Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances
Abstract
1. Introduction
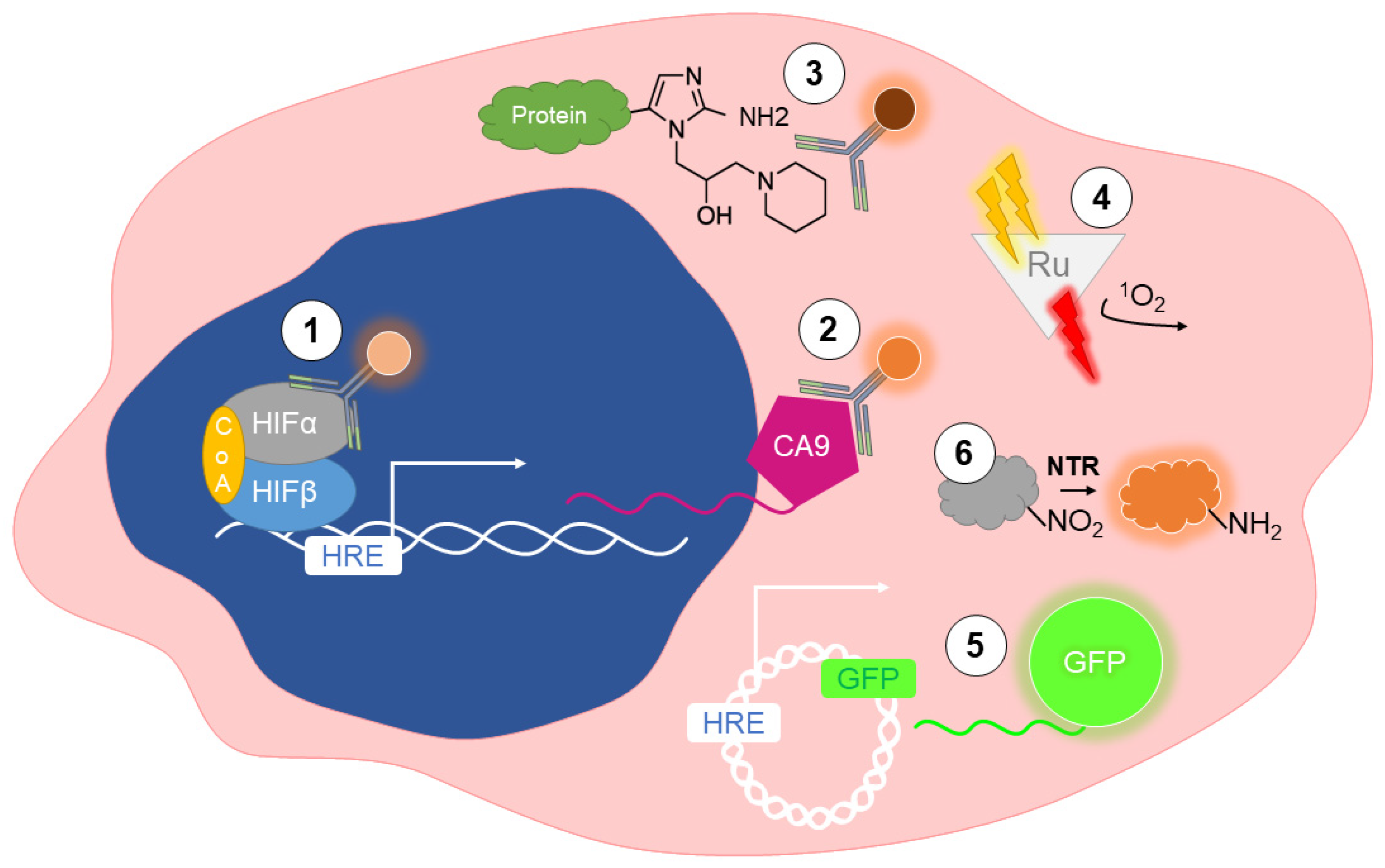
2. Detection of Hypoxia In Vitro
2.1. Immunolabeling of Endogenous Markers
2.2. Immunolabeling of Exogenous Markers
2.3. Phosphorescent Reporters
2.4. Fluorescent Reporters
2.5. Nitroreductase-Sensitive Fluorescent Probes
2.6. Noninvasive Optical Oxygen Sensors
2.7. Invasive Optical Oxygen Sensors
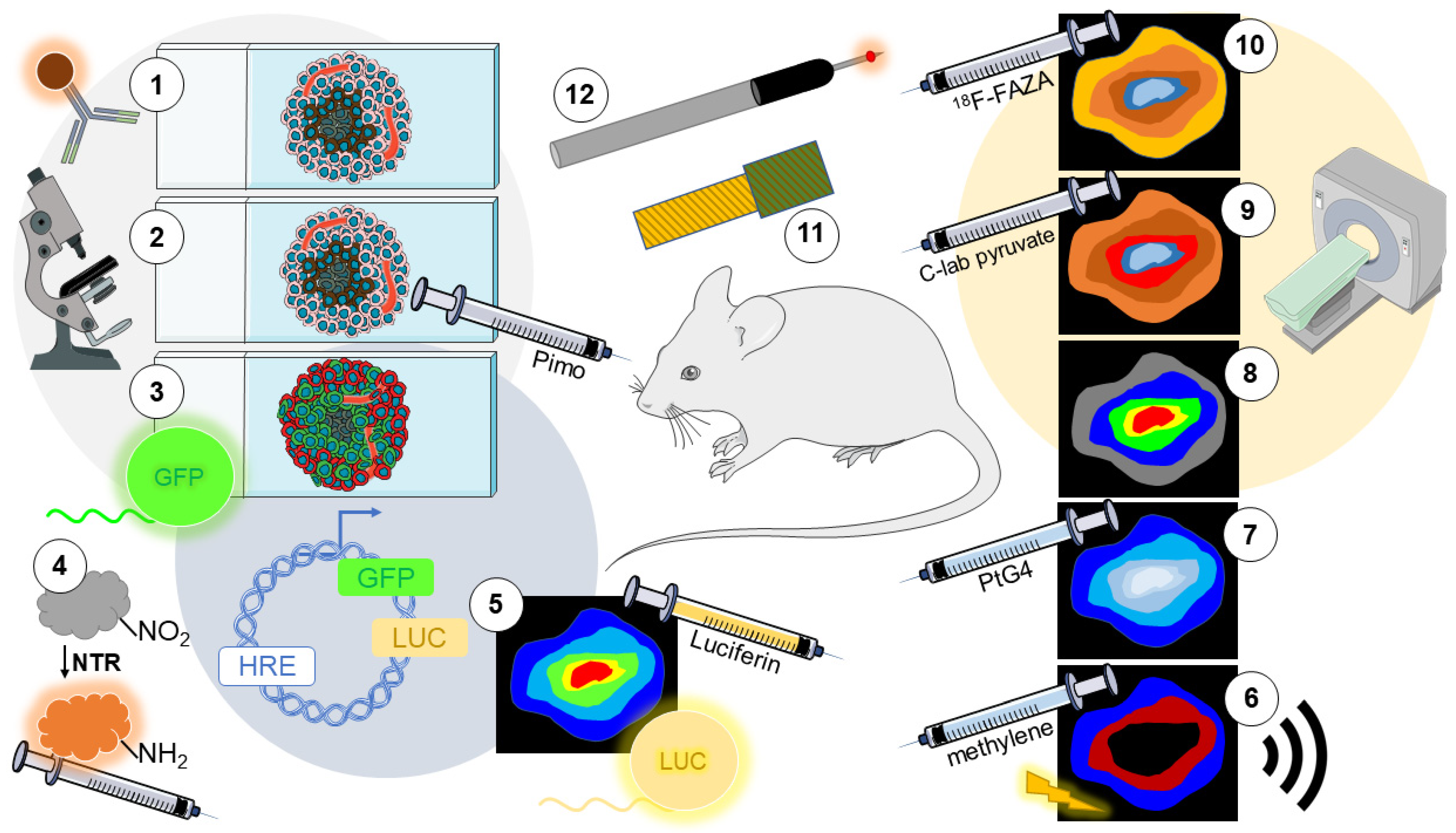
3. Detection of Hypoxia In Vivo
3.1. Immunolabeling of Endogenous Markers
3.2. Immunolabeling of Exogenous Markers
3.3. Fluorescent Reporters
3.4. Nitroreductase-Sensitive Fluorescent Probes
3.5. Bioluminescence Imaging (BLI) Reporters
3.6. Photoacoustic Imaging (PAI)
3.7. Cherenkov-Excited Luminescence Imaging (CELI)
3.8. Magnetic Resonance Imaging (MRI)
3.9. Electron Paramagnetic Resonance Imaging (EPRI)
3.10. Positron Emission Tomography (PET)
3.11. Electrochemical Oxygen Sensors
3.12. Invasive Optical Oxygen Sensors
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| HIF | Hypoxia-Inducible Factor |
| VHL | Von Hippel–Lindau |
| IHC | Immunohistochemical |
| IF | Immunofluorescent |
| GLUT-1 | Glucose Transporter 1 |
| MCT-1 | Monocarboxylate Transporter 1 |
| CA-IX | Carbonic Anhydrase IX |
| NITP | A2-Nitroimidazole |
| PO2 | O2 Partial Pressure |
| PLIM | Phosphorescence Lifetime Imaging |
| HRE | Hypoxia-Responsive Element |
| ODD | Oxygen-Dependent Degradation |
| NTR | Nitroreductase |
| NIR | Near-Infrared |
| BLI | Bioluminescent Imaging |
| PAI | Photoacoustic Imaging |
| PA | Photoacoustic |
| AuNR | Gold Nanorod |
| CELI | Cherenkov-Excited Luminescence Imaging |
| BOLD | Blood-Oxygen-Level Dependent |
| TOLD | Tumor Oxygenation Level-Dependent |
| PISTOL | Proton Imaging of Siloxanes to map Tissue Oxygen Levels |
| MR-CA | Magnetic Resonance Contrast Amplification |
| EPRI | Electron Paramagnetic Resonance Imaging |
| 18F-FMISO | 18F-fluoromisonidazole |
| 18F-FAZA | 18F-fluoroazomycin arabinoside |
| fMRI | Functional Magnetic Resonance Imaging |
References
- Cosse, J.-P. Tumour Hypoxia Affects the Responsiveness of Cancer Cells to Chemotherapy and Promotes Cancer Progression. Anti-Cancer Agents Med. Chem. 2008, 8, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Höckel, M.; Mayer, A. Detection and Characterization of Tumor Hypoxia Using pO2 Histography. Antioxid. Redox Signal 2007, 9, 1221–1236. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Mayer, A.; Briest, S.; Höckel, M. Oxygenation gain factor: A novel parameter characterizing the association between hemoglobin level and the oxygenation status of breast cancers. Cancer Res. 2003, 63, 7634–7637. [Google Scholar] [PubMed]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Walsh, J.C.; Lebedev, A.; Aten, E.; Madsen, K.; Marciano, L.; Kolb, H.C. The Clinical Importance of Assessing Tumor Hypoxia: Relationship of Tumor Hypoxia to Prognosis and Therapeutic Opportunities. Antioxid. Redox Signal. 2014, 21, 1516–1554. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A.; Höckel, M. Tumor Hypoxia and Malignant Progression. Methods Enzymol. 2004, 381, 335–354. [Google Scholar] [CrossRef]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Jiang, B.-H.; Rue, E.; Wang, G.L.; Roe, R.; Semenza, G.L. Dimerization, DNA Binding, and Transactivation Properties of Hypoxia-inducible Factor 1. J. Biol. Chem. 1996, 271, 17771–17778. [Google Scholar] [CrossRef]
- Wang, G.L.; Semenza, G.L. Purification and Characterization of Hypoxia-inducible Factor. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef]
- Ye, I.C.; Fertig, E.J.; Digiacomo, J.W.; Considine, M.; Godet, I.; Gilkes, D.M. Molecular Portrait of Hypoxia in Breast Cancer: A Prognostic Signature and Novel HIF-Regulated Genes. Mol. Cancer Res. 2018, 16, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Daly, L.A.; Brownridge, P.J.; Batie, M.; Rocha, S.; Sée, V.; Eyers, C.E. Oxygen-dependent changes in binding partners and post-translational modifications regulate the abundance and activity of HIF-1α/2α. Sci. Signal. 2021, 14, 6685. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Knoop, C.; Schlenger, K.; Vorndran, B.; Bauβnann, E.; Mitze, M.; Knapstein, P.G.; Vaupel, P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother. Oncol. 1993, 26, 45–50. [Google Scholar] [CrossRef]
- Hopf, H.W.; Hunt, T.K. Comparison of Clark Electrode and Optode for Measurement of Tissue Oxygen Tension. Adv. Exp. Med. Biol. 1994, 345, 841–847. [Google Scholar] [CrossRef]
