Treatment of Pheochromocytoma Cells with Recurrent Cycles of Hypoxia: A New Pseudohypoxic In Vitro Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Generation of Sub-Cell Lines by Treatment with Recurrent Hypoxia–Reoxygenation Cylces
2.3. Viability Assay
2.4. Proliferation Assay
2.5. Clonogenic Cell Survival Assay
2.6. Migration Assay
2.7. Invasion Assay
2.8. Adhesion Assay
2.9. Catecholamine Measurments
2.10. TCA Cycle Metabolites
2.11. TET Activity Assay
2.12. Global Methylation Assay
2.13. Pre-Treatment with Ascorbic Acid or 5-Octyl-alpha-ketoglutarate
2.14. RNA Isolation and qRT-PCR
2.15. RNA Sequencing
2.16. Bioinformatics Analysis
2.17. Statistical Analysis
3. Results
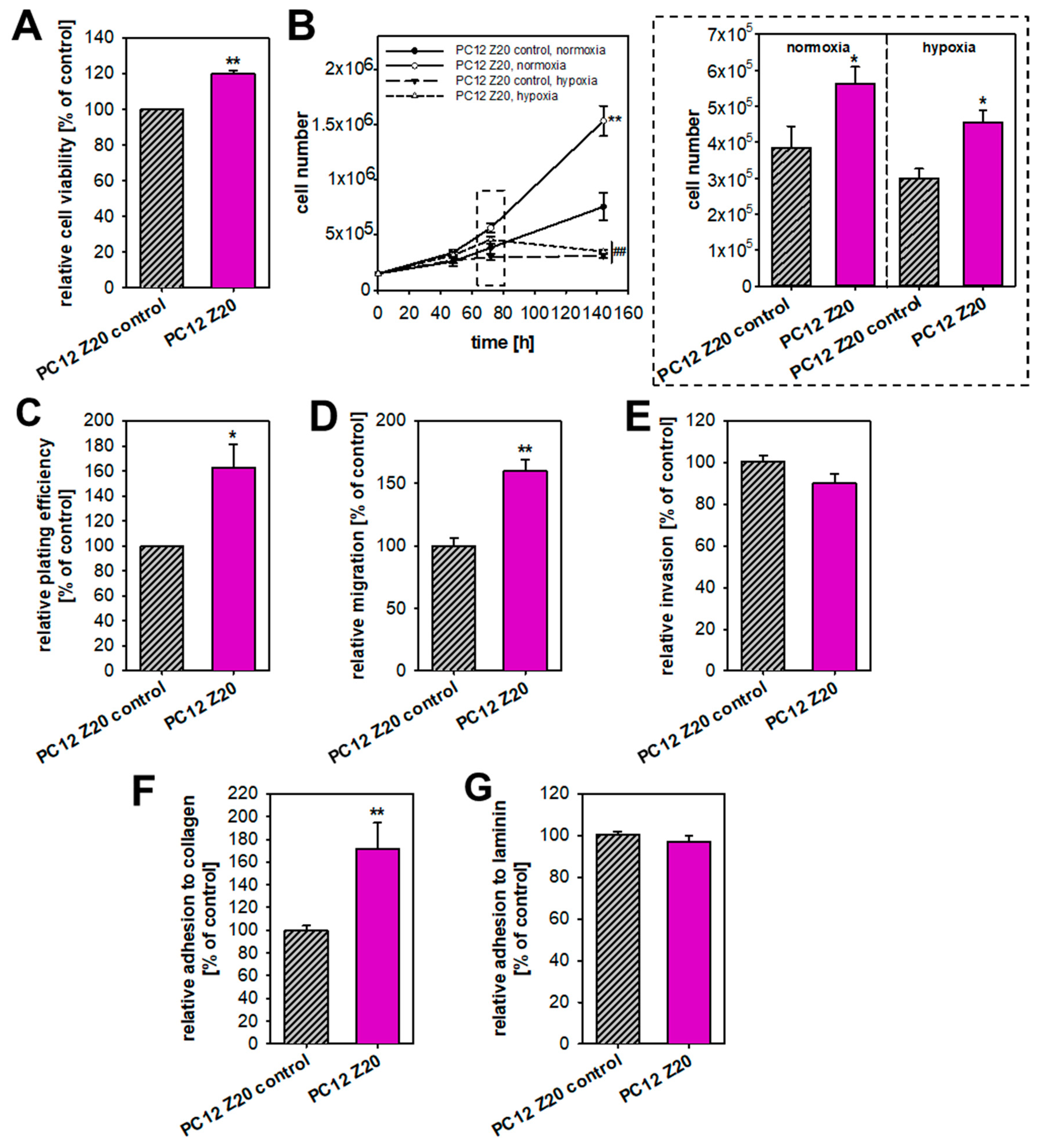
3.1. Recurrent Cycles of Hypoxia Lead to the Establishment of a Pro-Metastatic Phenotype with Enhanced Growth Characteristic under Normoxic Conditions
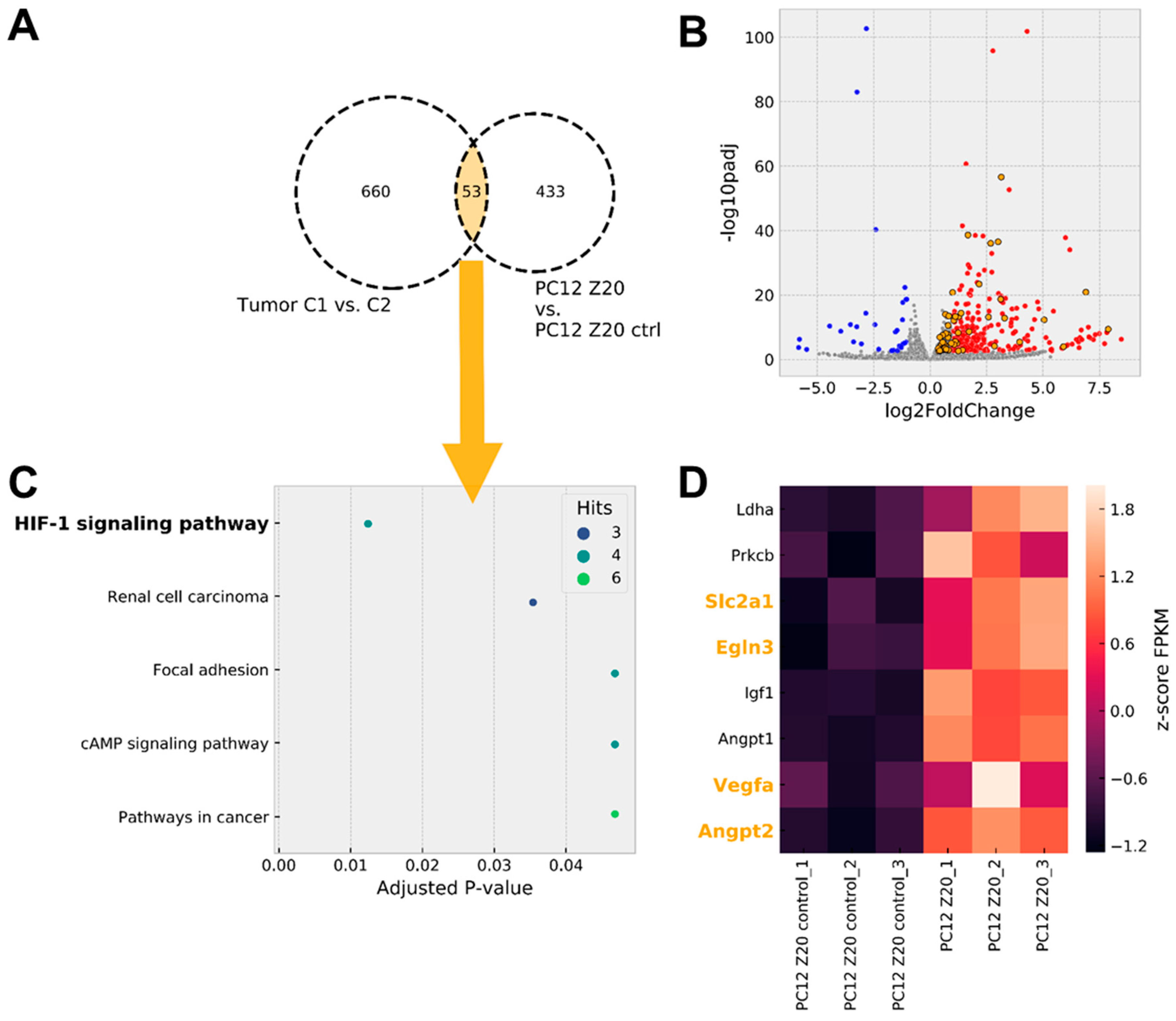
3.2. Gene Expression Analysis Revealed a Pseudohypoxic Gene Signature in PC12 Z20 Cells Compared with the Control
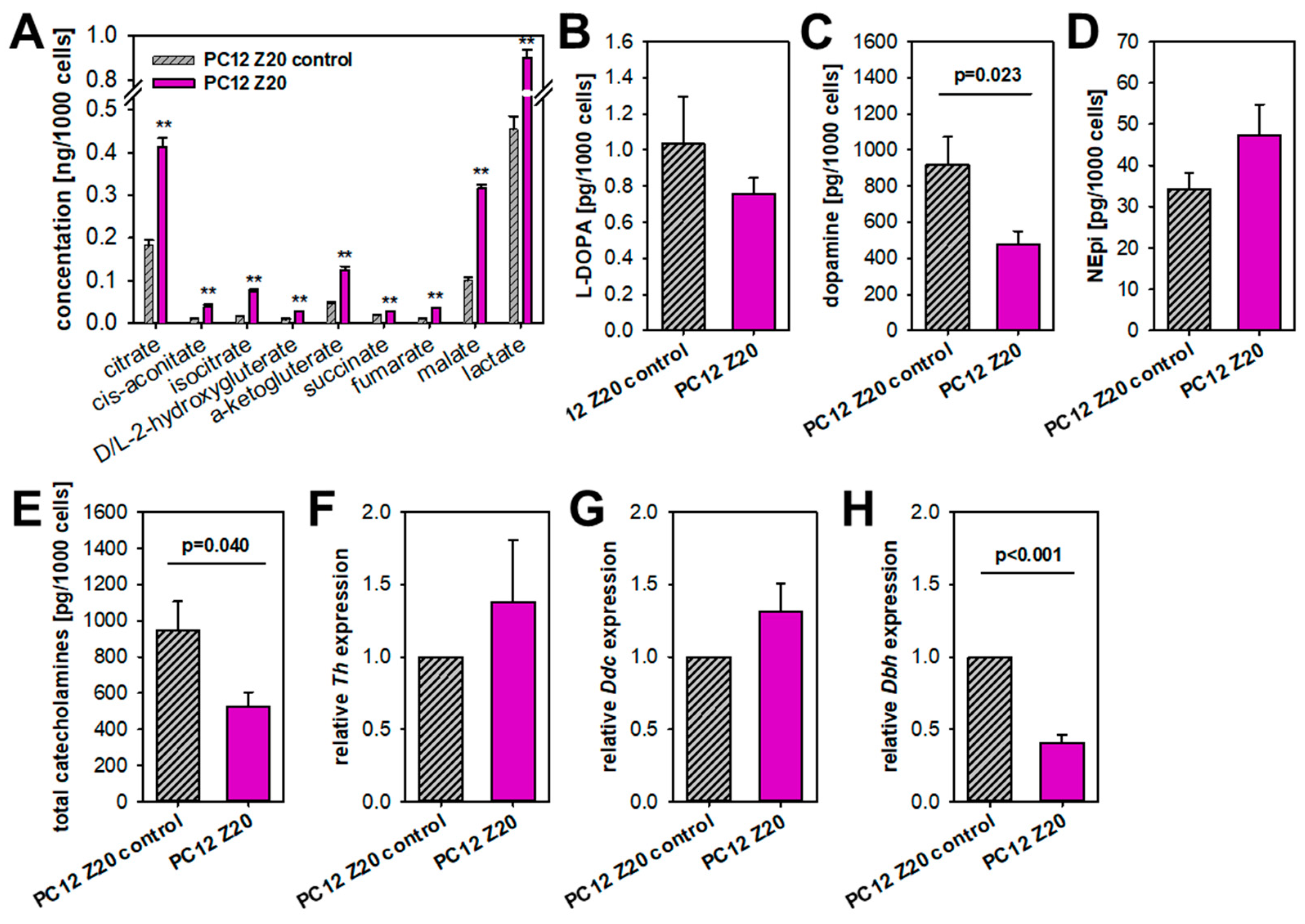
3.3. PC12 Z20 Cells Showed an Accumulation of TCA Cycle Metabolites and a Decrease in the Cellular Catecholamine Content
3.4. Epigenetic Changes in PC12 Z20 Cells
4. Discussion
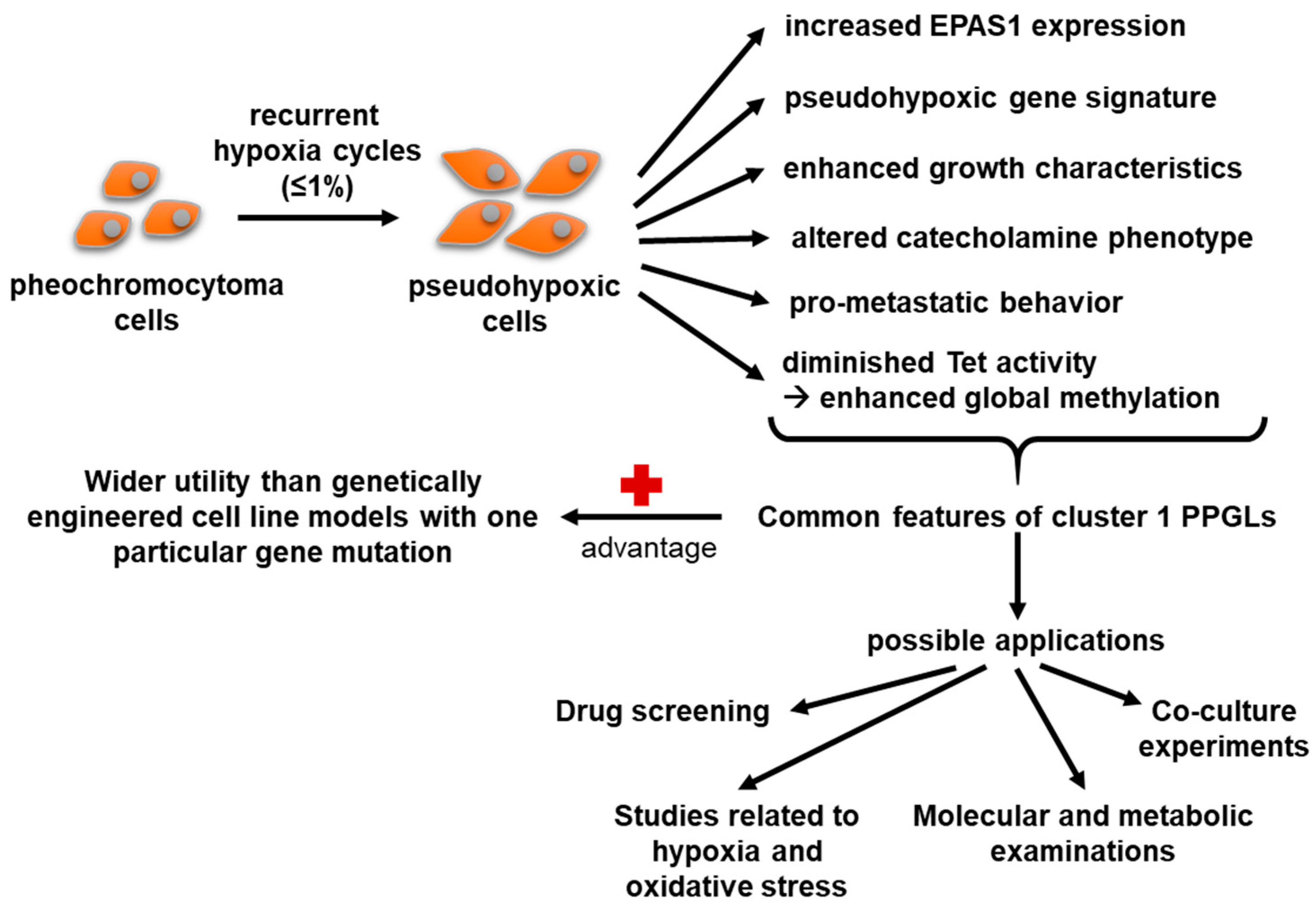
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crona, J.; Lamarca, A.; Ghosal, S.; Welin, S.; Skogseid, B.; Pacak, K. Genotype-phenotype correlations in pheochromocytoma and paraganglioma: A systematic review and individual patient meta-analysis. Endocr. Relat. Cancer 2019, 5, 539–550. [Google Scholar] [CrossRef]
