TFAM’s Contributions to mtDNA Replication and OXPHOS Biogenesis Are Genetically Separable
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Growth and Treatment
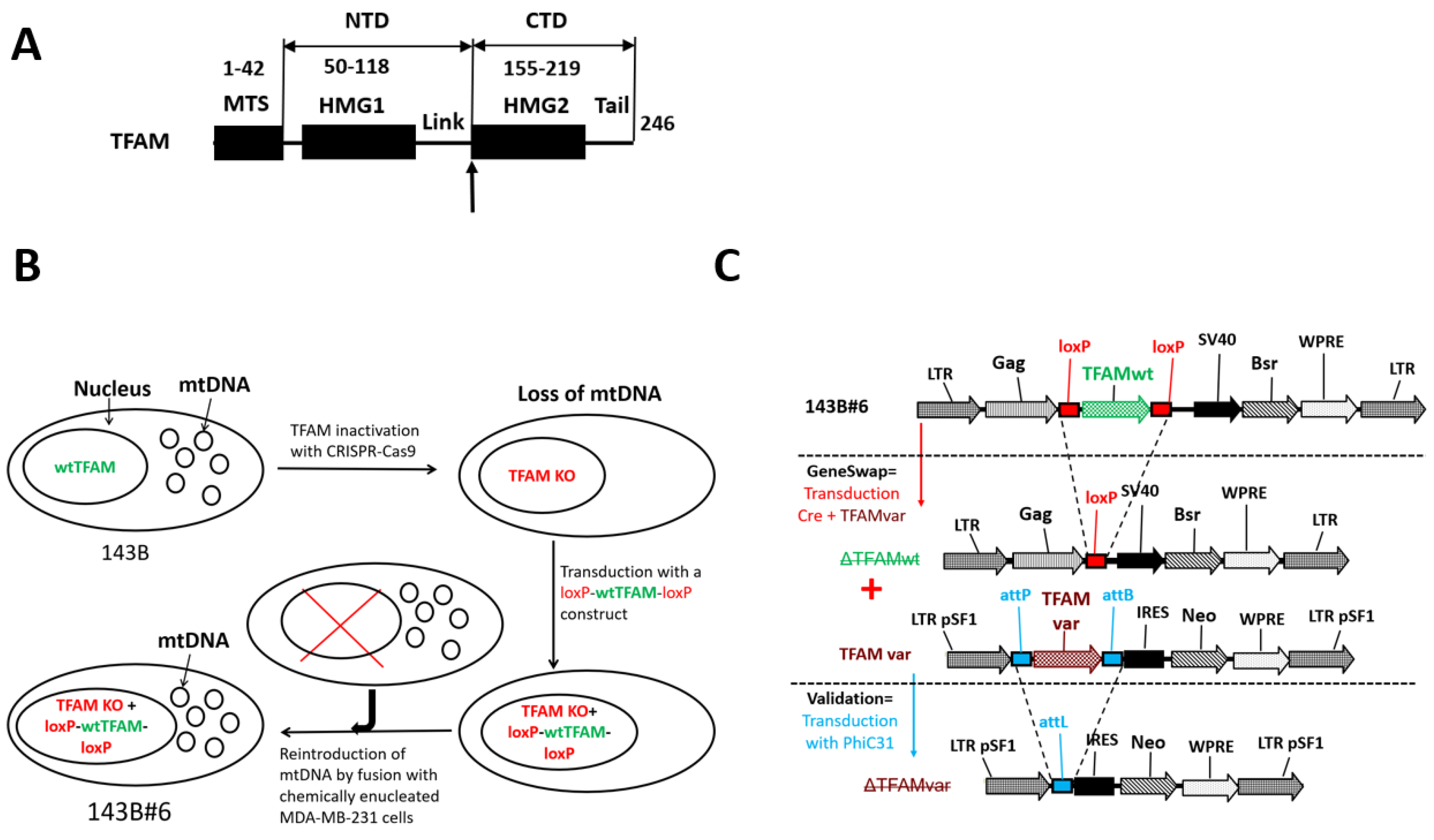
2.2. DNA Constructs
2.3. GeneSwap
2.4. Retrovirus Generation
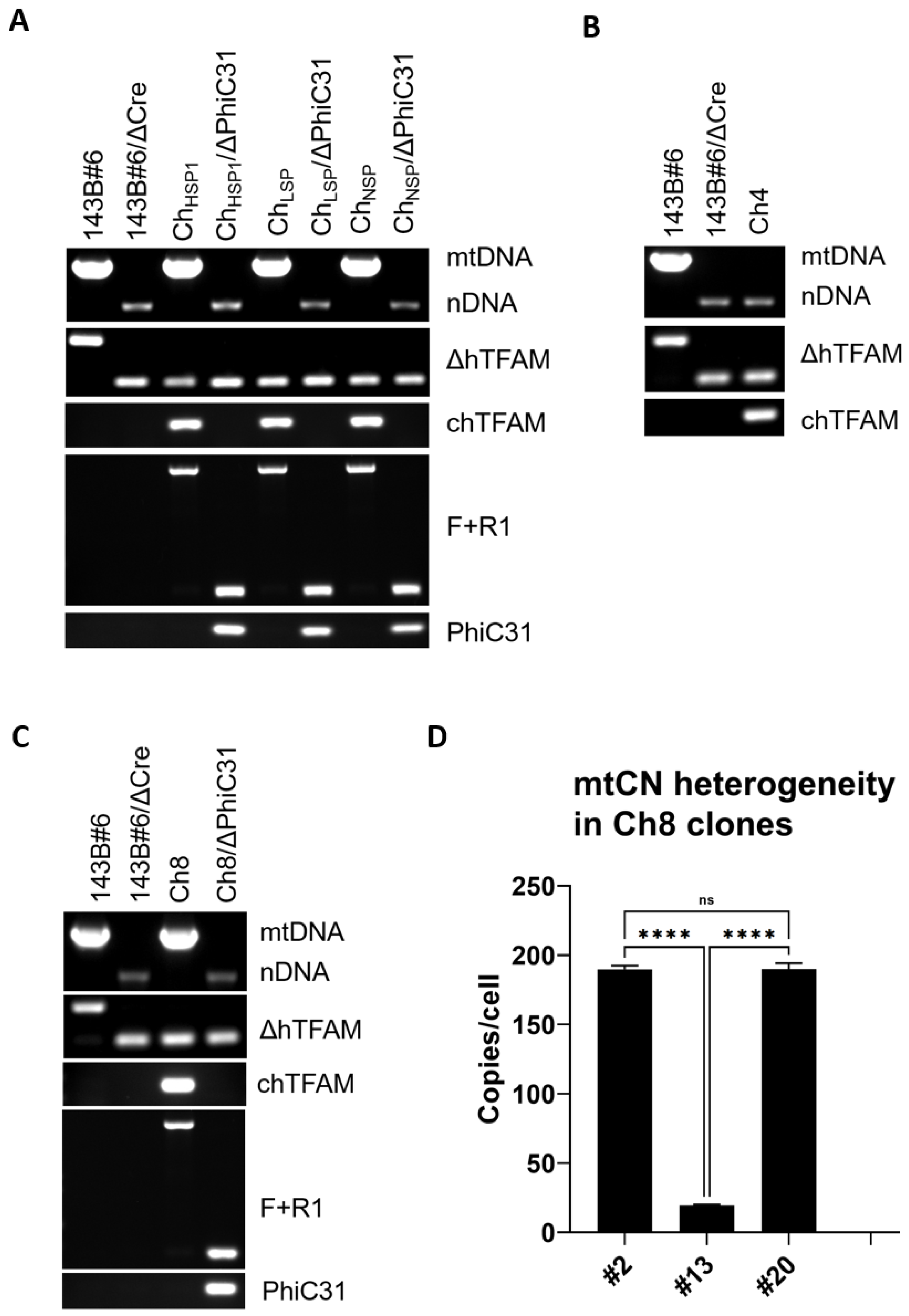
2.5. mtDNA Diagnostics
2.6. mtCN Determination by Direct Droplet Digital PCR (dddPCR)
2.7. PhiC31-Mediated Excision of Proviral Inserts
2.8. Genotyping of hTFAM Excision in 143B#6 Cells
2.9. Quantitation of Mitochondrial Transcripts
2.10. Cellular Respiration
2.11. Western Blotting
2.12. Amino Acid Alignments
2.13. Statistical Analyses
3. Results
3.1. A Limited Number of aa Substitutions Enable chTFAM to Support hmtDNA Replication
3.2. F53L, T174Q, and F181K Substitutions Do Not Enable chTFAM Functionality in hmtDNA Replication
3.3. Ch13 Chimeras Do Not Support hmtDNA Replication
3.4. Substitutions in TFAM Residues That Make DNA Contacts Are Responsible for chTFAM’s Inability to Rescue hmtDNA Replication
3.5. mtDNA Instability Mediated by the Ch8 TFAM Variant
3.6. TFAM’s Contributions to mtDNA Replication and Gene Expression Are Genetically Separable
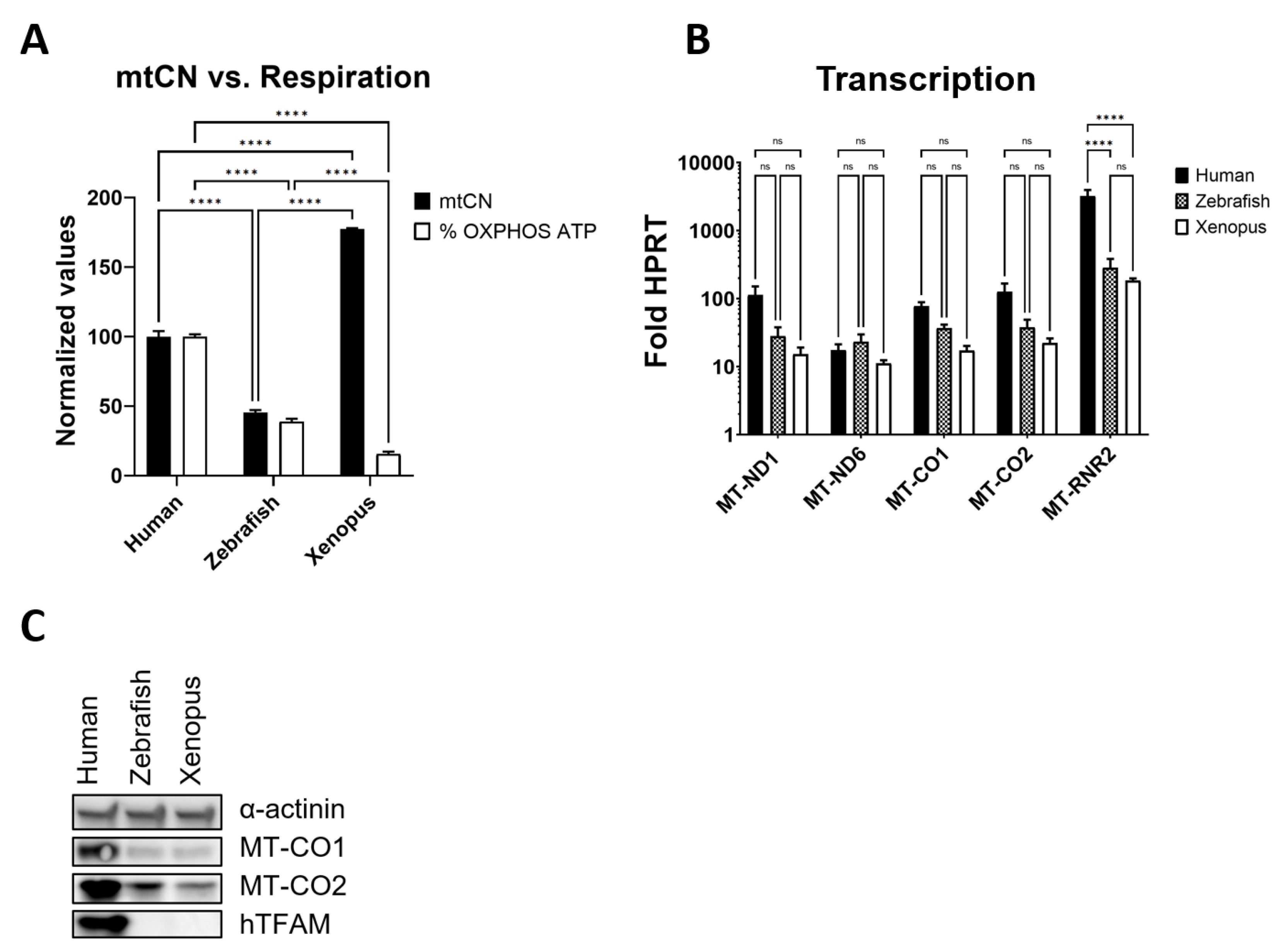
3.7. hmtDNA Maintenance and OXPHOS Biogenesis Are Separated in Frog TFAM