- Nordsmark, M.; Bentzen, S.M.; Overgaard, J. Measurement of Human Tumour Oxygenation Status by a Polarographic Needle Electrode: An analysis of inter- and intratumour heterogeneity. Acta Oncol. 1994, 33, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, D.; Dufies, M.; Dadone, B.; Durand, M.; Borchiellini, D.; Amiel, J.; Pouyssegur, J.; Rioux-Leclercq, N.; Pages, G.; Burel-Vandenbos, F.; et al. The two glycolytic markers GLUT1 and MCT1 correlate with tumor grade and survival in clear-cell renal cell carcinoma. PLoS ONE 2018, 13, e0193477. [Google Scholar] [CrossRef]
- Varia, M.A.; Calkins-Adams, D.P.; Rinker, L.H.; Kennedy, A.S.; Novotny, D.B.; Fowler, W.C.; Raleigh, J.A. Pimonidazole: A Novel Hypoxia Marker for Complementary Study of Tumor Hypoxia and Cell Proliferation in Cervical Carcinoma. Gynecol. Oncol. 1998, 71, 270–277. [Google Scholar] [CrossRef]
- Danhier, P.; Krishnamachary, B.; Bharti, S.; Kakkad, S.; Mironchik, Y.; Bhujwalla, Z.M. Combining Optical Reporter Proteins with Different Half-lives to Detect Temporal Evolution of Hypoxia and Reoxygenation in Tumors. Neoplasia 2015, 17, 871–881. [Google Scholar] [CrossRef]
- Godet, I.; Shin, Y.J.; Ju, J.A.; Ye, I.C.; Wang, G.; Gilkes, D.M. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat. Commun. 2019, 10, 4862. [Google Scholar] [CrossRef]
- Ju, J.A.; Godet, I.; Ye, I.C.; Byun, J.; Jayatilaka, H.; Lee, S.J.; Xiang, L.; Samanta, D.; Lee, M.H.; Wu, P.-H.; et al. Hypoxia Selectively Enhances Integrin α5β1 Receptor Expression in Breast Cancer to Promote Metastasis. Mol. Cancer Res. 2017, 15, 723–734. [Google Scholar] [CrossRef]
- Ju, J.A.; Godet, I.; DiGiacomo, J.W.; Gilkes, D.M. RhoB is regulated by hypoxia and modulates metastasis in breast cancer. Cancer Rep. 2020, 3, e1164. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Sobhanifar, S.; Aquino-Parsons, C.; Stanbridge, E.J.; Olive, P. Reduced Expression of Hypoxia-Inducible Factor-1α in Perinecrotic Regions of Solid Tumors. Cancer Res. 2005, 65, 7259–7266. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Kimura, T.; Sadahiro, H.; Kawano, H.; Takubo, K.; Suzuki, M.; Ikeda, E. Histological Characterization of the Tumorigenic “Peri-Necrotic Niche” Harboring Quiescent Stem-Like Tumor Cells in Glioblastoma. PLoS ONE 2016, 11, e0147366. [Google Scholar] [CrossRef]
- Grimes, D.R.; Jansen, M.; Macauley, R.J.; Scott, J.G.; Basanta, D. Evidence for hypoxia increasing the tempo of evolution in glioblastoma. Br. J. Cancer 2020, 123, 1562–1569. [Google Scholar] [CrossRef]
- Ohnishi, K.; Tani, T.; Bando, S.-I.; Kubota, N.; Fujii, Y.; Hatano, O.; Harada, H. Plastic induction of CD133AC133-positive cells in the microenvironment of glioblastoma spheroids. Int. J. Oncol. 2014, 45, 581–586. [Google Scholar] [CrossRef][Green Version]
- Miranda-Gonçalves, V.; Granja, S.; Martinho, O.; Honavar, M.; Pojo, M.; Costa, B.M.; Pires, M.M.; Pinheiro, C.; Cordeiro, M.; Bebiano, G.; et al. Hypoxia-mediated upregulation of MCT1 expression supports the glycolytic phenotype of glioblastomas. Oncotarget 2016, 7, 46335–46353. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin. Cancer Biol. 2009, 19, 12–16. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Wilson, M.C. The monocarboxylate transporter family-Role and regulation. IUBMB Life 2011, 64, 109–119. [Google Scholar] [CrossRef]
- Iommarini, L.; Porcelli, A.M.; Gasparre, G.; Kurelac, I. Non-Canonical Mechanisms Regulating Hypoxia-Inducible Factor 1 Alpha in Cancer. Front. Oncol. 2017, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.J.; Gulyas, S.; Mohindra, J.K. Hypoxia-dependent reduction of 1-(2-nitro-1-imidazolyl)-3-methoxy-2-propanol by Chinese hamster ovary cells and KHT tumor cells in vitro and in vivo. Cancer Res. 1976, 36, 3761–3765. [Google Scholar]
- Webster, L.; Hodgkiss, R.J.; Wilson, G.D. Simultaneous triple staining for hypoxia, proliferation, and DNA content in murine tumours. Cytometry 1995, 21, 344–351. [Google Scholar] [CrossRef]
- Hodgkiss, R.J.; Stratford, M.R.; Dennis, M.F.; Hill, S.A. Pharmacokinetics and binding of the bioreductive probe for hypoxia, NITP: Effect of route of administration. Br. J. Cancer 1995, 72, 1462–1468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laurent, J.; Frongia, C.; Cazales, M.; Mondesert, O.; Ducommun, B.; Lobjois, V. Multicellular tumor spheroid models to explore cell cycle checkpoints in 3D. BMC Cancer 2013, 13, 73. [Google Scholar] [CrossRef]
- Lo, L.-W.; Koch, C.J.; Wilson, D.F. Calibration of Oxygen-Dependent Quenching of the Phosphorescence of Pd-meso-tetra (4-Carboxyphenyl) Porphine: A Phosphor with General Application for Measuring Oxygen Concentration in Biological Systems. Anal. Biochem. 1996, 236, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, Y.; Yuan, W.; Zhou, X.; Qiu, X.; Kong, M.; Wang, Q.; Feng, W.; Li, F. Quantitative Mapping of Liver Hypoxia in Living Mice Using Time-Resolved Wide-Field Phosphorescence Lifetime Imaging. Adv. Sci. 2020, 7, 1902929. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Hirakawa, Y.; Hosaka, M.; Nangaku, M.; Tobita, S. Oxygen imaging of living cells and tissues using luminescent molecular probes. J. Photochem. Photobiol. C Photochem. Rev. 2017, 30, 71–95. [Google Scholar] [CrossRef]
