Front. Mol. Neurosci. 2017, 10, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Stukel, J.M.; Cebull, H.L.; Willits, R.K. Tuning the Mechanical Properties of Poly(Ethylene Glycol) Microgel-Based Scaffolds to Increase 3D Schwann Cell Proliferation. Macromol. Biosci. 2016, 16, 535–544. [Google Scholar] [CrossRef]
- López-Fagundo, C.; Bar-Kochba, E.; Livi, L.L.; Hoffman-Kim, D.; Franck, C. Three-dimensional traction forces of Schwann cells on compliant substrates. J. R. Soc. Interface 2014, 11, 20140247. [Google Scholar] [CrossRef] [Green Version]
- Manganas, P.; Kavatzikidou, P.; Kordas, A.; Babaliari, E.; Stratakis, E.; Ranella, A. The role of mechanobiology on the Schwann cell response: A tissue engineering perspective. Front. Cell. Neurosci. 2022, 16, 948454. [Google Scholar] [CrossRef]
- Rosso, G.; Wehner, D.; Schweitzer, C.; Möllmert, S.; Sock, E.; Guck, J.; Shahin, V. Matrix stiffness mechanosensing modulates the expression and distribution of transcription factors in Schwann cells. Bioeng. Transl. Med. 2022, 7, e10257. [Google Scholar] [CrossRef] [PubMed]

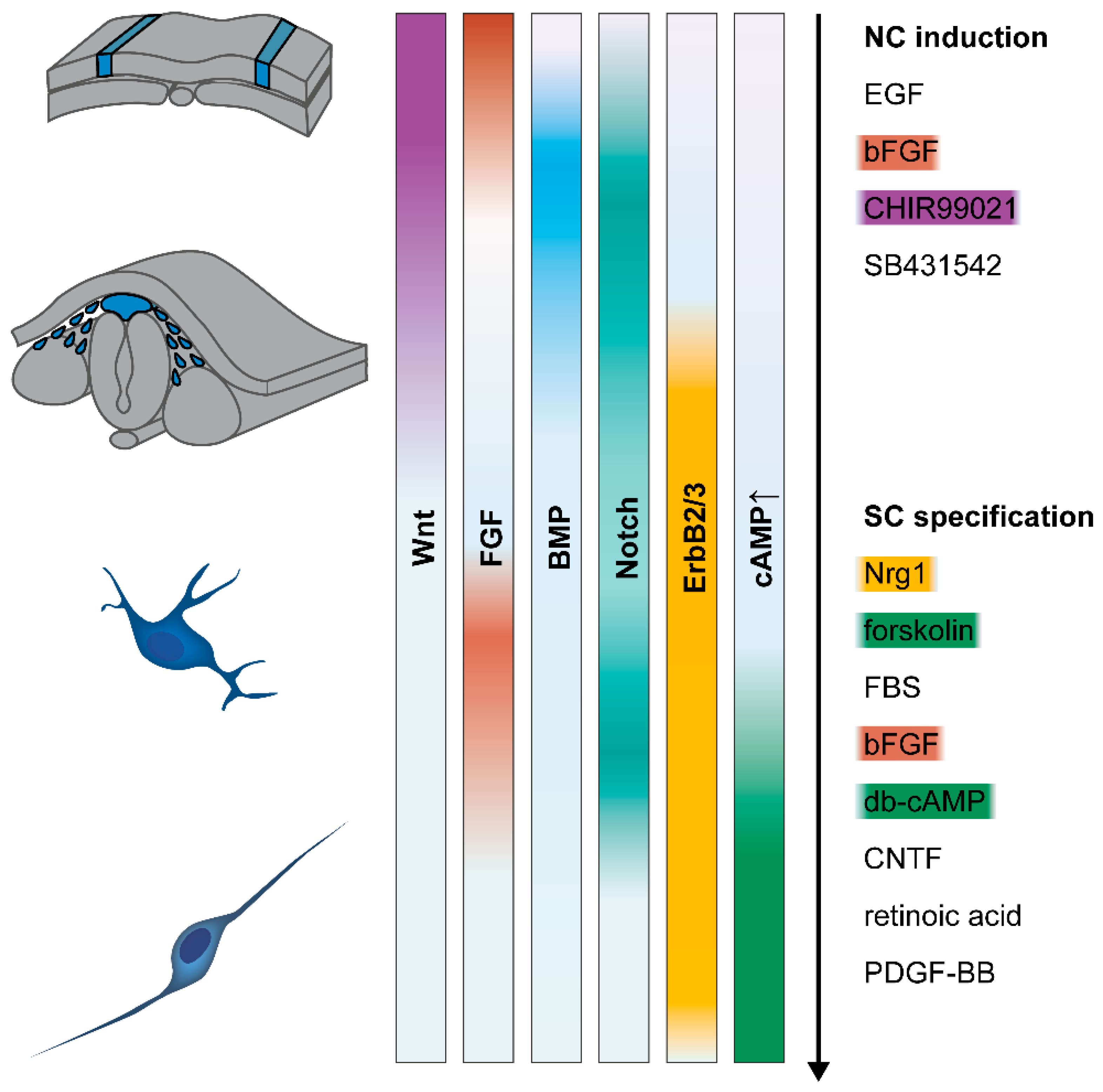
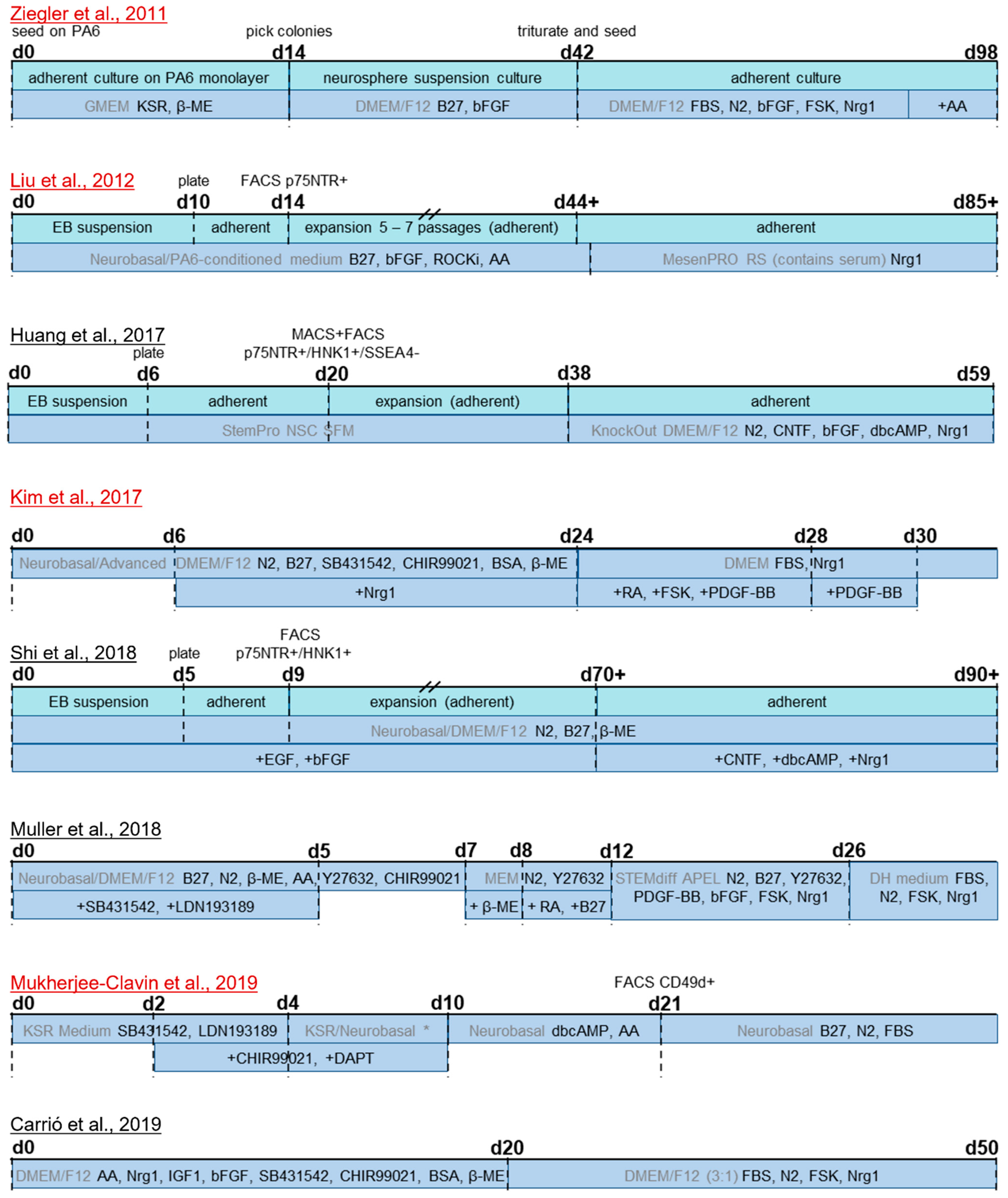
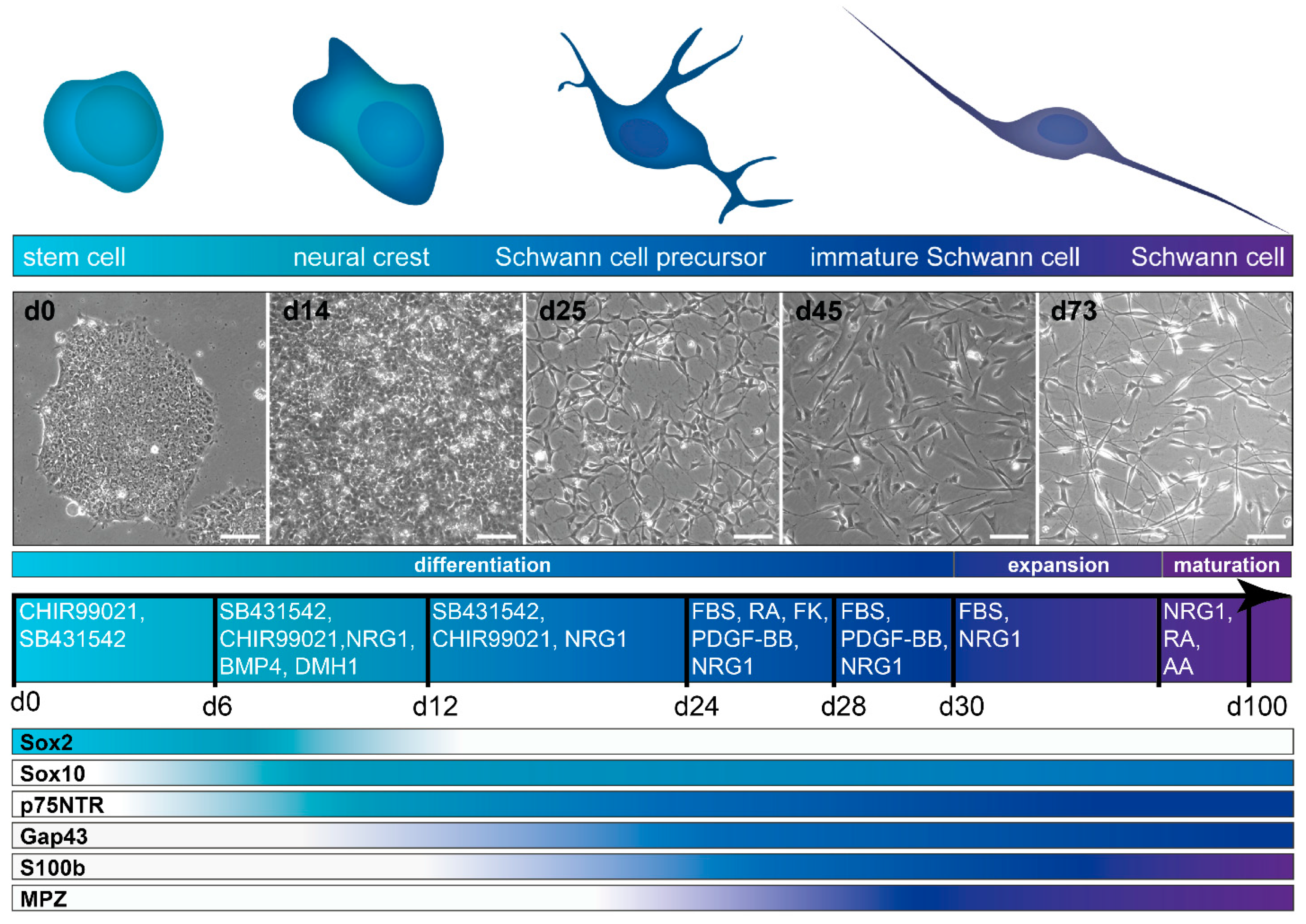
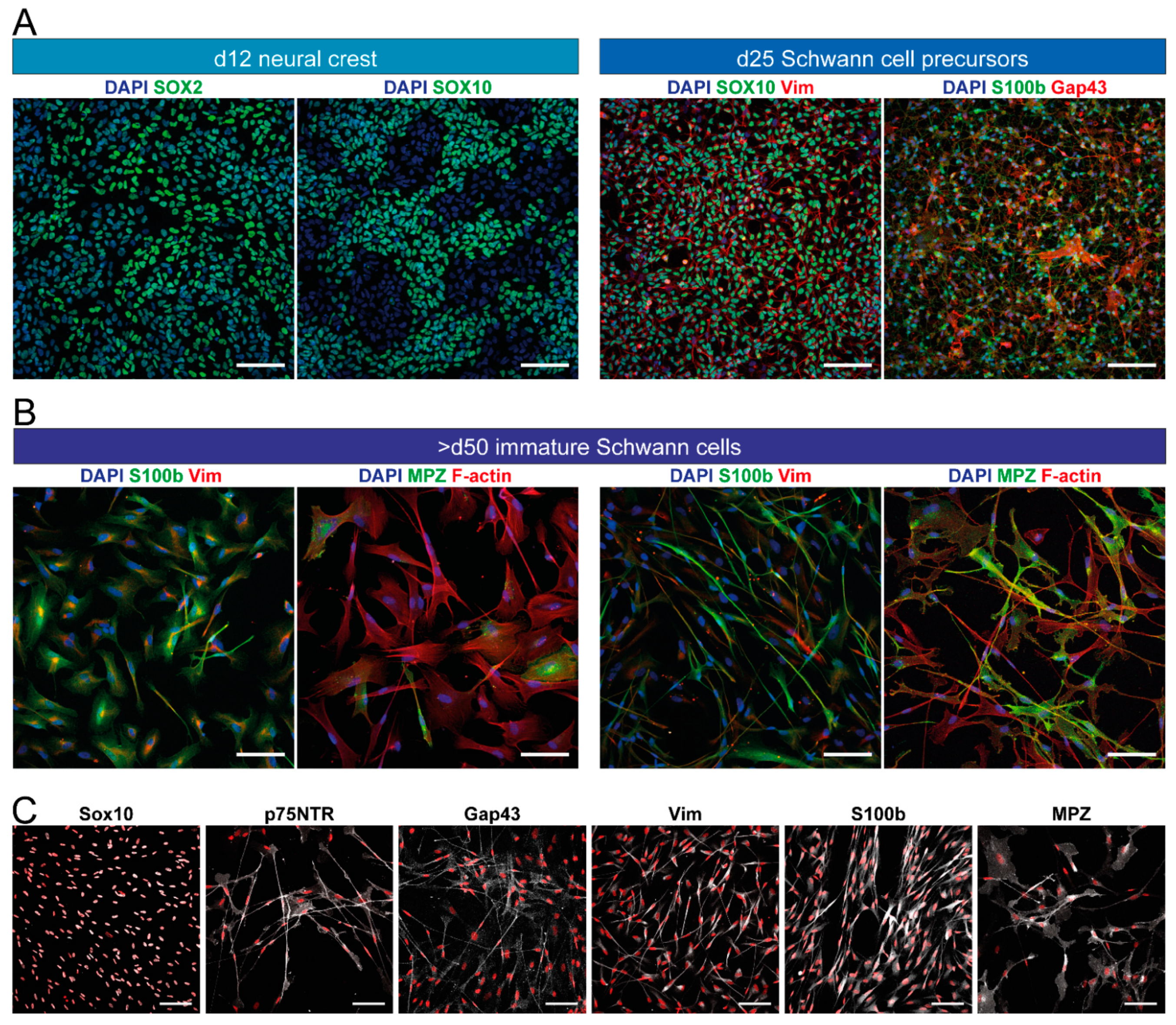
| Study | Cell Source (Number of Cell Lines) | Duration | Characterization | Schwann Cell Markers | Sorting Steps/Yield |
|---|---|---|---|---|---|
| Mukherjee-Clavin et al., 2019 [119] | hESC (1 control, 2 disease) hiPSC (2 control, 3 disease) | approx. 55 d (21 d to SCP, up to +80 d in culture; SC maturation after 55 d in total) | IF, RT-qPCR, microarray, RNA-seq, in vitro myelination assay, in vivo transplantation assay | PMP22, SOX10, MPZ, S100b, GFAP, GalC, MBP | 13.5% CD49d+ SCP at d21–23 (for hiPSC), are FACS purified for further culture; final SC yield not reported |
