Accelerated Evolution of Cytochrome c in Higher Primates, and Regulation of the Reaction between Cytochrome c and Cytochrome Oxidase by Phosphorylation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Site-Directed Mutagenesis
2.3. Analytical Ultracentrifuge Experiments
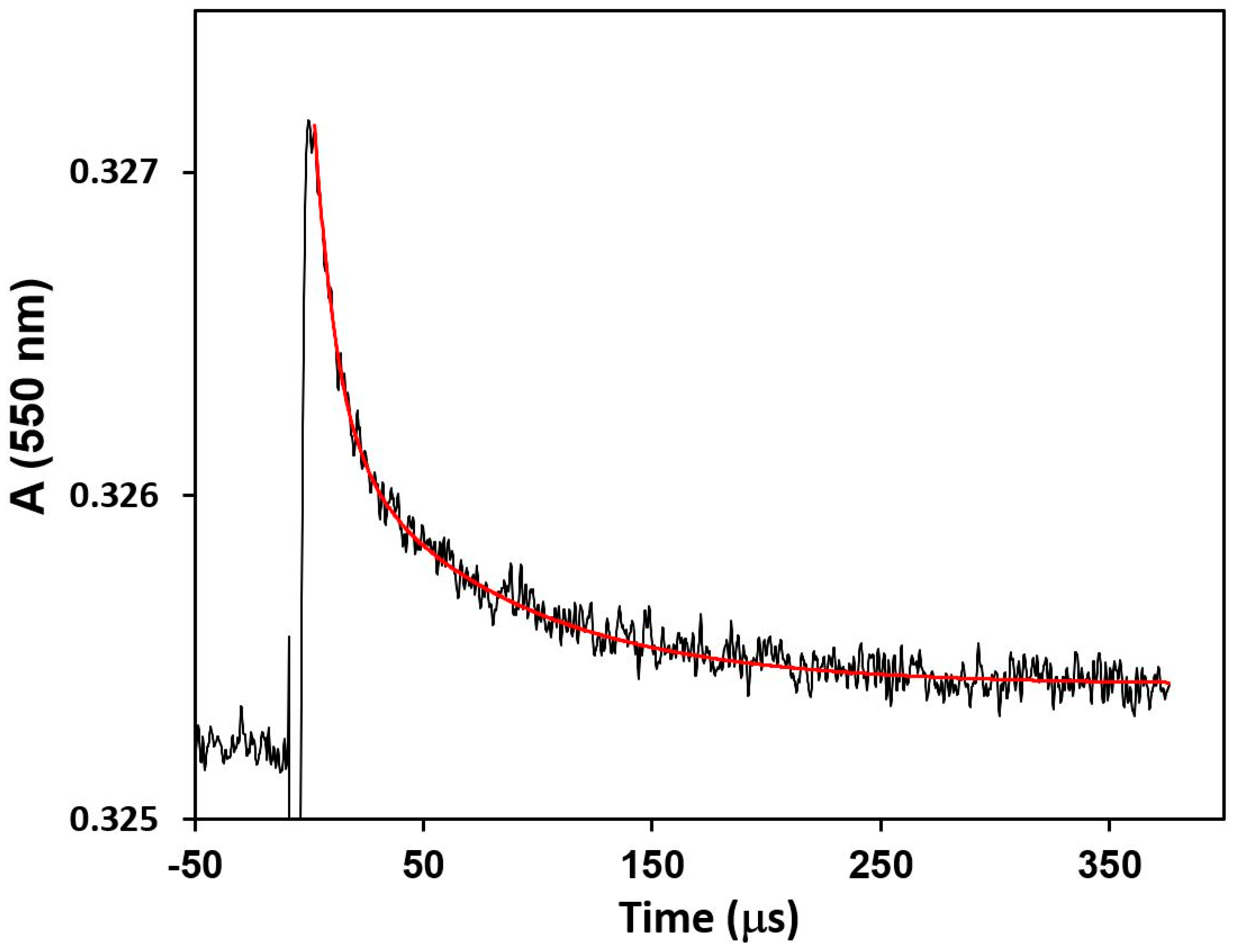
2.4. Flash Photolysis Experiments
kobs = kf[CcO]···kf = kobs/[CcO]
3. Results
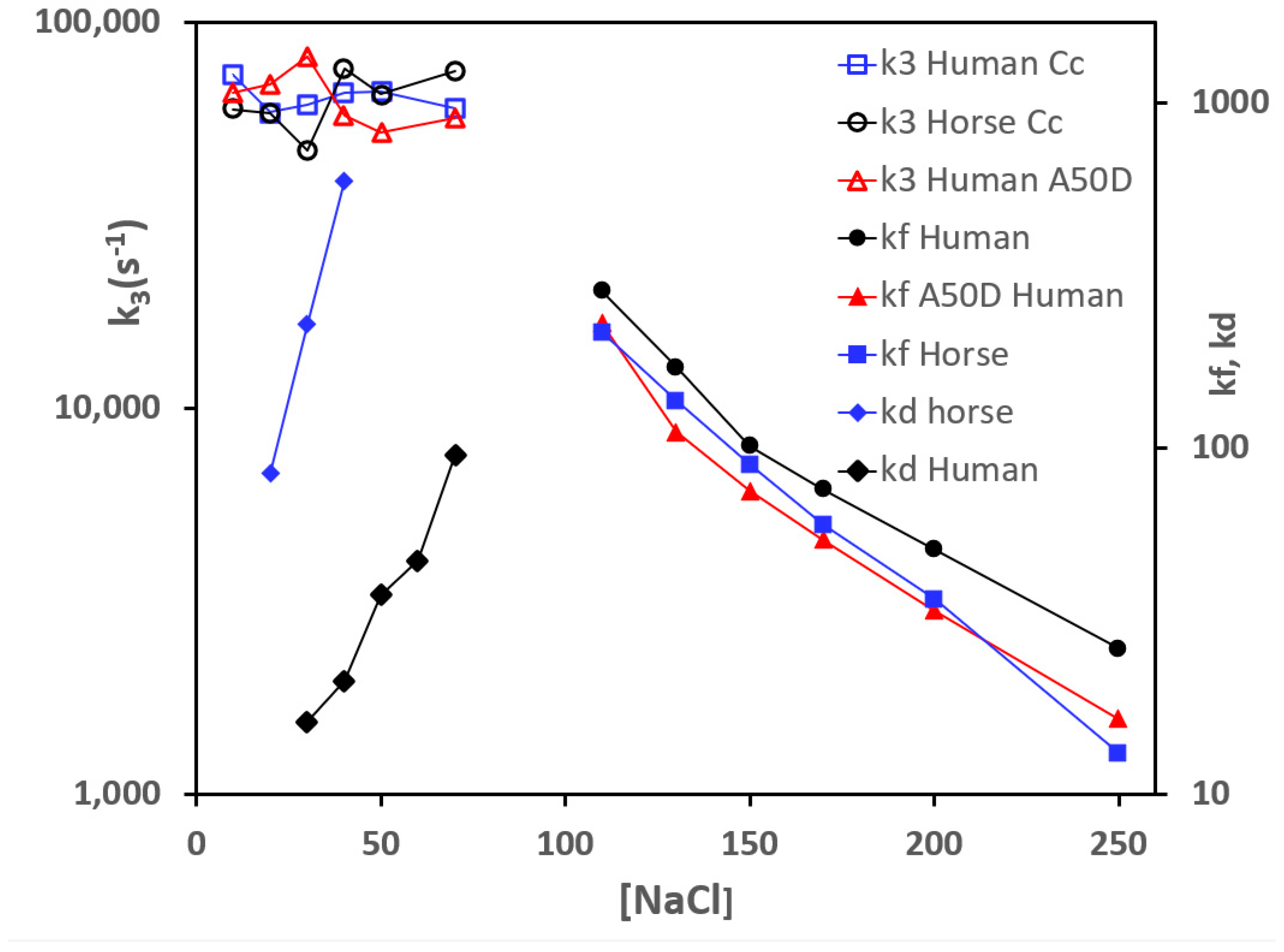
3.1. Effect of Mutations from Horse Cc to Human Cc on the Reaction with CcO
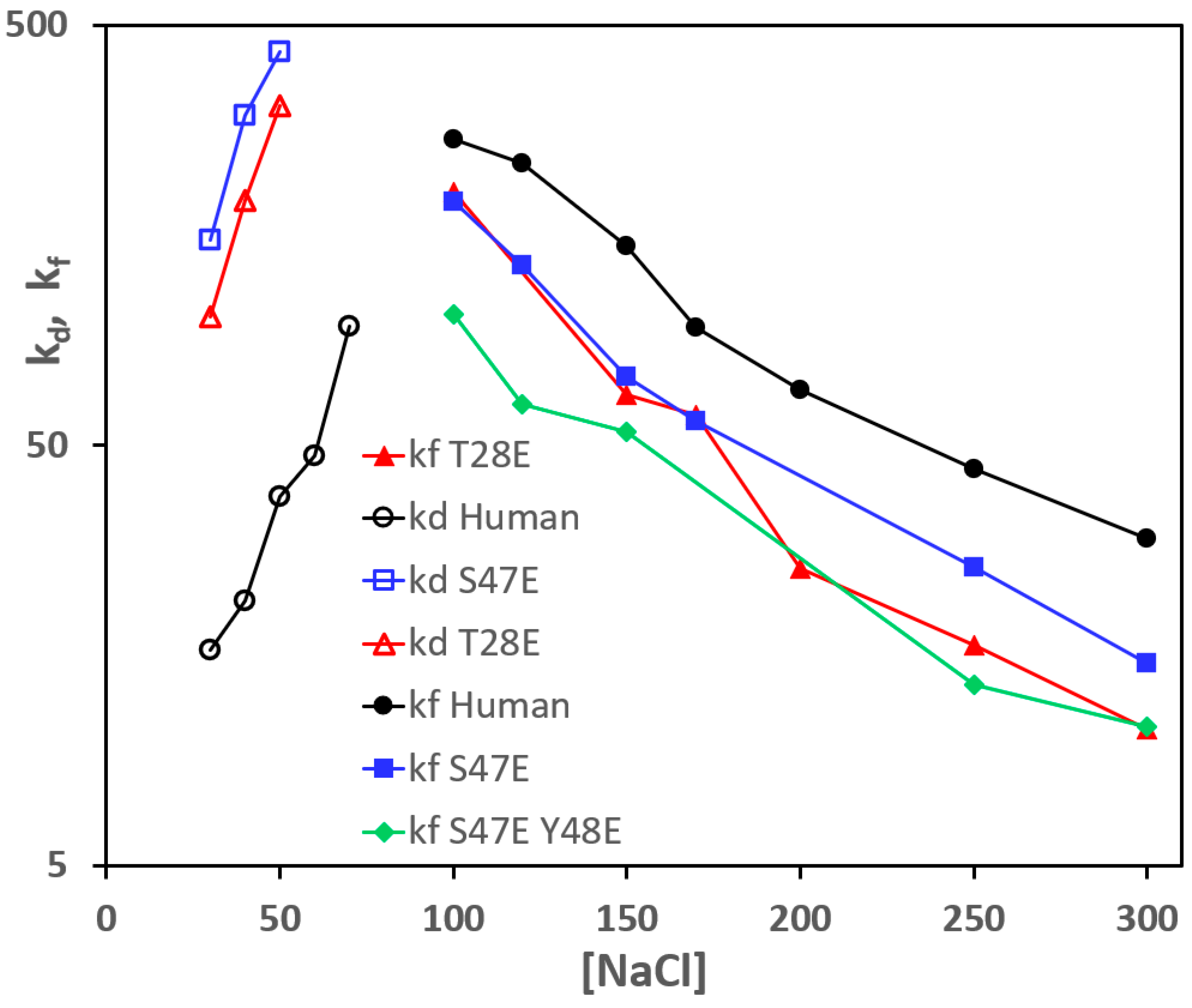
3.2. Effect of Phosphomimetic Mutations of Human Cc on the Reaction with CcO
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cc | cytochrome c |
| CcO | cytochrome c oxidase |
| Ru-39-Cc | (dimethylbipyridine)(bisbipyridine)ruthenium-Cys-39-cytochrome c |
| Ru-55-Cc | (dimethylbipyridine)(bisbipyridine)ruthenium-Lys-55-cytochrome c |
| 3CP | carboxyl-2,2,5,5-tetramethyl-1-pyrolidinyloxy free radical |
| Ru2Z | Ru(bipyrazine)2-(quaterpyridine) |
| Rb. sphaeroides | Rhodobacter sphaeroides |
References
- Fischer, W.; Hemp, J.; Johnson, J.; Jeanloz, R.; Freeman, K. Evolution of Oxygenic Photosynthesis. Annu. Rev. Earth Planet. Sci. 2016, 44, 647–683. [Google Scholar] [CrossRef]
- Dickerson, R. Structure and history of an ancient protein. Sci. Am. 1972, 226, 58. [Google Scholar] [CrossRef] [PubMed]
- Long, J.E.; Durham, B.; Okamura, M.; Millett, F. Role of specific lysine residues in binding cytochrome c2 to the Rhodobacter sphaeroides reaction center in optimal orientation for rapid electron transfer. Biochemistry 1989, 28, 6970–6974. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, M.; Cusanovich, M.; Meyer, T.; Axelrod, H.; Okamura, M. Double mutant studies identify electrostatic interactions that are important for docking cytochrome c(2) onto the bacterial reaction center. Biochemistry 2002, 41, 5807–5815. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, H.L.; Abresch, E.C.; Okamura, M.Y.; Yeh, A.P.; Rees, D.C.; Feher, G. X-ray structure determination of the cytochrome c2: Reaction center electron transfer complex from Rhodobacter sphaeroides. J. Mol. Biol. 2002, 319, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Stonehuerner, J.; Williams, J.B.; Millett, F. Interaction between cytochrome c and cytochrome b5. Biochemistry 1979, 18, 5422–5427. [Google Scholar] [CrossRef] [PubMed]
- Salemme, F. Hypothetical Structure For An Intermolecular Electron-Transfer Complex of Cytochrome-C and Cytochrome-b5. J. Mol. Biol. 1976, 102, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Willie, A.; Stayton, P.; Sligar, S.; Durham, B.; Millett, F. Genetic-Engineering of Redox Donor Sites—Measurement of Intracomplex Electron-Transfer between Ruthenium-65 Cytochrome-B5 and Cytochrome-C. Biochemistry 1992, 31, 7237–7242. [Google Scholar] [CrossRef]
