Cell Sources for Retinal Regeneration: Implication for Data Translation in Biomedicine of the Eye
Abstract
1. Introduction
2. The Main Structure of the Retina
3. Brief Characteristics of the Major Degenerative Disorders of the Retina
4. Intrinsic Retinal Regeneration Cell Sources and Their Implication for Biomedicine of the Eye
4.1. Retinal Ciliary Zone Cells
4.2. Ciliary Body Cells
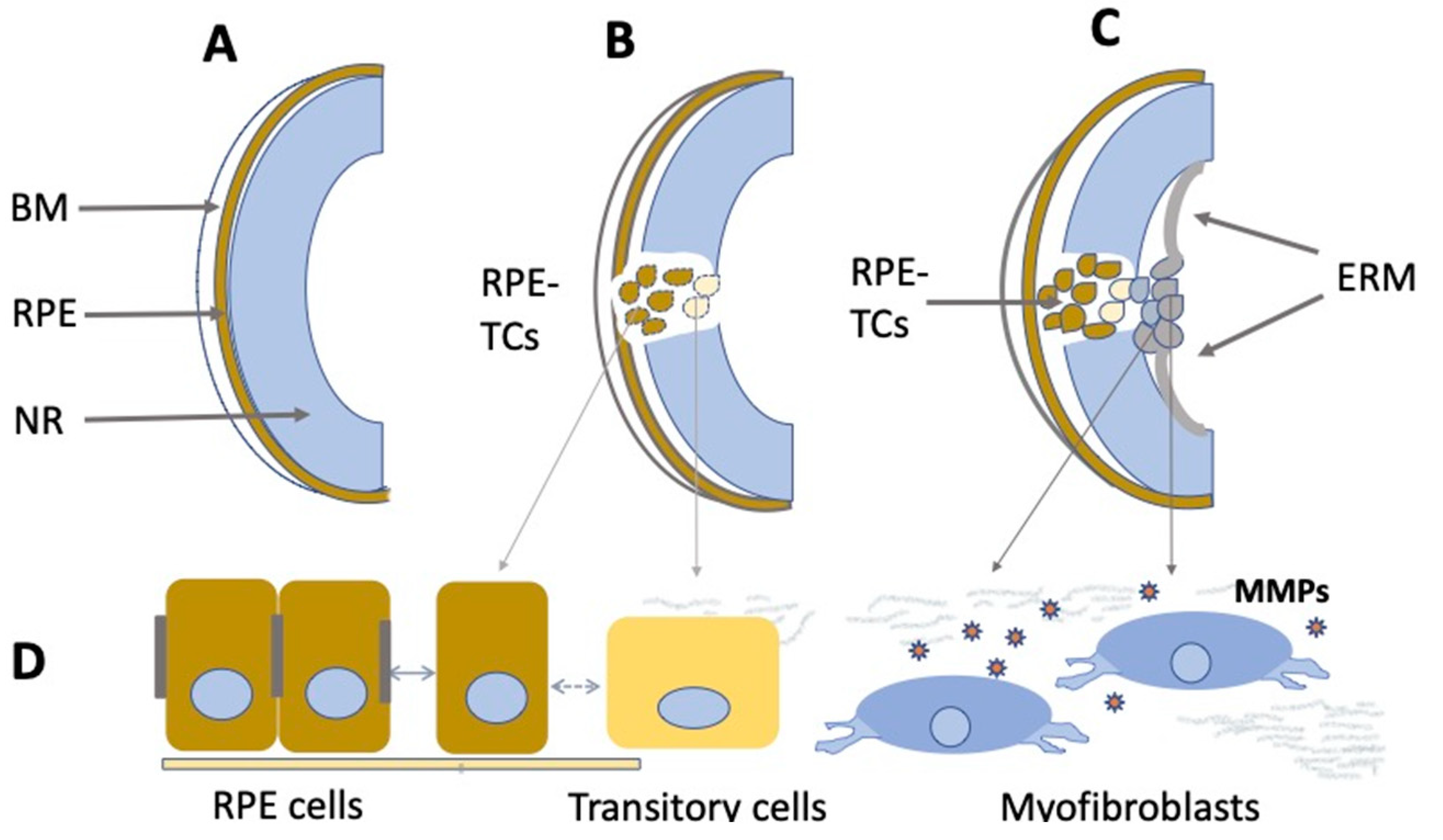
4.3. Retinal Pigment Epithelium Cells
4.4. Müller Glial Cells
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varma, R.; Vajaranant, T.S.; Burkemper, B.; Wu, S.; Torres, M.; Hsu, C.; Choudhury, F.; McKean-Codwin, P. Visual impairment and blindness in adults in the United States: Demographic and geographic variations from 2015 to 2050. JAMA Ophthalmol. 2016, 134, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Pardue, M.T.; Allen, R.S. Neuroprotective strategies for retinal disease. Prog. Retin. Eye Res. 2018, 65, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, A.; Close, J.; Reh, T.A. Retinal stem cells and regeneration. Int. J. Dev. Biol. 2004, 48, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Lamba, D.; Karl, M.; Reh, T. Neural regeneration and cell replacement: A view from the eye. Cell Stem Cell 2008, 2, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.O.; Reh, T.A. Regenerative medicine for retinal diseases: Activating endogenous repair mechanisms. Trends Mol. Med. 2010, 16, 193–202. [Google Scholar] [CrossRef]
- Wohl, S.G.; Schmeer, C.W.; Isenmann, S. Neurogenic potential of stem/progenitor-like cells in the adult mammalian eye. Prog. Retin. Eye Res. 2012, 31, 213–242. [Google Scholar] [CrossRef]
- Yu, H.; Vu, T.H.; Cho, K.S.; Guo, C.; Chen, D.F. Mobilizing endogenous stem cells for retinal repair. Transl. Res. 2014, 163, 387–398. [Google Scholar] [CrossRef]
- Madelaine, R.; Mourrain, P. Endogenous retinal neural stem cell reprogramming for neuronal regeneration. Neural. Reg. Res. 2017, 12, 1765–1767. [Google Scholar] [CrossRef]
- Grigoryan, E.N. Molecular Factors of the Maintenance and Activation of the Juvenile Phenotype of Cellular Sources for Eye Tissue Regeneration. Biochemistry 2018, 83, 1318–1331. [Google Scholar] [CrossRef]
- Grigoryan, E.N. Endogenous cell sources for eye retina regeneration in vertebrate animals and human. Russ. J. Dev. Biol. 2018, 49, 314–326. [Google Scholar] [CrossRef]
- Aladdad, A.M.; Kador, K.E. Adult Stem Cells, Tools for Repairing the Retina. Curr. Ophthalmol. Rep. 2019, 7, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.M.; Powner, M.B.; Carr, A.J.; Smart, M.J.; da Cruz, L.; Coffey, P.J. Stem cells in retinal regeneration: Past, present and future. Development 2013, 140, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- Sowden, J.C. ESC-derived retinal pigmented epithelial cell transplants in patients: So far, so good. Cell Stem Cell 2014, 15, 537–538. [Google Scholar] [CrossRef][Green Version]
- Jeon, S.; Oh, I.H. Regeneration of the retina: Toward stem cell therapy for degenerative retinal diseases. BMB Rep. 2015, 48, 193–199. [Google Scholar] [CrossRef]
- Nazari, H.; Zhang, L.; Zhu, D.; Chader, G.J.; Falabella, P.; Stefanini, F.; Rowland, T.; Clegg, D.O.; Kashani, A.H.; Hinton, D.R.; et al. Stem cell-based therapies for age-related macular degeneration: The promises and the challenges. Prog. Retin. Eye Res. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Singh, M.S.; Park, S.S.; Albini, T.A.; Canto-Soler, M.V.; Klassen, H.; MacLaren, R.E.; Takahashi, M.; Nagiel, A.; Schwartz, S.D.; Bharti, K. Retinal stem cell transplantation: Balancing safety and potential. Prog. Retin. Eye Res. 2020, 75, 100779. [Google Scholar] [CrossRef] [PubMed]
- German, O.L.; Vallese-Maurizi, H.; Soto, T.B.; Rotstein, N.P.; Politi, L.E. Retina stem cells, hopes and obstacles. World J. Stem Cells 2021, 13, 1446–1479. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt‘s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Kim, G.; Lee, R.D. Survival of Transplanted Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in a Human Recipient for 22 Months. JAMA Ophthalmol. 2017, 135, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- Han, I.C.; Bohrer, L.; Gibson-Corley, K.; Wiley, L.A.; Shrestha, A.; Harman, B.E.; Jiao, C.; Sohn, E.H.; Wendland, R.; Allen, B.N.; et al. Biocompatibility of Human Induced Pluripotent Stem Cell-Derived Retinal Progenitor Cell Grafts in Immunocompromised Rats. Cell Transplant. 2022, 31, 9636897221104451. [Google Scholar] [CrossRef]
- Masland, R.H. The Neuronal Organization of the Retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Alm, A. Ocular circulation. In Adler’s Physiology of the Eye: Clinical Application; Hart, W.M., Ed.; Mosby: St. Louis, MO, USA, 1992; pp. 198–227. [Google Scholar]
- Fernandes, A.-R.; Zielińska, A.; Sanchez-Lopez, E.; dos Santos, T.; Garcia, M.L.; Silva, A.M.; Karczewski, J.; Souto, E.B. Exudative versus Nonexudative Age-Related Macular Degeneration: Physiopathology and Treatment Options. Int. J. Mol. Sci. 2022, 23, 2592. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaini, H.; Schneiders, M.; Lundh, P.; Jeffery, G. Drusen are associated with local and distant disruptions to human retinal pigment epithelium cells. Exp. Eye Res. 2009, 88, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Patel, M.; Chan, C.C. Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 2009, 28, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chirco, K.; Sohn, E.; Stone, E.; Tucker, B.; Mullins, R. Structural and molecular changes in the aging choroid: Implications for age-related macular degeneration. Eye 2017, 31, 10. [Google Scholar] [CrossRef]
- Novikova, Y.P.; Gancharova, O.S.; Eichler, O.V.; Philippov, P.P.; Grigoryan, E.N. Preventive and Therapeutic Effects of SkQ1-Containing Visomitin Eye Drops against Light-Induced Retinal Degeneration. Biochemistry 2014, 79, 1101–1110. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Ciulla, T.A.; Ciardella, A.P.; Devin, F.; Dugel, P.U.; Eandi, C.M.; Masonson, H.; Monés, J.; Pearlman, J.A.; Quaranta-El Maftouhi, M.; et al. Dual antagonism of PDGF and VEGF in neovascular age-related macular degeneration: A phase IIb, multicenter, randomized controlled trial. Ophthalmology 2017, 124, 224–234. [Google Scholar] [CrossRef]
- Ammar, M.J.; Hsu, J.; Chiang, A.; Ho, A.C.; Regillo, C.D. Age-related macular degeneration therapy: A review. Curr. Opin. Ophthalmol. 2020, 31, 215–221. [Google Scholar] [CrossRef]
- Han, J.W.; Choi, J.; Kim, Y.S.; Kim, J.; Brinkmann, R.; Lyu, J.; Park, T.K. Comparison of the neuroinflammatory responses to selective retina therapy and continuous-wave laser photocoagulation in mouse eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 341–353. [Google Scholar] [CrossRef]
- Khanna, S.; Komati, R.; Eichenbaum, D.A.; Hariprasad, I.; Ciulla, T.A.; Hariprasad, S.M. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: A comparative review. BMJ Open Ophthalmol. 2019, 4, e000398. [Google Scholar] [CrossRef]
- Morescalchi, F.; Duse, S.; Gambicorti, E.; Romano, M.R.; Costagliola, C.; Semeraro, F. Proliferative Vitreoretinopathy after Eye Injuries: An Overexpression of Growth Factors and Cytokines Leading to a Retinal Keloid. Mediat. Inflamm. 2013, 2013, 269787. [Google Scholar] [CrossRef]
- Idrees, S.; Sridhar, J.; Kuriyan, A.E. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019, 59, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Shan, C.; Ma, L.; Liu, J.; Yang, N.; Zhao, J. Polarity and epithelial-mesenchymal transition of retinal pigment epithelial cells in proliferative vitreoretinopathy. Peer J. 2020, 8, e10136. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Midena, E.; Al-Shabrawey, M.; Mohammad, G. New Developments in the Pathophysiology and Management of Diabetic Retinopathy. J. Diabetes Res. 2013, 2013, 424258. [Google Scholar] [CrossRef]