- Fliedner, S.M.; Brabant, G.; Lehnert, H. Pheochromocytoma and paraganglioma: Genotype versus anatomic location as determinants of tumor phenotype. Cell Tissue Res. 2018, 372, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Cascón, A.; Remacha, L.; Calsina, B.; Robledo, M. Pheochromocytomas and paragangliomas: Bypassing cellular respiration. Cancers 2019, 11, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechmann, N.; Moskopp, M.L.; Ullrich, M.; Calsina, B.; Wallace, P.W.; Richter, S.; Friedemann, M.; Langton, K.; Fliedner, S.M.; Timmers, H.J. Hif2α supports pro-metastatic behavior in pheochromocytomas/paragangliomas. Endocr. Relat. Cancer 2020, 27, 625–640. [Google Scholar] [CrossRef]
- Favier, J.; Plouin, P.-F.; Corvol, P.; Gasc, J.-M. Angiogenesis and vascular architecture in pheochromocytomas: Distinctive traits in malignant tumors. Am. J. Pathol. 2002, 161, 1235–1246. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Huynh, T.; Pacak, K.; Brouwers, F.; Walther, M.; Linehan, W.; Munson, P.; Mannelli, M.; Goldstein, D.; Elkahloun, A. Distinct gene expression profiles in norepinephrine-and epinephrine-producing hereditary and sporadic pheochromocytomas: Activation of hypoxia-driven angiogenic pathways in von hippel–lindau syndrome. Endocr. Relat. Cancer 2004, 11, 897–911. [Google Scholar] [CrossRef] [Green Version]
- López-Jiménez, E.; Gómez-López, G.; Leandro-García, L.J.; Muñoz, I.; Schiavi, F.; Montero-Conde, C.; De Cubas, A.A.; Ramires, R.; Landa, I.; Leskelä, S. Research resource: Transcriptional profiling reveals different pseudohypoxic signatures in sdhb and vhl-related pheochromocytomas. Mol. Endocrinol. 2010, 24, 2382–2391. [Google Scholar] [CrossRef] [Green Version]