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reeves, R. High mobility group (HMG) proteins: Modulators of chromatin structure and DNA repair in mammalian cells. DNA Repair 2015, 36, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.B.; Kaiser, J.T.; Chan, D.C. The mitochondrial transcription and packaging factor Tfam imposes a U-turn on mitochondrial DNA. Nat. Struct. Mol. Biol. 2011, 18, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Zhou, Y.; Wu, H.; Du, S.; Hu, M.; Qi, J.; Li, W.; Guo, J.; Wu, Y.; Yang, L.; et al. Phase separation drives the self-assembly of mitochondrial nucleoids for transcriptional modulation. Nat. Struct. Mol. Biol. 2021, 28, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.B.; Lovely, G.A.; Phillips, R.; Chan, D.C. Distinct structural features of TFAM drive mitochondrial DNA packaging versus transcriptional activation. Nat. Commun. 2014, 5, 3077. [Google Scholar] [CrossRef] [PubMed]
- Larsson, N.G.; Wang, J.; Wilhelmsson, H.; Oldfors, A.; Rustin, P.; Lewandoski, M.; Barsh, G.S.; Clayton, D.A. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 1998, 18, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kozhukhar, N.; Alexeyev, M.F. Limited predictive value of TFAM in mitochondrial biogenesis. Mitochondrion 2019, 49, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Brinckmann, A.; Weiss, C.; Wilbert, F.; von Moers, A.; Zwirner, A.; Stoltenburg-Didinger, G.; Wilichowski, E.; Schuelke, M. Regionalized pathology correlates with augmentation of mtDNA copy numbers in a patient with myoclonic epilepsy with ragged-red fibers (MERRF-syndrome). PLoS ONE 2010, 5, e13513. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, M.I.; Falkenberg, M.; Rantanen, A.; Park, C.B.; Gaspari, M.; Hultenby, K.; Rustin, P.; Gustafsson, C.M.; Larsson, N.G. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004, 13, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Bonekamp, N.A.; Jiang, M.; Motori, E.; Garcia Villegas, R.; Koolmeister, C.; Atanassov, I.; Mesaros, A.; Park, C.B.; Larsson, N.G. High levels of TFAM repress mammalian mitochondrial DNA transcription in vivo. Life Sci. Alliance 2021, 4, e202101034. [Google Scholar] [CrossRef] [PubMed]
- Kozhukhar, N.; Spadafora, D.; Rodriguez, Y.A.R.; Alexeyev, M.F. A Method for In Situ Reverse Genetic Analysis of Proteins Involved mtDNA Replication. Cells 2022, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russel, D.W. Molecular Cloning. A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Khozhukhar, N.; Spadafora, D.; Rodriguez, Y.; Alexeyev, M. Elimination of Mitochondrial DNA from Mammalian Cells. Curr. Protoc. Cell Biol. 2018, 78, 20.11.1–20.11.14. [Google Scholar] [CrossRef] [PubMed]
- Kozhukhar, N.; Fant, A.; Alexeyev, M.F. Quantification of mtDNA content in cultured cells by direct droplet digital PCR. Mitochondrion 2021, 61, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Shokolenko, I.N.; Fayzulin, R.Z.; Katyal, S.; McKinnon, P.J.; Wilson, G.L.; Alexeyev, M.F. Mitochondrial DNA ligase is dispensable for the viability of cultured cells but essential for mtDNA maintenance. J. Biol. Chem. 2013, 288, 26594–26605. [Google Scholar] [CrossRef] [PubMed]