- Durham, B.; Fairris, J.L.; McLean, M.; Millett, F.; Scott, J.R.; Sligar, S.G.; Willie, A. Electron transfer from cytochrome b5 to cytochrome c. J. Bioenerg. Biomembr. 1995, 27, 331–340. [Google Scholar] [CrossRef]
- Kang, C.; Brautigan, D.; Osheroff, N.; Margoliash, E. Definition of Cytochrome-C Binding Domains by Chemical Modification—Reaction of Carboxydinitrophenyl-Cytochromes-C and Trinitrophenyl-Cytochromes-C with Bakers-Yeast Cytochrome-C Peroxidase. J. Biol. Chem. 1978, 253, 6502–6510. [Google Scholar] [CrossRef]
- Smith, M.B.; Millett, F. A 19F nuclear magnetic resonance study of the interaction between cytochrome c and cytochrome c peroxidase. Biochim. Biophys. Acta 1980, 626, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, H.; Kraut, J. Crystal structure of a complex between electron transfer partners, cytochrome c peroxidase and cytochrome c. Science 1992, 258, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mei, H.; Geren, L.; Miller, M.; Saunders, A.; Wang, X.; Waldner, J.; Pielak, G.; Durham, B.; Millett, F. Design of a ruthenium-cytochrome c derivative to measure electron transfer to the radical cation and oxyferryl heme in cytochrome c peroxidase. Biochemistry 1996, 35, 15107–15119. [Google Scholar] [CrossRef] [PubMed]
- Staudenmayer, N.; Smith, M.B.; Smith, H.T.; Spies, F.K.; Millett, F. An enzyme kinetics and 19F nuclear magnetic resonance study of selectively trifluoroacetylated cytochrome c derivatives. Biochemistry 1976, 15, 3198–3205. [Google Scholar] [CrossRef]
- Staudenmayer, N.; Ng, S.; Smith, M.B.; Millett, F. Effect of specific trifluoroacetylation of individual cytochrome c lysines on the reaction with cytochrome oxidase. Biochemistry 1977, 16, 600–604. [Google Scholar] [CrossRef]
- Smith, M.B.; Stonehuerner, J.; Ahmed, A.J.; Staudenmayer, N.; Millett, F. Use of specific trifluoroacetylation of lysine residues in cytochrome c to study the reaction with cytochrome b5, cytochrome c1, and cytochrome oxidase. Biochim. Biophys. Acta 1980, 592, 303–313. [Google Scholar] [CrossRef]
- Smith, H.T.; Staudenmayer, N.; Millett, F. Use of specific lysine modifications to locate the reaction site of cytochrome c with cytochrome oxidase. Biochemistry 1977, 16, 4971–4974. [Google Scholar] [CrossRef]
- Ferguson-Miller, S.; Brautigan, D.L.; Margoliash, E. Definition of cytochrome c binding domains by chemical modification. III. Kinetics of reaction of carboxydinitrophenyl cytochromes c with cytochrome c oxidase. J. Biol. Chem. 1978, 253, 149–159. [Google Scholar] [CrossRef]
- Ahmed, A.J.; Smith, H.T.; Smith, M.B.; Millett, F.S. Effect of specific lysine modification on the reduction of cytochrome c by succinate-cytochrome c reductase. Biochemistry 1978, 17, 2479–2483. [Google Scholar] [CrossRef]
- Speck, S.; Fergusonmiller, S.; Osheroff, N.; Margoliash, E. Definition of Cytochrome-C Binding Domains by Chemical Modification—Kinetics of Reaction with Beef Mitochondrial Reductase and Functional Organization of the Respiratory-Chain. Proc. Natl. Acad. Sci. USA 1979, 76, 155–159. [Google Scholar] [CrossRef]
