Enhancing In Vitro Production of the Tree Fern Cyathea delgadii and Modifying Secondary Metabolite Profiles by LED Lighting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
- (1)
- Fl—fluorescent lamps (Philips TL-D 36W/54)
- (2)
- B—100% blue LED light (430 nm)
- (3)
- R—100% red LED light (670 nm)
- (4)
- RB—combination of red and blue LED lights (70%/30%)
- (5)
- RBfR—combination of red, blue, and far-red (730 nm) LED lights (35%/15%/50%)
- (6)
- RBY—combination of red, blue, and yellow (600 nm) LED lights (35%/15%/50%)
- (7)
- RBUV—combination of red, blue, and UV (400 nm) LED lights (35%/15%/50%)
- (8)
- RBG—combination of red, blue, and green (528 nm) LED lights (35%/15%/50%)
- (9)
- Wh—white LED (1:1:1 2700 K:4500 K:5700 K)
2.2. Evaluation of Regenerative Ability
2.3. Content of Photosynthetic Pigments
2.4. Secondary Metabolite Extraction
2.5. LC-ESI-MS/MS Analysis of Phenolic Acids and Flavonoids
2.6. Antioxidant Activity Assays
2.7. Statistical Analysis
3. Results
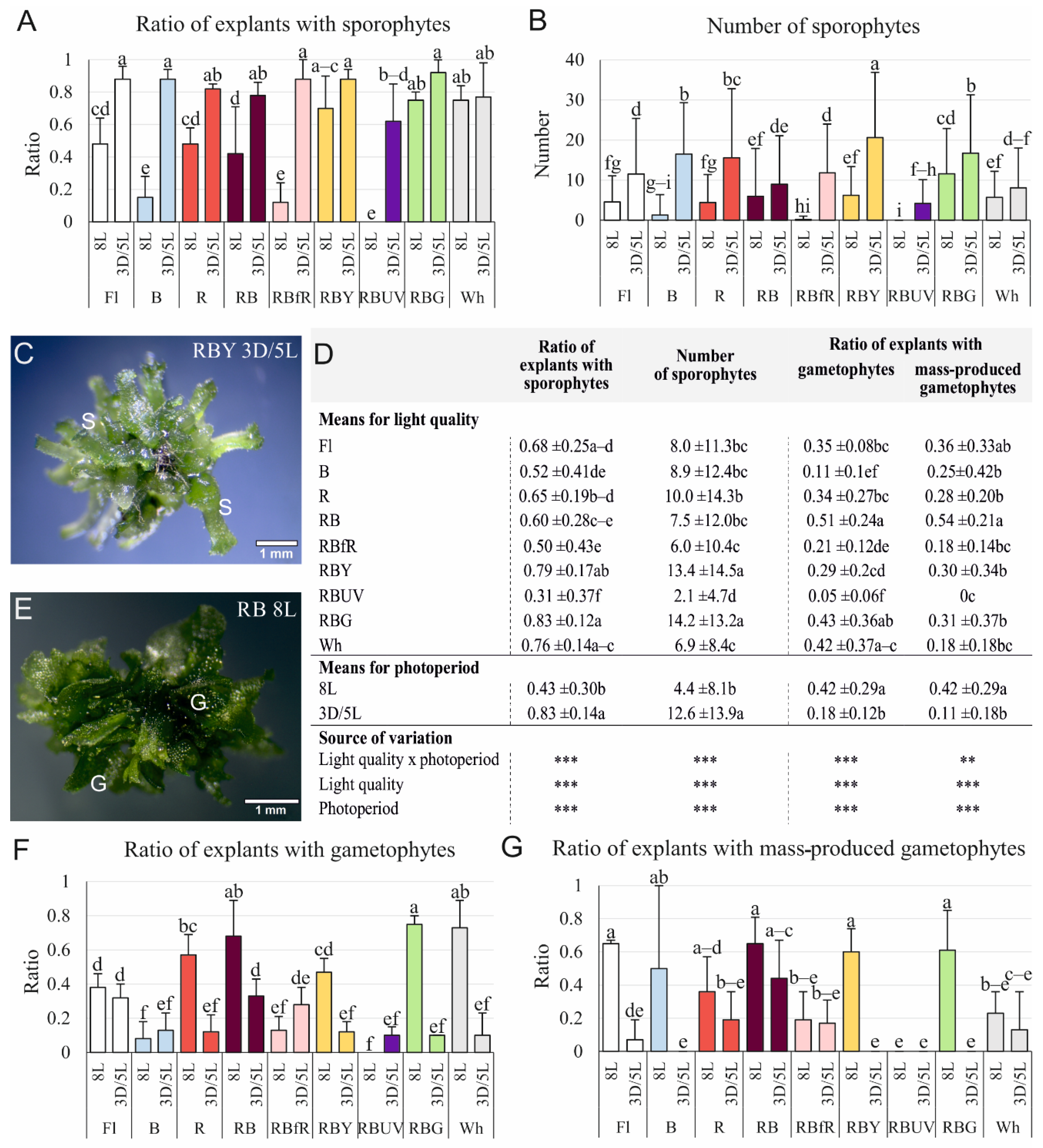
3.1. Efficiency of Sporophyte and Gametophyte Production on the Stipe Explants
3.2. Effect of Light Quality and Photoperiod on the Development of Sporophytes Obtained on the Stipe Explants
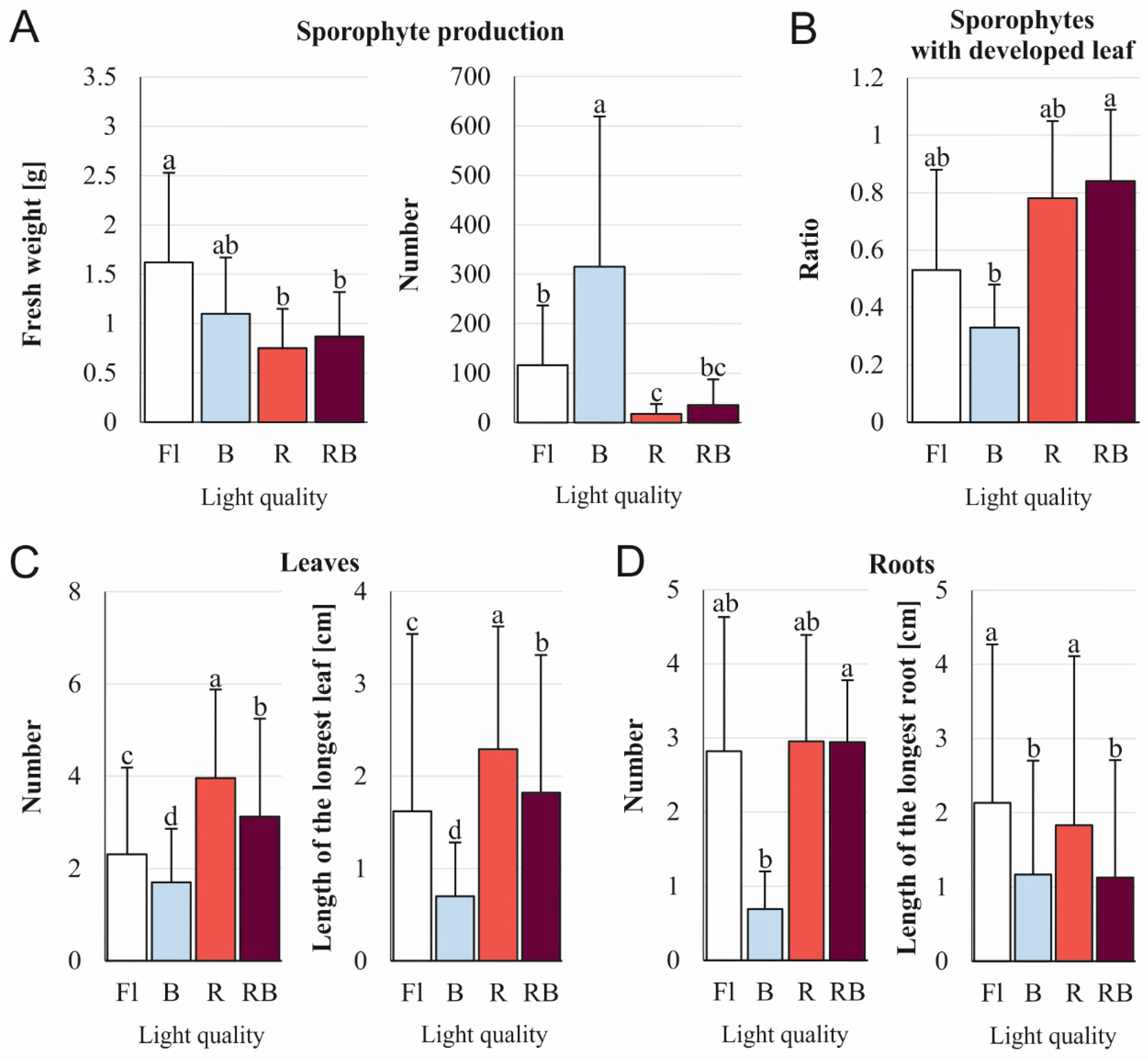
3.3. Effect of Light Quality on the Sporophyte Production on Whole Etiolated Sporophytes
3.4. Photosynthetic Pigment Content
3.5. Secondary Metabolite Profiles
3.6. Antioxidant Activity of Plants Extracts
4. Discussion
4.1. Effect of Light Conditions on the Production of Somatic Embryo-Derived Plantlets
4.2. Effect of Light Conditions on Plant Development
4.2.1. Leaves
4.2.2. Roots
4.3. Effect of Light Conditions on Fern Gametophyte Production
4.4. Effect of Light Quality on Content of Photosynthetic Pigments
4.5. Effect of Light Quality on Secondary Metabolite Production and Their Antioxidant Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, C.; Fu, Y.; Liu, G.; Liu, H. Low light intensity effects on the growth, photosynthetic characteristics, antioxidant capacity, yield and quality of wheat (Triticum aestivum L.) at different growth stages in BLSS. Adv. Space Res. 2014, 53, 1557–1566. [Google Scholar] [CrossRef]
- Huang, X.; Ouyang, X.; Deng, X.W. Beyond repression of photomorphogenesis: Role switching of COP/DET/FUS in light signaling. Curr. Opin. Plant Biol. 2014, 21, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Bula, R.J.; Morrow, R.C.; Tibbitts, T.W.; Barta, D.J.; Ignatius, R.W.; Martin, T.S. Light-emitting diodes as a radiation source for plants. HortScience 1991, 26, 203–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.D.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J. Am. Soc. Hort. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef] [Green Version]
- Yeh, N.; Chung, J.-P. High-brightness LEDs—Energy efficient lighting sources and their potential in indoor plant cultivation. Renew. Sustain. Energy Rev. 2009, 13, 2175–2180. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Tang, C. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tissue Organ Cult. 2010, 103, 155–163. [Google Scholar] [CrossRef]
- Chen, C.-C.; Agrawal, D.C.; Lee, M.-R.; Lee, R.-J.; Kuo, C.-L.; Wu, C.-R.; Tsay, H.-S.; Chang, H.-C. Influence of LED light spectra on in vitro somatic embryogenesis and LC–MS analysis of chlorogenic acid and rutin in Peucedanum japonicum Thunb.: A medicinal herb. Bot. Stud. 2016, 57, 9. [Google Scholar] [CrossRef] [Green Version]