- Bechmann, N.; Eisenhofer, G. Hypoxia-inducible factor 2α: A key player in tumorigenesis and metastasis of pheochromocytoma and paraganglioma? Exp. Clin. Endocrinol. Diabetes 2021. [Google Scholar] [CrossRef]
- Morin, A.; Goncalves, J.; Moog, S.; Castro-Vega, L.-J.; Job, S.; Buffet, A.; Fontenille, M.-J.; Woszczyk, J.; Gimenez-Roqueplo, A.-P.; Letouzé, E. Tet-mediated hypermethylation primes sdh-deficient cells for hif2α-driven mesenchymal transition. Cell Rep. 2020, 30, 4551–4566.e7. [Google Scholar] [CrossRef]
- Martinelli, S.; Maggi, M.; Rapizzi, E. Pheochromocytoma/paraganglioma preclinical models: Which to use and why? Endocr. Connect. 2020, 9, R251–R260. [Google Scholar] [CrossRef]
- Richter, S.; D’Antongiovanni, V.; Martinelli, S.; Bechmann, N.; Riverso, M.; Poitz, D.M.; Pacak, K.; Eisenhofer, G.; Mannelli, M.; Rapizzi, E. Primary fibroblast co-culture stimulates growth and metabolism in sdhb-impaired mouse pheochromocytoma mtt cells. Cell Tissue Res. 2018, 374, 473–485. [Google Scholar] [CrossRef]
- Tischler, A.S.; Greene, L.A.; Kwan, P.W.; Slayton, V.W. Ultrastructural effects of nerve growth factor on pc 12 pheochromocytoma cells in spinner culture. Cell Tissue Res. 1983, 228, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Ghayee, H.K.; Bhagwandin, V.J.; Stastny, V.; Click, A.; Ding, L.-H.; Mizrachi, D.; Zou, Y.S.; Chari, R.; Lam, W.L.; Bachoo, R.M. Progenitor cell line (hpheo1) derived from a human pheochromocytoma tumor. PLoS ONE 2013, 8, e65624. [Google Scholar] [CrossRef] [Green Version]