- Konig, B.; Osheroff, N.; Wilms, J.; Muijsers, A.; Dekker, H.; Margoliash, E. Mapping of the interaction domain for purified cytochrome-C1 on cytochrome-C. FEBS Lett. 1980, 111, 395–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieder, R.; Bosshard, H.R. Comparison of the binding sites on cytochrome c for cytochrome c oxidase, cytochrome bc1, and cytochrome c1. Differential acetylation of lysyl residues in free and complexed cytochrome c. J. Biol. Chem. 1980, 255, 4732–4739. [Google Scholar] [CrossRef] [PubMed]
- Rieder, R.; Bosshard, H. Cytochrome-BC1 and cytochrome-oxidase can bind to same surface domain of cytochrome-C molecule. FEBS Lett. 1978, 92, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Bosshard, H.; Zurrer, M.; Schagger, H.; Vonjagow, G. Binding of cytochrome-C to the cytochrome-BC1 complex (Complex-III) and its subunits cytochrome-C1 and cytochrome-B. Biochem. Biophys. Res. Commun. 1979, 89, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.T.; Ahmed, A.J.; Millett, F. Electrostatic interaction of cytochrome c with cytochrome c1 and cytochrome oxidase. J. Biol. Chem. 1981, 256, 4984–4990. [Google Scholar] [CrossRef] [PubMed]
- Hunte, C.; Koepke, J.; Lange, C.; Rossmanith, T.; Michel, H. Structure at 2.3 A resolution of the cytochrome bc(1) complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure 2000, 8, 669–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, C.; Hunte, C. Crystal structure of the yeast cytochrome bc(1) complex with its bound substrate cytochrome c. Proc. Natl. Acad. Sci. USA 2002, 99, 2800–2805. [Google Scholar] [CrossRef] [Green Version]
- Millett, F.; Havens, J.; Rajagukguk, S.; Durham, B. Design and use of photoactive ruthenium complexes to study electron transfer within cytochrome bc1 and from cytochrome bc1 to cytochrome c. Biochim. Biophys. Acta 2013, 1827, 1309–1319. [Google Scholar] [CrossRef] [Green Version]
- Engstrom, G.; Rajagukguk, R.; Saunders, A.J.; Patel, C.N.; Rajagukguk, S.; Merbitz-Zahradnik, T.; Xiao, K.; Pielak, G.J.; Trumpower, B.; Yu, C.A.; et al. Design of a ruthenium-labeled cytochrome c derivative to study electron transfer with the cytochrome bc1 complex. Biochemistry 2003, 42, 2816–2824. [Google Scholar] [CrossRef]
- Tian, H.; Sadoski, R.; Zhang, L.; Yu, C.; Yu, L.; Durham, B.; Millett, F. Definition of the interaction domain for cytochrome c on the cytochrome bc(1) complex—Steady-state and rapid kinetic analysis of electron transfer between cytochrome c and Rhodobacter sphaeroides cytochrome bc1 surface mutants. J. Biol. Chem. 2000, 275, 9587–9595. [Google Scholar] [CrossRef]
- Ferguson-Miller, S.; Babcock, G.T. Heme/Copper Terminal Oxidases. Chem. Rev. 1996, 96, 2889–2908. [Google Scholar] [CrossRef] [PubMed]