- Cao, X.; Allu, S.R.; Jiang, S.; Jia, M.; Gunn, J.R.; Yao, C.; LaRochelle, E.P.; Shell, J.R.; Bruza, P.; Gladstone, D.J.; et al. Tissue pO2 distributions in xenograft tumors dynamically imaged by Cherenkov-excited phosphorescence during fractionated radiation therapy. Nat. Commun. 2020, 11, 573. [Google Scholar] [CrossRef]
- Lee, Y.-E.K.; Ulbrich, E.E.; Kim, G.; Hah, H.; Strollo, C.; Fan, W.; Gurjar, R.; Koo, S.; Kopelman, R. Near Infrared Luminescent Oxygen Nanosensors with Nanoparticle Matrix Tailored Sensitivity. Anal. Chem. 2010, 82, 8446–8455. [Google Scholar] [CrossRef]
- Komatsu, H.; Yoshihara, K.; Yamada, H.; Kimura, Y.; Son, A.; Nishimoto, S.-I.; Tanabe, K. Ruthenium Complexes with Hydrophobic Ligands That Are Key Factors for the Optical Imaging of Physiological Hypoxia. Chem.—A Eur. J. 2013, 19, 1971–1977. [Google Scholar] [CrossRef]
- Wang, X.-H.; Peng, H.-S.; Cheng, K.; Liu, X.-M.; Liu, Y.-A.; Yang, W. Two-photon oxygen nanosensors based on a conjugated fluorescent polymer doped with platinum porphyrins. Methods Appl. Fluoresc. 2018, 6, 035008. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Byrne, A.; Burke, C.S.; Forster, R.J.; Keyes, T.E. Peptide-Bridged Dinuclear Ru(II) Complex for Mitochondrial Targeted Monitoring of Dynamic Changes to Oxygen Concentration and ROS Generation in Live Mammalian Cells. J. Am. Chem. Soc. 2014, 136, 15300–15309. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Ito, H.; Inoue, M.; Tabata, K.; Sato, Y.; Yamagata, K.; Kizaka-Kondoh, S.; Kadonosono, T.; Yano, S.; Inoue, M.; et al. High resolution imaging of intracellular oxygen concentration by phosphorescence lifetime. Sci. Rep. 2015, 5, 10657. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Colley, H.E.; Baggaley, E.; Sazanovich, I.V.; Green, N.H.; Weinstein, J.A.; Botchway, S.W.; MacNeil, S.; Haycock, J.W. Oxygen Mapping of Melanoma Spheroids using Small Molecule Platinum Probe and Phosphorescence Lifetime Imaging Microscopy. Sci. Rep. 2017, 7, 10743. [Google Scholar] [CrossRef]
- Chelushkin, P.S.; Shakirova, J.R.; Kritchenkov, I.S.; Baigildin, V.A.; Tunik, S.P. Phosphorescent NIR emitters for biomedicine: Applications, advances and challenges. Dalton Trans. 2021, 51, 1257–1280. [Google Scholar] [CrossRef]
- Borisov, S.M. Fundamentals of Quenched Phosphorescence O2 Sensing and Rational Design of Sensor Materials. In Detection Science; Royal Society of Chemistry: London, UK, 2018; pp. 1–18. [Google Scholar]
- Zhang, S.; Hosaka, M.; Yoshihara, T.; Negishi, K.; Iida, Y.; Tobita, S.; Takeuchi, T. Phosphorescent Light–Emitting Iridium Complexes Serve as a Hypoxia-Sensing Probe for Tumor Imaging in Living Animals. Cancer Res. 2010, 70, 4490–4498. [Google Scholar] [CrossRef]
- Fridman, I.B.; Ugolini, G.S.; VanDelinder, V.; Cohen, S.; Konry, T. High throughput microfluidic system with multiple oxygen levels for the study of hypoxia in tumor spheroids. Biofabrication 2021, 13, 035037. [Google Scholar] [CrossRef]
- Erapaneedi, R.; Belousov, V.V.; Schäfers, M.; Kiefer, F. A novel family of fluorescent hypoxia sensors reveal strong heterogeneity in tumor hypoxia at the cellular level. EMBO J. 2015, 35, 102–113. [Google Scholar] [CrossRef]
- Almoustafa, H.A.; Alshawsh, M.A.; Chik, Z. Targeted polymeric nanoparticle for anthracycline delivery in hypoxia-induced drug resistance in metastatic breast cancer cells. Anti-Cancer Drugs 2021, 32, 745–754. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Li, J.; Entenberg, D.; Xue, A.; Wang, W.; Condeelis, J. Direct visualization of the phenotype of hypoxic tumor cells at single cell resolution in vivo using a new hypoxia probe. IntraVital 2016, 5, e1187803. [Google Scholar] [CrossRef]
- Coralli, C.; Cemazar, M.; Kanthou, C.; Tozer, G.M.; Dachs, G.U. Limitations of the reporter green fluorescent protein under simulated tumor conditions. Cancer Res. 2001, 61, 4784–4790. [Google Scholar]