- Lopez, P.F.; Yan, Q.; Kohen, L.; Rao, N.A.; Spee, C.; Black, J.; Oganesian, A. Retinal pigment epithelial wound healing in vivo. Arch Ophthalmol. 1995, 113, 1437–1446. [Google Scholar] [CrossRef]
- Grigoryan, E.N. Pigment Epithelia of the Eye: Cell-Type Conversion in Regeneration and Disease. Life 2022, 12, 382. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Pach, J.; Gekeler, F. Therapeutic approaches for retinitis pigmentosa. Klin. Monbl. Augenheilkd. 2013, 230, 512–518. [Google Scholar]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Cholkar, K.; Trinh, H.M.; Pal, D.; Mitra, A.K. Discovery of novel inhibitors for the treatment of glaucoma. Expert Opin. Drug Discov. 2015, 10, 293–313. [Google Scholar] [CrossRef]
- Prum, B.E., Jr.; Herndon, L.W., Jr.; Moroi, S.E.; Mansberger, S.L.; Stein, J.D.; Lim, M.C.; Rosenberg, L.F.; Gedde, S.J.; Williams, R.D. Primary Angle Closure Preferred Practice Pattern® Guidelines. Ophthalmology 2016, 123, P1–P40. [Google Scholar] [CrossRef]
- Grigoryan, E.N. Potential Endogenous Cell Sources for Retinal Regeneration in Vertebrates and Humans: Progenitor Traits and Specialization. Biomedicines 2020, 8, 208. [Google Scholar] [CrossRef]
- Harris, W.A.; Perron, M. Molecular recapitulation: The growth of the vertebrate retina. Int. J. Dev. Biol. 1998, 42, 299–304. [Google Scholar]
- Hitchcock, P.F.; Raymond, P.A. The teleost retina as a model for developmental and regeneration biology. Zebrafish 2004, 1, 257–271. [Google Scholar] [CrossRef]
- Johns, P.R. Growth of the adult goldfish eye. III. Source of the new retinal cells. J. Comp. Neurol. 1977, 176, 343–357. [Google Scholar] [CrossRef]
- Mitashov, V.I.; Panova, I.G.; Koussoulakos, S. Transdifferentiation potencies of ciliary and pigment epithelium cells of lower vertebrates and mammals. Russ. J. Dev. Biol. 2004, 35, 395–403. [Google Scholar]
- Fischer, A.J.; Bosse, J.L.; El-Hodiri, H.M. The ciliary marginal zone (CMZ) in development and regeneration of the vertebrate eye. Exp. Eye Res. 2013, 116, 199–204. [Google Scholar] [CrossRef]
- Raymond, P.; Barthel, L.K.; Bernardos, R.L.; Perkowski, J.J. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev. Biol. 2006, 6, 36. [Google Scholar] [CrossRef]
- Borday, C.; Cabochette, P.; Parain, K.; Mazurier, N.; Janssens, S.; Tran, H.T.; Sekkali, B.; Bronchain, O.; Vleminckx, K.; Locker, M.; et al. Antagonistic crossregulation between Wnt and Hedgehog signaling pathways control post-embryonic retinal proliferation. Development 2012, 139, 3499–3509. [Google Scholar] [CrossRef]
- Cerveny, K.L.; Varga, M.; Wilson, S.W. Continued growth and circuit building in the anamniote visual system. Dev. Neurobiol. 2012, 72, 328–345. [Google Scholar] [CrossRef]
- Zuber, M.E.; Gestri, G.; Viczian, A.S.; Barsacchi, G.; Harris, W.A. Specification of the vertebrate eye by a network of eye field transcription factors. Development 2003, 130, 5155–5167. [Google Scholar] [CrossRef]
- Viczian, A.S.; Solessio, E.C.; Lyou, Y.; Zuber, M.E. Generation of functional eyes from pluripotent cells. PLoS Biol. 2009, 7, e1000174. [Google Scholar] [CrossRef]
- Wang, J.C.; Harris, W.A. The role of combinational coding by homeodomain and bhlh transcription factors in retinal cell fate specification. Dev. Biol. 2005, 285, 101–115. [Google Scholar] [CrossRef]
- Wehman, A.M.; Staub, W.; Meyers, J.R.; Raymond, P.A.; Baier, H. Genetic dissection of the zebrafish retinal stem-cell compartment. Dev. Biol. 2005, 281, 53–65. [Google Scholar] [CrossRef][Green Version]
- Otteson, D.C.; Hitchcock, P.F. Stem cells in the teleost retina: Persistent neurogenesis and injury-induced regeneration. Vis. Res. 2003, 43, 927–936. [Google Scholar] [CrossRef]
- Stenkamp, D.L. Neurogenesis in the Fish Retina. Int. Rev. Cytol. 2007, 259, 173–224. [Google Scholar]
- Perron, M.; Kanekar, S.; Vetter, M.L.; Harris, W.A. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev. Biol. 1998, 199, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Centanin, L.; Ander, J.J.; Hoeckendorf, B.; Lust, K.; Kellner, T.; Kraemer, I.; Urbany, C.; Hasel, E.; Harris, W.A.; Simons, B.D.; et al. Exclusive multipotency and preferential asymmetric divisions in post-embryonic neural stem cells of the fish retina. Development 2014, 141, 3472–3482. [Google Scholar] [CrossRef]
- Shi, D.; Tavhelidse, T.; Thumberger, T.; Wittbrodt, J.; Greb, T. Bifacial stem cell niches in fish and plants. Curr. Opin. Genet. Dev. 2017, 45, 28–33. [Google Scholar] [CrossRef]
- Wan, Y.; Almeida, A.D.; Rulands, S.; Chalour, N.; Muresan, L.; Wu, Y.; Simons, B.D.; He, J.; Harris, W.A. The ciliary marginal zone of the zebrafish retina: Clonal and time-lapse analysis of a continuously growing tissue. Development 2016, 143, 1099–1107. [Google Scholar] [CrossRef]
- Martinez-De Luna, R.I.; Kelly, L.E.; El-Hodiri, H.M. The Retinal Homeobox (Rx) gene is necessary for retinal regeneration. Dev. Biol. 2011, 353, 10–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pan, Y.; Kelly, L.E.; El-Hodiri, H.M. Identification of retinal homeobox (rax) gene-dependent genes by a microarray approach: The DNA endoglycosylase neil3 is a major downstream component of the rax genetic pathway. Dev. Dyn. 2018, 247, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Markitantova, Y.V.; Makar’ev, E.O.; Smirnova, Y.A.; Zinov’eva, R.D.; Mitashov, V.I. Analysis of the expression pattern of regulatory genes Pax6, Prox1, and Six3 during regeneration of eye structures in the newt. Biol. Bull. 2004, 31, 428–436. [Google Scholar] [CrossRef]
- Avdonin, P.P.; Markitantova, Y.V.; Zinov’eva, R.D.; Mitashov, V.I. Expression of regulatory genes Pax6, Otx2, Six3, and FGF2 during newt retina regeneration. Biol. Bull. 2008, 35, 355–361. [Google Scholar] [CrossRef]
- Avdonin, P.P.; Grigoryan, E.N.; Markitantova, Y.V. Transcriptional factor Pitx2: Localization during triton retina regeneration. Biol. Bull. 2010, 37, 231–235. [Google Scholar] [CrossRef]
- Miles, A.; Tropepe, V. Retinal Stem Cell ‘Retirement Plans’: Growth, Regulation and Species Adaptations in the Retinal Ciliary Marginal Zone. Int. J. Mol. Sci. 2021, 22, 6528. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Lust, K.; Wittbrodt, J. Igf signaling couples retina growth with body growth by modulating progenitor cell division. Development 2021, 148, dev199133. [Google Scholar] [CrossRef] [PubMed]
- Ghai, K.; Stanke, J.J.; Fischer, A.J. Patterning of the circumferential marginal zone of progenitors in the chicken retina. Brain Res. 2008, 1192, 76–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiyama, T.; Li, H.; Gupta, M.; Lin, Y.P.; Chuang, A.Z.; Otteson, D.C.; Wang, S.W. Distinct neurogenic potential in the retinal margin and the pars plana of mammalian eye. J. Neurosci. 2012, 32, 12797–12807. [Google Scholar] [CrossRef] [PubMed]
- Todd, L.; Suarez, L.; Squires, N.; Zelinka, C.P.; Gribbins, K.; Fischer, A.J. Comparative analysis of glucagonergic cells, glia and the circumferential marginal zone in the reptilian retina. J. Comp. Neurol. 2016, 1, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Prada, C.; Puga, J.; Perez-Mendez, L.; López, A.R.; Ramírez, G. Spatial and temporal patterns of neurogenesis in the chick retina. Eur. J. Neurosci. 1991, 3, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J. Neural regeneration in the chick retina. Prog. Retin. Eye Res. 2005, 24, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.J.; Reh, T.A. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev. Biol. 2000, 220, 197–210. [Google Scholar] [CrossRef]
- Fischer, A.J.; Dierks, B.D.; Reh, T.A. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development 2002, 129, 2283–2291. [Google Scholar] [CrossRef]
- Kubota, R.; Hokoc, J.N.; Moshiri, A.; McGuire, C.; Reh., T.A. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Brain Res. Dev. Brain Res. 2002, 134, 31–41. [Google Scholar] [CrossRef]