- Pawłowska, B.; Żupnik, M.; Szewczyk-Taranek, B.; Cioć, M. Impact of LED light sources on morphogenesis and levels of photosynthetic pigments in Gerbera jamesonii grown in vitro. Hortic. Environ. Biotechnol. 2018, 59, 115–123. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Prokopiuk, B.; Komsta, Ł.; Pawłowska, B.; Ekiert, H. The influence of light quality on the production of bioactive metabolites–verbascoside, isoverbascoside and phenolic acids and the content of photosynthetic pigments in biomass of Verbena officinalis L. cultured in vitro. J. Photochem. Photobiol. B Biol. 2020, 203, 111768. [Google Scholar] [CrossRef]
- Tryon, R. A revision of the genus Cyathea. Contrib. Gray Herb. Harvard Univ. 1976, 206, 19–98. [Google Scholar]
- Moran, R.C.; Klimas, S.; Carlsen, M. Low-trunk epiphytic ferns on tree ferns versus angiosperms in Costa Rica. Biotropica 2003, 35, 48–56. [Google Scholar] [CrossRef]
- CITES. Convention of International Trade in Endangered Species of Wild Fauna and Flora. 2021. Available online: https://cites.org/eng/app/appendices.php (accessed on 2 January 2022).
- Eleutério, A.A.; Pérez-Salicrup, D. Management of tree ferns (Cyathea spp.) for handicraft production in Cuetzalan, Mexico. Econ. Bot. 2006, 60, 182–186. [Google Scholar] [CrossRef]
- Liu, Y.; Wujisguleng, W.; Long, C. Food uses of ferns in China: A review. Acta Soc. Bot. Pol. 2012, 81, 263–270. [Google Scholar] [CrossRef]
- Goswami, H.K.; Sen, K.; Mukhopadhyay, R. Pteridophytes: Evolutionary boon as medicinal plants. Plant Genet. Resour. 2016, 14, 328–355. [Google Scholar] [CrossRef]
- Nath, K.; Talukdar, A.D.; Bhattacharya, M.K.; Bhowmik, D.; Chetri, S.; Choudhury, D.; Mitra, A.; Choudhury, N.A. Cyathea gigantea (Cyatheaceae) as an antimicrobial agent against multidrug resistant organisms. BMC Complement. Altern. 2019, 19, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janakiraman, N.; Johnson, M. Larvicidal potential of Cyathea species against Culex quinquefasciatus. Pharm. Biomed. Res. 2016, 3, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Ida, N.; Iwasaki, A.; Teruya, T.; Suenaga, K.; Kato-Noguchi, H. Tree fern Cyathea lepifera may survive by its phytotoxic property. Plants 2020, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Madhu Kiran, P.; Vijaya Raju, A.; Ganga Rao, B. Investigation of hepatoprotective activity of Cyathea gigantea (Wall. ex. Hook.) leaves against paracetamol-induced hepatotoxicity in rats. Asian Pac. J. Trop. Biomed. 2012, 2, 352–356. [Google Scholar] [CrossRef] [Green Version]
- Faizal, A.; Taufik, I.; Rachmani, A.F.; Prihartini Azar, A.W. Short communication: Antioxidant and antibacterial properties of tree fern Cyathea contaminans. Biodiversitas 2020, 21, 2201–2205. [Google Scholar] [CrossRef]
- Hiendlmeyer, R.; Randi, A.M. Response of spores and young gametophytes of Cyathea delgadii Sternb. (Cyatheaceae) and Blechnum brasiliense Desv. (Blechnaceae) to different light levels. Acta Bot. Bras. 2007, 21, 909–915. [Google Scholar] [CrossRef]
- Rechenmacher, C.; Schmitt, J.L.; Droste, A. Spore germination and gametophyte development of Cyathea atrovirens (Langsd. & Fisch.) Domin (Cyatheaceae) under different pH conditions. Braz. J. Biol. 2010, 70, 1155–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis Moura, I.; Simões-Costa, M.C.; Garcia, J.; Silva, M.J.; Duarte, M.C. In vitro culture of tree fern spores from Cyatheaceae and Dicksoniaceae families. Acta Hortic. 2012, 937, 455–461. [Google Scholar] [CrossRef]