| Kim et al., 2017 [121] | hESC (3) hiPSC (1) | 31 d to SC identity | IF, RT-qPCR, microarray, neurotrophic factors secretion (ELISA), in vitro myelination assay, in vivo transplantation assay | GAP43, SOX10, p75NTR, MPZ, S100b, PMP22, KROX20, GFAP | no sorting, 99% SOX10+ (SCP stage), final SC yield not reported |
| Liu et al., 2012 [111] Liu et al., 2014 [235] | hESC (2) hiPSC (1) | >85 d (14 d to NCC, at least 30 d expansion, 40 d to SC) | IF, RT-PCR/qPCR, microarray, in vitro myelination assay | GFAP, S100b, p75NTR, PMP22, MBP | FACS for p75NTR+ to purify NCC at d14, 85% S100b+ at d75 |
| Ziegler et al., 2011 [112] | hESC (1) | 98 d | IF, RT-PCR, neuron coculture (alignment with neurites) | GFAP, S100, p75NTR, Krox20, PMP22, MPZ, MBP | no sorting, 60% GFAP+/S100+ |
| Study | Cell Source (Number of Cell Lines) | Duration | Characterization | Schwann Cell Markers | Sorting Steps/Yield | Comments |
|---|---|---|---|---|---|---|
| Hörner et al., 2021 [118] | hiPSC (2) | 31 d to SC identity (maturation after 50 d in total) | IF, morphometric analysis, neuron coculture (alignment with neurites) | SOX10, S100b, Vimentin, GAP43, MPZ | no sorting, 90% SOX10+ SCP on d19, 83.4% S100b+/80.9% MPZ+ SC | protocol based on Kim et al., 2017 [121] (Table 1); with modifications (tuned activation of BMP signaling, further maturation step) |
| Carrió et al., 2019 [131] | hiPSC (2 control, 2 disease) | >50 d (20 d to NCC, 30 d to SC) | IF, RT-qPCR, neuron coculture (alignment with neurites for control SC) | SOX10, p75NTR, S100b, GAP43, MPZ, PLP, PMP22, KROX20 | no sorting, ~90% p75NTR+/HNK1+ NCC, final SC yield not reported | NCC protocol based on Menendez et al., 2013 [127] with modifications, disease hiPSC derived from primary neurofibroma tissue |
| Shi et al., 2018 [116] | hiPSC (2 control, 2 disease) | >100 d | IF, RT-qPCR, RNA-seq, in vitro myelination assay | GFAP, S100b, MPZ, MBP | FACS for p75NTR+/HNK1+ to purify NCC, final yield 74% S100b+ cells (4.8% for disease lines) | NCC protocol based on Li et al., 2015 [239] (cynomolgus monkey ESC), SC protocol based on Lee et al., 2007 [114] (hESC) |