- Contamine, V.; Picard, M. Maintenance and integrity of the mitochondrial genome: A plethora of nuclear genes in the budding yeast. Microbiol. Mol. Biol. Rev. 2000, 64, 281–315. [Google Scholar] [CrossRef] [PubMed]
- D’Erchia, A.M.; Atlante, A.; Gadaleta, G.; Pavesi, G.; Chiara, M.; De Virgilio, C.; Manzari, C.; Mastropasqua, F.; Prazzoli, G.M.; Picardi, E.; et al. Tissue-specific mtDNA abundance from exome data and its correlation with mitochondrial transcription, mass and respiratory activity. Mitochondrion 2015, 20, 13–21. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhukhar, N.; Alexeyev, M.F. TFAM’s Contributions to mtDNA Replication and OXPHOS Biogenesis Are Genetically Separable. Cells 2022, 11, 3754. https://doi.org/10.3390/cells11233754
Kozhukhar N, Alexeyev MF. TFAM’s Contributions to mtDNA Replication and OXPHOS Biogenesis Are Genetically Separable. Cells. 2022; 11(23):3754. https://doi.org/10.3390/cells11233754
Chicago/Turabian StyleKozhukhar, Natalya, and Mikhail F. Alexeyev. 2022. "TFAM’s Contributions to mtDNA Replication and OXPHOS Biogenesis Are Genetically Separable" Cells 11, no. 23: 3754. https://doi.org/10.3390/cells11233754
APA StyleKozhukhar, N., & Alexeyev, M. F. (2022). TFAM’s Contributions to mtDNA Replication and OXPHOS Biogenesis Are Genetically Separable. Cells, 11(23), 3754. https://doi.org/10.3390/cells11233754