- Amato, M.A.; Arnault, E.; Perron, M. Retinal stem cells in vertebrates: Parallels and divergences. Int. J. Dev. Biol. 2004, 48, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Fiore, L.; Carreño, C.O.; Medori, M.; Spelzini, G.; Sanchez, V.; Scicolone, G. NeurospheRes. obtained from the ciliary margin of the chicken eye possess positional values and retinal ganglion cells differentiated from them respond to EphA/ephrin-A system. Exp. Eye Res. 2022, 217, 108965. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.L.; Singhal, S.; Lawrence, J.M.; Khaw, P.T.; Limb, G.A. Distribution of Muller stem cells within the neural retina: Evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp. Eye Res. 2009, 89, 373–382. [Google Scholar] [CrossRef]
- Marcucci, F.; Murcia-Belmonte, V.; Coca, Y.; Ferreiro-Galve, S.; Kuwajima, N.; Khalid, S.; Ross, M.E.; Mason, C.; Herrera, E. The ciliary margin zone of the mammalian retina generates retinal ganglion cells. Cell Rep. 2016, 17, 3153–3164. [Google Scholar] [CrossRef]
- Martinez-Morales, J.R.; Signore, M.; Acampora, D.; Simeone, A.; Bovolenta, P. Otx genes are required for tissue specification in the developing eye. Development 2001, 128, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Horsford, D.J.; Nguyen, M.T.; Sellar, G.C.; Kothary, R.; Arnheiter, H.; McInnes, R.R. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development 2005, 132, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, A.; Ozone, C.; Nakano, T.; Saito, K.; Eiraku, M.; Sasai, Y. Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat. Commun. 2015, 6, 6286. [Google Scholar] [CrossRef] [PubMed]
- Belanger, M.C.; Robert, B.; Cayouette, M. Msx1-Positive progenitors in the retinal ciliary margin give rise to both neural and non-neural progenies in mammals. Dev. Cell 2017, 40, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Jian, Q.; Xu, H.; Xie, H.; Tian, C.; Zhao, T.; Yin, Z.Q. Activation of retinal stem cells in the proliferating marginal region of RCS rats during development of retinitis pigmentosa. Neurosci. Lett. 2009, 465, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Too, L.K.; Shen, W.; Mammo, Z.; Osaadon, P.; Gillies, M.C.; Simunovic, M.P. Surgical retinal explants as a source of retinal progenitor cells. Retina 2021, 41, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Ducournau, Y.; Boscher, C.; Adelman, R.A.; Guillaubey, C.; Schmidt-Morand, D.; Mosnier, J.F.; Ducournau, D. Proliferation of the ciliary epithelium with retinal neuronal and photoreceptor cell differentiation in human eyes with retinal detachment and proliferative vitreoretinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, A.P.; Lust, K.; Wittbrodt, J. Notch signaling patterns retinal composition by regulating atoh7 during post-embryonic growth. Development 2018, 145, dev169698. [Google Scholar] [CrossRef] [PubMed]
- Locker, M.; Agathocleous, M.; Amato, M.A.; Parain, K.; Harris, W.A.; Perron, M. Hedgehog signaling and the retina: Insights into the mechanisms controlling the proliferative properties of neural precursors. Genes Dev. 2006, 20, 3036–3048. [Google Scholar] [CrossRef]
- Moshiri, A.; McGuire, C.R.; Reh, T.A. Sonic hedgehog regulates proliferation of the retinal ciliary marginal zone in posthatch chicks. Dev. Dyn. 2005, 233, 66–75. [Google Scholar] [CrossRef]
- Liu, H.; Thurig, S.; Mohamed, O.; Dufort, D.; Wallace, V.A. Mapping canonical Wnt signaling in the developing and adult retina. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5088–5097. [Google Scholar] [CrossRef]
- Denayer, T.; Locker, M.; Borday, C.; Deroo, T.; Janssens, S.; Hecht, A.; van Roy, F.; Perron, M.; Vleminckx, K. Canonical Wnt signaling controls proliferation of retinal stem/progenitor cells in postembryonic xenopus eyes. Stem Cells 2008, 26, 2063–2074. [Google Scholar] [CrossRef]
- Kubo, F.; Nakagawa, S. Hairy1 acts as a node downstream of Wnt signaling to maintain retinal stem cell-like progenitor cells in the chick ciliary marginal zone. Development 2009, 136, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.R.; Hu, L.; Moses, A.; Kaboli, K.; Papandrea, A.; Raymond, P.A. β-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural. Dev. 2012, 7, 30. [Google Scholar] [CrossRef]
- Diacou, R.; Zhao, Y.; Zheng, D.; Cvekl, A.; Liu, W. Six3 and Six6 Are Jointly Required for the Maintenance of Multipotent Retinal Progenitors through Both Positive and Negative Regulation. Cell Rep. 2018, 25, 2510–2523. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; Omar, G.; Walton, N.A.; Verrill, T.A.; Unson, C.G. Glucagon-expressing neurons within the retina regulate the proliferation of neural progenitors in the circumferential marginal zone of the avian eye. J. Neurosci. 2005, 25, 10157–10166. [Google Scholar] [CrossRef] [PubMed]
- Angileri, K.M.; Gross, J.M. dnmt1 function is required to maintain retinal stem cells within the ciliary marginal zone of the zebrafish eye. Sci. Rep. 2020, 10, 11293. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.; Shine, L.; Heffernan, T.; Deeti, S.; Reynolds, A.L.; O’Connor, J.J.; Dillon, E.T.; Duffy, D.J.; Kolch, W.; Cagney, G.; et al. A brain-derived neurotrophic factor mimetic is sufficient to restore cone photoreceptor visual function in an inherited blindness model. Sci. Rep. 2017, 7, 11320. [Google Scholar] [CrossRef]
- Wallman, J.; Winawer, J. Homeostasis of eye growth and the question of myopia. Neuron 2004, 43, 447–468. [Google Scholar] [CrossRef]
- Karl, M.O.; Hayes, S.; Nelson, B.R.; Tan, K.; Buckingham, B.; Reh, T.A. Stimulation of neural regeneration in the mouse retina. Proc. Natl. Acad. Sci. USA 2008, 105, 19508–19513. [Google Scholar] [CrossRef] [PubMed]
- Napier, H.R.; Kidson, S.H. Molecular events in early development of the ciliary body: A question of folding. Exp. Eye Res. 2007, 84, 615–625. [Google Scholar] [CrossRef] [PubMed]
- McDougal, D.H.; Gamlin, P.D. Autonomic control of the eye. Comp. Physiol. 2015, 5, 439–473. [Google Scholar]
- Nickerson, P.E.; Emsley, J.G.; Myers, T.; Clarke, D.B. Proliferation and expression of progenitor and mature retinal phenotypes in the adult mammalian ciliary body after retinal ganglion cell injury. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5266–5275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ooto, S.; Akagi, T.; Kageyama, R.; Akita, J.; Mandai, M.; Honda, Y.; Takahashi, M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc. Natl. Acad. Sci. USA 2004, 101, 13654–13659. [Google Scholar] [CrossRef]
- Wohl, S.G.; Schmeer, C.W.; Kretz, A.; Witte, O.W.; Isenmann, S. Optic nerve lesion increases cell proliferation and nestin expression in the adult mouse eye in vivo. Exp. Neurol. 2009, 219, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Del Debbio, C.B.; Santos, M.F.; Yan, C.Y.; Ahmad, I.; Hamassaki, D.E. Rho GTPases control ciliary epithelium cells proliferation and progenitor profile induction in vivo. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2631–2641. [Google Scholar] [CrossRef]
- Ahmad, I.; Tang, L.; Pham, H. Identification of neural progenitors in the adult mammalian eye. Biochem. Biophys. Res. Commun. 2000, 270, 517–521. [Google Scholar] [CrossRef]
- Das, A.V.; James, J.; Rahnenfuhrer, J.; Thoreson, W.B.; Bhattacharya, S.; Zhao, X.; Ahmad, I. Retinal properties and potential of the adult mammalian ciliary epithelium stem cells. Vis. Res. 2005, 45, 1653–1666. [Google Scholar] [CrossRef] [PubMed]
- Lord-Grignon, J.; Abdouh, M.; Bernier, G. Identification of genes expressed in retinal progenitor/stem cell colonies isolated from the ocular ciliary body of adult mice. Gene Expr. Patterns 2006, 6, 992–999. [Google Scholar] [CrossRef]