| Muller et al., 2018 [120] | hiPSC (3) | 26 d to SC identity (44–63 d in total until experiment endpoint) | IF, neurite outgrowth assay | S100, GFAP, p75NTR, SOX10 | no sorting, yield not reported | protocol based on Kingham et al., 2007 [162]/Dezawa et al., 2001 [163] (rat ADSC/MSC, Table 4); transferred to hiPSC, with modifications for induction and maturation |
| Huang et al., 2017 [117] | hiPSC (not specified) | 59 d | IF, neurotrophic factors secretion (ELISA), in vivo transplantation assay | SOX10, S100b, GFAP | MACS for p75NTR+, FACS for HNK1+/SEEA4- to purify NCC, final SC yield not reported | protocol based on Lee et al., 2007 [114] (hESC), with modifications for NCC and SC induction |
| Sugiyama-Nakagiri et al., 2016 [237] | hiPSC (1) | 36 d (15 d to SKP, 21 d to SC) | IF | S100b | no sorting, yield not reported (97% SKP stage) | protocol to differentiate SKP from hiPSC, differentiation potential to SC lineage demonstrated |
| Kreitzer et al., 2013 [134] | hESC (1) hiPSC (4) | 8 d to NCC, not given for SC | IF | GFAP | FACS for p75NTR+/HNK1+ to purify NCC, no yield reported for SC | protocol for NCC, demonstration of spontaneous differentiation into GFAP+ putative SC in mixed cultures |
| Wang et al., 2011 [142] | hESC (2) hiPSC (5) | >22 d to NCC, not given for SC | IF | GFAP, S100b | picking of colonies, FACS for p75NTR+; no yield reported for SC | protocol for NCC, demonstration of differentiation capacity towards SC lineage |
| Lee et al., 2007 [114] Lee et al., 2010 [286] | hESC (3) | 28–35 d to NCC, >100 d to SC | IF | S100b, GFAP, MBP | FACS for p75NTR+/HNK1+ to purify NCC, final SC yield < 10% | protocol for NCC, demonstration of differentiation capacity towards SC |
| Study | Cell Source | Duration | Characterization | Schwann Cell Markers | Sorting Steps/Yield | Comments |
|---|---|---|---|---|---|---|
