Biomaterials and Cell-Based Regenerative Therapies for Intervertebral Disc Degeneration with a Focus on Biological and Biomechanical Functional Repair: Targeting Treatments for Disc Herniation
Abstract
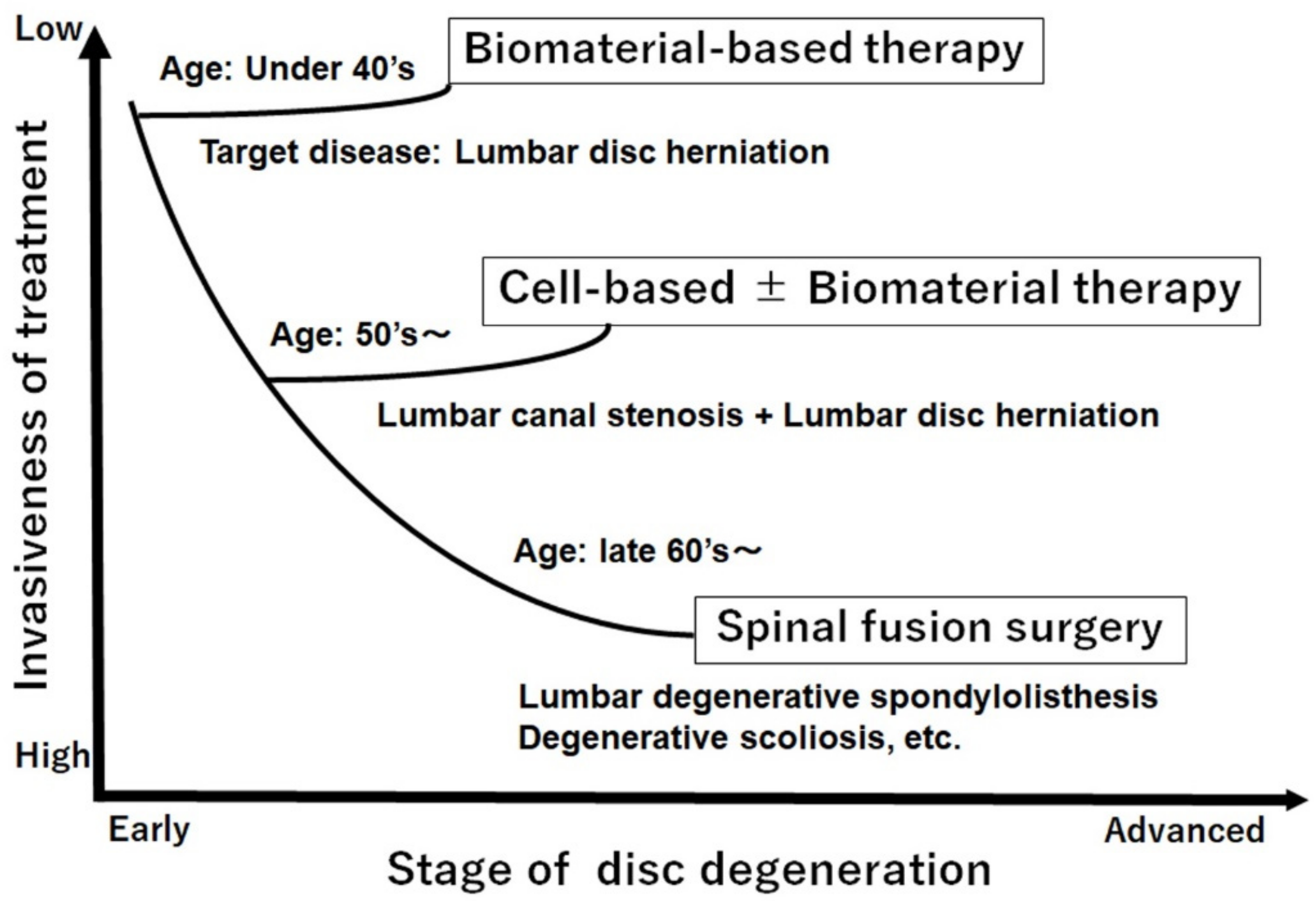
:1. Introduction
- cell-based IVD regeneration therapy,
- biomaterial-based IVD regeneration therapy, and
- disc regeneration/repair treatment for IVD herniation.
2. Cell-Based IVD Regeneration Therapy: Cell Transplantation
2.1. Autologous IVD-Derived Cells as Therapy for IVD Regeneration
2.2. MSC Therapy for IVD Regeneration
2.3. Use of Bone Marrow Aspirate Concentrate (BMAC) for IVD Regeneration Therapy
2.4. Problems of IVD Regeneration Therapy Using Cell-Only Transplantation
3. Biomaterial-Based IVD Regeneration Therapy: Soft Biomaterials Used to Regenerate Biological and Biomechanical Function
3.1. Soft Biomaterials for NP Repair and/or Regeneration
- being biocompatible, non-toxic, and safe in vivo;
- support cell survival;
- promote ECM formation;
- reduce inflammation; and
- inhibit pathological fibrosis [113].
3.2. Biological NP Repair and/or Regeneration Using Soft Biomaterials
3.3. Mechanism of IVD Regeneration Therapy Using Cell-Free Soft Biomaterials Alone
3.4. Effects of Biomaterials on Reduction in Pain Related to Damaged IVDs
3.5. Biomechanical Evaluation of Soft Biomaterials for NP Repair and/or Regeneration
3.6. Clinical Trial of Soft Biomaterials for Treating IVD Degeneration
4. Disc Regeneration and/or Repair Treatment for IVD Herniation
4.1. Adhesive Function of Soft Biomaterials after Discectomy or AF Injury
4.2. Evaluation of Biomechanical and Biological Regeneration by Soft Biomaterials in IVD after Discectomy or AF Injury
4.3. Clinical Application of Soft Biomaterial Therapy for IVD Herniation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marras, W.S.; Ferguson, S.A.; Burr, D.; Schabo, P.; Maronitis, A. Low back pain recurrence in occupational environments. Spine 2007, 32, 2387–2397. [Google Scholar] [CrossRef]
- Di Iorio, A.; Abate, M.; Guralnik, J.M.; Bandinelli, S.; Cecchi, F.; Cherubini, A.; Corsonello, A.; Foschini, N.; Guglielmi, M.; Lauretani, F.; et al. From chronic low back pain to disability, a multifactorial mediated pathway: The InCHIANTI study. Spine 2007, 32, E809–E815. [Google Scholar] [CrossRef]
- Mielenz, T.J.; Garrett, J.M.; Carey, T.S. Association of psychosocial work characteristics with low back pain outcomes. Spine 2008, 33, 1270–1275. [Google Scholar] [CrossRef]
- Yamada, K.; Sudo, H.; Iwasaki, K.; Sasaki, N.; Higashi, H.; Kameda, Y.; Ito, M.; Takahata, M.; Abumi, K.; Minami, A.; et al. Caspase 3 silencing inhibits biomechanical overload-induced intervertebral disk degeneration. Am. J. Pathol. 2014, 184, 753–764. [Google Scholar] [CrossRef]
- Priyadarshani, P.; Li, Y.; Yao, L. Advances in biological therapy for nucleus pulposus regeneration. Osteoarthr. Cartil. 2016, 24, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Tsujimoto, T.; Sudo, H.; Todoh, M.; Yamada, K.; Iwasaki, K.; Ohnishi, T.; Hirohama, N.; Nonoyama, T.; Ukeba, D.; Ura, K.; et al. An acellular bioresorbable ultra-purified alginate gel promotes intervertebral disc repair: A preclinical proof-of-concept study. eBioMedicine 2018, 37, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Frith, J.E.; Cameron, A.R.; Menzies, D.J.; Ghosh, P.; Whitehead, D.L.; Gronthos, S.; Zannettino, A.C.; Cooper-White, J.J. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials 2013, 34, 9430–9440. [Google Scholar] [CrossRef]
- Hunter, C.J.; Matyas, J.R.; Duncan, N.A. The notochordal cell in the nucleus pulposus: A review in the context of tissue engineering. Tissue Eng. 2003, 9, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Sudo, H.; Minami, A. Caspase 3 as a therapeutic target for regulation of intervertebral disc degeneration in rabbits. Arthritis Rheum. 2011, 63, 1648–1657. [Google Scholar] [CrossRef]
- Sudo, H.; Minami, A. Regulation of apoptosis in nucleus pulposus cells by optimized exogenous Bcl-2 overexpression. J. Orthop. Res. 2010, 28, 1608–1613. [Google Scholar] [CrossRef]
- Ju, D.G.; Kanim, L.E.; Bae, H.W. Intervertebral Disc Repair: Current Concepts. Glob. Spine J. 2020, 10, 130–136. [Google Scholar] [CrossRef]
- Park, J.B.; Lee, J.K.; Park, S.J.; Kim, K.W.; Riew, K.D. Mitochondrial involvement in fas-mediated apoptosis of human lumbar disc cells. J. Bone Jt. Surg. Am. 2005, 87, 1338–1342. [Google Scholar]
- Gruber, H.E.; Hanley, E.N., Jr. Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine 1998, 23, 751–757. [Google Scholar] [CrossRef]
- Kim, K.W.; Ha, K.Y.; Lee, J.S.; Rhyu, K.W.; An, H.S.; Woo, Y.K. The apoptotic effects of oxidative stress and antiapoptotic effects of caspase inhibitors on rat notochordal cells. Spine 2007, 32, 2443–2448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotz, J.C.; Chin, J.R. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine 2000, 25, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314. [Google Scholar] [CrossRef]
- Rannou, F.; Lee, T.S.; Zhou, R.H.; Chin, J.; Lotz, J.C.; Mayoux-Benhamou, M.A.; Barbet, J.P.; Chevrot, A.; Shyy, J.Y. Intervertebral disc degeneration: The role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am. J. Pathol. 2004, 164, 915–924. [Google Scholar] [CrossRef]
- Sakai, D.; Schol, J. Cell therapy for intervertebral disc repair: Clinical perspective. J. Orthop. Translat. 2017, 9, 8–18. [Google Scholar] [CrossRef]
- Tessier, S.; Risbud, M.V. Understanding embryonic development for cell-based therapies of intervertebral disc degeneration: Toward an effort to treat disc degeneration subphenotypes. Dev. Dyn. 2021, 250, 302–317. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Bach, F.C.; Tryfonidou, M.A.; Le Maitre, C.L.; Mwale, F.; Diwan, A.D.; Ito, K. Leaping the hurdles in developing regenerative treatments for the intervertebral disc from preclinical to clinical. JOR Spine 2018, 1, e1027. [Google Scholar] [CrossRef] [PubMed]
- Oehme, D.; Goldschlager, T.; Ghosh, P.; Rosenfeld, J.V.; Jenkin, G. Cell-Based Therapies Used to Treat Lumbar Degenerative Disc Disease: A Systematic Review of Animal Studies and Human Clinical Trials. Stem Cells Int. 2015, 2015, 946031. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Andersson, G.B. Stem cell therapy for intervertebral disc regeneration: Obstacles and solutions. Nat. Rev. Rheumatol. 2015, 11, 243–256. [Google Scholar] [CrossRef]
- Binch, A.L.A.; Fitzgerald, J.C.; Growney, E.A.; Barry, F. Cell-based strategies for IVD repair: Clinical progress and translational obstacles. Nat. Rev. Rheumatol. 2021, 17, 158–175. [Google Scholar] [CrossRef]
- Mochida, J.; Sakai, D.; Nakamura, Y.; Watanabe, T.; Yamamoto, Y.; Kato, S. Intervertebral disc repair with activated nucleus pulposus cell transplantation: A three-year, prospective clinical study of its safety. Eur. Cell Mater. 2015, 29, 202–212; discussion 212. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Mochida, J.; Iwashina, T.; Hiyama, A.; Omi, H.; Imai, M.; Nakai, T.; Ando, K.; Hotta, T. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials 2006, 27, 335–345. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Mochida, J.; Sakai, D.; Nakai, T.; Nishimura, K.; Kawada, H.; Hotta, T. Upregulation of the viability of nucleus pulposus cells by bone marrow-derived stromal cells: Significance of direct cell-to-cell contact in coculture system. Spine 2004, 29, 1508–1514. [Google Scholar] [CrossRef]