- Karlsson, H.; Fryknäs, M.; Larsson, R.; Nygren, P. Loss of cancer drug activity in colon cancer HCT-116 cells during spheroid formation in a new 3-D spheroid cell culture system. Exp. Cell Res. 2012, 318, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Stine, Z.E.; Nguyen, C.; Afzal, J.; Sun, P.; Hamaker, M.; Siegel, N.M.; Gouw, A.; Kang, B.-H.; Yu, S.-H.; et al. Tumorigenicity of hypoxic respiring cancer cells revealed by a hypoxia-cell cycle dual reporter. Proc. Natl. Acad. Sci. USA 2014, 111, 12486–12491. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Perry, J.; Jay, C.; Monget, D.; Rasburn, J.; Gould, F. Fluorogenic substrates for the detection of microbial nitroreductases. Lett. Appl. Microbiol. 2001, 33, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Tian, H.Y.; Zang, T.N.; Li, M.; Zhou, Y.; Zhang, J.F. Hypoxia imaging in cells and tumor tissues using a highly selective fluorescent nitroreductase probe. Sci. Rep. 2017, 7, 9174. [Google Scholar] [CrossRef] [PubMed]
- Piao, W.; Tsuda, S.; Tanaka, Y.; Maeda, S.; Liu, F.; Takahashi, S.; Kushida, Y.; Komatsu, T.; Ueno, T.; Terai, T.; et al. Development of Azo-Based Fluorescent Probes to Detect Different Levels of Hypoxia. Angew. Chem. Int. Ed. 2013, 52, 13028–13032. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nishikawa, M.; Mizukami, Y.; Kusamori, K.; Ogino, Y.; Nishimura, S.; Shimizu, K.; Konishi, S.; Takahashi, Y.; Takakura, Y. Control of polarization and tumoricidal activity of macrophages by multicellular spheroid formation. J. Control. Release 2018, 270, 177–183. [Google Scholar] [CrossRef]
- Jiao, S.; Yang, S.; Meng, X.; Wang, C. One step synthesis of red-emitting fluorescence turn-on probe for nitroreductase and its application to bacterial detection and oral cancer cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118637. [Google Scholar] [CrossRef]
- Wolff, P.; Heimann, L.; Liebsch, G.; Meier, R.J.; Gutbrod, M.; van Griensven, M.; Balmayor, E.R. Oxygen-distribution within 3-D collagen I hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2019, 95, 422–427. [Google Scholar] [CrossRef]
- Silva, C.J.P.; Liebsch, G.; Meier, R.J.; Gutbrod, M.S.; Balmayor, E.R.; Van Griensven, M. A New Non-invasive Technique for Measuring 3D-Oxygen Gradients in Wells During Mammalian Cell Culture. Front. Bioeng. Biotechnol. 2020, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.M.; Park, K.M.; Tang, V.; Xu, Y.; Pak, K.; Eisinger-Mathason, T.S.K.; Simon, M.C.; Gerecht, S. Intratumoral oxygen gradients mediate sarcoma cell invasion. Proc. Natl. Acad. Sci. USA 2016, 113, 9292–9297. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.C. Development of a pO2 Guided Fine Needle Tumor Biopsy Device. J. Med Devices 2021, 16, 021003. [Google Scholar] [CrossRef] [PubMed]
- Talks, K.L.; Turley, H.; Gatter, K.C.; Maxwell, P.; Pugh, C.; Ratcliffe, P.; Harris, A.L. The Expression and Distribution of the Hypoxia-Inducible Factors HIF-1α and HIF-2α in Normal Human Tissues, Cancers, and Tumor-Associated Macrophages. Am. J. Pathol. 2000, 157, 411–421. [Google Scholar] [CrossRef]
- Seelam, S.R.; Hong, M.K.; Lee, Y.-S.; Jeong, J.M. Comparative study of 2-nitroimidazole-fluorophore-conjugated derivatives with pimonidazole for imaging tumor hypoxia. J. Radiopharm. Mol. Probes 2019, 5, 101–112. [Google Scholar] [CrossRef]
- Kumagai, A.; Ando, R.; Miyatake, H.; Greimel, P.; Kobayashi, T.; Hirabayashi, Y.; Shimogori, T.; Miyawaki, A. A Bilirubin-Inducible Fluorescent Protein from Eel Muscle. Cell 2013, 153, 1602–1611. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Rocha, H.L.; Godet, I.; Kurtoglu, F.; Metzcar, J.; Konstantinopoulos, K.; Bhoyar, S.; Gilkes, D.M.; Macklin, P. A persistent invasive phenotype in post-hypoxic tumor cells is revealed by fate mapping and computational modeling. iScience 2021, 24, 102935. [Google Scholar] [CrossRef]
- Godet, I.; Mamo, M.; Thurnheer, A.; Rosen, D.M.; Gilkes, D.M. Post-Hypoxic Cells Promote Metastatic Recurrence after Chemotherapy Treatment in TNBC. Cancers 2021, 13, 5509. [Google Scholar] [CrossRef]
- Vermeer, J.A.F.; Ient, J.; Markelc, B.; Kaeppler, J.; Barbeau, L.M.O.; Groot, A.J.; Muschel, R.J.; Vooijs, M.A. A lineage-tracing tool to map the fate of hypoxic tumour cells. Dis. Model. Mech. 2020, 13, 044768. [Google Scholar] [CrossRef]
- Zheng, J.; Shen, Y.; Xu, Z.; Yuan, Z.; He, Y.; Wei, C.; Er, M.; Yin, J.; Chen, H. Near-infrared off-on fluorescence probe activated by NTR for in vivo hypoxia imaging. Biosens. Bioelectron. 2018, 119, 141–148. [Google Scholar] [CrossRef]
- Hettie, K.S.; Klockow, J.L.; Moon, E.J.; Giaccia, A.J.; Chin, F.T. A NIR fluorescent smart probe for imaging tumor hypoxia. Cancer Rep. 2021, 4, e1384. [Google Scholar] [CrossRef]
- Li, X.; Kim, J.; Yoon, J.; Chen, X. Cancer-Associated, Stimuli-Driven, Turn on Theranostics for Multimodality Imaging and Therapy. Adv. Mater. 2017, 29, 1606857. [Google Scholar] [CrossRef] [PubMed]
- Gorman, C.M.; Moffat, L.F.; Howard, B.H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol. Cell. Biol. 1982, 2, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Dunn, H.; Zhou, H.; Harada, H.; Hiraoka, M.; Mason, R.P.; Zhao, D. In vivo Bioluminescence Imaging of Tumor Hypoxia Dynamics of Breast Cancer Brain Metastasis in a Mouse Model. J. Vis. Exp. 2011, 56, e3175. [Google Scholar] [CrossRef] [PubMed]