- Bechmann, N.; Poser, I.; Seifert, V.; Greunke, C.; Ullrich, M.; Qin, N.; Walch, A.; Peitzsch, M.; Robledo, M.; Pacak, K. Impact of extrinsic and intrinsic hypoxia on catecholamine biosynthesis in absence or presence of hif2α in pheochromocytoma cells. Cancers 2019, 11, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechmann, N.; Hauser, S.; Hofheinz, F.; Kniess, T.; Pietzsch, J. Nitric oxide-releasing selective cyclooxygenase-2 inhibitors as promising radiosensitizers in melanoma cells in vitro. Ann Radiat Ther Oncol. 2017, 1, 1010. [Google Scholar]
- Bechmann, N.; Ehrlich, H.; Eisenhofer, G.; Ehrlich, A.; Meschke, S.; Ziegler, C.G.; Bornstein, S.R. Anti-tumorigenic and anti-metastatic activity of the sponge-derived marine drugs aeroplysinin-1 and isofistularin-3 against pheochromocytoma in vitro. Marinedrugs 2018, 16, 172. [Google Scholar] [CrossRef] [Green Version]
- Richter, S.; Peitzsch, M.; Rapizzi, E.; Lenders, J.W.; Qin, N.; De Cubas, A.A.; Schiavi, F.; Rao, J.U.; Beuschlein, F.; Quinkler, M. Krebs cycle metabolite profiling for identification and stratification of pheochromocytomas/paragangliomas due to succinate dehydrogenase deficiency. J. Clin. Endocrinol. Metab. 2014, 99, 3903–3911. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. Star: Ultrafast universal rna-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. Featurecounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for rna-seq data with deseq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative html5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, N.; De Cubas, A.A.; Garcia-Martin, R.; Richter, S.; Peitzsch, M.; Menschikowski, M.; Lenders, J.W.; Timmers, H.J.; Mannelli, M.; Opocher, G. Opposing effects of hif1α and hif2α on chromaffin cell phenotypic features and