- Babcock, G.T.; Wikström, M. Oxygen activation and the conservation of energy in cell respiration. Nature 1992, 356, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.P.; Hibdon, S.; Liu, R.Q.; Durham, B.; Millett, F. Intracomplex electron transfer between ruthenium-cytochrome c derivatives and cytochrome c oxidase. Biochemistry 1993, 32, 8492–8498. [Google Scholar] [CrossRef] [PubMed]
- Geren, L.M.; Beasley, J.R.; Fine, B.R.; Saunders, A.J.; Hibdon, S.; Pielak, G.J.; Durham, B.; Millett, F. Design of a ruthenium-cytochrome c derivative to measure electron transfer to the initial acceptor in cytochrome c oxidase. J. Biol. Chem. 1995, 270, 2466–2472. [Google Scholar] [CrossRef] [Green Version]
- Zaslavsky, D.L.; Smirnova, I.A.; Siletsky, S.A.; Kaulen, A.D.; Millett, F.; Konstantinov, A.A. Rapid kinetics of membrane potential generation by cytochrome c oxidase with the photoactive Ru(II)-tris-bipyridyl derivative of cytochrome c as electron donor. FEBS Lett. 1995, 359, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Hill, B.C. The reaction of the electrostatic cytochrome c-cytochrome oxidase complex with oxygen. J. Biol. Chem. 1991, 266, 2219–2226. [Google Scholar] [CrossRef]
- Wang, K.; Geren, L.; Zhen, Y.; Ma, L.; Ferguson-Miller, S.; Durham, B.; Millett, F. Mutants of the CuA site in cytochrome c oxidase of Rhodobacter sphaeroides: II. Rapid kinetic analysis of electron transfer. Biochemistry 2002, 41, 2298–2304. [Google Scholar] [CrossRef]
- Zhen, Y.; Schmidt, B.; Kang, U.; Antholine, W.; Ferguson-Miller, S. Mutants of the Cu-A site in cytochrome c oxidase of Rhodobacter sphaeroides: I. Spectral and functional properties. Biochemistry 2002, 41, 2288–2297. [Google Scholar] [CrossRef]
- Osheroff, N.; Brautigan, D.; Margoliash, E. Definition of enzymic interaction domains on cytochrome-c—Purification and activity of singly substituted carboxydinitrophenyl-lysine 7, 25, 73, 86, and 99 cytochromes-C. J. Biol. Chem. 1980, 255, 8245–8251. [Google Scholar] [CrossRef]
- Tsukihara, T.; Aoyama, H.; Yamashita, E.; Tomizaki, T.; Yamaguchi, H.; Shinzawa-Itoh, K.; Nakashima, R.; Yaono, R.; Yoshikawa, S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science 1996, 272, 1136–1144. [Google Scholar] [CrossRef]
- Iwata, S.; Ostermeier, C.; Ludwig, B.; Michel, H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature 1995, 376, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qin, L.; Ferguson-Miller, S. Crystallographic and online spectral evidence for role of conformational change and conserved water in cytochrome oxidase proton pump. Proc. Natl. Acad. Sci. USA 2011, 108, 1284–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.; Hiser, C.; Mulichak, A.; Garavito, R.M.; Ferguson-Miller, S. Identification of conserved lipid/detergent-binding sites in a high-resolution structure of the membrane protein cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2006, 103, 16117–16122. [Google Scholar] [CrossRef] [Green Version]