- Cecic, I.; Chan, D.A.; Sutphin, P.D.; Ray, P.; Gambhir, S.S.; Giaccia, A.J.; Graves, E.E. Oxygen Sensitivity of Reporter Genes: Implications for Preclinical Imaging of Tumor Hypoxia. Mol. Imaging 2007, 6, 219–228. [Google Scholar] [CrossRef]
- Inoue, Y.; Kiryu, S.; Watanabe, M.; Tojo, A.; Ohtomo, K. Timing of Imaging after D-Luciferin Injection Affects the Longitudinal Assessment of Tumor Growth Using In Vivo Bioluminescence Imaging. Int. J. Biomed. Imaging 2010, 2010, 471408. [Google Scholar] [CrossRef]
- Cheng, M.H.Y.; Mo, Y.; Zheng, G. Nano versus Molecular: Optical Imaging Approaches to Detect and Monitor Tumor Hypoxia. Adv. Health Mater. 2021, 10, e2001549. [Google Scholar] [CrossRef]
- Shao, Q.; Morgounova, E.; Jiang, C.; Choi, J.; Bischof, J.; Ashkenazi, S. In vivophotoacoustic lifetime imaging of tumor hypoxia in small animals. J. Biomed. Opt. 2013, 18, 076019. [Google Scholar] [CrossRef]
- Knox, H.J.; Kim, T.W.; Zhu, Z.; Chan, J. Photophysical Tuning of N-Oxide-Based Probes Enables Ratiometric Photoacoustic Imaging of Tumor Hypoxia. ACS Chem. Biol. 2018, 13, 1838–1843. [Google Scholar] [CrossRef]
- Umehara, Y.; Kageyama, T.; Son, A.; Kimura, Y.; Kondo, T.; Tanabe, K. Biological reduction of nitroimidazole-functionalized gold nanorods for photoacoustic imaging of tumor hypoxia. RSC Adv. 2019, 9, 16863–16868. [Google Scholar] [CrossRef]
- Nyayapathi, N.; Xia, J. Photoacoustic imaging of breast cancer: A mini review of system design and image features. J. Biomed. Opt. 2019, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Gunn, J.R.; Allu, S.R.; Bruza, P.; Jiang, S.; Vinogradov, S.A.; Pogue, B.W. Implantable sensor for local Cherenkov-excited luminescence imaging of tumor pO2 during radiotherapy. J. Biomed. Opt. 2020, 25, 112704. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Allu, S.R.; Jiang, S.; Gunn, B.J.R.; Yao, C.; Xin, J.; Bruza, P.; Gladstone, S.D.J.; Jarvis, L.A.; Tian, J.; et al. High-Resolution pO2 Imaging Improves Quantification of the Hypoxic Fraction in Tumors During Radiation Therapy. Int. J. Radiat. Oncol. 2021, 109, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, J.S.; Edwards, A.D.; Azzopardi, D.; Reynolds, E.O. Magnetic resonance and near infrared spectroscopy for investigation of perinatal hypoxic-ischaemic brain injury. Arch. Dis. Child. 1989, 64, 953–963. [Google Scholar] [CrossRef]
- Byrne, P.; Welch, R.; Johnson, M.; Darrah, J.; Piper, M. Serial magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 1990, 117, 694–700. [Google Scholar] [CrossRef]
- Ishige, N.; Pitts, L.H.; Berry, I.; Carlson, S.G.; Nishimura, M.C.; Moseley, M.E.; Weinstein, P.R. The Effect of Hypoxia on Traumatic Head Injury in Rats: Alterations in Neurologic Function, Brain Edema, and Cerebral Blood Flow. J. Cereb. Blood Flow Metab. 1987, 7, 759–767. [Google Scholar] [CrossRef]
- Mirabello, V.; Cortezon-Tamarit, F.; Pascu, S.I. Oxygen Sensing, Hypoxia Tracing and in Vivo Imaging with Functional Metalloprobes for the Early Detection of Non-communicable Diseases. Front. Chem. 2018, 6, 27. [Google Scholar] [CrossRef]
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642. [Google Scholar] [CrossRef]
- Virani, N.; Kwon, J.; Zhou, H.; Mason, R.; Berbeco, R.; Protti, A. In vivo hypoxia characterization using blood oxygen level dependent magnetic resonance imaging in a preclinical glioblastoma mouse model. Magn. Reson. Imaging 2021, 76, 52–60. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Quiros-Gonzalez, I.; O’Connor, J.P.; Abeyakoon, O.; Parker, G.; Williams, K.J.; Gilbert, F.; Bohndiek, E.S. Oxygen Enhanced Optoacoustic Tomography (OE-OT) Reveals Vascular Dynamics in Murine Models of Prostate Cancer. Theranostics 2017, 7, 2900–2913. [Google Scholar] [CrossRef]
- Howe, F.A.; Robinson, S.P.; McIntyre, D.J.O.; Stubbs, M.; Griffiths, J.R. Issues in flow and oxygenation dependent contrast (FLOOD) imaging of tumours. NMR Biomed. 2001, 14, 497–506. [Google Scholar] [CrossRef]
