1. Introduction
Testicular and spermatic cord torsion are relatively common, representing a surgical emergency. This pathology occurs in 1 out of 4000 males younger than 25 years [
1]. It accounts for approximately 10% to 15% of acute scrotal disease in children and results in an orchiectomy rate of 42% in boys undergoing surgery for testicular torsion [
1]. This event generally takes place antenatally/in the early postnatal period as an entire cord twisting or in older children and adults as a twisting of the cord within the tunica vaginalis [
2]. In both situations, the first phase of spermatic cord twist is characterized by the increase in venous pressure and congestion, followed by a second phase in which there is a decrease in arterial blood flow with ischemia. However, a wide range of diverse events may induce acute scrotum pain or dysfunction, such as epididymo-orchitis, idiopathic scrotal edema, infection, torsion of the appendix testicle or appendix epididymis, torsion of the spermatic cord, trauma, tumor, and varicocele [
2]. Therefore, despite most patients not requiring urgent intervention, a significant minority present testicular torsion. However, in the case of testicular torsion, the viability of the testis decreases 6 h after the onset of symptoms, which necessarily requires a prompt diagnosis. The risk of testicular loss or unnecessary surgery calls for the necessity of novel diagnostic techniques; for instance, a recent study focused on the role of mean platelet volume in the diagnosis of testicular torsion [
1]. By now, the only safe approach to resolve testicular torsion is surgery, which should be performed as soon as possible, and testicular viability is secondary to both the number of spermatic cord turns and the post-derotation recovery. Testis vascularization originates from the spermatic cord as well as from the cremasteric artery and gubernaculum. In fact, during testicular torsion, the third path of blood supply to the testis, i.e., the gubernaculum, is generally twisted and ischemic. Real therapeutic doubt arises from the ability to predict which testes left in place after derotation will be viable and functional in the long term.
The gubernaculum testis is an elongated conical structure composed of loose extracellular matrix and mesenchymal cells, mainly including fibroblasts and muscle cells, which have the function to pull the testes through the abdomen and the inguinal canal down into the scrotum. From a histological examination, it has been reported that the gubernaculum consists of moderately vascular, fibrofatty tissue with a core of essentially longitudinally oriented collagenous fibers surrounded by mature fatty tissue [
3]. Insulin-like 3 peptide (INSL3) is a hormone produced by the gonads and is the ligand of the relaxin family peptide receptor 2 (RXFP2), a G protein-coupled receptor (GPCR) [
4]. In males, INSL3 is primarily produced by Leydig cells, and RXFP2 has been shown to be highly expressed in the gubernaculum. During embryogenesis, testicular descent consists of two phases: the transabdominal and inguinoscrotal phases. The INSL3/RXFP2 axis has an essential role in the development of the gubernaculum for the initial transabdominal descent of the testis and in the maintenance of reproductive health in men [
5,
6]. Despite the inguinoscrotal phase being mainly mediated by androgens, some studies have also shown the involvement of INSL3/RXFP2 [
6]. In addition, it has been shown that rat gubernacular cells show an increased production of cAMP upon stimulation with INSL3 and that bilateral cryptorchidism in INSL3- and RXFP2-deficient mice is linked with the impaired development of the gubernaculum [
4]. During testicular migration, the gubernaculum undergoes extensive remodeling, which contributes to the testicular descent toward the scrotum. A study showed that during 15 to 16 weeks of gestation, collagen fibers that were composed of the gubernaculum were sparser and more embedded in a loose extracellular matrix; then, at 28 weeks of gestation, the number of fibers gradually increased, making the gubernaculum mostly collagenous. Fibroblasts largely predominate over other cell types and decrease in number with gestational age, whereas smooth muscle cells are restricted to the walls of blood vessels. Striated muscle cells are generally detected at the scrotal end of the gubernaculum, decreasing in number with age [
7]. Usually, after birth, the muscles and connective tissues obliterate any opening and the gubernaculum testis becomes the scrotal ligament (SL), persisting in adults as a fibrous residual structure only individualized as microscopic connective fiber. The SL is typically described as a ‘‘Y-shape’’ ligament with the testis and epididymis as proximal insertions and the scrotum as a distal attachment. However, in some cases, authors have shown the absence of attachment to the adult scrotum, supporting the involution of the gubernaculum after birth and therefore the possible absence of SL [
8].