| Kim et al., 2020 [224] | human skin fibroblasts | 35 d | IF, RT-qPCR, WB, neurotrophic factors secretion (ELISA), neurite outgrowth assay, in vitro myelination assay, in vivo transplantation assay | SOX10, GFAP, p75NTR, GAP43, S100b, PMP22, MPZ, MBP | SCP colony picking on d18, 95% SOX10+ SCP, 95% S100b+ SC | based on Kim et al., 2017 [121] (Table 1); but with direct conversion from fibroblasts instead of hiPSC |
| Saulite et al., 2018 [201] | human dermis MSC | 8 d | IF, RT-qPCR | Sox10, p75NTR, GFAP, S100b, MBP | no sorting, 20–40% MBP+ | protocol based on Dezawa et al., 2001 [163] (rat MSC, Table 4) |
| Bajpai et al., 2017 [230] | human epidermal keratinocytes (neonate foreskin) | 7d to NCC, 35d to SC | IF, RT-qPCR | MPZ, PMP22, GFAP, S100b, Krox20 | no sorting, 94% S100b+ | clonal variability regarding differentiation capacity: 62.5% of clones could acquire SC fate |
| Mazzara et al., 2017 [177] | human skin fibroblasts, rodent skin fibroblasts (mouse embryonal/neonatal/adult, rat neonatal) | 21 d | IF, RT-qPCR, RNA-Seq, in vitro myelination assay, neurite outgrowth assay | S100, O4, MPZ, GFAP, MBP | 12.3% S100b+/O4+ (mouse embryonal)/5% (human) d14 → purification by FACS for O4+ | full characterization only done on rodent cells, but human derived cells were also shown to induce neurite outgrowth and align with neurites |
| Cai et al., 2017 [202] | human MSC | 56 d | IF, WB, neurotrophic factors secretion (ELISA), neurite outgrowth assay, in vitro myelination assay, in vivo transplantation assay | p75NTR, S100, MBP | no sorting, 84.9% S100+/p75NTR+ | protocol based on Dezawa et al., 2001 [163] (rat MSC, Table 4)/Zhang et al., 2009 [174] (human MSC, Table 3); maturation step: coculture with rat primary neurons for SC fate commitment |
| Jung et al., 2016 [203] | human tonsil-derived MSC | 16 d | IF, RT-qPCR, WB, neurite outgrowth assay, in vitro myelination assay, in vivo transplantation assay | p75NTR, S100b, Krox20, GFAP | no sorting, 67.6% p75NTR+ | protocol based on Dezawa et al., 2001 [163] (rat MSC, Table 4)/Zhang et al., 2009 [174] (human MSC, Table 3) |