- White, D.A.; Zhang, Z.; Li, L.; Gerberich, J.; Stojadinovic, S.; Peschke, P.; Mason, R.P. Developing oxygen-enhanced magnetic resonance imaging as a prognostic biomarker of radiation response. Cancer Lett. 2016, 380, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kodibagkar, V.D.; Wang, X.; Torres, J.P.; Gulaka, P.; Mason, R.P. Proton imaging of siloxanes to map tissue oxygenation levels (PISTOL): A tool for quantitative tissue oximetry. NMR Biomed. 2008, 21, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Shankar, R.V.; Kodibagkar, V.D. A faster PISTOL for 1 H MR-based quantitative tissue oximetry. NMR Biomed. 2019, 32, e4076. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, W.; Ma, Q.; Lu, Y.; Xu, Y.; Bian, K.; Liu, F.; Shi, C.; Wang, H.; Shi, Y.; et al. Hemoglobin-mediated biomimetic synthesis of paramagnetic O2-evolving theranostic nanoprobes for MR imaging-guided enhanced photodynamic therapy of tumor. Theranostics 2020, 10, 11607–11621. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cabral, H.; Song, B.; Aoki, I.; Chen, Z.; Nishiyama, N.; Huang, Y.; Kataoka, K.; Mi, P. Nanoprobe-Based Magnetic Resonance Imaging of Hypoxia Predicts Responses to Radiotherapy, Immunotherapy, and Sensitizing Treatments in Pancreatic Tumors. ACS Nano 2021, 15, 13526–13538. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Matsumoto, S.; Yasui, H.; Saito, K.; Devasahayam, N.; Subramanian, S.; Mitchell, J.B. Electron Paramagnetic Resonance Imaging of Tumor pO2. Radiat. Res. 2012, 177, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Gertsenshteyn, I.; Giurcanu, M.; Vaupel, P.; Halpern, H. Biological validation of electron paramagnetic resonance (EPR) image oxygen thresholds in tissue. J. Physiol. 2021, 599, 1759–1767. [Google Scholar] [CrossRef]
- Kishimoto, S.; Brender, J.R.; Chandramouli, G.V.R.; Saida, Y.; Yamamoto, K.; Mitchell, J.B.; Krishna, M.C. Hypoxia-Activated Prodrug Evofosfamide Treatment in Pancreatic Ductal Adenocarcinoma Xenografts Alters the Tumor Redox Status to Potentiate Radiotherapy. Antioxid. Redox Signal. 2021, 35, 904–915. [Google Scholar] [CrossRef]
- Kishimoto, S.; Matsumoto, K.-I.; Saito, K.; Enomoto, A.; Matsumoto, S.; Mitchell, J.B.; Devasahayam, N.; Krishna, M.C. Pulsed Electron Paramagnetic Resonance Imaging: Applications in the Studies of Tumor Physiology. Antioxid. Redox Signal. 2018, 28, 1378–1393. [Google Scholar] [CrossRef]
- Bobko, A.A.; Eubank, T.D.; Driesschaert, B.; Khramtsov, V.V. In Vivo EPR Assessment of pH, pO2, Redox Status, and Concentrations of Phosphate and Glutathione in the Tumor Microenvironment. J. Vis. Exp. 2018, 133, e56624. [Google Scholar] [CrossRef]
- Yasui, H.; Kawai, T.; Matsumoto, S.; Saito, K.; Devasahayam, N.; Mitchell, J.B.; Camphausen, K.; Inanami, O.; Krishna, M.C. Quantitative imaging of pO2 in orthotopic murine gliomas: Hypoxia correlates with resistance to radiation. Free Radic. Res. 2017, 51, 861–871. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, N.-T.; Barth, E.D.; Lee, T.-H.; Chen, C.-T.; Epel, B.; Halpern, H.J.; Lo, L.-W. Highly sensitive electron paramagnetic resonance nanoradicals for quantitative intracellular tumor oxymetric images. Int. J. Nanomed. 2019, 14, 2963–2971. [Google Scholar] [CrossRef]
- Ashkenazi, S.; Cho, D.; Song, C.W. Scanning Tissue Oxygen Needle Probe. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1269, pp. 51–55. [Google Scholar]
- Reeves, K.M.; Song, P.N.; Angermeier, A.; Della Manna, D.; Li, Y.; Wang, J.; Yang, E.S.; Sorace, A.G.; Larimer, B.M. 18F-FMISO PET Imaging Identifies Hypoxia and Immunosuppressive Tumor Microenvironments and Guides Targeted Evofosfamide Therapy in Tumors Refractory to PD-1 and CTLA-4 Inhibition. Clin. Cancer Res. 2021, 28, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Yamaguchi, S.; Shiga, T.; Kuge, Y.; Tamaki, N. The Roles of Hypoxia Imaging Using 18F-Fluoromisonidazole Positron Emission Tomography in Glioma Treatment. J. Clin. Med. 2019, 8, 1088. [Google Scholar] [CrossRef]
- Peeters, S.G.; Zegers, C.M.; Lieuwes, N.G.; van Elmpt, W.; Eriksson, J.; van Dongen, G.A.; Dubois, L.; Lambin, P. A Comparative Study of the Hypoxia PET Tracers [18F]HX4, [18F]FAZA, and [18F]FMISO in a Preclinical Tumor Model. Int. J. Radiat. Oncol. 2015, 91, 351–359. [Google Scholar] [CrossRef]
- Quartuccio, N.; Young AIMN Working Group; Laudicella, R.; Mapelli, P.; Guglielmo, P.; Pizzuto, D.A.; Boero, M.; Arnone, G.; Picchio, M. Hypoxia PET imaging beyond 18F-FMISO in patients with high-grade glioma: 18F-FAZA and other hypoxia radiotracers. Clin. Transl. Imaging 2020, 8, 11–20. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Kolb, H.C.; Walsh, J.C.; Zhang, J.; Guan, Y. 18F-HX4 hypoxia imaging with PET/CT in head and neck cancer: A comparison with 18F-FMISO. Nucl. Med. Commun. 2012, 33, 1096–1102. [Google Scholar] [CrossRef]
- More, K.N.; Lee, J.Y.; Kim, D.-Y.; Cho, N.-C.; Pyo, A.; Yun, M.; Kim, H.S.; Kim, H.; Ko, K.; Park, J.-H.; et al. Acetazolamide-based [18F]-PET tracer: In vivo validation of carbonic anhydrase IX as a sole target for imaging of CA-IX expressing hypoxic solid tumors. Bioorganic Med. Chem. Lett. 2018, 28, 915–921. [Google Scholar] [CrossRef]