- Meisel, H.J.; Ganey, T.; Hutton, W.C.; Libera, J.; Minkus, Y.; Alasevic, O. Clinical experience in cell-based therapeutics: Intervention and outcome. Eur. Spine J. 2006, 15 (Suppl. 3), 397–405. [Google Scholar] [CrossRef] [Green Version]
- Okuma, M.; Mochida, J.; Nishimura, K.; Sakabe, K.; Seiki, K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: An in vitro and in vivo experimental study. J. Orthop. Res. 2000, 18, 988–997. [Google Scholar] [CrossRef]
- Watanabe, K.; Mochida, J.; Nomura, T.; Okuma, M.; Sakabe, K.; Seiki, K. Effect of reinsertion of activated nucleus pulposus on disc degeneration: An experimental study on various types of collagen in degenerative discs. Connect. Tissue Res. 2003, 44, 104–108. [Google Scholar] [CrossRef]
- Ganey, T.; Libera, J.; Moos, V.; Alasevic, O.; Fritsch, K.G.; Meisel, H.J.; Hutton, W.C. Disc chondrocyte transplantation in a canine model: A treatment for degenerated or damaged intervertebral disc. Spine 2003, 28, 2609–2620. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Johnson, T.L.; Leslie, K.; Ingram, J.A.; Martin, D.; Hoelscher, G.; Banks, D.; Phieffer, L.; Coldham, G.; Hanley, E.N., Jr. Autologous intervertebral disc cell implantation: A model using Psammomys obesus, the sand rat. Spine 2002, 27, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhuang, Y.; Li, C.Q.; Liu, L.T.; Zhou, Y. Regeneration of the intervertebral disc with nucleus pulposus cell-seeded collagen II/hyaluronan/chondroitin-6-sulfate tri-copolymer constructs in a rabbit disc degeneration model. Spine 2011, 36, 2252–2259. [Google Scholar] [CrossRef]
- Hohaus, C.; Ganey, T.M.; Minkus, Y.; Meisel, H.J. Cell transplantation in lumbar spine disc degeneration disease. Eur. Spine J. 2008, 17 (Suppl. 4), 492–503. [Google Scholar] [CrossRef] [Green Version]
- Iwashina, T.; Mochida, J.; Sakai, D.; Yamamoto, Y.; Miyazaki, T.; Ando, K.; Hotta, T. Feasibility of using a human nucleus pulposus cell line as a cell source in cell transplantation therapy for intervertebral disc degeneration. Spine 2006, 31, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H.J.; Siodla, V.; Ganey, T.; Minkus, Y.; Hutton, W.C.; Alasevic, O.J. Clinical experience in cell-based therapeutics: Disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol. Eng. 2007, 24, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Mochida, J.; Okuma, M.; Nishimura, K.; Sakabe, K. Nucleus pulposus allograft retards intervertebral disc degeneration. Clin. Orthop. Relat. Res. 2001, 389, 94–101. [Google Scholar] [CrossRef]
- Ruan, D.K.; Xin, H.; Zhang, C.; Wang, C.; Xu, C.; Li, C.; He, Q. Experimental intervertebral disc regeneration with tissue-engineered composite in a canine model. Tissue Eng. Part. A 2010, 16, 2381–2389. [Google Scholar] [CrossRef]
- Coric, D.; Pettine, K.; Sumich, A.; Boltes, M.O. Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 Joint Spine Section Meeting. J. Neurosurg. Spine 2013, 18, 85–95. [Google Scholar] [CrossRef]
- Gorensek, M.; Jaksimovic, C.; Kregar-Velikonja, N.; Gorensek, M.; Knezevic, M.; Jeras, M.; Pavlovcic, V.; Cor, A. Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol. Biol. Lett. 2004, 9, 363–373. [Google Scholar]
- Acosta, F.L., Jr.; Metz, L.; Adkisson, H.D.; Liu, J.; Carruthers-Liebenberg, E.; Milliman, C.; Maloney, M.; Lotz, J.C. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng. Part. A 2011, 17, 3045–3055. [Google Scholar] [CrossRef] [Green Version]
- Omlor, G.W.; Bertram, H.; Kleinschmidt, K.; Fischer, J.; Brohm, K.; Guehring, T.; Anton, M.; Richter, W. Methods to monitor distribution and metabolic activity of mesenchymal stem cells following in vivo injection into nucleotomized porcine intervertebral discs. Eur. Spine J. 2010, 19, 601–612. [Google Scholar] [CrossRef] [Green Version]
- Sakai, D.; Mochida, J.; Yamamoto, Y.; Nomura, T.; Okuma, M.; Nishimura, K.; Nakai, T.; Ando, K.; Hotta, T. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: A potential therapeutic model for disc degeneration. Biomaterials 2003, 24, 3531–3541. [Google Scholar] [CrossRef]
- Serigano, K.; Sakai, D.; Hiyama, A.; Tamura, F.; Tanaka, M.; Mochida, J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J. Orthop. Res. 2010, 28, 1267–1275. [Google Scholar] [CrossRef]
- Vadala, G.; Sowa, G.; Hubert, M.; Gilbertson, L.G.; Denaro, V.; Kang, J.D. Mesenchymal stem cells injection in degenerated intervertebral disc: Cell leakage may induce osteophyte formation. J. Tissue Eng. Regen. Med. 2012, 6, 348–355. [Google Scholar] [CrossRef]
- Wei, A.; Tao, H.; Chung, S.A.; Brisby, H.; Ma, D.D.; Diwan, A.D. The fate of transplanted xenogeneic bone marrow-derived stem cells in rat intervertebral discs. J. Orthop. Res. 2009, 27, 374–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Leung, V.Y.; Luk, K.D.; Chan, D.; Cheung, K.M. Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol. Ther. 2009, 17, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Guo, X.; Xu, P.; Kang, L.L.; Li, J. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin. Orthop. Relat. Res. 2005, 430, 219–226. [Google Scholar] [CrossRef]
- Allon, A.A.; Aurouer, N.; Yoo, B.B.; Liebenberg, E.C.; Buser, Z.; Lotz, J.C. Structured coculture of stem cells and disc cells prevent disc degeneration in a rat model. Spine J. 2010, 10, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Zhao, X.; Liu, H.; Zhang, H.; Chen, X.; Shi, R.; Liu, X.; Zhao, X.; Zhang, W.; Wang, B. Transplantation of mesenchymal stem cells and nucleus pulposus cells in a degenerative disc model in rabbits: A comparison of 2 cell types as potential candidates for disc regeneration. J. Neurosurg. Spine 2011, 14, 322–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, T.; Ueda, Y.; Miyazaki, K.; Koizumi, M.; Takakura, Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: A report of two case studies. Spine 2010, 35, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Soler, R.; Morera, C.; Alberca, M.; Sanchez, A.; Garcia-Sancho, J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: A pilot study. Transplantation 2011, 92, 822–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Wu, J.; Liu, J.; Ebraheim, M.; Castillo, S.; Liu, X.; Tang, T.; Ebraheim, N.A. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J. 2010, 10, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, M.; Bunger, C.E.; Zou, X.; Foldager, C.; Jorgensen, H.S. Autologous stem cell therapy maintains vertebral blood flow and contrast diffusion through the endplate in experimental intervertebral disc degeneration. Spine 2011, 36, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Barczewska, M.; Wojtkiewicz, J.; Habich, A.; Janowski, M.; Adamiak, Z.; Holak, P.; Matyjasik, H.; Bulte, J.W.; Maksymowicz, W.; Walczak, P. MR monitoring of minimally invasive delivery of mesenchymal stem cells into the porcine intervertebral disc. PLoS ONE 2013, 8, e74658. [Google Scholar] [CrossRef]
- Yuan, M.; Yeung, C.W.; Li, Y.Y.; Diao, H.; Cheung, K.M.C.; Chan, D.; Cheah, K.; Chan, P.B. Effects of nucleus pulposus cell-derived acellular matrix on the differentiation of mesenchymal stem cells. Biomaterials 2013, 34, 3948–3961. [Google Scholar] [CrossRef]
- Crevensten, G.; Walsh, A.J.; Ananthakrishnan, D.; Page, P.; Wahba, G.M.; Lotz, J.C.; Berven, S. Intervertebral disc cell therapy for regeneration: Mesenchymal stem cell implantation in rat intervertebral discs. Ann. Biomed. Eng. 2004, 32, 430–434. [Google Scholar] [CrossRef]