Human chorionic gonadotropin (hCG) is a glycoprotein hormone that belongs to the gonadotropin hormone family, which also includes the luteinizing hormone (LH) and the follicle-stimulating hormone (FSH) and binds to the LH receptor (LHR). HCG is clinically used in post-operative patients, more specifically cryptorchid patients, to achieve higher testicular volume and function [
9].
Interestingly, it has been reported that, when treated with hCG, gubernaculum components alter significantly and become rich in vessels [
10].
Although the positive effect of hCG treatment on the gubernaculum in cryptorchid patients is partially known, its clinical use in other andrological pathologies, including testicular torsion, has not been applied. Thus, with this study, we show that gubernacular tissues that have been derived from patients affected by testicular torsion can be cultured and amplified in vitro in order to test the efficiency of hCG therapy, which can also be used in this pathology to recover testicle functionality. In addition, the union of biological and clinical approaches may help in the identification of a best personalized posology prior to administration to the patient.
2. Materials and Methods
This prospective observational study was approved by the Pediatric Fertility Lab internal review board (09/2020).
The Ethical Committee of “Azienda Ospedaliera Universitaria Integrata” (AOUI) of Verona, Italy, had already approved the program “Fertility Potential (FePo) 2.0” (N. 3072 CESC), regarding the study of fertility preservation in pediatric and adolescent patients, this being the basis of the study aimed at the improvement of fertility potential in males.
Since May 2019, at our Fertility Lab Tissue Biobank, we have been collecting scrotal fat and testicular and gubernacular tissue from pediatric and adult patients operated on for testicular pathologies. Each patient is cataloged with a progressive number and identification. The tissue database is composed of samples from patients aged between 1 month and 50 years.
2.1. Patients
Informed consent was obtained from all the parents and patient compliance was 100%. The internal IRB approved the study. At our center, we collect samples of patients with primary azoospermia, patients with genetic syndromes associated with azoospermia, patients with undescended testis, patients with neoplasms, and patients with testicular neoplasms. In addition, patients with testicular torsion or testicular trauma are included.
2.2. Inclusion Criteria
We considered all patients with testicular torsion that were clinically diagnosed and treated within 24 h of symptom onset and surgically treated within 2 h after hospital arrival in order to avoid bias related to hospital coordination.
2.3. Exclusion Criteria
We excluded patients treated for torsion that had definitely occurred at least 24 h before symptom onset, either reported in history or showing an absence of pain on palpation. Moreover, patients reporting testicular trauma immediately before a suspected torsion or this being considered as the main cause of their torsion were excluded.
2.4. Surgical Procedure
All patients underwent surgery by the same operator under general anesthesia. All procedures were performed with a trans-scrotal incision. All testes were delivered, the torsion was resolved, and then 3 micro-biopsies were performed to verify the vitality. If necessary (no bleeding), the testes were removed. In cases with partial testicular viability (bleeding and/or good parenchymal color), the testes were left in place. All patients underwent gubernaculum biopsies to deduce more information about vascularization. No other procedures were performed on the contralateral testes.
2.5. Drugs and Chemicals
Highly purified human chorionic gonadotropin (hCG) extracted from pregnant women’s urine was kindly provided by IBSA Italia. As reported in the results section, we treated the cells in vitro with 100 UI/mL hCG once a week for 4 weeks and then analyzed cell proliferation after the end of the 5th week. We chose this therapeutic scheme because it was very similar to the scheme used in clinics for certain pediatric testicular pathologies. This hormonal treatment is also suggested in adulthood for infertility with different dosages.