- Millett, F.; de Jong, C.; Paulson, L.; Capaldi, R.A. Identification of specific carboxylate groups on cytochrome c oxidase that are involved in binding cytochrome c. Biochemistry 1983, 22, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, P.; Watmough, N.J.; Greenwood, C.; Saraste, M. Electron transfer between cytochrome c and the isolated CuA domain: Identification of substrate-binding residues in cytochrome c oxidase. Biochemistry 1995, 34, 5824–5830. [Google Scholar] [CrossRef] [PubMed]
- Witt, H.; Malatesta, F.; Nicoletti, F.; Brunori, M.; Ludwig, B. Tryptophan 121 of subunit II is the electron entry site to cytochrome-c oxidase in Paracoccus denitrificans. Involvement of a hydrophobic patch in the docking reaction. J. Biol. Chem. 1998, 273, 5132–5136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, H.; Malatesta, F.; Nicoletti, F.; Brunori, M.; Ludwig, B. Cytochrome-c-binding site on cytochrome oxidase in Paracoccus denitrificans. Eur. J. Biochem. 1998, 251, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhen, Y.; Sadoski, R.; Grinnell, S.; Geren, L.; Ferguson-Miller, S.; Durham, B.; Millett, F. Definition of the interaction domain for cytochrome c on cytochrome c oxidase. Ii. Rapid kinetic analysis of electron transfer from cytochrome c to Rhodobacter sphaeroides cytochrome oxidase surface mutants. J. Biol. Chem. 1999, 274, 38042–38050. [Google Scholar] [CrossRef] [Green Version]
- Drosou, V.; Malatesta, F.; Ludwig, B. Mutations in the docking site for cytochrome c on the Paracoccus heme aa3 oxidase. Electron entry and kinetic phases of the reaction. Eur. J. Biochem. 2002, 269, 2980–2988. [Google Scholar] [CrossRef]
- Zhen, Y.; Hoganson, C.W.; Babcock, G.T.; Ferguson-Miller, S. Definition of the interaction domain for cytochrome c on cytochrome c oxidase. I. Biochemical, spectral, and kinetic characterization of surface mutants in subunit ii of Rhodobacter sphaeroides cytochrome aa(3). J. Biol. Chem. 1999, 274, 38032–38041. [Google Scholar] [CrossRef]
- Cao, J.; Shapleigh, J.; Gennis, R.; Revzin, A.; Ferguson-Miller, S. The gene encoding cytochrome c oxidase subunit II from Rhodobacter sphaeroides; comparison of the deduced amino acid sequence with sequences of corresponding peptides from other species. Gene 1991, 101, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Roberts, V.A.; Pique, M.E. Definition of the interaction domain for cytochrome c on cytochrome c oxidase. III. Prediction of the docked complex by a complete, systematic search. J. Biol. Chem. 1999, 274, 38051–38060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharlau, M.; Geren, L.; Zhen, E.Y.; Ma, L.; Rajagukguk, R.; Ferguson-Miller, S.; Durham, B.; Millett, F. Definition of the Interaction Domain and Electron Transfer Route between Cytochrome c and Cytochrome Oxidase. Biochemistry 2019, 58, 4125–4135. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Shinzawa-Itoh, K.; Baba, J.; Aoe, S.; Shimada, A.; Yamashita, E.; Kang, J.; Tateno, M.; Yoshikawa, S.; Tsukihara, T. Complex structure of cytochrome c-cytochrome c oxidase reveals a novel protein-protein interaction mode. EMBO J. 2017, 36, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Darga, L.; Goodman, M.; Czelusniak, J. Evolution of cytochrome-c investigated by the maximum parsimony method. J. Mol. Evol. 1981, 17, 197–213. [Google Scholar] [CrossRef]
- Pierron, D.; Wildman, D.; Huttemann, M.; Letellier, T.; Grossman, L.; Kadenbach, B. Evolution of the Couple Cytochrome c and Cytochrome c Oxidase in Primates. Mitochondrial Oxidative Phosphorylation Nucl.-Encoded Genes Enzym. Regul. Pathophysiol. 2012, 748, 185–213. [Google Scholar]
- Pierron, D.; Opazo, J.C.; Heiske, M.; Papper, Z.; Uddin, M.; Chand, G.; Wildman, D.E.; Romero, R.; Goodman, M.; Grossman, L.I. Silencing, Positive Selection and Parallel Evolution: Busy History of Primate Cytochromes c. PLoS ONE 2011, 6, e26269. [Google Scholar] [CrossRef] [Green Version]
- Grossman, L.; Schmidt, T.; Wildman, D.; Goodman, M. Molecular evolution of aerobic energy metabolism in primates. Mol. Phylogen. Evol. 2001, 18, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Kalpage, H.; Wan, J.; Morse, P.; Zurek, M.; Turner, A.; Khobeir, A.; Yazdi, N.; Hakim, L.; Liu, J.; Vaishnav, A.; et al. Cytochrome c phosphorylation: Control of mitochondrial electron transport chain flux and apoptosis. Int. J. Biochem. Cell Biol. 2020, 121, 105704. [Google Scholar] [CrossRef]
- Huttemann, M.; Helling, S.; Sanderson, T.; Sinkler, C.; Samavati, L.; Mahapatra, G.; Varughese, A.; Lu, G.; Liu, J.; Ramzan, R.; et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: Cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 598–609. [Google Scholar] [CrossRef]
- Huttemann, M.; Lee, I.; Grossman, L.; Doan, J.; Sanderson, T.; Kadenbach, B. Phosphorylation of Mammalian Cytochrome c and Cytochrome c Oxidase in the Regulation of Cell Destiny: Respiration, Apoptosis, and Human Disease. Mitochondrial Oxidative Phosphorylation Nuclear-Encoded Genes Enzym. Regul. Pathophysiol. 2012, 748, 237–264. [Google Scholar]
- Kalpage, H.; Vaishnav, A.; Liu, J.; Varughese, A.; Wan, J.; Turner, A.; Ji, Q.; Zurek, M.; Kapralov, A.; Kagan, V.; et al. Serine-47 phosphorylation of cytochrome c in the mammalian brain regulates cytochrome c oxidase and caspase-3 activity. FASEB J. 2019, 33, 13503–13514. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, G.; Varughese, A.; Ji, Q.; Lee, I.; Liu, J.; Vaishnav, A.; Sinkler, C.; Kapralov, A.; Moraes, C.; Sanderson, T.; et al. Phosphorylation of Cytochrome c Threonine 28 Regulates Electron Transport Chain Activity in Kidney implications for amp kinase. J. Biol. Chem. 2017, 292, 64–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olteanu, A.; Patel, C.; Dedmon, M.; Kennedy, S.; Linhoff, M.; Minder, C.; Potts, P.; Deshmukh, M.; Pielak, G. Stability and apoptotic activity of recombinant human cytochrome c. Biochem. Biophys. Res. Commun. 