- Elabd, C.; Centeno, C.J.; Schultz, J.R.; Lutz, G.; Ichim, T.; Silva, F.J. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: A long-term safety and feasibility study. J. Transl. Med. 2016, 14, 253. [Google Scholar] [CrossRef] [Green Version]
- Noriega, D.C.; Ardura, F.; Hernandez-Ramajo, R.; Martin-Ferrero, M.A.; Sanchez-Lite, I.; Toribio, B.; Alberca, M.; Garcia, V.; Moraleda, J.M.; Sanchez, A.; et al. Intervertebral Disc Repair by Allogeneic Mesenchymal Bone Marrow Cells: A Randomized Controlled Trial. Transplantation 2017, 101, 1945–1951. [Google Scholar] [CrossRef]
- Ganey, T.; Hutton, W.C.; Moseley, T.; Hedrick, M.; Meisel, H.J. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: Experiments in a canine model. Spine 2009, 34, 2297–2304. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.H.; Lee, J.H.; Jin, E.S.; Min, J.K.; Jeon, S.R.; Choi, K.H. Regeneration of intervertebral discs in a rat disc degeneration model by implanted adipose-tissue-derived stromal cells. Acta Neurochir. 2010, 152, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Kim, Y.S.; Kim, B.K.; Kim, E.H.; Kim, J.H.; Do, B.R.; Hwang, S.J.; Hwang, J.Y.; Lee, Y.K. Transplantation of human adipose-derived stem cells in a rabbit model of traumatic degeneration of lumbar discs. World Neurosurg. 2012, 78, 364–371. [Google Scholar] [CrossRef]
- Liang, C.Z.; Li, H.; Tao, Y.Q.; Peng, L.H.; Gao, J.Q.; Wu, J.J.; Li, F.C.; Hua, J.M.; Chen, Q.X. Dual release of dexamethasone and TGF-beta3 from polymeric microspheres for stem cell matrix accumulation in a rat disc degeneration model. Acta Biomater. 2013, 9, 9423–9433. [Google Scholar] [CrossRef]
- Kumar, H.; Ha, D.H.; Lee, E.J.; Park, J.H.; Shim, J.H.; Ahn, T.K.; Kim, K.T.; Ropper, A.E.; Sohn, S.; Kim, C.H.; et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res. Ther. 2017, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wang, J.; Fang, W.; Tao, Y.; Zhao, T.; Xia, K.; Liang, C.; Hua, J.; Li, F.; Chen, Q. Genipin cross-linked type II collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater. 2018, 71, 496–509. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, F.; Tian, H.; Guan, K.; Zhao, G.; Shan, J.; Ren, D. Differentiation of adipose-derived stem cells toward nucleus pulposus-like cells induced by hypoxia and a three-dimensional chitosan-alginate gel scaffold in vitro. Chin. Med. J. 2014, 127, 314–321. [Google Scholar] [PubMed]
- Miyamoto, T.; Muneta, T.; Tabuchi, T.; Matsumoto, K.; Saito, H.; Tsuji, K.; Sekiya, I. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res. Ther. 2010, 12, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Nan, L.P.; Zhou, S.F.; Liu, Y.; Wang, Z.Y.; Wang, J.C.; Feng, X.M.; Zhang, L. Injectable Hydrogel Combined with Nucleus Pulposus-Derived Mesenchymal Stem Cells for the Treatment of Degenerative Intervertebral Disc in Rats. Stem Cells Int. 2019, 2019, 8496025. [Google Scholar] [CrossRef]
- Chen, J.; Lee, E.J.; Jing, L.; Christoforou, N.; Leong, K.W.; Setton, L.A. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PLoS ONE 2013, 8, 75548. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Rahaman, M.N.; Bal, B.S. Modulating notochordal differentiation of human induced pluripotent stem cells using natural nucleus pulposus tissue matrix. PLoS ONE 2014, 9, 100885. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Fu, S.; Rahaman, M.N.; Mao, J.J.; Bal, B.S. Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration. J. Biomed. Mater. Res. A 2015, 103, 1053–1059. [Google Scholar] [CrossRef]
- Tang, R.; Jing, L.; Willard, V.P.; Wu, C.L.; Guilak, F.; Chen, J.; Setton, L.A. Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells. Stem Cell Res. Ther. 2018, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Z.; Chen, P.; Ma, C.Y.; Li, C.; Au, T.Y.K.; Tam, V.; Peng, Y.; Wu, R.; Cheung, K.M.C.; et al. Directed Differentiation of Notochord-like and Nucleus Pulposus-like Cells Using Human Pluripotent Stem Cells. Cell Rep. 2020, 30, 2791–2806 e2795. [Google Scholar] [CrossRef] [Green Version]
- Xia, K.; Zhu, J.; Hua, J.; Gong, Z.; Yu, C.; Zhou, X.; Wang, J.; Huang, X.; Yu, W.; Li, L.; et al. Intradiscal Injection of Induced Pluripotent Stem Cell-Derived Nucleus Pulposus-Like Cell-Seeded Polymeric Microspheres Promotes Rat Disc Regeneration. Stem Cells Int. 2019, 2019, 6806540. [Google Scholar] [CrossRef]
- Oldershaw, R.A.; Baxter, M.A.; Lowe, E.T.; Bates, N.; Grady, L.M.; Soncin, F.; Brison, D.R.; Hardingham, T.E.; Kimber, S.J. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat. Biotechnol. 2010, 28, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Hernandez, M.E.; Khan, N.M.; Trochez, C.M.; Yoon, T.; Maye, P.; Presciutti, S.M.; Gibson, G.; Drissi, H. Derivation of notochordal cells from human embryonic stem cells reveals unique regulatory networks by single cell-transcriptomics. J. Cell Physiol. 2020, 235, 5241–5255. [Google Scholar] [CrossRef] [PubMed]
- Winzi, M.K.; Hyttel, P.; Dale, J.K.; Serup, P. Isolation and characterization of node/notochord-like cells from mouse embryonic stem cells. Stem Cells Dev. 2011, 20, 1817–1827. [Google Scholar] [CrossRef] [Green Version]
- Ukeba, D.; Yamada, K.; Tsujimoto, T.; Ura, K.; Nonoyama, T.; Iwasaki, N.; Sudo, H. Bone Marrow Aspirate Concentrate Combined with in Situ Forming Bioresorbable Gel Enhances Intervertebral Disc Regeneration in Rabbits. J. Bone Jt. Surg. Am. 2021, 103, e31. [Google Scholar] [CrossRef]
- Pettine, K.A.; Murphy, M.B.; Suzuki, R.K.; Sand, T.T. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells 2015, 33, 146–156. [Google Scholar] [CrossRef]
- Pettine, K.A.; Suzuki, R.K.; Sand, T.T.; Murphy, M.B. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int. Orthop. 2017, 41, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Serhan, H. Advancements in the Treatment of Degenerative Disc Disease. Hamdan Med. J. 2018, 11, 175–183. [Google Scholar] [CrossRef]
- Otsuru, S.; Gordon, P.L.; Shimono, K.; Jethva, R.; Marino, R.; Phillips, C.L.; Hofmann, T.J.; Veronesi, E.; Dominici, M.; Iwamoto, M.; et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood 2012, 120, 1933–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwale, F.; Wang, H.T.; Roughley, P.; Antoniou, J.; Haglund, L. Link N and mesenchymal stem cells can induce regeneration of the early degenerate intervertebral disc. Tissue Eng. Part. A 2014, 20, 2942–2949. [Google Scholar] [CrossRef] [Green Version]
- Sakai, D.; Mochida, J.; Iwashina, T.; Watanabe, T.; Nakai, T.; Ando, K.; Hotta, T. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: Potential and limitations for stem cell therapy in disc regeneration. Spine 2005, 30, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Mochida, J.; Iwashina, T.; Omi, H.; Watanabe, T.; Serigano, K.; Tamura, F.; Sakai, D. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J. Orthop. Res. 2008, 26, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Yim, R.L.; Lee, J.T.; Bow, C.H.; Meij, B.; Leung, V.; Cheung, K.M.; Vavken, P.; Samartzis, D. A systematic review of the safety and efficacy of mesenchymal stem cells for disc degeneration: Insights and future directions for regenerative therapeutics. Stem Cells Dev. 2014, 23, 2553–2567. [Google Scholar] [CrossRef]
- Centeno, C.; Markle, J.; Dodson, E.; Stemper, I.; Williams, C.J.; Hyzy, M.; Ichim, T.; Freeman, M. Treatment of lumbar degenerative disc disease-associated radicular pain with culture-expanded autologous mesenchymal stem cells: A pilot study on safety and efficacy. J. Transl. Med. 2017, 15, 197. [Google Scholar] [CrossRef] [Green Version]