| Sakaue et al., 2015 [215] | human epidermal NCC from hair bulge explants | 21–30 d | IF, RT-qPCR, microarray, neuron coculture (alignment with neurites) | Sox10, p75NTR, Krox20, S100b, GFAP, MPZ, MBP | no sorting, 90% Krox20+ | protocol based on Dezawa et al., 2001 [163] (rat MSC, Table 4) |
| Martens et al., 2014 [217] | human dental pulp stem cells | 18 d | IF, in vitro myelination assay, neurite outgrowth assay, neurotrophic factors secretion (ELISA) | P75, GFAP, S100 | no sorting, yield not reported | protocol based on Dezawa et al., 2001 [163] (rat MSC, Table 4) |
| Thoma et al., 2014 [228] | human foreskin fibroblasts | 39 d | IF, microarray, neurite outgrowth, in vitro myelination assay | Sox10, Krox20, PLP, GFAP, S100b, GalC, MBP | no sorting, 60% PLP+ | |
| Tomita et al., 2013 [204] | human ADSC | 18 d | IF, WB, morphometric analysis, neurotrophic factors secretion (ELISA), neurite outgrowth assay, in vivo transplantation assay | p75NTR, GFAP, S100 | no sorting, yield not reported | protocol based on Dezawa et al., 2001 [163] (rat MSC, Table 4) |
| Razavi et al., 2012 [205] | human ADSC | 16 d | IF, RT-qPCR | GFAP, S100 | no sorting, 90% GFAP+/S100+ | protocol based on Dezawa et al., 2001 [163] (rat MSC, Table 4)/Zhang et al., 2009 [174] (human MSC, Table 3) |
| Matsuse et al., 2010 [173] | human umbilical cord Wharton’s jelly-derived MSC | 8 d | IF, RT-PCR, in vivo transplantation assay | Sox10, Krox20, GFAP, p75NTR, S100b, MPZ | no sorting, 98% MPZ+ | protocol based on Dezawa et al., 2001 [163] (rat MSC, Table 4) |
| Zhang et al., 2009 [174] | human umbilical cord blood derived MSC | 24 d | IF, WB, neurite outgrowth assay | GFAP, S100 | no sorting, 60.8% GFAP+/S100+ | protocol based on Dezawa et al., 2001 [163] (rat MSC, Table 4)/Xu et al., 2008 [212] (rat ADSC, Table 4) → neurosphere induction |
| Shimizu et al., 2007 [206] | human MSC | 11 d | IF, in vivo transplantation assay | S100, MPZ, p75NTR, GFAP, O4 | no sorting, yield not reported | protocol from Dezawa et al., 2001 [163] (rat MSC, Table 4) transferred to human MSC |