- MacNeil, A.; Pearson, R.A.; MacLaren, R.E.; Smith, A.J.; Sowden, J.C.; Ali, R.R. Comparative analysis of progenitor cells isolated from the iris, pars plana, and ciliary body of the adult porcine eye. Stem Cells 2007, 25, 2430–2438. [Google Scholar] [CrossRef]
- Martinez-Navarrete, G.C.; Angulo, A.; Martin-Nieto, J.; Cuenca, N. Gradual morphogenesis of retinal neurons in the peripheral retinal margin of adult monkeys and humans. J. Comp. Neurol. 2008, 511, 557–580. [Google Scholar] [CrossRef] [PubMed]
- Tropepe, V.; Coles, B.L.; Chiasson, B.J.; Horsford, D.J.; Elia, A.J.; McInnes, R.R.; van der Kooy, D. Retinal stem cells in the adult mammalian eye. Science 2000, 287, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Coles, B.L.; Angenieux, B.; Inoue, T.; del Rio-Tsonis, K.; Spence, J.R.; McInnes, R.R.; Arsenijevic, Y.; van der Kooy, D. Facile isolation and the characterization of human retinal stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 15772–15777. [Google Scholar] [CrossRef] [PubMed]
- Cicero, S.A.; Johnson, D.; Reyntjens, S.; Frase, S.; Connell, S.; Chow, L.M.; Baker, S.J.; Sorrentino, B.P.; Dyer, M.A. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc. Natl. Acad. Sci. USA 2009, 106, 6685–6690. [Google Scholar] [CrossRef] [PubMed]
- Gualdoni, S.; Baron, M.; Lakowski, J.; Decembrini, S.; Smith, A.J.; Pearson, R.A.; Ali, R.R.; Sowden, J.C. Adult ciliary epithelial cells, previously identified as retinal stem cells with potential for retinal repair, fail to differentiate into new rod photoreceptors. Stem Cells 2010, 28, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Coles, B.L.; Dorval, K.; Bremner, R.; Bessho, Y.; Kageyama, R.; Hino, S.l.; Matsuoka, M.; Craft, C.M.; McInnes, R.R.; et al. Maximizing functional photoreceptor differentiation from adult human retinal stem cells. Stem Cells 2010, 28, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Ballios, B.J.; Clarke, L.; Coles, B.L.; Shoichet, M.S.; van der Kooy, D. The adult retinal stem cell is a rare cell in the ciliary epithelium whose progeny can differentiate into photoreceptors. Biol. Open 2012, 1, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Nogales, M.; Murcia-Belmonte, V.; Chen, H.Y.; Herrera, E. The peripheral eye: A neurogenic area with potential to treat retinal pathologies? Prog. Retin. Eye Res. 2019, 68, 110–123. [Google Scholar] [CrossRef]
- Guduric-Fuchs, J.; Chen, W.; Price, H.; Archer, D.B.; Cogliati, T. RPE and neuronal differentiation of allotransplantated porcine ciliary epithelium-derived cells. Mol. Vis. 2011, 17, 2580. [Google Scholar] [PubMed]
- Ahmad, I.; Das, A.V.; James, J.; Bhattacharya, S.; Zhao, X. Neural stem cells in the mammalian eye: Types and regulation. Semin. Cell Dev. Biol. 2004, 15, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Das, A.V.; James, J.; Zhao, X.; Rahnenführer, J.; Ahmad, I. Identification of c-Kit receptor as a regulator of adult neural stem cells in the mammalian eye: Interactions with Notch signaling. Dev. Biol. 2004, 273, 87–105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pang, J.; Le, L.; Zhou, Y.; Tu, R.; Hou, Q.; Tsuchiya, D.; Thomas, N.; Wang, Y.; Yu, Z.; Alexander, R.; et al. NOTCH Signaling Controls Ciliary Body Morphogenesis and Secretion by Directly Regulating Nectin Protein Expression. Cell Rep. 2021, 34, 108603. [Google Scholar] [CrossRef] [PubMed]
- Jasty, S.; Krishnakumar, S. Profiling of DNA and histone methylation reveals epigenetic-based regulation of gene expression during retinal differentiation of stem/progenitor cells isolated from the ciliary pigment epithelium of human cadaveric eyes. Brain Res. 2016, 165115, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Hicks, D.; Hamel, C.P. The Retinal Pigment Epithelium in Health and Disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, S.; Zou, C.J.; Levine, E.M. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp. Eye Res. 2014, 123, 141–150. [Google Scholar] [CrossRef]
- Lakkaraju, A.; Umapathy, A.; Tan, L.X.; Daniele, L.; Philp, N.J.; Boesze-Battaglia, K.; Williams, D.S. The cell biology of the retinal pigment epithelium. Prog. Retin. Eye Res. 2020, 78, 100846. [Google Scholar] [CrossRef] [PubMed]
- Koster, C.; Wever, K.E.; Wagstaff, P.E.; van den Hirk, K.T.; Hooijmans, C.R.; Bergen, A.A. A Systematic Review on Transplantation Studies of the Retinal Pigment Epithelium in Animal Models. Int. J. Mol. Sci. 2020, 21, 2719. [Google Scholar] [CrossRef] [PubMed]
- Caceres, P.S.; Rodriguez-Boulan, E. Retinal Pigment Epithelium Polarity in Health and Blinding Diseases. Curr. Opin. Cell Biol. 2020, 62, 37–45. [Google Scholar] [CrossRef]
- Markitantova, Y.; Simirskii, V. Inherited Eye Diseases with Retinal Manifestations through the Eyes of Homeobox Genes. Int. J. Mol. Sci. 2020, 21, 1602. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, E.N.; Markitantova, Y.V. Molecular Strategies for Transdifferentiation of Retinal Pigment Epithelial Cells in Amphibians and Mammals In Vivo. Russ. J. Dev. Biol. 2021, 52, 20–43. [Google Scholar] [CrossRef]
- George, S.; Lu, F.; Rao, M.; Leach, L.; Gross, J.M. The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems. Prog. Retin. Eye Res. 2021, 85, 100969. [Google Scholar] [CrossRef] [PubMed]
- Rizzolo, L.J.; Nasonkin, I.O.; Adelman, R.A. Retinal Cell Transplantation, Biomaterials, and In Vitro Models for Developing Next-generation Therapies of Age-related Macular Degeneration. Stem Cells Transl. Med. 2022, 11, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, E.N. Self-Organization of the Retina during Eye Development, Retinal Regeneration In Vivo, and in Retinal 3D Organoids In Vitro. Biomedicines 2022, 10, 1458. [Google Scholar] [CrossRef]
- Hanovice, N.J.; Leach, L.L.; Slater, K.; Gabriel, A.E.; Romanovicz, D.; Shao, E.; Collery, R.; Burton, E.A.; Lathrop, K.L.; Link, B.A.; et al. Regeneration of the zebrafish retinal pigment epithelium after widespread genetic ablation. PLoS Genet. 2019, 15, e1007939. [Google Scholar] [CrossRef]
- Leach, L.L.; Hanovice, N.J.; George, S.M.; Gabriel, A.E.; Gross, J.M. The immune response is a critical regulator of zebrafish retinal pigment epithelium regeneration. Proc. Natl. Acad. Sci. USA 2021, 118, 2017198118. [Google Scholar] [CrossRef]
- Keefe, J.R. An analysis of urodelean retinal regeneration. I–IV. J. Exp. Zool. 1973, 184, 185–257. [Google Scholar] [CrossRef]
- Mitashov, V.I. Mechanisms of retina regeneration in vertebrates. Int. J. Dev. Biol. 1996, 40, 833–844. [Google Scholar] [PubMed]
- Mitashov, V.I. Retinal regeneration in amphibians. Int. J. Dev. Biol. 1997, 41, 893. [Google Scholar]
- Chiba, C.; Mitashov, V.I. Cellular and molecular events in the adult newt retinal regeneration. In Strategies for Retinal Tissue Repair and Regeneration in Vertebrates: From Fish to Human; Chiba, C., Ed.; Research Signpost: Kerala, India, 2007; pp. 15–33. [Google Scholar]
- Yasumuro, H.; Sakurai, K.; Toyama, F.; Maruo, F.; Chiba, C. Implications of a Multi-Step Trigger of Retinal Regeneration in the Adult Newt. Biomedicines 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Maki, N.; Suetsugu-Maki, R.; Tarui, H.; Agata, K.; del Rio-Tsonis, K.; Tsonis, P.A. Expression of stem cell pluripotency factors during regeneration in newts. Dev. Dyn. 2009, 238, 1613–1616. [Google Scholar] [CrossRef]
- Islam, M.R.; Nakamura, K.; Casco-Robles, M.M.; Kunahong, A.; Inami, W.; Toyama, F.; Maruo, F.; Chiba, C. The newt reprograms mature RPE cells into a unique multipotent state for retinal regeneration. Sci. Rep. 2014, 4, 6043. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, J.; Chiba, C. Immunohistochemical analysis of Musashi-1 expression during retinal regeneration of adult newt. Neurosci. Lett. 2009, 450, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Makar’ev, E.O.; Zinov’eva, R.D.; Mitashov, V.I. Expression of regulatory homeobox genes during retina regeneration in adult newts. Izv Akad Nauk Ser Biol. 2002. 6, 663–667.