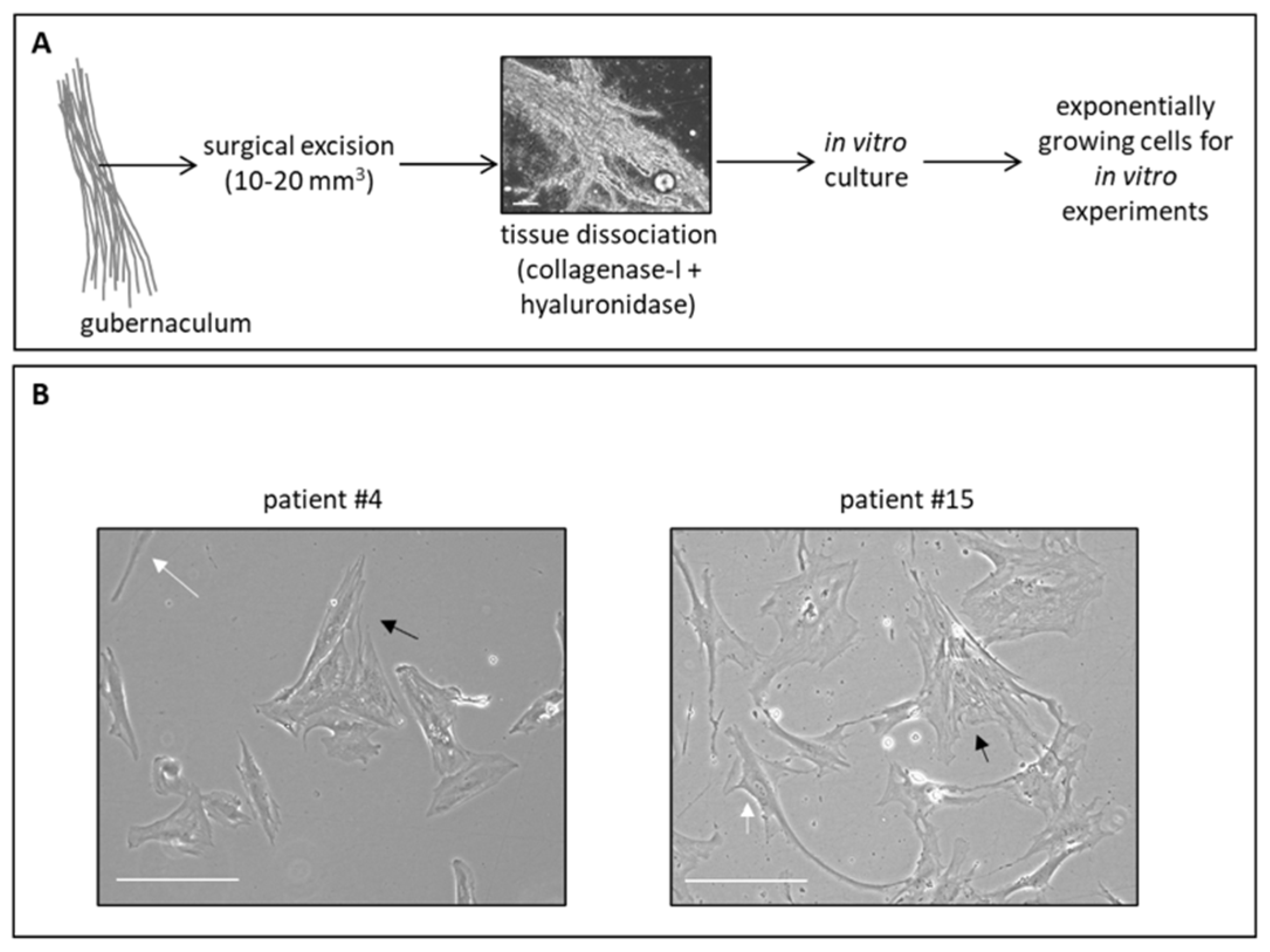
2.6. Gubernacular Tissue Processing and In Vitro Culture
The gubernacula were processed by firstly cutting the piece of tissue (about 10–20 mm3) with a scalpel in 500 μL of phosphate-buffered saline (PBS) on a Petri dish in sterility under the biological hood. Then, the fragmented tissues were enzymatically disaggregated with 1X collagenase type I and hyaluronidase (both from Sigma-Aldrich, St. Louis, MO, USA, Merck) resuspended in 200 μL of a complete cell culture medium and incubated at 37 °C for 1 h, or until complete homogenization of the tissue. Cell viability was evaluated with a trypan blue assay and was variably around 20–40%. Finally, the cell suspension was grown in DMEM-Glutamax supplemented with 10% fetal bovine serum (FBS), 4.5 g/L glucose, and 50 μg/mL gentamicin sulfate (all from Gibco/Life Technologies, Waltham, MA, USA) and maintained at 37 °C with 5% CO2 in Corning Primaria cell culture flasks (Corning, New York, NY, USA).