| Study | Cell Source | Duration | Characterization | Schwann Cell Markers | Sorting Steps/Yield | Comments |
|---|---|---|---|---|---|---|
| Pan et al., 2021 [223] | mouse embryonic fibroblasts | >49 d | IF | S100b, GFAP | FACS purification for NCC, SC yield not reported | |
| Xie et al., 2017 [207] | rat ADSC | 21 d | IF, RT-qPCR, WB, in vitro myelination assay, neurotrophic factors secretion (ELISA) | S100, GFAP, MBP | purification of ADSCs by FACS for CD44+, 89.5% S100+ | combination of defined components and olfactory ensheathing cell conditioned medium |
| La Bierlein De Rosa et al., 2017 [208] | mouse MSC | 24 d | IF, neurite outgrowth assay, morphometric analysis | S100, S100b, p75NTR | no sorting, 23.1% S100b+, 52% p75NTR+ | based on Dezawa et al., 2001 [163] |
| Shea et al., 2010 [210] | rat MSC | 50 d (29 d + 21 d coculture) | IF, RT-qPCR, in vitro myelination assay | S100b, p75NTR, Sox10, GFAP, MPZ, MBP | no sorting, 98.9% S100b+, 97.9% p75NTR + (after DRG neuron coculture) | based on Dezawa et al., 2001 [163]; Xu et al., 2008 [212] (neurosphere induction); addition of maturation step: coculture with rat primary neurons for SC fate commitment |
| Wakao et al., 2010 [265] | cynomolgus monkey MSC | 9 d | IF, RT-PCR, in vivo transplantation assay | p75NTR, GFAP, MPZ, GFAP, Krox20, MBP | 99% p75NTR+ | protocol from Dezawa et al., 2001 [163] transferred to monkey MSC |
| Xu et al., 2008 [212] | rat ADSC | ca. 16 d (not precisely stated) | IF, neurite outgrowth assay, in vitro myelination assay | GFAP, S100, p75NTR | no sorting, 35% p75NTR+ (higher amount S100+/GFAP+) | combines neurospheres induction with Dezawa method |
| Kingham et al., 2007 [162] | rat ADSC | 18 d | IF, WB, neurite outgrowth assay | GFAP, S100, p75NTR | no sorting, 42.9% GFAP+/S100+; 81.5% spindle-like morphology | protocol similar to Dezawa et al., 2001 [163]; with modifications (longer differentiation time, higher forskolin concentration) |