- Markitantova, Y.V.; Avdonin, P.P.; Grigoryan, E.N.; Zinovieva, R.D. Identification of the pitx1 embryogenesis regulatory gene in a regenerating newt retina. Dokl. Biol. Sci. 2010, 435, 421–424. [Google Scholar] [CrossRef]
- Casco-Robles, M.M.; Islam, M.R.; Inami, W.; Tanaka, H.V.; Kunahong, A.; Yasumuro, H.; Hanzawa, S.; Casco-Robles, R.M.; Toyama, F.; Maruo, F.; et al. Turning the fate of reprogramming cells from retinal disorder to regeneration by Pax6 in newts. Sci. Rep. 2016, 6, 33761. [Google Scholar] [CrossRef]
- Kaneko, Y.; Hirota, K.; Matsumoto, G.; Hanyu, Y. Expression pattern of a newt Notch homologue in regenerating newt retina. Brain Res. Dev. Brain Res. 2001, 31, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Chiba, C. Evidence for Notch signaling involvement in retinal regeneration of adult newt. Brain Res. 2007, 1136, 28–42. [Google Scholar] [CrossRef]
- Mercer, S.E.; Cheng, C.H.; Atkinson, D.L.; Krcmery, J.; Guzman, C.E.; Kent, D.T.; Zukor, K.; Marx, K.A.; Odelberg, S.J.; Simon, H.G. Multi-tissue microarray analysis identifies a molecular signature of regeneration. PLoS ONE 2012, 7, e52375. [Google Scholar] [CrossRef] [PubMed]
- Sherpa, T.; Lankford, T.; McGinn, T.E.; Hunter, S.S.; Frey, R.A.; Sun, C.; Ryan, M.; Robison, B.D.; Stenkamp, D.L. Retinal regeneration is facilitated by the presence of surviving neurons. Dev. Neurobiol. 2014, 74, 851–876. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.R.; Aycinena, J.-C.; del Rio-Tsonis, K. FGF-Hedgehog Interdependence During Retina Regeneration. Dev. Dyn. 2007, 236, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Kidd, A.R., 3rd; Thomas, J.L.; Poss, K.D.; Hyde, D.R.; Raymond, P.A.; Thummel, R. FGF signaling regulates rod photoreceptor cell maintenance and regeneration zebrafish. Exp. Eye Res. 2011, 93, 726–734. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fukui, L.; Henry, J.J. FGF signaling is required for lens regeneration in Xenopus laevis. Biol. Bull. 2011, 221, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Susaki, K.; Chiba, C. MEK mediates in vitro neural transdifferentiation of the adult newt retinal pigment epithelium cells: Is FGF2 an induction factor? Cell Res. 2007, 20, 364–679. [Google Scholar] [CrossRef] [PubMed]
- Markitantova, Y.V.; Avdonin, P.P.; Grigoryan, E.N. FGF2 signaling pathway components in tissues of the posterior eye sector in the adult newt Pleurodeles waltl. Biol. Bull. 2014, 41, 297–305. [Google Scholar] [CrossRef]
- Dvoriantchikova, D.; Seemungal, R.J.; Ivanov, D. The epigenetic basis for the impaired ability of adult murine retinal pigment epithelium cells to regenerate retinal tissue. Sci. Rep. 2019, 9, 3860. [Google Scholar] [CrossRef]
- Yoshii, C.; Ueda, Y.; Okamoto, M.; Araki, M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: Transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev. Biol. 2007, 303, 45–56. [Google Scholar] [CrossRef]
- Vergara, M.N.; del Rio-Tsonis, K. Retina regeneration in the Xenopus laevis tadpole: A new model system. Mol. Vis. 2009, 15, 1000–1013. [Google Scholar]
- Arresta, E.; Bernardini, S.; Bernardini, E.; Filoni, S.; Cannata, S.M. Pigmented epithelium to retinal transdifferentiation and Pax6 expression in larval Xenopus laevis. J. Exp. Zool. A Comp. Exp. Biol. 2005, 303, 958–967. [Google Scholar] [CrossRef]
- Nabeshima, A.; Nishibayashi, C.; Ueda, Y.; Ogino, H.; Araki, M. Loss of cell-extracellular matrix interaction triggers retinal regeneration accompanied by Rax and Pax6 activation. Genesis 2013, 51, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Andreazzoli, M.; Gestri, G.; Angeloni, D.; Menna, E.; Barsacchi, G. Role of Xrx1 in Xenopus eye and anterior brain development. Development. 1999, 126, 2451–2460. [Google Scholar] [CrossRef]
- Sakaguchi, D.S.; Janick, L.M.; Reh, T.A. Basic Fibroblast Growth Factor (FGF-2) induced transdifferentiation of retinal pigment epithelium: Generation of retinal neurons and glia. Dev. Dyn. 1997, 209, 387–398. [Google Scholar] [CrossRef]
- Naitoh, H.; Suganuma, Y.; Ueda, Y.; Sato, T.; Hiramuki, Y.; Fujisawa-Sehara, A.; Taketani, S.; Araki, M. Upregulation of matrix metalloproteinase triggers transdifferentiation of retinal pigmented epithelial cells in Xenopus laevis: A Link between inflammatory response and regeneration. Dev. Neurobiol. 2017, 77, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Ochi, H. Regeneration enhancers: A clue to reactivation of developmental genes. Dev. Growth Differ. 2020, 62, 343–354. [Google Scholar] [CrossRef]
- Coulombre, J.L.; Coulombre, A.J. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev. Biol. 1965, 12, 79–92. [Google Scholar] [CrossRef]
- Park, C.M.; Hollenberg, M.J. Growth factor-induced retinal regeneration in vivo. Int. Rev. Cytol. 1993, 146, 49–74. [Google Scholar] [PubMed]
- Haynes, T.; Luz-Madrigal, A.; Reis, E.S.; Echeverri Ruiz, N.P.; Grajales-Esquivel, E.; Tzekou, A.; Tsonis, P.A.; Lambris, J.D.; del Rio-Tsonis, K. Complement anaphylatoxin C3a is a potent inducer of embryonic chick retina regeneration. Nat. Commun. 2013, 4, 2312. [Google Scholar] [CrossRef]
- Zhu, J.; Luz-Madrigal, A.; Haynes, T.; Zavada, J.; Burke, A.K.; del Rio-Tsonis, K. Catenin Inactivation is a Pre-Requisite for Chick Retina Regeneration. PLoS ONE 2014, 9, e101748. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.R.; Madhavan, M.; Aycinena, J.-C.; del Rio-Tsonis, K. Retina regeneration in the chick embryo is not induced by spontaneous Mitf downregulation but requiRes. FGF/FGFR/MEK/Erk dependent upregulation of Pax6. Mol. Vis. 2007, 13, 57–65. [Google Scholar]
- Steinfeld, J.; Steinfeld, I.; Bausch, A.; Coronato, N.; Hampel, M.-L.; Depner, H.; Layer, P.G.; Vogel-Höpker, A. BMP-induced reprogramming of the neural retina into retinal pigment epithelium requiRes. Wnt signalling. Biol. Open 2017, 6, 979–992. [Google Scholar]
- Tangeman, J.A.; Luz-Madrigal, A.; Sreeskandarajan, S.; Grajales-Esquivel, E.; Liu, L.; Liang, C.H.; Tsonis, P.A.; del Rio-Tsonis, K. Transcriptome Profiling of Embryonic Retinal Pigment Epithelium Reprogramming. Genes 2021, 12, 840. [Google Scholar] [CrossRef]
- Luz-Madrigal, A.; Grajales-Esquivel, E.; Tangeman, J.; Kosse, S.; Liu, L.; Wang, K.; Fausey, A.; Liang, C.; Tsonis, P.A.; del Rio-Tsonis, K. DNA demethylation is a driver for chick retina regeneration. Epigenetics 2020, 15, 998–1019. [Google Scholar] [CrossRef]
- Xia, H.; Krebs, M.P.; Kaushal, S.; Scott, E.W. Enhanced retinal pigment epithelium regeneration after injury in MRL/MpJ mice. Exp. Eye Res. 2011, 93, 862–872. [Google Scholar] [CrossRef]
- Kampik, D.; Basche, M.; Luhmann, U.F.O.; Nishiguchi, K.M.; Williams, J.A.E.; Greenwood, J.; Moss, S.E.; Han, H.; Azam, S.; Duran, Y.; et al. In situ regeneration of retinal pigment epithelium by gene transfer of E2F2: A potential strategy for treatment of macular degenerations. Gene Ther. 2017, 24, 810–818. [Google Scholar] [CrossRef]
- Grigoryan, E.N.; Novikova, Y.P.; Kilina, O.V.; Philippov, P.P. New method of in vitro culturing of pigment retinal epithelium in the structure of the posterior eye sector of adult rat. Bull. Exp. Biol. Med. 2007, 144, 618–625. [Google Scholar] [CrossRef]
- Hadziahmetovic, M.; Dentchev, T.; Song, Y.; Haddad, N.; He, X.; Hahn, P.; Pratico, D.; Wen, R.; Harris, Z.L.; Lambris, J.D.; et al. Ceruloplasmin/ hephaestin knockout mice model morphologic and molecular features. of AMD. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2728–2736. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, R.; Fruttiger, M.; Douglas, R.H.; Martinez-Barbera, J.P.; Greenwood, J.; Moss, S.E. Genetic ablation of retinal pigment epithelial cells reveals the adaptive response of the epithelium and impact on photoreceptors. Proc. Natl. Acad. Sci. USA 2009, 106, 18728–18733. [Google Scholar] [CrossRef]
- Kolomeyer, A.M.; Zarbin, M.A. Trophic factors in the pathogenesis and therapy for retinal degenerative diseases. Surv. Ophthalmol. 2014, 59, 134–165. [Google Scholar] [CrossRef]