Before hCG treatment, cells were maintained for 16 h in an FBS-free medium as FBS generally contains FSH and LH at different concentrations from lot-to-lot, as reported by different manufacturers’ sheets. Then, cells were treated with the indicated doses and for the indicated time (see figure legends) with hCG by keeping cells in the medium without FBS.
Bright field cell images were acquired with an inverted microscope (Axio Vert. A1, Zeiss, Oberkochen, Germany).
2.7. RNA Extraction and qPCR
RNA extraction and real-time quantitative PCR (qPCR) were performed as previously described [
11]. The positive control cells were MCF7 (breast cancer cell line) and the negative control cells were PaCa44 (pancreatic cancer cell line), as previously described [
12]. Briefly, total RNA was extracted from 1 × 10
5 cells using a Single Cell RNA Purification Kit (Norgen Biotek, Thorold, ON, Canada) and 0.5 μg of RNA was reverse-transcribed using first-strand cDNA synthesis. The quality of RNA was evaluated by running 0.5 μg of RNA for each sample on an agarose gel. Real-time quantification was performed in triplicate samples by SYBR-Green detection chemistry with the GoTaq qPCR Master Mix (Promega, Madison, WI, USA) on a QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The primers used were:
LHR forward, 5′-GCTGCGATTAAGACATGCCA-3′,
LHR reverse, 5′-AGAAGGCCACCACATTGAGA-3′; Androgen Receptor (
AR) forward, 5′-CCCACTTGTGTCAAAAGCGA-3′,
AR reverse, 5′-GCAGCTTCCACATGTGAGAG-3′; and Ribosomal Protein Lateral Stalk Subunit P0 (
RPLP0) forward, 5’-ACATGTTGCTGGCCAATAAGGT-3’,
RPLP0 reverse, 5’-CCTAAAGCCTGGAAAAAGGAGG-3’. The negative control samples were without cDNA. The cycling conditions used were: 95 °C for 10 min, 40 cycles at 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s, and 60 °C for 15 s. The average cycle threshold of each triplicate was analyzed according to the 2
−ΔΔCt method.
RPLP0 gene expression was used as an endogenous control to standardize mRNA expression. All reactions were performed in three independent experiments.
2.8. Cell Proliferation Assay
Gubernacular cells were plated in 96-well cell culture plates (5 × 103 cells/well) and incubated at 37 °C with 5% CO2. Cell viability was measured by crystal violet assay (Merck Millipore) according to the manufacturer’s protocol, and absorbance was measured by spectrophotometric analysis (A595 nm). The crystal violet assay is designed to work with adherent cells, and when cells die, they detach from the surface of the plate. Thus, this assay is suitable for in vitro cell proliferation and cell cytotoxicity studies, as reported in several manufacturers’ sheets. At each treatment time point, we added 25 mL of medium containing solvent or hCG to the control or treated cells, respectively, in the wells. Gubernacular cells derived from patients affected by cryptorchidism with normal testicular viability were used as a control. Three independent biological replicates were performed, each in triplicate.
2.9. Statistical Analysis
ANOVA (post hoc Tukey) analysis by GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA) or Student’s t-test (two-tailed) were conducted. The p-values < 0.05, 0.01, or 0.001 were considered significantly different (for details, see figure legends).
4. Discussion
Testicular torsion is a disease that necessarily requires a prompt diagnosis and treatment alongside a surgeon’s decision on whether to preserve the testes or not. A negative long-term effect of a damaged testicle left in place could be an alteration of the fertility potential of the subject. At present, the only safe approach to treat testicular torsion is timely surgery, albeit no adjuvant therapeutic approach is currently considered in the post-operative phase. As chorionic gonadotropin is a treatment generally used to improve fertility potential in infertile men and increase testicular trophism in post-operative cryptorchid patients, we hypothesized that the administration of this treatment in patients with testicular torsion may improve testicle health. Starting from this assumption, our study focused on the in vitro validation of these observations in order to understand if this approach could also be leveraged in this therapeutic field. In particular, we focused our attention on the gubernaculum because it is known to have an important role not only during testicle descent but also as one of the major sources of testis vascularization. Indeed, it has been demonstrated that gubernacular components become rich in vessels when treated with gonadotropin [
10]. In addition, during testicular torsion, the gubernaculum is generally twisted and ischemic, thus representing an important element that can be exploited to analyze the viability of the testicle and the possible response to the treatment with chorionic gonadotropin.