- Das, S.; Dutta Choudhury, M.; Mazumder, P.B. Research article in vitro propagation of Cyathea gigantea (wall ex. Hook)-A tree fern. Int. J. Recent Sci. Res. 2013, 4, 221–224. [Google Scholar]
- Marcon, C.; Silveira, T.; Schmitt, J.L.; Droste, A. Abiotic environmental conditions for germination and development of gametophytes of Cyathea phalerata Mart. (Cyatheaceae). Acta Bot. Brasilica 2017, 31, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Rybczyński, J.J.; Mikuła, A. Tree ferns biotechnology: From spores to sporophytes. In Working with Ferns: Issues and Applications; Fernández, H., Ed.; Springer: New York, NY, USA, 2011; pp. 135–146. [Google Scholar]
- Bonomo, M.C.; Martínez, O.G.; Tanco, M.E.; Cardozo, R.; Avilés, Z. Spores germination and gametophytes of Alsophila odonelliana (Cyatheaceae) in different sterile media. Pyton 2013, 82, 119–126. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, G.; Li, H.; Cao, H.; Mo, X.; Gui, M.; Zhou, X.; Jiang, Y.; Li, S.; Wang, J. In vitro propagation of the endangered tree fern Cibotium barometz through formation of green globular bodies. Plant Cell Tiss. Organ Cult. 2017, 128, 369–379. [Google Scholar] [CrossRef]
- Mikuła, A.; Pożoga, M.; Tomiczak, K.; Rybczyński, J.J. Somatic embryogenesis in ferns: A new experimental system. Plant Cell Rep. 2015, 34, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, J.; Joshi, S.D. In vitro study of effects of growth hormones on sporophyte development of Cyathea spinulosa. Int. J. Biodivers. Conserv. 2014, 6, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.P.; Khare, P.B. In vitro conservation of some threatened and economically important ferns belonging to the Indian subcontinent. J. Bot. 2014, 2014, 949028. [Google Scholar] [CrossRef] [Green Version]
- Mikuła, A.; Pożoga, M.; Grzyb, M.; Rybczyński, J.J. An unique system of somatic embryogenesis in the tree fern Cyathea delgadii Sternb.: The importance of explant type, and physical and chemical factors. Plant Cell Tiss. Organ Cult. 2015, 123, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Grzyb, M.; Mikuła, A. Explant type and stress treatment determine the uni-and multicellular origin of somatic embryos in the tree fern Cyathea delgadii Sternb. Plant Cell Tiss. Organ Cult. 2019, 136, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Grzyb, M.; Kalandyk, A.; Waligórski, P.; Mikuła, A. The content of endogenous hormones and sugars in the process of early somatic embryogenesis in the tree fern Cyathea delgadii Sternb. Plant Cell Tiss. Organ Cult. 2017, 129, 387–397. [Google Scholar] [CrossRef]
- Gauer Medeiros, L.; Marcon, C.; Silveira, T.; Schmitt, J.L.; Droste, A. Looking for the conservation and sustainable use of Cyathea corcovadensis (Raddi) Domin (Cyatheaceae): The influence of environmental factors on gametophytes. Braz. J. Bot. 2017, 40, 13–20. [Google Scholar] [CrossRef]
- Donaher, D.J.; Partanen, C.R. The role of light in the interrelated processes of morphogenesis and photosynthesis in the fern gametophyte. Physiol. Plant. 1971, 25, 461–468. [Google Scholar] [CrossRef]