- Grigoryan, E.N. Shared triggering mechanisms of retinal regeneration in lower vertebrates and retinal rescue in higher ones. In Tissue Regeneration—From Basic Biology to Clinical Application; Davies, J., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 145–164. [Google Scholar]
- Al-Hussaini, H.; Kam, J.H.; Vugler, A.; Semo, M.; Jeffery, G. Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo. Mol. Vis. 2008, 14, 1784–1791. [Google Scholar]
- Kokkinopoulos, I.; Shahabi, G.; Colman, A.; Jeffery, G. Mature peripheral RPE cells have an intrinsic capacity to proliferate; a potential regulatory mechanism for age-related cell loss. PLoS ONE 2011, 6, e18921. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, B.; Sorgente, N. Pathogenesis of proliferative vitreoretinopathy. Modulation of retinal pigment epithelial cell functions by vitreous and macrophages. Dev. Ophthalmol. 1989, 16, 1–53. [Google Scholar] [PubMed]
- Abe, T.; Sato, M.; Tamai, M. Dedifferentiation of the retinal pigment epithelium compared to the proliferative membranes of proliferative vitreoretinopathy. Curr. Eye Res. 1998, 17, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, S.; Liu, L.; Kaplan, H.J. Epithelial-mesenchymal transition and proliferation of retinal pigment epithelial cells initiated upon loss of cell-cell contact. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2755–2763. [Google Scholar] [CrossRef]
- Wang, L.C.; Hung, K.H.; Hsu, C.C.; Chen, S.J.; Li, W.Y.; Lin, T.C. Assessment of retinal pigment epithelial cells in epiretinal membrane formation. J. Chin. Med. Assoc. 2015, 78, 370–373. [Google Scholar] [CrossRef]
- Wu, J.; Chen, X.; Liu, X.; Huang, S.; He, C.; Chen, B.; Liu, Y. Autophagy regulates TGF-beta2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells. Mol. Med. Rep. 2018, 17, 3607–3614. [Google Scholar]
- Garweg, J.G.; Tappeiner, C.; Halberstadt, M. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Surv. Ophthalmol. 2013, 58, 321–329. [Google Scholar] [CrossRef]
- Tamiya, S.; Kaplan, H.J. Role of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Exp. Eye Res. 2016, 142, 26–31. [Google Scholar] [CrossRef]
- Han, J.W.; Lyu, J.; Park, Y.J.; Jang, S.-Y.; Park, T.K. Wnt/β-catenin signaling mediates regeneration of retinal pigment epithelium after laser photocoagulation in mouse eye. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8314–8324. [Google Scholar] [CrossRef]
- Kent, D.; Sheridan, C. Choroidal neovascularization: A wound healing perspective. Mol. Vis. 2003, 9, 747–755. [Google Scholar]
- Ishikawa, K.; Kannan, R.; Hinton, D.R. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp. Eye Res. 2016, 142, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Philp, N.J.; Nachmias, V.T. Polarized distribution of integrin and fibronectin in retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1275–1280. [Google Scholar]
- Huang, X.; Wei, Y.; Ma, H.; Zhang, S. Vitreous-induced cytoskeletal rearrangements via the Rac1 GTPase-dependent signaling pathway in human retinal pigment epithelial cells. Biochem. Biophys. Res. Commun. 2012, 419, 395–400. [Google Scholar] [CrossRef]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Imamichi, Y.; Menke, A. Signaling pathways involved in collagen-induced disruption of the E-cadherin complex during epithelial-mesenchymal transition. Cells Tissues Organs 2007, 185, 180–190. [Google Scholar] [CrossRef]
- Sheridan, C.; Hiscott, P.; Grierson, I. Retinal Pigment Epithelium Differentiation and Dedifferentiation. In Vitreo-Retinal Surgery; Kirchhof, B., Wong, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 101–119. [Google Scholar]
- Vadigepalli, R.; Chakravarthula, P.; Zak, D.E.; Schwaber, J.S.; Gonye, G.E. PAINT: A promoter analysis and interaction network generation tool for gene regulatory network identification. OMICS 2003, 7, 235–252. [Google Scholar] [CrossRef]
- Nazarieh, M.; Wiese, A.; Will, T.; Hamed, M.; Helms, V. Identification of key player genes in gene regulatory networks. BMC Syst. Biol. 2016, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Benayoun, B.A.; Caburet, S.; Veitia, R.A. Forkhead transcription factors: Key players in health and disease. Trends Genet. 2011, 27, 224–232. [Google Scholar] [CrossRef]
- Chen, X.; Muller, G.A.; Quaas, M.; Fischer, M.; Han, N.; Stutchbury, B.; Sharrocks, A.D.; Engeland, K. The forkhead transcription factor FOXM1 controls cell cycle-dependent gene expression through an atypical chromatin binding mechanism. Mol. Cell Biol. 2013, 33, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Dodsworth, B.T.; Sidders, B.; Gutteridge, A.; Michaelides, C.; Duckworth, J.K.L.; Whiting, P.J.; Benn, C.L. A FOXM1 dependent mesenchymal-epithelial transition in retinal pigment epithelium cells. PLoS ONE 2015, 10, e0130379. [Google Scholar] [CrossRef]
- Shu, D.Y.; Butcher, E.; Saint-Geniez, M. EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4271. [Google Scholar] [CrossRef]
- Hua, X.; Liu, X.; Ansari, D.O.; Lodish, H.F. Synergistic cooperation of TFE3 and smad proteins in TGF-beta induced transcription of the plasminogen activator inhibitor-1 gene. Genes Dev. 1998, 12, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Massague, J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 2004, 118, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.H.; Vadigepalli, R.; Chakravarthula, P.; Gonye, G.E.; Philp, N.J.; Grunwald, G.B. Transcriptional regulatory network analysis during epithelial-mesenchymal transformation of retinal pigment epithelium. Mol. Vis. 2008, 14, 1414–1428. [Google Scholar] [PubMed]
- Kaneko, H.; Terasaki, H. Biological Involvement of MicroRNAs in Proliferative Vitreoretinopathy. Transl. Vis. Sci. Technol. 2017, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.D.; Reibaldi, M.; Avitabile, T.; Bucolo, C.; Salomone, S.; Rejdak, R.; Nowomiejska, K.; Tripodi, S.; Posarelli, C.; Ragusa, M.; et al. MicroRNAs in the Vitreous Humor of Patients with Retinal Detachment and a Different Grading of Proliferative Vitreoretinopathy: A Pilot Study. Transl. Vis. Sci. Technol. 2020, 9, 23. [Google Scholar] [CrossRef]
- Boles, N.C.; Fernandes, M.; Swigut, T.; Srinivasan, R.; Schiff, L.; Rada-Iglesias, A.; Wang, Q.; Saini, J.S.; Kiehl, T.; Stern, J.H.; et al. Epigenomic and Transcriptomic Changes During Human RPE EMT in a Stem Cell Model of Epiretinal Membrane Pathogenesis and Prevention by Nicotinamide. Stem Cell Rep. 2020, 14, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Yamanaka, O.; Okada, Y.; Tanaka, S.; Miyamoto, T.; Sumioka, T.; Kitano, A.; Shirai, K.; Ikeda, K. TGF in fibroproliferative diseases in the eye. Front. Biosci. 2009, 1, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Chen, Z.; Shao, Y.; Li, X. The roles of signaling pathways in epithelial-to-mesenchymal transition of PVR. Mol. Vis. 2015, 21, 706–710. [Google Scholar]
- Dai, Y.; Dai, C.; Sun, T. Inflammatory mediators of proliferative vitreoretinopathy: Hypothesis and review. Int. Ophthalmol. 2020, 40, 1587–1601. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Scott, R.A.H.; Wallace, G.; Berry, M.; Logan, A.; Blanch, R.J. Inflammatory and Fibrogenic Factors in Proliferative Vitreoretinopathy Development. Transl. Vis. Sci. Technol. 2020, 9, 23. [Google Scholar] [CrossRef]
- Kita, T.; Hata, Y.; Arita, R.; Kawahara, S.; Miura, M.; Nakao, S.; Mochizuki, Y.; Enaida, H.; Goto, Y.; Shimokawa, H.; et al. Role of TGF- β in proliferative vitreoretinal diseases and ROCK as a therapeutic target. Proc. Natl. Acad. Sci. USA 2008, 105, 17504–17509. [Google Scholar] [CrossRef] [PubMed]
- Korthagen, N.M.; van Bilsen, K.; Swagemakers, S.M.; van de Peppel, J.; Bastiaans, J.; van der Spek, P.J.; van Hagen, P.M.; Dik, W.A. Retinal pigment epithelial cells display specific transcriptional responses upon TNF-alpha stimulation. Br. J. Ophthalmol. 2015, 99, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, Z.; Chen, Y. Regulation of TGF-signaling by Smad7. Acta Biochim. Biophys. Sin. 2009, 41, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Schiff, L.; Boles, N.C.; Fernandes, M.; Nachmani, B.; Gentile, R.; Blenkinsop, T.A. P38 inhibition reverses TGFb1 and TNFa induced contraction in a model of proliferative vitreoretinopathy. Commun. Biol. 2019, 2, 162. [Google Scholar] [CrossRef] [PubMed]