- Leung, V.Y.; Aladin, D.M.; Lv, F.; Tam, V.; Sun, Y.; Lau, R.Y.; Hung, S.C.; Ngan, A.H.; Tang, B.; Lim, C.T.; et al. Mesenchymal stem cells reduce intervertebral disc fibrosis and facilitate repair. Stem Cells 2014, 32, 2164–2177. [Google Scholar] [CrossRef]
- Oehme, D.; Ghosh, P.; Shimmon, S.; Wu, J.; McDonald, C.; Troupis, J.M.; Goldschlager, T.; Rosenfeld, J.V.; Jenkin, G. Mesenchymal progenitor cells combined with pentosan polysulfate mediating disc regeneration at the time of microdiscectomy: A preliminary study in an ovine model. J. Neurosurg. Spine 2014, 20, 657–669. [Google Scholar] [CrossRef] [Green Version]
- Hee, H.T.; Ismail, H.D.; Lim, C.T.; Goh, J.C.; Wong, H.K. Effects of implantation of bone marrow mesenchymal stem cells, disc distraction and combined therapy on reversing degeneration of the intervertebral disc. J. Bone Jt. Surg. Br. 2010, 92, 726–736. [Google Scholar] [CrossRef]
- Ukeba, D.; Sudo, H.; Tsujimoto, T.; Ura, K.; Yamada, K.; Iwasaki, N. Bone marrow mesenchymal stem cells combined with ultra-purified alginate gel as a regenerative therapeutic strategy after discectomy for degenerated intervertebral discs. eBioMedicine 2020, 53, 102698. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.R.; Rodeo, S.; Bhutani, N.; Goodrich, L.R.; Huard, J.; Irrgang, J.; LaPrade, R.F.; Lattermann, C.; Lu, Y.; Mandelbaum, B.; et al. Optimizing Clinical Use of Biologics in Orthopaedic Surgery: Consensus Recommendations From the 2018 AAOS/NIH U-13 Conference. J. Am. Acad. Orthop. Surg. 2019, 27, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Kregar Velikonja, N.; Urban, J.; Frohlich, M.; Neidlinger-Wilke, C.; Kletsas, D.; Potocar, U.; Turner, S.; Roberts, S. Cell sources for nucleus pulposus regeneration. Eur. Spine J. 2014, 23 (Suppl. 3), 364–374. [Google Scholar] [CrossRef]
- Huang, Y.C.; Leung, V.Y.; Lu, W.W.; Luk, K.D. The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc. Spine J. 2013, 13, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tao, Y.; Liang, C.; Han, B.; Li, F.; Chen, G.; Chen, Q. Influence of hypoxia in the intervertebral disc on the biological behaviors of rat adipose- and nucleus pulposus-derived mesenchymal stem cells. Cells Tissues Organs 2013, 198, 266–277. [Google Scholar] [CrossRef]
- Li, Y.Y.; Diao, H.J.; Chik, T.K.; Chow, C.T.; An, X.M.; Leung, V.; Cheung, K.M.; Chan, B.P. Delivering mesenchymal stem cells in collagen microsphere carriers to rabbit degenerative disc: Reduced risk of osteophyte formation. Tissue Eng. Part. A 2014, 20, 1379–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobajima, S.; Vadala, G.; Shimer, A.; Kim, J.S.; Gilbertson, L.G.; Kang, J.D. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008, 8, 888–896. [Google Scholar] [CrossRef]
- Bowles, R.D.; Setton, L.A. Biomaterials for intervertebral disc regeneration and repair. Biomaterials 2017, 129, 54–67. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Huang, B.; Liu, L.T.; Liu, M.H.; Wang, J.; Li, C.Q.; Zhang, Z.F.; Chu, T.W.; Xiong, C.J. Utilization of stem cells in alginate for nucleus pulposus tissue engineering. Tissue Eng. Part. A 2014, 20, 908–920. [Google Scholar] [CrossRef]
- Leckie, S.K.; Sowa, G.A.; Bechara, B.P.; Hartman, R.A.; Coelho, J.P.; Witt, W.T.; Dong, Q.D.; Bowman, B.W.; Bell, K.M.; Vo, N.V.; et al. Injection of human umbilical tissue-derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J. 2013, 13, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Bertram, H.; Kroeber, M.; Wang, H.; Unglaub, F.; Guehring, T.; Carstens, C.; Richter, W. Matrix-assisted cell transfer for intervertebral disc cell therapy. Biochem. Biophys. Res. Commun. 2005, 331, 1185–1192. [Google Scholar] [CrossRef]
- Embree, M.C.; Chen, M.; Pylawka, S.; Kong, D.; Iwaoka, G.M.; Kalajzic, I.; Yao, H.; Shi, C.; Sun, D.; Sheu, T.J.; et al. Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nat. Commun. 2016, 7, 13073. [Google Scholar] [CrossRef] [Green Version]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Waskow, C. Maintaining What Is Already There: Strategies to Rectify HSC Transplantation Dilemmas. Cell Stem Cell 2015, 17, 258–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicari, B.M.; Rubin, J.P.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; Wyse, A.; et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 2014, 6, 234ra258. [Google Scholar] [CrossRef] [Green Version]
- Abbushi, A.; Endres, M.; Cabraja, M.; Kroppenstedt, S.N.; Thomale, U.W.; Sittinger, M.; Hegewald, A.A.; Morawietz, L.; Lemke, A.J.; Bansemer, V.G.; et al. Regeneration of intervertebral disc tissue by resorbable cell-free polyglycolic acid-based implants in a rabbit model of disc degeneration. Spine 2008, 33, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Pattappa, G.; Li, Z.; Peroglio, M.; Wismer, N.; Alini, M.; Grad, S. Diversity of intervertebral disc cells: Phenotype and function. J. Anat. 2012, 221, 480–496. [Google Scholar] [CrossRef]
- Schmitz, T.C.; Salzer, E.; Crispim, J.F.; Fabra, G.T.; LeVisage, C.; Pandit, A.; Tryfonidou, M.; Maitre, C.L.; Ito, K. Characterization of biomaterials intended for use in the nucleus pulposus of degenerated intervertebral discs. Acta Biomater. 2020, 114, 1–15. [Google Scholar] [CrossRef]
- Maroudas, A.; Stockwell, R.A.; Nachemson, A.; Urban, J. Factors involved in the nutrition of the human lumbar intervertebral disc: Cellularity and diffusion of glucose in vitro. J. Anat. 1975, 120, 113–130. [Google Scholar] [PubMed]
- Iatridis, J.C.; Setton, L.A.; Weidenbaum, M.; Mow, V.C. Alterations in the mechanical behavior of the human lumbar nucleus pulposus with degeneration and aging. J. Orthop. Res. 1997, 15, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, W.; Elliott, D.M. Effects of degeneration on the biphasic material properties of human nucleus pulposus in confined compression. Spine 2005, 30, 724–729. [Google Scholar] [CrossRef]
- Huang, Y.C.; Hu, Y.; Li, Z.; Luk, K.D.K. Biomaterials for intervertebral disc regeneration: Current status and looming challenges. J. Tissue Eng. Regen. Med. 2018, 12, 2188–2202. [Google Scholar] [CrossRef]
- Nachemson, A.L.; Evans, J.H. Some mechanical properties of the third human lumbar interlaminar ligament (ligamentum flavum). J. Biomech 1968, 1, 211–220. [Google Scholar] [CrossRef]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Hydrogels in acellular and cellular strategies for intervertebral disc regeneration. J. Tissue Eng. Regen. Med. 2013, 7, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Iatridis, J.C.; Nicoll, S.B.; Michalek, A.J.; Walter, B.A.; Gupta, M.S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: What needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013, 13, 243–262. [Google Scholar] [CrossRef] [Green Version]
- Carl, A.; Ledet, E.; Yuan, H.; Sharan, A. New developments in nucleus pulposus replacement technology. Spine J. 2004, 4, 325S–329S. [Google Scholar] [CrossRef] [PubMed]
- Coric, D.; Mummaneni, P.V. Nucleus replacement technologies. J. Neurosurg. Spine 2008, 8, 115–120. [Google Scholar] [CrossRef]
- Goins, M.L.; Wimberley, D.W.; Yuan, P.S.; Fitzhenry, L.N.; Vaccaro, A.R. Nucleus pulposus replacement: An emerging technology. Spine J. 2005, 5, 317S–324S. [Google Scholar] [CrossRef]