- Wada, M.; Sei, H. Phytochrome-mediated phototropism in Adiantum cuneatum young leaves. J. Plant Res. 1994, 107, 181–186. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Kadota, A. Photosynthesis-dependent but neochrome1-independent light positioning of chloroplasts and nuclei in the fern Adiantum capillus-veneris. Plant Physiol. 2011, 155, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Doi, M.; Wada, M.; Shimazaki, K.-I. The fern Adiantum capillus-veneris lacks stomatal responses to blue light. Plant Cell Physiol. 2006, 47, 748–755. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Khasanah, N.M. Root anatomy and growth responses of soybean (Glycine max (L.) Merr.) ‘Wilis’ to NaCl stress. In Proceedings of the 1st International Conference on Tropical Agriculture, Yogyakarta, Indonesia, 25–26 October 2016; Isnansetyo, A., Nuringtyas, T.R., Eds.; Springer: Cham, Switzerland, 2017; pp. 209–218. [Google Scholar]
- Pietrzak, W.; Nowak, R.; Gawlik-Dziki, U.; Lemieszek, M.K.; Rzeski, W. LC-ESI-MS/MS identification of biologically active phenolic compounds in mistletoe berry extracts from different host trees. Molecules 2017, 22, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olech, M.; Nowacka-Jechalke, N.; Masłyk, M.; Martyna, A.; Pietrzak, W.; Kubiński, K.; Załuski, D.; Nowak, R. Polysaccharide-rich fractions from Rosa rugosa Thunb.-Composition and chemopreventive potential. Molecules 2019, 24, 1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.-T.; Lee, H.-L.; Chiang, S.-H.; Lin, F.-I.; Chang, C.-Y. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J. Food Drug Anal. 2001, 9, 96–101. [Google Scholar] [CrossRef]
- Szewczyk, K.; Bogucka-Kocka, A.; Vorobets, N.; Grzywa-Celińska, A.; Granica, S. Phenolic composition of the leaves of Pyrola rotundifolia L. and their antioxidant and cytotoxic activity. Molecules 2020, 25, 1749. [Google Scholar] [CrossRef] [Green Version]
- Deba, F.; Xuan, T.D.; Yasuda, M.; Tawata, S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control 2008, 19, 346–352. [Google Scholar] [CrossRef]
- Hutchinson, M.J.; Senaratna, T.; Sahi, S.V.; Saxena, P.K. Light mediates endogenous plant growth substances in thidiazuron-induced somatic embryogenesis in geranium hypocotyl cultures. J. Plant Biochem. Biotechnol. 2000, 9, 1–6. [Google Scholar] [CrossRef]
- Gatica, A.M.; Arrieta, G.; Espinoza, A.M. Direct somatic embryogenesis in Coffea arabica L. CVS. caturra and catuaí: Effect of triacontanol, light condition, and medium consistency. Agron. Costarricense 2008, 32, 139–147. [Google Scholar]
- Park, S.-Y.; Yeung, E.C.; Paek, K.-Y. Endoreduplication in Phalaenopsis is affected by light quality from light-emitting diodes during somatic embryogenesis. Plant Biotechnol. Rep. 2010, 4, 303–309. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kumar Tewari, R.; Hahn, E.-J.; Paek, K.-Y. Photon flux density and light quality induce changes in growth, stomatal development, photosynthesis and transpiration of Withania Somnifera (L.) Dunal. plantlets. Plant Cell Tiss. Organ Cult. 2007, 90, 141–151. [Google Scholar] [CrossRef]
- Aalifar, M.; Arab, M.; Aliniaeifard, S.; Dianati, S.; Mehrjerdi, M.Z.; Limpens, E.; Serek, M. Embryogenesis efficiency and genetic stability of Dianthus caryophyllus embryos in response to different light spectra and plant growth regulators. Plant Cell Tiss. Organ Cult. 2019, 139, 479–492. [Google Scholar] [CrossRef]
- De Castro, K.M.; Batista, D.S.; Fortini, E.A.; Silva, T.D.; Felipe, S.H.S.; Fernandes, A.M.; de Jesus Sousa, R.M.; de Queiroz Nascimento, L.S.; Campos, V.R.; Grazul, R.M.; et al. Photoperiod modulates growth, morphoanatomy, and linalool content in Lippia alba L. (Verbenaceae) cultured in vitro. Plant Cell Tiss. Organ Cult. 2019, 139, 139–153. [Google Scholar] [CrossRef]