- Gammon, S.T.; Pisaneschi, F.; Bandi, M.L.; Smith, M.G.; Sun, Y.; Rao, Y.; Muller, F.; Wong, F.; De Groot, J.; Ackroyd, J.; et al. Mechanism-Specific Pharmacodynamics of a Novel Complex-I Inhibitor Quantified by Imaging Reversal of Consumptive Hypoxia with [18F]FAZA PET In Vivo. Cells 2019, 8, 1487. [Google Scholar] [CrossRef]
- Shimizu, Y.; Motomura, A.; Takakura, H.; Tamaki, N.; Kuge, Y.; Ogawa, M. Accumulation of hypoxia imaging probe “18F-FMISO” in macrophages depends on macrophage polarization in addition to hypoxic state. Ann. Nucl. Med. 2019, 33, 362–367. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.; Malik, V.; Johnston, C.; Reynolds, J.V.; O’Sullivan, J. Can the Efficacy of [18F]FDG-PET/CT in Clinical Oncology Be Enhanced by Screening Biomolecular Profiles? Pharmaceuticals 2019, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Grassi, I.; Chiti, A.; Nanni, C.; Cicoria, G.; Toschi, L.; Fonti, C.; Lodi, F.; Mattioli, S.; Fanti, S. PET radiopharmaceuticals for imaging of tumor hypoxia: A review of the evidence. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 365–384. [Google Scholar]
- Qlark, L.C. Monitor and control of blood and tissue oxygen tensions. Trans.—Am. Soc. Artif. Intern. Organs 1956, 2, 41–48. [Google Scholar]
- Marland, J.R.; Gray, M.E.; Dunare, C.; Blair, E.O.; Tsiamis, A.; Sullivan, P.; González-Fernández, E.; Greenhalgh, S.N.; Gregson, R.; Clutton, R.E.; et al. Real-time measurement of tumour hypoxia using an implantable microfabricated oxygen sensor. Sens. Bio-Sensing Res. 2020, 30, 100375. [Google Scholar] [CrossRef]
- Rivas, L.; Dulay, S.; Miserere, S.; Pla, L.; Marin, S.B.; Parra, J.; Eixarch, E.; Gratacós, E.; Illa, M.; Mir, M.; et al. Micro-needle implantable electrochemical oxygen sensor: Ex-vivo and in-vivo studies. Biosens. Bioelectron. 2020, 153, 112028. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.E.; Marland, J.; Dunare, C.; Blair, E.O.; Meehan, J.; Tsiamis, A.; Kunkler, I.H.; Murray, A.F.; Argyle, D.; Dyson, A.; et al. In vivo validation of a miniaturized electrochemical oxygen sensor for measuring intestinal oxygen tension. Am. J. Physiol. Liver Physiol. 2019, 317, G242–G252. [Google Scholar] [CrossRef]
- Springett, R.; Swartz, H.M. Measurements of Oxygen In Vivo: Overview and Perspectives on Methods to Measure Oxygen Within Cells and Tissues. Antioxid. Redox Signal. 2007, 9, 1295–1302. [Google Scholar] [CrossRef]
- Owen, J.; Logan, K.; Nesbitt, H.; Able, S.; Vasilyeva, A.; Bluemke, E.; Kersemans, V.; Smart, S.; Vallis, K.A.; McHale, A.P.; et al. Orally administered oxygen nanobubbles enhance tumor response to sonodynamic therapy. Nano Sel. 2022, 3, 394–401. [Google Scholar] [CrossRef]
- Lukina, M.; Orlova, A.; Shirmanova, M.; Shirokov, D.; Pavlikov, A.; Neubauer, A.; Studier, H.; Becker, W.; Zagaynova, E.; Yoshihara, T.; et al. Interrogation of metabolic and oxygen states of tumors with fiber-based luminescence lifetime spectroscopy. Opt. Lett. 2017, 42, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.F.; Dupuis, N.P.; Kusumoto, T.; Liu, F.; Menon, K.; Teicher, B.A. Increased Tumor Oxygenation and Radiation Sensitivity in two Rat Tumors by A Hemoglobin-Based, Oxygen-Carrying Preparation. Artif. Cells Blood Substit. Biotechnol. 1995, 23, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-J.; Li, C.; Lv, H.-B.; Zhao, C.; Yu, J.-M.; Wang, G.-H.; Luo, Y.-X.; Li, Y.; Xiao, M.; Yin, J.; et al. Comparing CT perfusion with oxygen partial pressure in a rabbit VX2 soft-tissue tumor model. J. Radiat. Res. 2014, 55, 183–190. [Google Scholar] [CrossRef]
- Doss, M.; Zhang, J.J.; Bélanger, M.-J.; Stubbs, J.B.; Hostetler, E.D.; Alpaugh, K.; Kolb, H.C.; Yu, J.Q. Biodistribution and radiation dosimetry of the hypoxia marker 18F–HX4 in monkeys and humans determined by using whole-body PET/CT. Nucl. Med. Commun. 2010, 31, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K. The Underpinnings of the BOLD Functional Magnetic Resonance Imaging Signal. J. Neurosci. 2003, 23, 3963–3971. [Google Scholar] [CrossRef]
- Rickard, A.G.; Palmer, G.M.; Dewhirst, M.W. Clinical and Pre-clinical Methods for Quantifying Tumor Hypoxia. In Hypoxia and Cancer Metastasis; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1136, pp. 19–41. [Google Scholar]
- D’Alonzo, R.A.; Gill, S.; Rowshanfarzad, P.; Keam, S.; MacKinnon, K.M.; Cook, A.M.; Ebert, M.A. In vivo noninvasive preclinical tumor hypoxia imaging methods: A review. Int. J. Radiat. Biol. 2021, 97, 593–631. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Farooq, A.; Dhou, S.; Majdalawieh, A.F. Bioluminescence Imaging Applications in Cancer: A Comprehensive Review. IEEE Rev. Biomed. Eng. 2021, 14, 307–326. [Google Scholar] [CrossRef]
- Torres, J.P.; López-Larrubia, P.; Ballesteros, P.; Cerdán, S. Imaging tumor hypoxia by magnetic resonance methods. NMR Biomed. 2010, 24, 1–16. [Google Scholar] [CrossRef]