- Pelletier, M.H.; Cohen, C.S.; Ducheyne, P.; Walsh, W.R. Restoring Segmental Biomechanics Through Nucleus Augmentation: An In Vitro Study. Clin. Spine Surg. 2016, 29, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Schutgens, E.M.; Tryfonidou, M.A.; Smit, T.H.; Oner, F.C.; Krouwels, A.; Ito, K.; Creemers, L.B. Biomaterials for intervertebral disc regeneration: Past performance and possible future strategies. Eur. Cell Mater. 2015, 30, 210–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, C.T.; Hoyland, J.A.; Fujii, K.; Pandit, A.; Iatridis, J.C.; Grad, S. Critical aspects and challenges for intervertebral disc repair and regeneration-Harnessing advances in tissue engineering. JOR Spine 2018, 1, e1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, S.M.; Curran, J.M.; Chen, R.; Vaughan-Thomas, A.; Hunt, J.A.; Freemont, A.J.; Hoyland, J.A. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Biomaterials 2006, 27, 4069–4078. [Google Scholar] [CrossRef]
- El-Amin, S.F.; Lu, H.H.; Khan, Y.; Burems, J.; Mitchell, J.; Tuan, R.S.; Laurencin, C.T. Extracellular matrix production by human osteoblasts cultured on biodegradable polymers applicable for tissue engineering. Biomaterials 2003, 24, 1213–1221. [Google Scholar] [CrossRef]
- Endres, M.; Abbushi, A.; Thomale, U.W.; Cabraja, M.; Kroppenstedt, S.N.; Morawietz, L.; Casalis, P.A.; Zenclussen, M.L.; Lemke, A.J.; Horn, P.; et al. Intervertebral disc regeneration after implantation of a cell-free bioresorbable implant in a rabbit disc degeneration model. Biomaterials 2010, 31, 5836–5841. [Google Scholar] [CrossRef]
- Feng, G.; Jin, X.; Hu, J.; Ma, H.; Gupte, M.J.; Liu, H.; Ma, P.X. Effects of hypoxias and scaffold architecture on rabbit mesenchymal stem cell differentiation towards a nucleus pulposus-like phenotype. Biomaterials 2011, 32, 8182–8189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.Y.; Kim, H.N.; Lee, S.J.; Song, J.E.; Kwon, S.Y.; Chung, J.W.; Lee, D.; Khang, G. Effect of pore sizes of PLGA scaffolds on mechanical properties and cell behaviour for nucleus pulposus regeneration in vivo. J. Tissue Eng. Regen. Med. 2017, 11, 44–57. [Google Scholar] [CrossRef]
- Mizuno, H.; Roy, A.K.; Vacanti, C.A.; Kojima, K.; Ueda, M.; Bonassar, L.J. Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine 2004, 29, 1290–1297; discussion 1297–1298. [Google Scholar] [CrossRef]
- Xin, L.; Xu, W.; Yu, L.; Fan, S.; Wang, W.; Yu, F.; Wang, Z. Effects of annulus defects and implantation of poly(lactic-co-glycolic acid) (PLGA)/fibrin gel scaffolds on nerves ingrowth in a rabbit model of annular injury disc degeneration. J. Orthop. Surg. Res. 2017, 12, 73. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, C.; Zhong, F.; Fan, S.; Wang, W.; Wang, Z. Minimal invasive annulotomy for induction of disc degeneration and implantation of poly (lactic-co-glycolic acid) (PLGA) plugs for annular repair in a rabbit model. Eur. J. Med. Res. 2016, 21, 7. [Google Scholar] [CrossRef] [Green Version]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Collin, E.C.; Grad, S.; Zeugolis, D.I.; Vinatier, C.S.; Clouet, J.R.; Guicheux, J.J.; Weiss, P.; Alini, M.; Pandit, A.S. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials 2011, 32, 2862–2870. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.T.; Hwang, P.Y.; Jeong, C.G.; Jing, L.; Chen, J.; Setton, L.A. Photocrosslinkable laminin-functionalized polyethylene glycol hydrogel for intervertebral disc regeneration. Acta Biomater. 2014, 10, 1102–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Anseth, K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.T.; Hwang, Y.; Chen, A.C.; Varghese, S.; Sah, R.L. Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels. Biomaterials 2012, 33, 6682–6690. [Google Scholar] [CrossRef] [Green Version]
- Raeber, G.P.; Lutolf, M.P.; Hubbell, J.A. Molecularly engineered PEG hydrogels: A novel model system for proteolytically mediated cell migration. Biophys. J. 2005, 89, 1374–1388. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [Green Version]
- Scholz, B.; Kinzelmann, C.; Benz, K.; Mollenhauer, J.; Wurst, H.; Schlosshauer, B. Suppression of adverse angiogenesis in an albumin-based hydrogel for articular cartilage and intervertebral disc regeneration. Eur. Cell Mater. 2010, 20, 24–36; discussion 27–36. [Google Scholar] [CrossRef]
- Benz, K.; Stippich, C.; Osswald, C.; Gaissmaier, C.; Lembert, N.; Badke, A.; Steck, E.; Aicher, W.K.; Mollenhauer, J.A. Rheological and biological properties of a hydrogel support for cells intended for intervertebral disc repair. BMC Musculoskelet. Disord. 2012, 13, 54. [Google Scholar] [CrossRef] [Green Version]
- Benz, K.; Stippich, C.; Fischer, L.; Mohl, K.; Weber, K.; Lang, J.; Steffen, F.; Beintner, B.; Gaissmaier, C.; Mollenhauer, J.A. Intervertebral disc cell- and hydrogel-supported and spontaneous intervertebral disc repair in nucleotomized sheep. Eur. Spine J. 2012, 21, 1758–1768. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.D.; Fussell, G.; Sarkar, S.; Lowman, A.M.; Marcolongo, M. Synthesis and recovery characteristics of branched and grafted PNIPAAm-PEG hydrogels for the development of an injectable load-bearing nucleus pulposus replacement. Acta Biomater. 2010, 6, 1319–1328. [Google Scholar] [CrossRef]
- Halloran, D.O.; Grad, S.; Stoddart, M.; Dockery, P.; Alini, M.; Pandit, A.S. An injectable cross-linked scaffold for nucleus pulposus regeneration. Biomaterials 2008, 29, 438–447. [Google Scholar] [CrossRef]
- Attia, M.; Santerre, J.P.; Kandel, R.A. The response of annulus fibrosus cell to fibronectin-coated nanofibrous polyurethane-anionic dihydroxyoligomer scaffolds. Biomaterials 2011, 32, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.C.; Ahrens, M.; Sherman, J.E.; Martz, E.O. The restoration of lumbar intervertebral disc load distribution: A comparison of three nucleus replacement technologies. Spine 2010, 35, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, B.; Guo, F.; Du, J.; Gu, P.; Lin, X.; Yang, W.; Zhang, H.; Lu, M.; Huang, Y.; et al. Injectable silk fibroin/polyurethane composite hydrogel for nucleus pulposus replacement. J. Mater. Sci Mater. Med. 2012, 23, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Mauth, C.; Bono, E.; Haas, S.; Paesold, G.; Wiese, H.; Maier, G.; Boos, N.; Graf-Hausner, U. Cell-seeded polyurethane-fibrin structures—a possible system for intervertebral disc regeneration. Eur. Cell Mater. 2009, 18, 27–38; discussion 29–38. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Gong, M.S.; Park, J.H.; Moon, S.I.; Wall, I.B.; Kim, H.W.; Lee, J.H.; Knowles, J.C. Silk fibroin-polyurethane blends: Physical properties and effect of silk fibroin content on viscoelasticity, biocompatibility and myoblast differentiation. Acta Biomater. 2013, 9, 8962–8971. [Google Scholar] [CrossRef]
- Yang, X.; Li, X. Nucleus pulposus tissue engineering: A brief review. Eur. Spine J. 2009, 18, 1564–1572. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, C.M.; Ray, R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 2001, 55, 141–150. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-small je, Ukrainian-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Li, W.J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Persson, C.; Hilborn, J.; Engqvist, H. Synthesis and characterization of injectable composites of poly[D,L-lactide-co-(epsilon-caprolactone)] reinforced with beta-TCP and CaCO3 for intervertebral disk augmentation. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, L.; Remund, T.; Bao, J.; Neufeld, D.; Fong, H.; Deng, Y. Tissue engineering of annulus fibrosus using electrospun fibrous scaffolds with aligned polycaprolactone fibers. J. Biomed. Mater. Res. A 2011, 99, 564–575. [Google Scholar] [CrossRef]