| Roth et al., 2007 [240] | mouse ESC | 22–30 d | IF, RT-qPCR, neurite outgrowth assay, in vitro myelination assay | S100, GFAP, PMP22, MBP | no sorting, no yield reported | |
| McKenzie et al., 2006 [221] Biernaskie et al., 2006 [277] | human and rodent SKP (mouse embryo skin, rat neonate skin, human foreskin) | 28–42 d | IF, in vitro myelination assay, in vivo transplantation assay | S100b, PMP22, GFAP, p75NTR, MPZ, MBP | manual SC colony picking after 2–3 weeks, >95% (rodent, after expansion of picked SC colonies; not stated for human cells) | differentiation potential demonstrated for human SKP, but purification and extensive characterization mainly done with rodent SKP |
| Dezawa et al., 2001 [163] | rat MSC | 11 d | IF, in vivo transplantation assay | p75NTR, S100, GFAP, O4 (MBP in vivo) | no sorting, yield not reported | first report of in vitro differentiation of SC-like cells from stem cell source |
| Study | Cell Source | 3D Culture Matrix | Comparison to 2D | Comments |
|---|---|---|---|---|
| Podder et al., 2022 [352] | keratinocyte- derived NC-like cells from human epidermis | electrospun polycaprolactone (PCL) aligned fibers functionalized with Nrg1 | Nrg1-functionalized fibers improved differentiation | proliferation was reduced in 3D compared to 2D, expression of S100b/PLP1 increased when fibers were functionalized with Nrg1 |
| Entezari et al., 2022 [374] | human MSC from olfactory mucosa | 3D-printed PCL/ polypyrrole (PPy) conductive scaffolds | PPy-coated PCL scaffolds improved differentiation | coculture with PC12 cells, SC differentiation in 3D, SC marker expression and neurotrophic factor secretion increased on PPy/PCL compared to only PCL and 2D |
| Muller et al., 2018 [120] | human iPSC | collagen sponge | - | skin model, including hiPSC-derived sensory neurons, SC differentiation in 2D, matured in 3D |
| Bayat et al., 2016 [326] | human endometrial stem cells | fibrin gel | 3D culture in fibrin gel improved differentiation | |