- Ishida, W.; Mori, Y.; Lakos, G.; Sun, L.; Shan, F.; Bowes, S.; Josiah, S.; Lee, W.C.; Singh, J.; Ling, L.E.; et al. Intracellular TGF-β receptor blockade abrogates smad-dependent fibroblast activation in vitro and in vivo. J. Investig. Dermatol. 2006, 126, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Pannu, J.; Nakerakanti, S.; Smith, E.; Ten Dijke, P.; Trojanowska, M. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J. Biol. Chem. 2007, 282, 10405–10413. [Google Scholar] [CrossRef]
- Amemiya, K.; Haruta, M.; Takahashi, M.; Kosaka, M.; Eguchi, G. Adult human retinal pigment epithelial cells capable of differentiating into neurons. Biochem. Biophys. Res. Commun. 2004, 316, 1–5. [Google Scholar] [CrossRef]
- Engelhardt, M.; Bogdahn, U.; Aigner, L. Adult retinal pigment epithelium cells express neural progenitor properties and the neuronal precursor protein doublecortin. Brain Res. 2005, 1040, 98–111. [Google Scholar] [CrossRef]
- Milyushina, L.A.; Verdiev, B.I.; Kuznetsova, A.V.; Aleksandrova, M.A. Expression of multipotent and retinal markers in pigment epithelium of adult human in vitro. Bull. Exp. Biol. Med. 2012, 153, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Wang, A.; Liu, Y.; Liu, H.; Yue, F.; Abulaiti, X.; Zhang, C.; Li, L. Differentiation of adult human retinal pigment epithelial cells into dopaminergic-like cells in vitro and in the recipient monkey brain. Mol. Med. 2019, 25, 9. [Google Scholar] [CrossRef] [PubMed]
- Sakami, S.; Etter, P.; Reh, T.A. Activin signaling limits the competence for retinal regeneration from the pigmented epithelium. Mech. Dev. 2008, 125, 106–116. [Google Scholar] [CrossRef]
- Milyushina, L.A.; Kuznetsova, A.V.; Grigoryan, E.N.; Aleksandrova, M.A. Phenotypic plasticity of retinal pigment epithelium cells of adult human eye in vitro. Bull. Exp. Biol. Med. 2011, 151, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.V.; Kurinov, A.M.; Aleksandrova, M.A. Cell models to study regulation of cell transformation in pathologies of retinal pigment epithelium. J. Ophthalmol. 2014, 2014, 801787. [Google Scholar] [CrossRef] [PubMed]
- Shafei, E.V.; Kurinov, A.M.; Kuznetsova, A.V.; Aleksandrova, M.A. Reprogramming of human retinal pigment epithelial cells under the effect of bFGF in vitro. Bull. Exp. Biol. Med. 2017, 163, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.M. Epithelial phenotype and the RPE: Is the answer blowing in the Wnt? Prog. Retin. Eye Res. 2008, 27, 579–595. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Chen, Y.; Liu, J.Y.; Lu, H.; Wang, W.; Lu, X.; Dean, K.C.; Gao, L.; Kaplan, H.J.; et al. Sphere-induced reprogramming of RPE cells into dual-potential RPE stem-like cells. EBioMedicine 2020, 52, 102618. [Google Scholar] [CrossRef] [PubMed]
- Salero, E.; Blenkinsop, T.A.; Corneo, B.; Harris, A.; Rabin, D.; Stern, J.H.; Temple, S. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 2012, 10, 88–95. [Google Scholar] [CrossRef]
- Chen, H.C.; Zhu, Y.T.; Chen, S.Y.; Tseng, S.C. Wnt signaling induces epithelial-mesenchymal transition with proliferation in ARPE-19 cells upon loss of contact inhibition. Lab. Investig. 2012, 92, 676–687. [Google Scholar] [CrossRef]
- Saini, J.S.; Temple, S.; Stern, J.H. Human Retinal Pigment Epithelium Stem Cell (RPESC). Adv. Exp. Med. Biol. 2016, 854, 557–562. [Google Scholar]
- Coffey, P. Untapping the potential of human retinal pigmented epithelial cells. Cell Stem Cell 2012, 10, 1–2. [Google Scholar] [CrossRef][Green Version]
- Pandey, R.S.; Krebs, M.P.; Bolisetty, M.T.; Charette, J.R.; Naggert, J.K.; Robson, P.; Nishina, P.M.; Carter, G.W. Single-Cell RNA Sequencing Reveals Molecular Features of Heterogeneity in the Murine Retinal Pigment Epithelium. Int. J. Mol. Sci. 2022, 23, 10419. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Bok, D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol. Vis. 2001, 7, 14–19. [Google Scholar]
- Blenkinsop, T.A.; Salero, E.; Stern, J.H.; Temple, S. The culture and maintenance of functional retinal pigment epithelial monolayers from adult human eye. Methods Mol. Biol. 2013, 945, 45–55. [Google Scholar] [PubMed]
- Blenkinsop, T.A.; Saini, J.S.; Maminishkis, A.; Bharti, K.; Wan, Q.; Banzon, T.; Lotfi, M.; Davis, J.; Singh, D.; Rizzolo, L.J.; et al. Human Adult Retinal Pigment Epithelial Stem Cell-Derived RPE Monolayers Exhibit Key Physiological Characteristics of Native Tissue. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7085–7099. [Google Scholar] [CrossRef]
- Samuel, W.; Jaworski, C.; Postnikova, O.A.; Kutty, R.K.; Duncan, T.; Tan, L.X.; Poliakov, E.; Lakkaraju, A.; Redmond, T.M. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Mol. Vis. 2017, 23, 60–89. [Google Scholar] [PubMed]
- Lenkowski, J.R.; Raymond, P.A. Muller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog. Retin. Eye Res. 2014, 40, 94–123. [Google Scholar] [CrossRef]
- Goldman, D. Muller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef]
- Hamon, A.; Roger, J.E.; Yang, X.J.; Perron, M. Muller glial cell-dependent regeneration of the neural retina: An overview across vertebrate model systems. Dev. Dyn. 2016, 245, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Chohan, A.; Singh, U.; Kumar, A.; Kaur, J. Müller stem cell dependent retinal regeneration. Clin. Chim. Acta 2017, 464, 160–164. [Google Scholar] [CrossRef]
- Gao, H.; Huang, X.; Chen, X.; Xu, H. Müller Glia-Mediated Retinal Regeneration. Mol. Neurobiol. 2021, 58, 2342–2361. [Google Scholar] [CrossRef]
- Martins, R.R.; Zamzam, M.; Tracey-White, D.; Moosajee, M.; Thummel, R.; Henriques, C.M.; MacDonald, R.B. Müller Glia maintain their regenerative potential despite degeneration in the aged zebrafish retina. Aging Cell 2022, 21, e13597. [Google Scholar] [CrossRef] [PubMed]
- Otteson, D.C. Talkin’ about my (re)generation: The who of intrinsic retinal stem cells. Neuroscience 2017, 34627, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Devoldere, J.; Peynshaert, K.; De Smedt, S.C.; Remaut, K. Müller cells as a target for retinal therapy. Drug Discov. Today 2019, 24, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- de Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Gallego, B.I.; Triviño, A.; Ramírez, J.M. Retinal macroglial responses in health and disease. BioMed Res. Int. 2016, 2016, 2954721. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. New functions of Müller cells. Glia 2013, 61, 651–678. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.A. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis. Neurosci. 2000, 17, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Thummel, R.; Kassen, S.C.; Enright, J.M.; Nelson, C.M.; Montgomery, J.E.; Hyde, D.R. Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp. Eye Res. 2008, 87, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Barthel, L.K.; Raymond, P.A. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development 2013, 140, 4510–4521. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Hitchcock, P. Inflammation Regulates the Multi-Step Process of Retinal Regeneration in Zebrafish. Cells 2021, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Lahne, M.; Nagashima, M.; Hyde, D.R.; Hitchcock, P.F. Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu. Rev. Vis. Sci. 2020, 6, 171–193. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; McGuire, C.R.; Dierks, B.D.; Reh, T.A. Insulin and fibroblast growth factor 2 activate a neurogenic program in Müller glia of the chicken retina. J. Neurosci. 2002, 22, 9387–9398. [Google Scholar] [CrossRef]
- Todd, L.; Suarez, L.; Quinn, C.; Fischer, A.J. Retinoic Acid-signaling regulates the proliferative and neurogenic capacity of Müller glia-derived progenitor cells in the avian retina. Stem Cells 2018, 36, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, S.; Harpavat, S.; Trimarchi, J.; Cai, L.; Huang, H.; Kuo, W.P.; Weber, G.; Lee, K.; Fraioli, R.E.; Cho, S.H.; et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004, 2, E247. [Google Scholar] [CrossRef]