- Koepsell, L.; Zhang, L.; Neufeld, D.; Fong, H.; Deng, Y. Electrospun nanofibrous polycaprolactone scaffolds for tissue engineering of annulus fibrosus. Macromol. Biosci. 2011, 11, 391–399. [Google Scholar] [CrossRef]
- Martin, J.T.; Milby, A.H.; Chiaro, J.A.; Kim, D.H.; Hebela, N.M.; Smith, L.J.; Elliott, D.M.; Mauck, R.L. Translation of an engineered nanofibrous disc-like angle-ply structure for intervertebral disc replacement in a small animal model. Acta Biomater. 2014, 10, 2473–2481. [Google Scholar] [CrossRef] [Green Version]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, A.; Shen, B.; Williams, L.; Diwan, A. Mesenchymal stem cells: Potential application in intervertebral disc regeneration. Transl. Pediatr. 2014, 3, 71–90. [Google Scholar] [CrossRef]
- Bron, J.L.; Vonk, L.A.; Smit, T.H.; Koenderink, G.H. Engineering alginate for intervertebral disc repair. J. Mech. Behav. Biomed. Mater. 2011, 4, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.I.; Akintoye, S.O.; Nicoll, S.B. Photo-crosslinked alginate hydrogels support enhanced matrix accumulation by nucleus pulposus cells in vivo. Osteoarthr. Cartil. 2009, 17, 1377–1384. [Google Scholar] [CrossRef] [Green Version]
- Chou, A.I.; Nicoll, S.B. Characterization of photocrosslinked alginate hydrogels for nucleus pulposus cell encapsulation. J. Biomed. Mater. Res. A 2009, 91, 187–194. [Google Scholar] [CrossRef]
- Guo, J.F.; Jourdian, G.W.; MacCallum, D.K. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect. Tissue Res. 1989, 19, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.; Haug, A. Biosynthesis of alginate. 1. Composition and structure of alginate produced by Azotobacter vinelandii (Lipman). Carbohydr. Res. 1971, 17, 287–296. [Google Scholar] [CrossRef]
- Leone, G.; Torricelli, P.; Chiumiento, A.; Facchini, A.; Barbucci, R. Amidic alginate hydrogel for nucleus pulposus replacement. J. Biomed. Mater. Res. A 2008, 84, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gunn, J.; Chen, M.H.; Cooper, A.; Zhang, M. On-site alginate gelation for enhanced cell proliferation and uniform distribution in porous scaffolds. J. Biomed. Mater. Res. A 2008, 86, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Melrose, J.; Smith, S.; Ghosh, P.; Taylor, T.K. Differential expression of proteoglycan epitopes and growth characteristics of intervertebral disc cells grown in alginate bead culture. Cells Tissues Organs 2001, 168, 137–146. [Google Scholar] [CrossRef]
- Nunamaker, E.A.; Purcell, E.K.; Kipke, D.R. In vivo stability and biocompatibility of implanted calcium alginate disks. J. Biomed. Mater. Res. A 2007, 83, 1128–1137. [Google Scholar] [CrossRef] [Green Version]
- Ura, K.; Yamada, K.; Tsujimoto, T.; Ukeba, D.; Iwasaki, N.; Sudo, H. Ultra-purified alginate gel implantation decreases inflammatory cytokine levels, prevents intervertebral disc degeneration, and reduces acute pain after discectomy. Sci. Rep. 2021, 11, 638. [Google Scholar] [CrossRef]
- Naqvi, S.M.; Buckley, C.T. Differential response of encapsulated nucleus pulposus and bone marrow stem cells in isolation and coculture in alginate and chitosan hydrogels. Tissue Eng Part. A 2015, 21, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Chen, C.; Liu, W.; Fu, Q.; Han, Z.; Li, Y.; Feng, S.; Li, X.; Qi, C.; Wu, J.; et al. Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration. Biomaterials 2015, 59, 53–65. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Hunter, C.J. Developing an alginate/chitosan hybrid fiber scaffold for annulus fibrosus cells. J. Biomed. Mater. Res. A 2007, 82, 701–710. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Malhotra, N.R.; Weng, L.; Chen, W.; Mauck, R.L.; Elliott, D.M. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds. Eur. Spine J. 2007, 16, 1892–1898. [Google Scholar] [CrossRef] [Green Version]
- Gruber, H.E.; Hoelscher, G.L.; Leslie, K.; Ingram, J.A.; Hanley, E.N., Jr. Three-dimensional culture of human disc cells within agarose or a collagen sponge: Assessment of proteoglycan production. Biomaterials 2006, 27, 371–376. [Google Scholar] [CrossRef]
- Gupta, A.; Bhat, S.; Jagdale, P.R.; Chaudhari, B.P.; Lidgren, L.; Gupta, K.C.; Kumar, A. Evaluation of three-dimensional chitosan-agarose-gelatin cryogel scaffold for the repair of subchondral cartilage defects: An in vivo study in a rabbit model. Tissue Eng. Part. A 2014, 20, 3101–3111. [Google Scholar] [CrossRef] [Green Version]
- Hunt, N.C.; Grover, L.M. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol. Lett. 2010, 32, 733–742. [Google Scholar] [CrossRef]
- Lazebnik, M.; Singh, M.; Glatt, P.; Friis, L.A.; Berkland, C.J.; Detamore, M.S. Biomimetic method for combining the nucleus pulposus and annulus fibrosus for intervertebral disc tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, N.L.; Sen, S.; Huang, A.H.; Elliott, D.M.; Mauck, R.L. Engineered disc-like angle-ply structures for intervertebral disc replacement. Spine 2010, 35, 867–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilwani, R.K.; Bader, D.L.; Chowdhury, T.T. Biomechanical Conditioning Enhanced Matrix Synthesis in Nucleus Pulposus Cells Cultured in Agarose Constructs with TGFbeta. J. Funct. Biomater. 2012, 3, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part. B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- Colombini, A.; Ceriani, C.; Banfi, G.; Brayda-Bruno, M.; Moretti, M. Fibrin in intervertebral disc tissue engineering. Tissue Eng. Part. B Rev. 2014, 20, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Diederichs, S.; Baral, K.; Tanner, M.; Richter, W. Interplay between local versus soluble transforming growth factor-beta and fibrin scaffolds: Role of cells and impact on human mesenchymal stem cell chondrogenesis. Tissue Eng. Part. A 2012, 18, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Eyrich, D.; Brandl, F.; Appel, B.; Wiese, H.; Maier, G.; Wenzel, M.; Staudenmaier, R.; Goepferich, A.; Blunk, T. Long-term stable fibrin gels for cartilage engineering. Biomaterials 2007, 28, 55–65. [Google Scholar] [CrossRef]
- Ho, S.T.; Cool, S.M.; Hui, J.H.; Hutmacher, D.W. The influence of fibrin based hydrogels on the chondrogenic differentiation of human bone marrow stromal cells. Biomaterials 2010, 31, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kaplan, K.M.; Wertzel, A.; Peroglio, M.; Amit, B.; Alini, M.; Grad, S.; Yayon, A. Biomimetic fibrin-hyaluronan hydrogels for nucleus pulposus regeneration. Regen. Med. 2014, 9, 309–326. [Google Scholar] [CrossRef]
- Likhitpanichkul, M.; Dreischarf, M.; Illien-Junger, S.; Walter, B.A.; Nukaga, T.; Long, R.G.; Sakai, D.; Hecht, A.C.; Iatridis, J.C. Fibrin-genipin adhesive hydrogel for annulus fibrosus repair: Performance evaluation with large animal organ culture, in situ biomechanics, and in vivo degradation tests. Eur. Cell Mater. 2014, 28, 25–37; discussion 28–37. [Google Scholar] [CrossRef]
- Ma, K.; Titan, A.L.; Stafford, M.; Zheng, C.; Levenston, M.E. Variations in chondrogenesis of human bone marrow-derived mesenchymal stem cells in fibrin/alginate blended hydrogels. Acta Biomater. 2012, 8, 3754–3764. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Cho, H.; Gil, E.S.; Mandal, B.B.; Min, B.H.; Kaplan, D.L. Silk-fibrin/hyaluronic acid composite gels for nucleus pulposus tissue regeneration. Tissue Eng Part. A 2011, 17, 2999–3009. [Google Scholar] [CrossRef] [Green Version]