- Macedo, A.F.; Leal-Costa, M.V.; Tavares, E.S.; Lage, C.L.S.; Esquibel, M.A. The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Cybularz-Urban, T.; Hanus-Fajerska, E.; Świderski, A. Effect of light wavelength on in vitro organogenesis of a Cattleya hybrid. Acta Biol. Crac. Ser. Bot. 2007, 49, 113–118. [Google Scholar]
- Ryu, J.H.; Seo, K.S.; Choi, G.L.; Rha, E.S.; Lee, S.C.; Choi, S.K.; Kang, S.-Y.; Bae, C.-H. Effects of LED Light illumination on germination, growth and anthocyanin content of dandelion (Taraxacum officinale). Korean J. Plant Res. 2012, 25, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-C.; Lin, C.-C. Red light-emitting diode light irradiation improves root and leaf formation in difficult-to-propagate Protea cynaroides L. plantlets in vitro. HortScience 2012, 47, 1490–1494. [Google Scholar] [CrossRef] [Green Version]
- Lazzarini, L.E.S.; Bertolucci, S.K.V.; Pacheco, F.V.; dos Santos, J.; Silva, S.T.; de Carvalho, A.A.; Pinto, J.E.B.P. Quality and intensity of light affect Lippia gracilis Schauer plant growth and volatile compounds in vitro. Plant Cell Tiss. Organ Cult. 2018, 135, 367–379. [Google Scholar] [CrossRef]
- Tester, M.; Morris, C. The penetration of light through soil. Plant Cell Environ. 1987, 10, 281–286. [Google Scholar] [CrossRef]
- Silva-Navas, J.; Moreno-Risueno, M.A.; Manzano, C.; Pallero-Baena, M.; Navarro-Neila, S.; Téllez-Robledo, B.; Garcia-Mina, J.M.; Baigorri, R.; Gallego, F.J.; del Pozo, J.C. D-Root: A system for cultivating plants with the roots in darkness or under different light conditions. Plant J. 2015, 84, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Pérez, J.C.; Shackel, K.A.; Sutter, E.G. Effects of in vitro-formed roots and acclimatization on water status and gas exchange of tissue-cultured apple shoots. J. Amer. Soc. Hort. Sci. 1995, 120, 435–440. [Google Scholar] [CrossRef]
- Kircher, S.; Schopfer, P. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 11217–11221. [Google Scholar] [CrossRef] [Green Version]
- Sassi, M.; Lu, Y.; Zhang, Y.; Wang, J.; Dhonukshe, P.; Blilou, I.; Dai, M.; Li, J.; Gong, X.; Jaillais, Y.; et al. COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1-and PIN2-dependent auxin transport in Arabidopsis. Development 2012, 139, 3402–3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, N.; Wu, Q.; Shen, Z.; Xia, K.; Cui, J. Effects of light quality on the chloroplastic ultrastructure and photosynthetic characteristics of cucumber seedlings. Plant Growth Regul. 2014, 73, 227–235. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Z.; Yang, Y.; Yijie, F. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tiss. Organ Cult. 2011, 106, 1–10. [Google Scholar] [CrossRef]
- Silva, S.T.; Bertolucci, S.K.V.; da Cunha, S.H.B.; Lazzarini, L.E.S.; Tavares, M.C.; Pinto, J.E.B.P. Effect of light and natural ventilation systems on the growth parameters and carvacrol content in the in vitro cultures of Plectranthus amboinicus (Lour.) Spreng. Plant Cell Tiss. Organ Cult. 2017, 129, 501–510. [Google Scholar] [CrossRef]
- Wu, H.; Liu, X.-G.; Ji, H.; Chen, L.-Q. Effects of light, macronutrients, and sucrose on germination and development of the endangered fern Adiantum reniforme var. sinense (Adiantaceae). Sci. Hortic. 2010, 125, 417–421. [Google Scholar] [CrossRef]
- Suetsugu, N.; Wada, M. Cryptogam blue-light photoreceptors. Curr. Opin. Plant Biol. 2003, 6, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Kanegae, T.; Nozue, K.; Fukuda, S. Cryptogam phytochromes. Plant Cell Environ. 1997, 20, 685–690. [Google Scholar] [CrossRef]