- Roesch, K.; Jadhav, A.P.; Trimarchi, J.M.; Stadler, M.B.; Roska, B.; Sun, B.B.; Cepko, C.L. The transcriptome of retinal Müller glial cells. J. Comp. Neurol. 2008, 509, 225–238. [Google Scholar] [CrossRef]
- Too, L.K.; Gracie, G.; Hasic, E.; Iwakura, J.H.; Cherepanoff, S. Adult human retinal Müller glia display distinct peripheral and macular expression of CD117 and CD44 stem cell-associated proteins. Acta Histochem. 2017, 119, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.; Jayaram, H.; Singhal, S.; Jones, M.F.; Limb, G.A. Differences between the neurogenic and proliferative abilities of Müller glia with stem cell characteristics and the ciliary epithelium from the adult human eye. Exp. Eye Res. 2011, 93, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Wiedemann, P. Muller glial cells in retinal disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef]
- Bringmann, A.; Iandiev, I.; Pannicke, T.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Osborne, N.N.; Reichenbach, A. Cellular signaling and factors involved in Muller cell gliosis: Neuroprotective and detrimental effects. Prog. Retin. Eye Res. 2009, 28, 423–451. [Google Scholar] [CrossRef]
- Sardar Pasha, S.P.B.; Münch, R.; Schäfer, P.; Oertel, P.; Sykes, A.M.; Zhu, Y.; Karl, M.O. Retinal cell death dependent reactive proliferative gliosis in the mouse retina. Sci. Rep. 2017, 7, 9517. [Google Scholar] [CrossRef]
- Bringmann, A.; Francke, M.; Reichenbach, A. Müller Cells in Retinopathies. Adv. Mol. Cell Biol. 2003, 31, 1117–1132. [Google Scholar]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Close, J.L.; Liu, J.; Gumuscu, B.; Reh, T.A. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. Glia 2006, 54, 94–104. [Google Scholar] [CrossRef]
- Osakada, F.; Ooto, S.; Akagi, T.; Mandai, M.; Akaike, A.; Takahashi, M. Wnt signaling promotes regeneration in the retina of adult mammals. J. Neurosci. 2007, 27, 4210–4219. [Google Scholar] [CrossRef]
- Wan, J.; Zheng, H.; Xiao, H.L.; She, Z.J.; Zhou, G.M. Sonic hedgehog promotes stem-cell potential of Müller glia in the mammalian retina. Biochem. Biophys. Res. Commun. 2007, 363, 347–354. [Google Scholar] [CrossRef]
- Del Debbio, C.B.; Balasubramanian, S.; Parameswaran, S.; Chaudhuri, A.; Qiu, F.; Ahmad, I. Notch and Wnt signaling mediated rod photoreceptor regeneration by Müller cells in adult mammalian retina. PLoS ONE 2010, 5, 12425. [Google Scholar] [CrossRef]
- Liu, B.; Hunte, D.J.; Rooker, S.; Chan, A.; Paulus, Y.M.; Leucht, P.; Nomoto, H.Y.; Helms, J.A. Wnt signaling promotes Müller cell proliferation and survival after injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Limb, G.A.; Salt, T.E.; Munro, P.M.; Moss, S.E.; Khaw, P.T. In vitro characterization of a spontaneously immortalized human Muller cell line (MIO-M1). Investig. Ophthalmol. Vis. Sci. 2002, 43, 864–869. [Google Scholar]
- Lawrence, J.M.; Singhal, S.; Bhatia, B.; Keegan, D.J.; Reh, T.A.; Luthert, P.J.; Khaw, P.T.; Limb, G.A. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells 2007, 25, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, B.A.; Feenstra, D.J.; Mohr, S. Müller cells and diabetic retinopathy. Vis. Res. 2017, 139, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.J.; Anderson, N.M.; Jiang, Y.; Bahouth, S.; Steinle, J.J. Role of β-Adrenergic Receptor Regulation of TNF-α and Insulin Signaling in Retinal Müller Cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9527–9533. [Google Scholar] [CrossRef] [PubMed]
- Insua, M.F.; Simón, M.V.; Garelli, A.; de Los Santos, B.; Rotstein, N.P.; Politi, L.E. Trophic factors and neuronal interactions regulate the cell cycle and Pax6 expression in Müller stem cells. J. Neurosci. Res. 2008, 86, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Wohl, S.G.; Reh, T.A. The microRNA expression profile of mouse Müller glia in vivo and in vitro. Sci. Rep. 2016, 6, 35423. [Google Scholar] [CrossRef] [PubMed]
- Kara, N.; Kent, M.R.; Didiano, D.; Rajaram, K.; Patton, J.G. The miR-216a-Dot1l Regulatory Axis Is Necessary and Sufficient for Muller Glia Reprogramming during Retina Regeneration. Cell Rep. 2019, 28, 2037–2047.e4. [Google Scholar] [CrossRef] [PubMed]
- Wohl, S.G.; Hooper, M.J.; Reh, T.A. MicroRNAs miR-25, let-7 and miR-124 regulate the neurogenic potential of Müller glia in mice. Development 2019, 146, dev179556. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, F.; Okuno, M.; Tanaka, T.; Sanuki, R. Overexpression of neural miRNAs miR-9/9* and miR-124 suppresses differentiation to Müller glia and promotes differentiation to neurons in mouse retina in vivo. Genes Cells 2020, 25, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Greene, K.M.; Stamer, W.D.; Liu, Y. The role of microRNAs in glaucoma. Exp. Eye Res. 2022, 215, 108909. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Iwagawa, T.; Ochiai, G.; Koso, H.; Nakauchi, H.; Nagasaki, M.; Suzuki, Y.; Watanabe, S. Analysis of Muller glia specific genes and their histone modification using Hes1-promoter driven EGFP expressing mouse. Sci. Rep. 2017, 7, 3578. [Google Scholar] [CrossRef] [PubMed]
- Dvoriantchikova, G.; Seemungal, R.J.; Ivanov, D. Development and epigenetic plasticity of murine Müller glia. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.; Grant, A.R.; Cornblath, E.; Goldman, D. Analysis of DNA methylation reveals a partial reprogramming of the Muller glia genome during retina regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 19814–19819. [Google Scholar] [CrossRef] [PubMed]
- Jorstad, N.L.; Wilken, M.S.; Grimes, W.N.; Wohl, S.G.; Vanden Bosch, L.S.; Yoshimatsu, T.; Wong, R.O.; Rieke, F.; Reh, T.A. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature 2017, 548, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Fausett, B.V.; Gumerson, J.D.; Goldman, D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J. Neurosci. 2008, 28, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Wang, J.; Boyd, P.; Wang, F.; Santiago, C.; Jiang, L.; Yoo, S.; Lahne, M.; Todd, L.J.; Jia, M.; et al. Gene regulatory networks controlling vertebrate retinal regeneration. Science 2020, 370, eabb8598. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Pons, S.A.; Nakagawa, S.; Bahima, E.G.; Fernandez-Blanco, A.; Pesaresi, M.; D’Antin, J.C.; Sebastian-Perez, R.; Greco, D.; Domínguez-Sala, E.; Gómez-Riera, R.; et al. Müller glia fused with adult stem cells undergo neural differentiation in human retinal models. eBioMedicine 2022, 77, 103914. [Google Scholar] [CrossRef] [PubMed]
- Sanges, D.; Simonte, G.; Di Vicino, U.; Romo, N.; Pinilla, I.; Nicolás, M.; Cosma, M.P. Reprogramming Muller glia via in vivo cell fusion regenerates murine photoreceptors. J. Clin. Investig. 2016, 126, 3104–3116. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mikrani, R.; Zubair, H.M.; Taleb, A.; Naveed, M.; Baig, M.F.A.; Zhang, Q.; Li, C.; Habib, M.; Cui, X.; et al. Systemic and local delivery of mesenchymal stem cells for heart renovation: Challenges and innovations. Eur. J. Pharmacol. 2020, 876, 173049. [Google Scholar] [CrossRef] [PubMed]
- González Fleitas, M.F.; Devouassoux, J.D.; Aranda, M.L.; Calanni, J.S.; Chianelli, M.S.; Dorfman, D.; Rosenstein, R.E. Enriched environment provides neuroprotection against experimental glaucoma. J. Neurochem. 2020, 152, 103–121. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoryan, E.N. Cell Sources for Retinal Regeneration: Implication for Data Translation in Biomedicine of the Eye. Cells 2022, 11, 3755. https://doi.org/10.3390/cells11233755
Grigoryan EN. Cell Sources for Retinal Regeneration: Implication for Data Translation in Biomedicine of the Eye. Cells. 2022; 11(23):3755. https://doi.org/10.3390/cells11233755
Chicago/Turabian StyleGrigoryan, Eleonora N. 2022. "Cell Sources for Retinal Regeneration: Implication for Data Translation in Biomedicine of the Eye" Cells 11, no. 23: 3755. https://doi.org/10.3390/cells11233755
APA StyleGrigoryan, E. N. (2022). Cell Sources for Retinal Regeneration: Implication for Data Translation in Biomedicine of the Eye. Cells, 11(23), 3755. https://doi.org/10.3390/cells11233755