- Pirvu, T.; Blanquer, S.B.; Benneker, L.M.; Grijpma, D.W.; Richards, R.G.; Alini, M.; Eglin, D.; Grad, S.; Li, Z. A combined biomaterial and cellular approach for annulus fibrosus rupture repair. Biomaterials 2015, 42, 11–19. [Google Scholar] [CrossRef]
- Schek, R.M.; Michalek, A.J.; Iatridis, J.C. Genipin-crosslinked fibrin hydrogels as a potential adhesive to augment intervertebral disc annulus repair. Eur. Cell Mater. 2011, 21, 373–383. [Google Scholar] [CrossRef]
- Stern, S.; Lindenhayn, K.; Schultz, O.; Perka, C. Cultivation of porcine cells from the nucleus pulposus in a fibrin/hyaluronic acid matrix. Acta Orthop. Scand. 2000, 71, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Buser, Z.; Kuelling, F.; Liu, J.; Liebenberg, E.; Thorne, K.J.; Coughlin, D.; Lotz, J.C. Biological and biomechanical effects of fibrin injection into porcine intervertebral discs. Spine 2011, 36, E1201–E1209. [Google Scholar] [CrossRef] [Green Version]
- Long, R.G.; Ferguson, S.J.; Benneker, L.M.; Sakai, D.; Li, Z.; Pandit, A.; Grijpma, D.W.; Eglin, D.; Zeiter, S.; Schmid, T.; et al. Morphological and biomechanical effects of annulus fibrosus injury and repair in an ovine cervical model. JOR Spine 2020, 3, e1074. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zeiter, S.; Schmid, T.; Sakai, D.; Iatridis, J.C.; Zhou, G.; Richards, R.G.; Alini, M.; Grad, S.; Li, Z. Effect of the CCL5-Releasing Fibrin Gel for Intervertebral Disc Regeneration. Cartilage 2020, 11, 169–180. [Google Scholar] [CrossRef]
- Yin, W.; Pauza, K.; Olan, W.J.; Doerzbacher, J.F.; Thorne, K.J. Intradiscal injection of fibrin sealant for the treatment of symptomatic lumbar internal disc disruption: Results of a prospective multicenter pilot study with 24-month follow-up. Pain Med. 2014, 15, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Omlor, G.W.; Lorenz, S.; Nerlich, A.G.; Guehring, T.; Richter, W. Disc cell therapy with bone-marrow-derived autologous mesenchymal stromal cells in a large porcine disc degeneration model. Eur. Spine J. 2018, 27, 2639–2649. [Google Scholar] [CrossRef]
- Chen, Y.C.; Su, W.Y.; Yang, S.H.; Gefen, A.; Lin, F.H. In situ forming hydrogels composed of oxidized high molecular weight hyaluronic acid and gelatin for nucleus pulposus regeneration. Acta Biomater. 2013, 9, 5181–5193. [Google Scholar] [CrossRef]
- Chung, C.; Beecham, M.; Mauck, R.L.; Burdick, J.A. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials 2009, 30, 4287–4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, I.E.; Huang, A.H.; Sengupta, S.; Kestle, S.; Burdick, J.A.; Mauck, R.L. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthr. Cartil. 2009, 17, 1639–1648. [Google Scholar] [CrossRef] [Green Version]
- Kenne, L.; Gohil, S.; Nilsson, E.M.; Karlsson, A.; Ericsson, D.; Helander Kenne, A.; Nord, L.I. Modification and cross-linking parameters in hyaluronic acid hydrogels--definitions and analytical methods. Carbohydr. Polym. 2013, 91, 410–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogan, G.; Soltes, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Su, W.Y.; Chen, Y.C.; Lin, F.H. Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta Biomater. 2010, 6, 3044–3055. [Google Scholar] [CrossRef]
- Peroglio, M.; Grad, S.; Mortisen, D.; Sprecher, C.M.; Illien-Junger, S.; Alini, M.; Eglin, D. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur. Spine J. 2012, 21 (Suppl. 6), 839–849. [Google Scholar] [CrossRef]
- Woiciechowsky, C.; Abbushi, A.; Zenclussen, M.L.; Casalis, P.; Kruger, J.P.; Freymann, U.; Endres, M.; Kaps, C. Regeneration of nucleus pulposus tissue in an ovine intervertebral disc degeneration model by cell-free resorbable polymer scaffolds. J. Tissue Eng. Regen. Med. 2014, 8, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Kazezian, Z.; Sakai, D.; Pandit, A. Hyaluronic Acid Microgels Modulate Inflammation and Key Matrix Molecules toward a Regenerative Signature in the Injured Annulus Fibrosus. Adv. Biosyst. 2017, 1, e1700077. [Google Scholar] [CrossRef] [PubMed]
- Reitmaier, S.; Kreja, L.; Gruchenberg, K.; Kanter, B.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Perugini, V.; Santin, M.; Ignatius, A.; et al. In vivo biofunctional evaluation of hydrogels for disc regeneration. Eur. Spine J. 2014, 23, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Martin, J.T.; Elliott, D.M.; Smith, L.J.; Mauck, R.L. Phenotypic stability, matrix elaboration and functional maturation of nucleus pulposus cells encapsulated in photocrosslinkable hyaluronic acid hydrogels. Acta Biomater. 2015, 12, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Tsaryk, R.; Gloria, A.; Russo, T.; Anspach, L.; De Santis, R.; Ghanaati, S.; Unger, R.E.; Ambrosio, L.; Kirkpatrick, C.J. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater. 2015, 20, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Mohd Isa, I.L.; Abbah, S.A.; Kilcoyne, M.; Sakai, D.; Dockery, P.; Finn, D.P.; Pandit, A. Implantation of hyaluronic acid hydrogel prevents the pain phenotype in a rat model of intervertebral disc injury. Sci. Adv. 2018, 4, eaaq0597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omlor, G.W.; Nerlich, A.G.; Lorenz, H.; Bruckner, T.; Richter, W.; Pfeiffer, M.; Guhring, T. Injection of a polymerized hyaluronic acid/collagen hydrogel matrix in an in vivo porcine disc degeneration model. Eur. Spine J. 2012, 21, 1700–1708. [Google Scholar] [CrossRef] [Green Version]
- Bron, J.L.; Koenderink, G.H.; Everts, V.; Smit, T.H. Rheological characterization of the nucleus pulposus and dense collagen scaffolds intended for functional replacement. J. Orthop. Res. 2009, 27, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Bron, J.L.; Mulder, H.W.; Vonk, L.A.; Doulabi, B.Z.; Oudhoff, M.J.; Smit, T.H. Migration of intervertebral disc cells into dense collagen scaffolds intended for functional replacement. J. Mater. Sci. Mater. Med. 2012, 23, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Yang, S.H.; Su, W.Y.; Chen, Y.C.; Yang, K.C.; Cheng, W.T.; Wu, S.C.; Lin, F.H. Thermosensitive chitosan-gelatin-glycerol phosphate hydrogels as a cell carrier for nucleus pulposus regeneration: An in vitro study. Tissue Eng. Part. A 2010, 16, 695–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, N.R.; Han, W.M.; Beckstein, J.; Cloyd, J.; Chen, W.; Elliott, D.M. An injectable nucleus pulposus implant restores compressive range of motion in the ovine disc. Spine 2012, 37, E1099–E1105. [Google Scholar] [CrossRef] [Green Version]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part. B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Strange, D.G.; Oyen, M.L. Composite hydrogels for nucleus pulposus tissue engineering. J. Mech Behav. Biomed. Mater. 2012, 11, 16–26. [Google Scholar] [CrossRef]
- Wang, L.; Stegemann, J.P. Thermogelling chitosan and collagen composite hydrogels initiated with beta-glycerophosphate for bone tissue engineering. Biomaterials 2010, 31, 3976–3985. [Google Scholar] [CrossRef] [Green Version]
- Wilke, H.J.; Heuer, F.; Neidlinger-Wilke, C.; Claes, L. Is a collagen scaffold for a tissue engineered nucleus replacement capable of restoring disc height and stability in an animal model? Eur. Spine J. 2006, 15 (Suppl. 3), 433–438. [Google Scholar] [CrossRef] [Green Version]