tumor cell proliferation: Insights from myc-associated factor x. Int. J. Cancer 2014, 135, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.M.; Bouchier-Hayes, D.J.; Condron, C.M.; Toomey, D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat. Rev. 2003, 29, 297–307. [Google Scholar] [CrossRef]
- Fankhauser, M.; Bechmann, N.; Lauseker, M.; Goncalves, J.; Favier, J.; Klink, B.; William, D.; Gieldon, L.; Maurer, J.; Spöttl, G. Synergistic highly potent targeted drug combinations in different pheochromocytoma models including human tumor cultures. Endocrinology 2019, 160, 2600–2617. [Google Scholar] [CrossRef] [Green Version]
- Swinson, D.E.; Jones, J.L.; Richardson, D.; Wykoff, C.; Turley, H.; Pastorek, J.; Taub, N.; Harris, A.L.; O’Byrne, K.J. Carbonic anhydrase ix expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non–small-cell lung cancer. J. Clin. Oncol. 2003, 21, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Huynh, T.-T.; Elkahloun, A.; Morris, J.C.; Bratslavsky, G.; Linehan, W.M.; Zhuang, Z.; Balgley, B.M.; Lee, C.S.; Mannelli, M. Differential expression of the regulated catecholamine secretory pathway in different hereditary forms of pheochromocytoma. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1223–E1233. [Google Scholar] [CrossRef] [Green Version]
- Björklund, P.; Backman, S. Epigenetics of pheochromocytoma and paraganglioma. Mol. Cell Endocrinol. 2018, 469, 92–97. [Google Scholar] [CrossRef]