2003, 312, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Lind, M.; Pielak, G. Characterization of horse cytochrome c expressed in Escherichia coli. Protein Expr. Purif. 2001, 22, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Margoliash, E.; Frohwirt, N. Spectrum of horse-heart cytochrome c. Biochem. J. 1959, 71, 570–572. [Google Scholar] [CrossRef]
- Blair, D.F.; Bocian, D.F.; Babcock, G.T.; Chan, S.I. Evidence for modulation of the heme absorptions of cytochrome c oxidase by metal-metal interactions. Biochemistry 1982, 21, 6928–6935. [Google Scholar] [CrossRef]
- Blair, D.; Martin, C.; Gelles, J.; Wang, H.; Brudvig, G.; Stevens, T.; Chan, S. The metal centers of cytochrome-C oxidase–Structures and interactions. Chem. Scr. 1983, 21, 43–53. [Google Scholar]
- Hazzard, J.; Rong, S.; Tollin, G. Ionic-strength dependence of the kinetics of electron-transfer from bovine mitochondrial cytochrome-c to bovine cytochrome-c-oxidase. Biochemistry 1991, 30, 213–222. [Google Scholar] [CrossRef]
- Schroedl, N.; Hartzell, C. Oxidative titrations of reduced cytochrome AA3—Influence of cytochrome-c and carbon-monoxide on midpoint potential values. Biochemistry 1977, 16, 4966–4971. [Google Scholar] [CrossRef]
- Zaslavsky, D.; Sadoski, R.; Wang, K.; Durham, B.; Gennis, R.; Millett, F. Single electron reduction of cytochrome c oxidase compound F: Resolution of partial steps by transient spectroscopy. Biochemistry 1998, 37, 14910–14916. [Google Scholar] [CrossRef] [PubMed]
- Osheroff, N.; Speck, S.; Margoliash, E.; Veerman, E.; Wilms, J.; Konig, B.; Muijsers, A. The reaction of primate cytochromes-c with cytochrome-c oxidase—Analysis of the polarographic assay. J. Biol. Chem. 1983, 258, 5731–5738. [Google Scholar] [CrossRef] [PubMed]
- Pecina, P.; Borisenko, G.; Belikova, N.; Tyurina, Y.; Pecinova, A.; Lee, I.; Samhan-Arias, A.; Przyklenk, K.; Kagan, V.; Huttemann, M. Phosphomimetic Substitution of Cytochrome c Tyrosine 48 Decreases Respiration and Binding to Cardiolipin and Abolishes Ability to Trigger Downstream Caspase Activation. Biochemistry 2010, 49, 6705–6714. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Salomon, A.; Yu, K.; Doan, J.; Grossman, L.; Huttemann, M. New prospects for an old enzyme: Mammalian cytochrome c is tyrosine-phosphorylated in vivo. Biochemistry 2006, 45, 9121–9128. [Google Scholar] [CrossRef] [Green Version]
- Guerra-Castellano, A.; Diaz-Quintana, A.; Perez-Mejias, G.; Elena-Real, C.; Gonzalez-Arzola, K.; Garcia-Maurino, S.; De la Rosa, M.; Diaz-Moreno, I. Oxidative stress is tightly regulated by cytochrome c phosphorylation and respirasome factors in mitochondria. Proc. Natl. Acad. Sci. USA 2018, 115, 7955–7960. [Google Scholar] [CrossRef] [Green Version]