- Stieb, S.; Eleftheriou, A.; Warnock, G.; Guckenberger, M.; Riesterer, O. Longitudinal PET imaging of tumor hypoxia during the course of radiotherapy. Eur. J. Pediatr. 2018, 45, 2201–2217. [Google Scholar] [CrossRef]
- Lee, C.-T.; Boss, M.-K.; Dewhirst, M.W. Imaging Tumor Hypoxia to Advance Radiation Oncology. Antioxid. Redox Signal. 2014, 21, 313–337. [Google Scholar] [CrossRef]
- Daponte, A.; Ioannou, M.; Mylonis, I.; Simos, G.; Minas, M.; E Messinis, I.; Koukoulis, G. Prognostic significance of Hypoxia-Inducible Factor 1 alpha(HIF-1alpha) expression in serous ovarian cancer: An immunohistochemical study. BMC Cancer 2008, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.; Mazure, N.M.; Hofman, V.; Ammadi, E.R.; Ortholan, C.; Bonnetaud, C.; Havet, K.; Venissac, N.; Mograbi, B.; Mouroux, J.; et al. High levels of carbonic anhydrase IX in tumour tissue and plasma are biomarkers of poor prognostic in patients with non-small cell lung cancer. Br. J. Cancer 2010, 102, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Nordsmark, M.; Loncaster, J.; Aquino-Parsons, C.; Chou, S.-C.; Gebski, V.; West, C.; Lindegaard, J.C.; Havsteen, H.; Davidson, S.E.; Hunter, R.; et al. The prognostic value of pimonidazole and tumour pO2 in human cervix carcinomas after radiation therapy: A prospective international multi-center study. Radiother. Oncol. 2006, 80, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Dunwoodie, S.L. The Role of Hypoxia in Development of the Mammalian Embryo. Dev. Cell 2009, 17, 755–773. [Google Scholar] [CrossRef]
- Chen, P.-S.; Chiu, W.-T.; Hsu, P.-L.; Lin, S.-C.; Peng, I.-C.; Wang, C.-Y.; Tsai, S.-J. Pathophysiological implications of hypoxia in human diseases. J. Biomed. Sci. 2020, 27, 63. [Google Scholar] [CrossRef]
- Digiacomo, J.W.; Gilkes, D.M. Tumor Hypoxia as an Enhancer of Inflammation-Mediated Metastasis: Emerging Therapeutic Strategies. Target. Oncol. 2018, 13, 157–173. [Google Scholar] [CrossRef]
- Digiacomo, J.W.; Gilkes, D.M. Therapeutic Strategies to Block the Hypoxic Response. In Hypoxia and Cancer Metastasis; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1136, pp. 141–157. [Google Scholar]
| Method | Detection | Live | Direct | Readout | Scale | Single Cell Res | Non -Inv | Dyna-mic | Temp. Res |
In Vitro | Animal | Human | Processing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endogenous markers | LM, FM, FC | N | N | +/− | μm [26] | Y | NA | N | NA | Y | Y | Y | Fixation Staining |
| Exogenous markers | LM, FM, FC | N | N | +/− | μm [66] | Y | NA | N | NA | Y | Y | Y | Fixation Staining |
| Fluorescent Reporter | FM, FC, Fluorescent imager | Y | N | +/− [79] | μm [79] | Y | Y | Y | ms [79] | Y | Y | N | Fixation Dissociation |
| NTR-sensitive Fluorescence | FM, FC, Fluorescent imager | Y | N | +/− [79] | μm [73] | Y | Y | Y | ms [79] | Y | Y | N | Fixation Dissociation |
| Phosphorescence CELI | FM, Fluorescent imager | Y | Y | pO2 [79] | μm [79] | N | Y | Y | s [128] | Y | Y | N | Pre-exposure |
| PAI | Ultrasound | Y | N | sO2 [79] | μm [79,129] | N | Y | Y | ms [79] | Y | Y | N | Pre-injection |
| BLI | Luminescent imager | Y | N | Intensity Gradient [130] | mm [77] | N | Y | Y | min [129] | Y | Y | N | Pre-injection |
| MRI | MRI machine | Y | N | B: deoxyHb [90] T: [O2(s)] [90] | mm [131] | N | Y | Y | s-min [128] | N | Y | Y | Pre-injection |
| EPRI | EPRI machine Spin tracers | Y | Y | pO2 [99] | mm [129,131] | N | Y | Y | min-hr [128,129] | N | Y | N | Pre-injection |
| PET | Radiolabeled Tracers | Y | N | radiotracer [129] | mm [131,132] | N | Y | Y | min-hr [133] | N | Y | Y | Pre-injection |
| Clark Electrode | Current meter | Y | Y | pO2 [121] | μm [121] | N | N | Y | s [121] | N | Y | Y | Implant/ Insertion |
| Invasive optical probes | Optical Detector | Y | Y | pO2 [121] | μm [121] | N | N | Y | ms [121] | Y | Y | Y | Insertion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godet, I.; Doctorman, S.; Wu, F.; Gilkes, D.M. Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances. Cells 2022, 11, 686. https://doi.org/10.3390/cells11040686
Godet I, Doctorman S, Wu F, Gilkes DM. Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances. Cells. 2022; 11(4):686. https://doi.org/10.3390/cells11040686
Chicago/Turabian StyleGodet, Inês, Steven Doctorman, Fan Wu, and Daniele M. Gilkes. 2022. "Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances" Cells 11, no. 4: 686. https://doi.org/10.3390/cells11040686
APA StyleGodet, I., Doctorman, S., Wu, F., & Gilkes, D. M. (2022). Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances. Cells, 11(4), 686. https://doi.org/10.3390/cells11040686