- Gray, M.; Turnbull, A.K.; Ward, C.; Meehan, J.; Martínez-Pérez, C.; Bonello, M.; Pang, L.Y.; Langdon, S.P.; Kunkler, I.H.; Murray, A. Development and characterisation of acquired radioresistant breast cancer cell lines. Radiat. Oncol. 2019, 14, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kuwahara, Y.; Roudkenar, M.H.; Urushihara, Y.; Saito, Y.; Tomita, K.; Roushandeh, A.M.; Sato, T.; Kurimasa, A.; Fukumoto, M. Clinically relevant radioresistant cell line: A simple model to understand cancer radioresistance. Med. Mol. Morphol. 2017, 50, 195–204. [Google Scholar] [CrossRef]
- McDermott, M.; Eustace, A.; Busschots, S.; Breen, L.; Clynes, M.; O’Donovan, N.; Stordal, B. In vitro development of chemotherapy and targeted therapy drug-resistant cancer cell lines: A practical guide with case studies. Front. Oncol. 2014, 4, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmquist-Mengelbier, L.; Fredlund, E.; Löfstedt, T.; Noguera, R.; Navarro, S.; Nilsson, H.; Pietras, A.; Vallon-Christersson, J.; Borg, Å.; Gradin, K. Recruitment of hif-1α and hif-2α to common target genes is differentially regulated in neuroblastoma: Hif-2α promotes an aggressive phenotype. Cancer Cell 2006, 10, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, K.; Jolly, M.K. Acute vs. Chronic vs. Cyclic hypoxia: Their differential dynamics, molecular mechanisms, and effects on tumor progression. Biomolecules 2019, 9, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Pang, Y.; Zhu, B.; Uher, O.; Caisova, V.; Huynh, T.-T.; Taieb, D.; Vanova, K.H.; Ghayee, H.K.; Neuzil, J. Therapeutic targeting of sdhb-mutated pheochromocytoma/paraganglioma with pharmacologic ascorbic acid. Clin. Cancer Res. 2020, 26, 3868–3880. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Son, B.; Lee, S.; Youn, H.; Kim, E.; Kim, W.; Youn, B. The role of tumor microenvironment in therapeutic resistance. Oncotarget 2017, 8, 3933. [Google Scholar] [CrossRef] [Green Version]