| Martens et al., 2014 [217] | human dental pulp stem cells | collagen gel | - | SC differentiation in 2D, cocultures with rat DRG neurons in 3D |
| Ren et al., 2013 [272] | human ESC | electrospun polyethersulfone (PES) fiber matrices | aligned fibers improved differentiation | hESC differentiation to NCC in 2D, then 2 weeks SC differentiation in 2D, then 2 more weeks either 2D or 3D; comparison of different fiber topographies |
| Uz et al., 2017 [246] | rat MSC | gelatin based porous conduits | best results were obtained with largest pore size and lowest stiffness | comparison between ladder-like, macroporous, and nanofibrous structures (different pore sizes and elastic moduli) |
| Xue et al., 2017 [356] | rat MSC | electrospun PCL fibers | aligned fibers improved differentiation | cocultures with PC12 cells and chick DRG neurons, comparison of fiber alignment and diameters, differentiation further enhanced by coating aligned fibers with laminin |
| Chen et al., 2015 [363] | rat MSC | fibrin matrix | increased expression of myelin-related markers and neurotrophin secretion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hörner, S.J.; Couturier, N.; Gueiber, D.C.; Hafner, M.; Rudolf, R. Development and In Vitro Differentiation of Schwann Cells. Cells 2022, 11, 3753. https://doi.org/10.3390/cells11233753
Hörner SJ, Couturier N, Gueiber DC, Hafner M, Rudolf R. Development and In Vitro Differentiation of Schwann Cells. Cells. 2022; 11(23):3753. https://doi.org/10.3390/cells11233753
Chicago/Turabian StyleHörner, Sarah Janice, Nathalie Couturier, Daniele Caroline Gueiber, Mathias Hafner, and Rüdiger Rudolf. 2022. "Development and In Vitro Differentiation of Schwann Cells" Cells 11, no. 23: 3753. https://doi.org/10.3390/cells11233753
APA StyleHörner, S. J., Couturier, N., Gueiber, D. C., Hafner, M., & Rudolf, R. (2022). Development and In Vitro Differentiation of Schwann Cells. Cells, 11(23), 3753. https://doi.org/10.3390/cells11233753