- Grill, R. Induction of two-dimensional growth by red and green light in the fern Anemia phyllitidis L. Sw. J. Plant Physiol. 1987, 131, 363–371. [Google Scholar] [CrossRef]
- Sobota, A.E.; Partanen, C.R. The growth and division of cells in relation to morphogenesis in fern gametophytes: I. Photomorphogenetic studies in Pteridium aquilinum. Can. J. Bot. 1965, 44, 497–506. [Google Scholar] [CrossRef]
- Niranjan, A.R.S.; Singh, I.P.; Roy, S.K. Effect of UV-irradiation on the growth and differentiation of the gametophyte in the fern Cheilanthes rufa D. Proc. Indian Natl. 1983, 3, 257–262. [Google Scholar]
- Randi, A.M.; Freitas, M.C.A.; Rodrigues, A.C.; Maraschin, M.; Torres, M.A. Acclimation and photoprotection of young gametophytes of Acrostichum danaeifolium to UV-B stress. Photosynthetica 2014, 52, 50–56. [Google Scholar] [CrossRef]
- Fan, X.X.; Zang, J.; Xu, Z.G.; Guo, S.R.; Jiao, X.L.; Liu, X.Y.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.-H.; Kim, S.-K.; Lee, K.-H.; Park, J.-Y.; Nam, M.-W.; Choi, D.-H.; Lee, H.-I. LED light for in vitro and ex vitro efficient growth of economically important highbush blueberry (Vaccinium corymbosum L.). Acta Physiol. Plant. 2016, 38, 152. [Google Scholar] [CrossRef]
- Ye, S.; Shao, Q.; Xu, M.; Li, S.; Wu, M.; Tan, X.; Su, L. Effects of light quality on morphology, enzyme activities, and bioactive compound contents in Anoectochilus roxburghii. Front. Plant Sci. 2017, 8, 857. [Google Scholar] [CrossRef] [Green Version]
- Cioć, M.; Szewczyk, A.; Żupnik, M.; Kalisz, A.; Pawłowska, B. LED lighting affects plant growth, morphogenesis and phytochemical contents of Myrtus communis L. in vitro. Plant Cell Tiss. Organ Cult. 2018, 132, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Khayatnezhad, M.; Gholamin, R.; Jamaati-e-Somarin, S.; Zabihi-e-Mahmoodabad, R. The leaf chlorophyll content and stress resistance relationship considering in corn cultivars (Zea mays). Adv. Environ. Biol. 2011, 5, 118–122. [Google Scholar]
- Cioć, M.; Pawłowska, B. Leaf response to different light spectrum compositions during micropropagation of Gerbera axillary shoots. Agronomy 2020, 10, 1832. [Google Scholar] [CrossRef]
- Liu, X.Y.; Guo, S.R.; Xu, Z.G.; Jiao, X.L.; Takafumi, T. Regulation of chloroplast ultrastructure, cross-section anatomy of leaves, and morphology of stomata of cherry tomato by different light irradiations of light-emitting diodes. HortScience 2011, 46, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.-X.; Wang, X.-Z.; Gao, L.-H.; Chen, Q.-Y.; Qu, M. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Simlat, M.; Ślęzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.-C. Long-term effects of red-and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksman-Caldentey, K.-M.; Inzé, D. Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant Sci. 2004, 9, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. BBA Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Raghuvanshi, R.; Bhardwaj, P.; Sood, H.; Saxena, S.; Chaurasia, O.P. Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B Biol. 2018, 183, 258–265. [Google Scholar] [CrossRef]