- Seifert, V.; Richter, S.; Bechmann, N.; Bachmann, M.; Ziegler, C.G.; Pietzsch, J.; Ullrich, M. Hif2alpha-associated pseudohypoxia promotes radioresistance in pheochromocytoma: Insights from 3d models. Cancers 2021, 13, 385. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helm, J.; Drukewitz, S.; Poser, I.; Richter, S.; Friedemann, M.; William, D.; Mohr, H.; Nölting, S.; Robledo, M.; Bornstein, S.R.; et al. Treatment of Pheochromocytoma Cells with Recurrent Cycles of Hypoxia: A New Pseudohypoxic In Vitro Model. Cells 2022, 11, 560. https://doi.org/10.3390/cells11030560
Helm J, Drukewitz S, Poser I, Richter S, Friedemann M, William D, Mohr H, Nölting S, Robledo M, Bornstein SR, et al. Treatment of Pheochromocytoma Cells with Recurrent Cycles of Hypoxia: A New Pseudohypoxic In Vitro Model. Cells. 2022; 11(3):560. https://doi.org/10.3390/cells11030560
Chicago/Turabian StyleHelm, Jana, Stephan Drukewitz, Isabel Poser, Susan Richter, Markus Friedemann, Doreen William, Hermine Mohr, Svenja Nölting, Mercedes Robledo, Stefan R. Bornstein, and et al. 2022. "Treatment of Pheochromocytoma Cells with Recurrent Cycles of Hypoxia: A New Pseudohypoxic In Vitro Model" Cells 11, no. 3: 560. https://doi.org/10.3390/cells11030560
APA StyleHelm, J., Drukewitz, S., Poser, I., Richter, S., Friedemann, M., William, D., Mohr, H., Nölting, S., Robledo, M., Bornstein, S. R., Eisenhofer, G., & Bechmann, N. (2022). Treatment of Pheochromocytoma Cells with Recurrent Cycles of Hypoxia: A New Pseudohypoxic In Vitro Model. Cells, 11(3), 560. https://doi.org/10.3390/cells11030560