To accomplish the aims of this study, we first optimized a protocol to culture and amplify in vitro gubernacular cells. The potentiality to use gubernaculum for these in vitro studies was twofold: first, the testicle, which is suffering due to torsion, could be completely preserved and surgically untouched; second, from our preliminary data (not shown), the culture of gubernacular tissue provides a higher yield of in vitro growing cells in comparison to testicular cells, which propagate more slowly. Indeed, we demonstrated that the disruption of a small portion of gubernacular tissue harvested during surgery in pediatric patients allows the obtainment of viable primary cells that possess the ability to grow in culture. However, as we also obtained tissue from a patient who underwent orchiectomy, here, we revealed that there was a good correlation between the clinical evaluation of testicle health and the ability of gubernacular cells to grow in vitro; indeed, the isolated cells derived from patient #12, whose testicle had been removed, were not able to grow in vitro, in line with the surgeon’s decision to perform orchiectomy due to the presence of a necrotic testicle. Thus, this initial evidence suggests that the in vitro culture of the gubernaculum may be exploited as an evaluation of the state of the testicle, evidencing the viability of the organ and thereafter confirming to the surgeon whether there is a necessity or not to remove a testicle that showed a border-line health appearance.
Further evidence that emerged from our data was that gubernacular cells not only expressed the androgen receptor (
AR) but also the luteinizing hormone receptor (
LHR), which is essential to assess a possible response to gonadotropin, being its specific receptor. It is interesting to highlight that, for the control patient, who was affected by cryptorchidism, there was an expression of high levels
LHR mRNA, further supporting that the gubernaculum is a candidate tissue for the response to hCG therapy. Afterward, we treated gubernacular cells derived from patients #4 and #15 with a dosage and timing of treatment that was as close as possible to the posology that is provided to patients. Indeed, we treated in vitro cultured cells with 100UI/mL of hCG every week for 4 weeks and we showed that both patients’ gubernacular cells increased their proliferation after hormonal stimuli. Interestingly, when stimulated, the cells of patient #4 showed a significantly higher proliferation in comparison to the cells of patient #15, confirming the different temporal windows within which the two cell types started to exponentially grow (
Table 1).
This study represents the first step toward a broader in vitro study and potential future clinical validations of an hCG-based therapeutic approach for patients affected by testicular torsion. However, some limits may be reported: (i) The number of patients would be implemented in the future to further corroborate the obtained results. However, as a preliminary study, our outcome was to evaluate the effectiveness of hormonal treatment on some representative cases and it is necessary to take into account that in vitro evaluations can hardly be performed on a large set of patients. (ii) A surgical biopsy must be performed correctly; however, due to torsion, the difference between the gubernaculum, scrotal fat, etc., was not always clear, also considering the edema and the scrotal hematoma. (iii) In this study, we considered as a control patient a 2-year-old cryptorchid child whose tissue had been taken from our tissue biobank and processed following the same protocol of the other patients; the response to the hCG treatment of the control patient was considered representative of a control group presenting a healthy testicle and having a good rate of response to hormonal stimulation. (iv) A future step would be to compare the in vitro response to the treatment with clinical information that would be obtained by analyzing testicular trophism and vascularization at the end of therapeutic treatment on the same patient. (v) It would be necessary to delineate a cut-off of therapy responder patients in order to propose orchiectomy after treatment.
Clinically, to decide whether an injured testicle should be kept or removed during surgery, it will be fundamental to recognize the quality of the testicle in order to predict how it (and the gubernaculum) will respond to therapy. Finally, it is noteworthy that the data on in vitro proliferation analysis were obtained by treating the cells with a single standardized dosage. Therefore, a future goal would be to evaluate how the cells of each patient proliferate under different conditions of treatment with chorionic gonadotropin and to identify the dose and timing at which the gubernaculum of a given patient responds more. This information will permit proposing a tailored therapy for each patient.