- Yap, E.S.P.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Vaswani, A.; Magana, A.A.; Morre, J.; Maier, C.S. Plant growth and metabolic changes in ‘Super Hot’ chili fruit (Capsicum annuum) exposed to supplemental LED lights. Plant Sci. 2021, 305, 110826. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Gu, M.; Chen, X.; Chen, X.; Yang, J.; Zhao, F.; Ye, N. Exploration of the effects of different blue LED light intensities on flavonoid and lipid metabolism in tea plants via transcriptomics and metabolomics. Int. J. Mol. Sci. 2020, 21, 4606. [Google Scholar] [CrossRef]
- Hiraoka, A.; Hasegawa, M. Flavonoid glycosides from five Cyathea species. Bot. Mag. 1975, 88, 127–130. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]




| Parameter | Method of Calculation |
|---|---|
| Experiment with Stipe Explants | |
| Ratio of explants with sporophytes | the number of explants with regenerated sporophytes divided by the total number of stipe explants used for culture initiation |
| Number of sporophytes | the total number of obtained sporophytes divided by the sum of cultured explants |
| Ratio of explants with gametophytes | the number of explants with regenerated gametophytes divided by the total number of explants used for culture initiation |
| Ratio of explants with mass-produced gametophytes | the number of explants that produced gametophytes with total surface equal or higher than 4 mm2, per total number of explants with gametophytes |
| Ratio of sporophytes with a developed leaf | the number of sporophytes with at least 1 leaf at crosier stadium or older per sum of all sporophytes (including these with only a leaf primordium) |
| Ratio of sporophytes with a developed leaf blade | the number of sporophytes with at least 1 leaf with the leaf blade older than crosier stadium per total number of sporophytes with a developed leaf |
| Length of the longest leaf | the sum of the length of the longest leaf from each sporophyte per the sum of obtained sporophytes |
| Number of roots | the sum of roots divided by the total number of sporophytes |
| Length of the roots | the sum of root length divided by the total number of sporophytes |
| Experiment with whole sporophytes | |
| Fresh weight | weight of total proliferated plants obtained from a single initial sporophyte |
| Number of sporophytes | the sum of newly-formed sporophytes per the total number of initial sporophytes |
| Ratio of sporophytes with a developed leaf | the sum of sporophytes with at least 1 leaf at crosier stadium or older per sum of all sporophytes (including these with only a leaf primordium) |
| Number of leaves | the sum of leaves produced by sporophytes divided by the total number of sporophytes |
| Length of the longest leaf | the length of the longest leaf of each sporophyte divided by the total number of sporophytes |
| Number of roots | the sum of roots divided by the total number of sporophytes |
| Length of the longest root | the sum of the length of the longest root of each sporophyte divided by the total number of sporophytes |
| Compound | Retention Time [min] | [M-H]- [m/z] | Fragment Ions [m/z] | Collision Energy [eV] | Light Conditions | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dark | Fl | B | R | RB | Greenhouse | |||||
| Compound Content [µg/mg of Dry Weight] | ||||||||||
| Phenolic acids | ||||||||||
| Protocatechuic acid | 8.46 | 152.9 | 80.9 107.8 | −26 −38 | 0.0058 ± 0.0002 d | 0.0098 ± 0.0006 a | 0.0048 ± 0.0001 e | 0.0067 ± 0.0006 c | 0.0076 ± 0.0003 b | 0.0033 ± 0.0001 f |
| Trans-5-O-caffeoylquinic acid | 9.32 | 352.9 | 190.8 84.9 | −24 −60 | 0.3698 ± 0.0075 e | 0.7004 ± 0.0064 c | 0.7420 ± 0.0562 b | 0.5733 ± 0.0143 d | 1.0129 ± 0.0306 a | 0.0028 ± 0.0003 f |