- Olloqui-Sariego, J.; Perez-Mejias, G.; Marquez, I.; Guerra-Castellano, A.; Calvente, J.; de la Rosa, M.; Andreu, R.; Diaz-Moreno, I. Electric field-induced functional changes in electrode-immobilized mutant species of human cytochrome c. Biochim. Biophys. Acta-Bioenerg. 2022, 1863, 148570. [Google Scholar] [CrossRef]
- Kalpage, H.A.; Vaishnav, A.; Liu, J.; Lee, I.; Mahapatra, G.; Wan, J.; Kalpage, H.A.; Vaishnav, A.; Liu, J.; Lee, I.; et al. Regulation of Respiration and Apoptosis by Cytochrome c Threonine 58 Phosphorylation. Sci. Rep. 2019, 9, 15815. [Google Scholar]

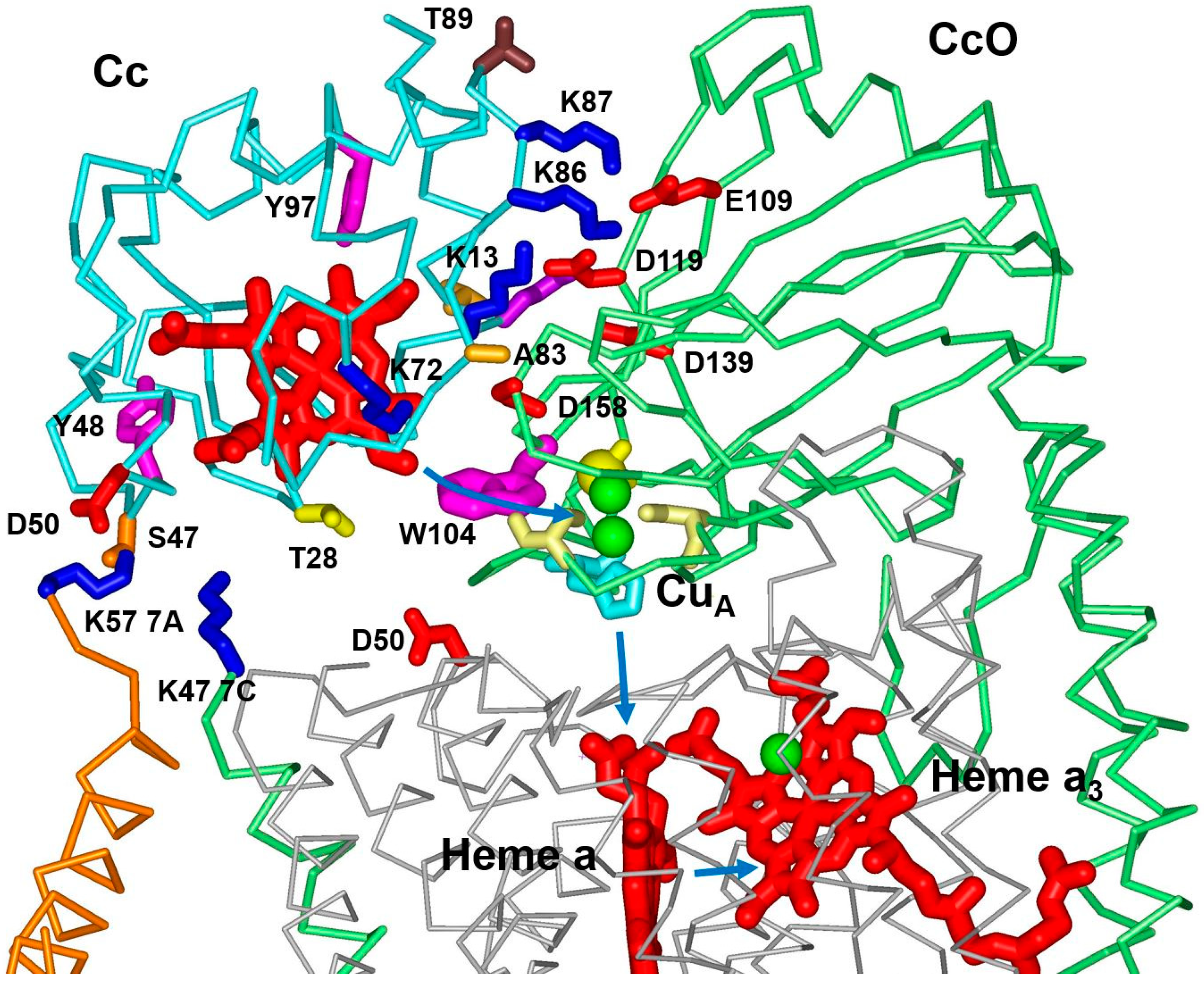
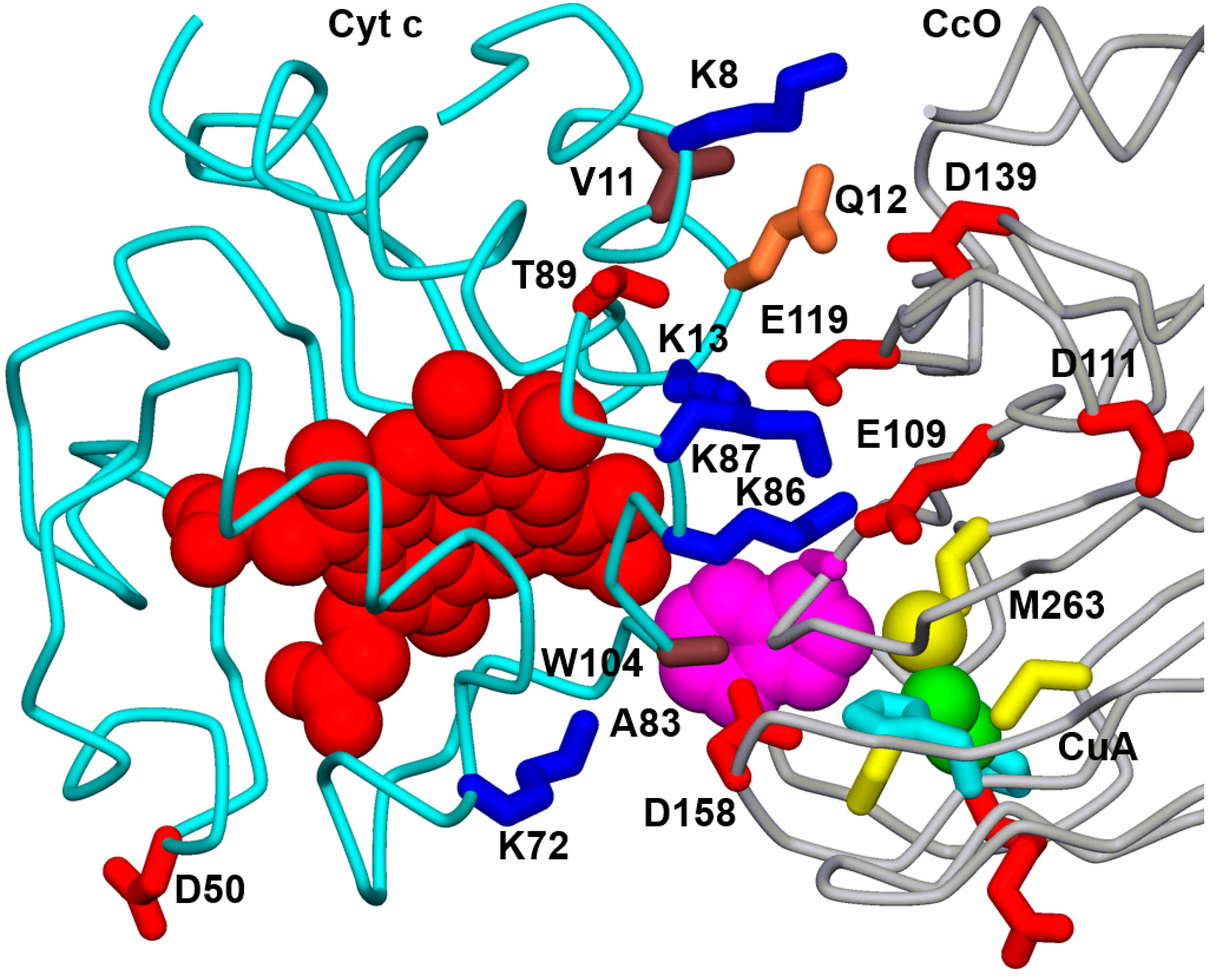
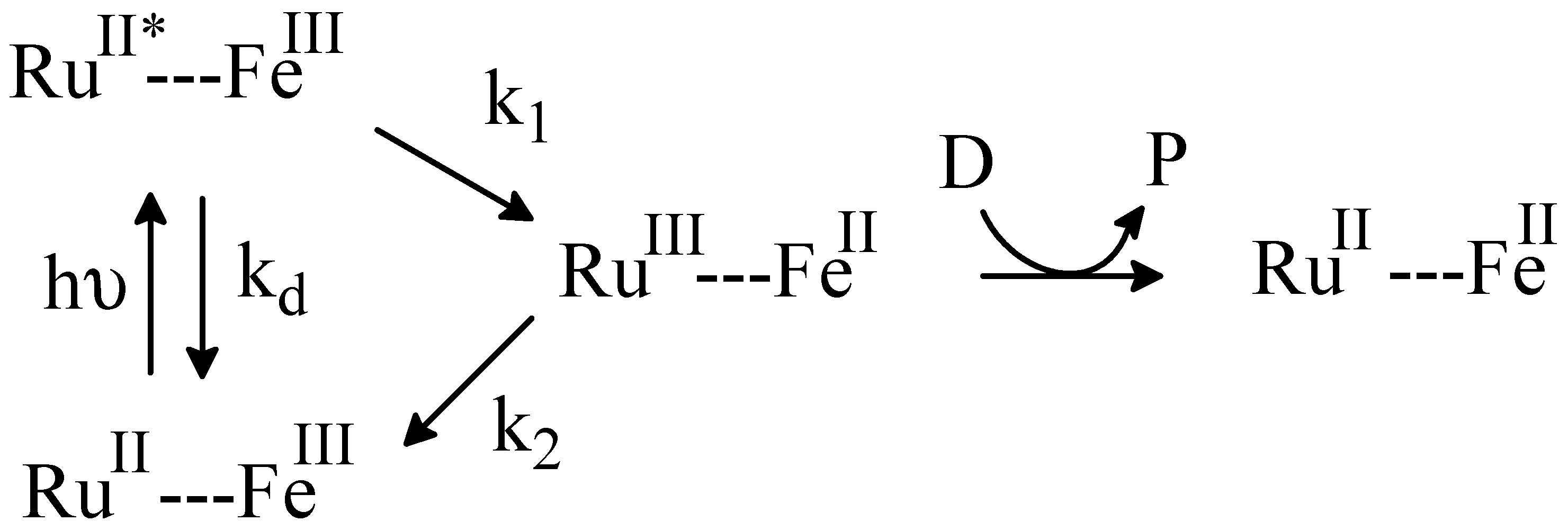
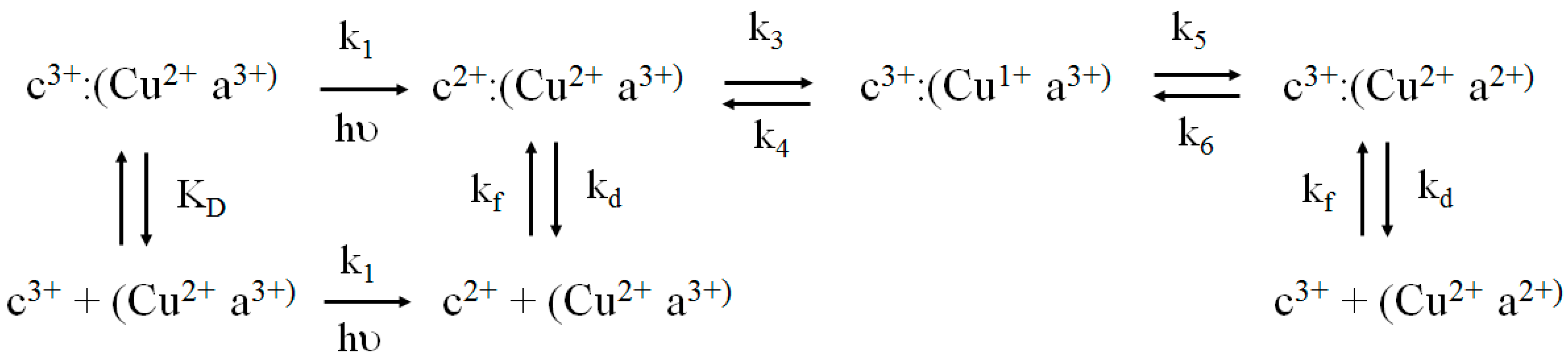
| Cyt c | Kd (μM) | Cyt c | Kd (μM) |
|---|---|---|---|
| Horse | 28.4 | Horse | 28.4 |
| Human | 4.7 | Human | 4.7 |
| Horse V11I | 23.4 | Human I11V | 2.9 |
| Horse Q12M | 14.3 | Human M12Q | 1.7 |
| Horse D50A | 11.8 | Human A50D | 15.7 |
| Horse A83V | 6.9 | Human V83A | 5.9 |
| Horse T89E | >35 | Human E89T | 2.1 |
| Cyt c | Kd (μM) | kd (s−1) | kf (μM−1 s−1) |
|---|---|---|---|
| Human | 4.7 | 1203 | 256 |
| Human T28E | 18.0 | 3456 | 192 |
| Human S47E | 24.0 | 4344 | 181 |
| Human Y48E | 10.5 | ||
| Human Y97E | 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brand, S.E.; Scharlau, M.; Geren, L.; Hendrix, M.; Parson, C.; Elmendorf, T.; Neel, E.; Pianalto, K.; Silva-Nash, J.; Durham, B.; et al. Accelerated Evolution of Cytochrome c in Higher Primates, and Regulation of the Reaction between Cytochrome c and Cytochrome Oxidase by Phosphorylation. Cells 2022, 11, 4014. https://doi.org/10.3390/cells11244014
Brand SE, Scharlau M, Geren L, Hendrix M, Parson C, Elmendorf T, Neel E, Pianalto K, Silva-Nash J, Durham B, et al. Accelerated Evolution of Cytochrome c in Higher Primates, and Regulation of the Reaction between Cytochrome c and Cytochrome Oxidase by Phosphorylation. Cells. 2022; 11(24):4014. https://doi.org/10.3390/cells11244014
Chicago/Turabian StyleBrand, Sue Ellen, Martha Scharlau, Lois Geren, Marissa Hendrix, Clayre Parson, Tyler Elmendorf, Earl Neel, Kaila Pianalto, Jennifer Silva-Nash, Bill Durham, and et al. 2022. "Accelerated Evolution of Cytochrome c in Higher Primates, and Regulation of the Reaction between Cytochrome c and Cytochrome Oxidase by Phosphorylation" Cells 11, no. 24: 4014. https://doi.org/10.3390/cells11244014
APA StyleBrand, S. E., Scharlau, M., Geren, L., Hendrix, M., Parson, C., Elmendorf, T., Neel, E., Pianalto, K., Silva-Nash, J., Durham, B., & Millett, F. (2022). Accelerated Evolution of Cytochrome c in Higher Primates, and Regulation of the Reaction between Cytochrome c and Cytochrome Oxidase by Phosphorylation. Cells, 11(24), 4014. https://doi.org/10.3390/cells11244014