| Cis-5-O-caffeoylquinic acid | 10.45 | 352.9 | 190.8 84.9 | −24 −60 | 0.0940 ± 0.0065 d | 0.2361 ± 0.0140 a | 0.0413 ± 0.0014 e | 0.1268 ± 0.0043 b | 0.1073 ± 0.0049 c | 0.0006 ± 0.0001 f |
| Caffeic acid | 11.40 | 178.7 | 88.9 134.9 | −46 −16 | 0.0019 ± 0.0002 b | 0.0037 ± 0.0001 a | nd | 0.0012 ± 0.0001 c | nd | nd |
| Flavonoid aglycones | ||||||||||
| Prunetin | 21.98 | 282.8 | 267.7 238.7 | −20 −26 | nd | 0.00017 ± 0.000005 c | 0.00020 ± 0.00002 b | 0.00016 ± 0.000004 d | 0.00024 ± 0.000015 a | nd |
| Flavonoid glycosides | ||||||||||
| Quercetin 3-O-rutinoside (Rutin) | 11.99 | 608.7 | 299.6 270.9 | −46 −60 | BQL | 0.0346 ± 0.0028 c | 0.0603 ± 0.0005 a | 0.0081 ± 0.0001 d | 0.0571 ± 0.0004 b | nd |
| Quercetin 3-O-glucoside (Isoquercetin) | 13.00 | 462.7 | 299.7 270.7 | −30 −44 | nd | 0.0327 ± 0.0022 c | 0.1142 ± 0.0034 a | BQL | 0.0483 ± 0.0009 b | nd |
| Kaempferol 3-O-rutinoside (Nicotiflorin) | 13.31 | 592.7 | 284.8 226.7 | −38 −68 | 0.0005 ± 0.00003 e | 0.0476 ± 0.0011 c | 0.0742 ± 0.0020 a | 0.0273 ± 0.0017 d | 0.0686 ± 0.0021 b | nd |
| Kaempferol 3-O-glucoside (Astragalin) | 14.66 | 446.7 | 226.8 254.8 | −54 −40 | nd | 0.0360 ± 0.0004 b | 0.0728 ± 0.0039 a | BQL | 0.0316 ± 0.0011 c | BQL |
| Quercetin 3-O-rhamnoside (Quercitrin) | 14.83 | 446.7 | 299.7 270.7 | −30 −40 | BQL | 0.0091 ± 0.0009 a | 0.0049 ± 0.0003 b | BQL | 0.0021 ± 0.0002 c | nd |
| Naringenin-7-O-glucoside | 15.12 | 432.7 | 270.8 118.9 | −22 −64 | 0.0007 ± 0.00004 d | 0.0183 ± 0.0004 a | 0.0144 ± 0.0005 b | 0.0136 ± 0.0005 c | 0.0143 ± 0.0006 b | nd |
| Antioxidant Activity | Light Conditions | |||||
|---|---|---|---|---|---|---|
| Dark | Fl | B | R | RB | Greenhouse | |
| DPPH (EC50 mg/mL) | 0.72 ± 0.78 ab | 0.08 ± 0.07 a | 0.88 ± 0.14 b | 0.39 ± 0.20 ab | 0.36 ± 0.39 ab | 0.03 ± 0.01 a |
| ABTS (EC50 mg/mL) | 1.87 ± 0.13 cd | 0.59 ± 0.10 a | 2.24 ± 0.50 d | 1.43 ± 0.25 bc | 1.32 ± 0.17 b | 0.35 ± 0.15 a |
| CHEL (EC50 mg/mL) | 1.65 ± 0.15 b | 1.42 ± 0.12 b | 1.15 ± 0.11 a | 1.50 ± 0.10 b | 2.15 ± 0.20 c | 2.70 ± 0.18 d |
| β-Carotene/linoleic acid (EC50 μg/mL) | 52.10 ± 0.16 d | 31.70 ± 0.12 b | 72.45 ± 0.75 e | 32.17 ± 0.20 b | 34.85 ± 0.13 c | 22.68 ± 0.39 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewicz, W.; Cioć, M.; Dos Santos Szewczyk, K.; Grzyb, M.; Pietrzak, W.; Pawłowska, B.; Mikuła, A. Enhancing In Vitro Production of the Tree Fern Cyathea delgadii and Modifying Secondary Metabolite Profiles by LED Lighting. Cells 2022, 11, 486. https://doi.org/10.3390/cells11030486
Tomaszewicz W, Cioć M, Dos Santos Szewczyk K, Grzyb M, Pietrzak W, Pawłowska B, Mikuła A. Enhancing In Vitro Production of the Tree Fern Cyathea delgadii and Modifying Secondary Metabolite Profiles by LED Lighting. Cells. 2022; 11(3):486. https://doi.org/10.3390/cells11030486
Chicago/Turabian StyleTomaszewicz, Wojciech, Monika Cioć, Katarzyna Dos Santos Szewczyk, Małgorzata Grzyb, Wioleta Pietrzak, Bożena Pawłowska, and Anna Mikuła. 2022. "Enhancing In Vitro Production of the Tree Fern Cyathea delgadii and Modifying Secondary Metabolite Profiles by LED Lighting" Cells 11, no. 3: 486. https://doi.org/10.3390/cells11030486
APA StyleTomaszewicz, W., Cioć, M., Dos Santos Szewczyk, K., Grzyb, M., Pietrzak, W., Pawłowska, B., & Mikuła, A. (2022). Enhancing In Vitro Production of the Tree Fern Cyathea delgadii and Modifying Secondary Metabolite Profiles by LED Lighting. Cells, 11(3), 486. https://doi.org/10.3390/cells11030486