- Takeoka, Y.; Yurube, T.; Morimoto, K.; Kunii, S.; Kanda, Y.; Tsujimoto, R.; Kawakami, Y.; Fukase, N.; Takemori, T.; Omae, K.; et al. Reduced nucleotomy-induced intervertebral disc disruption through spontaneous spheroid formation by the Low Adhesive Scaffold Collagen (LASCol). Biomaterials 2020, 235, 119781. [Google Scholar] [CrossRef]
- Friedmann, A.; Baertel, A.; Schmitt, C.; Ludtka, C.; Milosevic, J.; Meisel, H.J.; Goehre, F.; Schwan, S. Intervertebral Disc Regeneration Injection of a Cell-Loaded Collagen Hydrogel in a Sheep Model. Int. J. Mol. Sci. 2021, 22, 4248. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Shang, J.; Liu, H.; Yuan, Y.; Guo, Y.; Huang, B.; Zhou, Y. Repairing the ruptured annular fibrosus by using type I collagen combined with citric acid, EDC and NHS: An in vivo study. Eur. Spine J. 2017, 26, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Sloan, S.R., Jr.; Wipplinger, C.; Kirnaz, S.; Navarro-Ramirez, R.; Schmidt, F.; McCloskey, D.; Pannellini, T.; Schiavinato, A.; Hartl, R.; Bonassar, L.J. Combined nucleus pulposus augmentation and annulus fibrosus repair prevents acute intervertebral disc degeneration after discectomy. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, A.; Mehr, M.; Aebli, N.; Baur, M.; Ferguson, S.J.; Stoyanov, J.V. Influence of different commercial scaffolds on the in vitro differentiation of human mesenchymal stem cells to nucleus pulposus-like cells. Eur. Spine J. 2012, 21 (Suppl. 6), 826–838. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.H.; Yang, S.H.; Liu, C.C.; Gefen, A.; Lin, F.H. Thermosensitive hydrogel made of ferulic acid-gelatin and chitosan glycerophosphate. Carbohydr. Polym. 2013, 92, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, N.; Yamane, S.T.; Majima, T.; Kasahara, Y.; Minami, A.; Harada, K.; Nonaka, S.; Maekawa, N.; Tamura, H.; Tokura, S.; et al. Feasibility of polysaccharide hybrid materials for scaffolds in cartilage tissue engineering: Evaluation of chondrocyte adhesion to polyion complex fibers prepared from alginate and chitosan. Biomacromolecules 2004, 5, 828–833. [Google Scholar] [CrossRef]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Hughes, N.; Hunt, J.A.; Freemont, A.J.; Hoyland, J.A. Human mesenchymal stem cell differentiation to NP-like cells in chitosan-glycerophosphate hydrogels. Biomaterials 2008, 29, 85–93. [Google Scholar] [CrossRef]
- Roughley, P.; Hoemann, C.; DesRosiers, E.; Mwale, F.; Antoniou, J.; Alini, M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials 2006, 27, 388–396. [Google Scholar] [CrossRef]
- Smith, L.J.; Gorth, D.J.; Showalter, B.L.; Chiaro, J.A.; Beattie, E.E.; Elliott, D.M.; Mauck, R.L.; Chen, W.; Malhotra, N.R. In vitro characterization of a stem-cell-seeded triple-interpenetrating-network hydrogel for functional regeneration of the nucleus pulposus. Tissue Eng. Part A 2014, 20, 1841–1849. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Zhang, K.; Liu, G.; Ding, W.; Zhao, C.; Xie, Y.; Yuan, J.; Sun, X.; Li, H.; Liu, C.; et al. Sox9 gene transfer enhanced regenerative effect of bone marrow mesenchymal stem cells on the degenerated intervertebral disc in a rabbit model. PLoS ONE 2014, 9, e93570. [Google Scholar] [CrossRef] [Green Version]
- Reza, A.T.; Nicoll, S.B. Characterization of novel photocrosslinked carboxymethylcellulose hydrogels for encapsulation of nucleus pulposus cells. Acta Biomater. 2010, 6, 179–186. [Google Scholar] [CrossRef]
- Lin, H.A.; Varma, D.M.; Hom, W.W.; Cruz, M.A.; Nasser, P.R.; Phelps, R.G.; Iatridis, J.C.; Nicoll, S.B. Injectable cellulose-based hydrogels as nucleus pulposus replacements: Assessment of in vitro structural stability, ex vivo herniation risk, and in vivo biocompatibility. J. Mech. Behav. Biomed. Mater. 2019, 96, 204–213. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, A.A.; Boyes, V.L.; Sammon, C.; Le Maitre, C.L. Thermally triggered injectable hydrogel, which induces mesenchymal stem cell differentiation to nucleus pulposus cells: Potential for regeneration of the intervertebral disc. Acta Biomater. 2016, 36, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Vadala, G.; Russo, F.; Musumeci, M.; D’Este, M.; Cattani, C.; Catanzaro, G.; Tirindelli, M.C.; Lazzari, L.; Alini, M.; Giordano, R.; et al. Clinically relevant hydrogel-based on hyaluronic acid and platelet rich plasma as a carrier for mesenchymal stem cells: Rheological and biological characterization. J. Orthop. Res. 2017, 35, 2109–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.A.; Gupta, M.S.; Varma, D.M.; Gilchrist, M.L.; Nicoll, S.B. Lower crosslinking density enhances functional nucleus pulposus-like matrix elaboration by human mesenchymal stem cells in carboxymethylcellulose hydrogels. J. Biomed. Mater. Res. A 2016, 104, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Wachs, R.A.; Hoogenboezem, E.N.; Huda, H.I.; Xin, S.; Porvasnik, S.L.; Schmidt, C.E. Creation of an injectable in situ gelling native extracellular matrix for nucleus pulposus tissue engineering. Spine J. 2017, 17, 435–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Drapeau, S.; Howard, S.A.; Thonar, E.J.; Anderson, D.G. Transplantation of goat bone marrow stromal cells to the degenerating intervertebral disc in a goat disc injury model. Spine 2011, 36, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, H.T.J.; Hodson, N.; Baird, P.; Richardson, S.M.; Hoyland, J.A. Acidic pH promotes intervertebral disc degeneration: Acid-sensing ion channel -3 as a potential therapeutic target. Sci. Rep. 2016, 6, 37360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.C.; Urban, J.P.; Luk, K.D. Intervertebral disc regeneration: Do nutrients lead the way? Nat. Rev. Rheumatol. 2014, 10, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Newell, N.; Little, J.P.; Christou, A.; Adams, M.A.; Adam, C.J.; Masouros, S.D. Biomechanics of the human intervertebral disc: A review of testing techniques and results. J. Mech. Behav. Biomed. Mater. 2017, 69, 420–434. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Schmidt, H.; Reitmaier, S.; Graichen, F.; Shirazi-Adl, A. Review of the fluid flow within intervertebral discs - How could in vitro measurements replicate in vivo? J. Biomech. 2016, 49, 3133–3146. [Google Scholar] [CrossRef]
- Sakai, D.; Nakamura, Y.; Nakai, T.; Mishima, T.; Kato, S.; Grad, S.; Alini, M.; Risbud, M.V.; Chan, D.; Cheah, K.S.; et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat. Commun. 2012, 3, 1264. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Kenichiro, M.; Ito, Y.M.; Inage, F.; Isoe, T.; Yokota, N.; Sugita, O.; Sato, N.; Tha, K.K.; Iwasaki, N.; et al. Exploratory clinical trial on the safety and capability of dMD-001. in lumbar disc herniation: Study protocol for a first-in-human pilot study. Contemp. Clin. Trials Commun. 2021, 23, 100805. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, M.; Ishikawa, T.; Kamoda, H.; Suzuki, M.; Murakami, K.; Shibayama, M.; Orita, S.; Eguchi, Y.; Arai, G.; Sakuma, Y.; et al. ISSLS prize winner: Disc dynamic compression in rats produces long-lasting increases in inflammatory mediators in discs and induces long-lasting nerve injury and regeneration of the afferent fibers innervating discs: A pathomechanism for chronic discogenic low back pain. Spine 2012, 37, 1810–1818. [Google Scholar] [CrossRef]
- Burke, J.G.; Watson, R.W.; McCormack, D.; Dowling, F.E.; Walsh, M.G.; Fitzpatrick, J.M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J. Bone Joint. Surg. Br. 2002, 84, 196–201. [Google Scholar] [CrossRef]
- Freemont, A.J.; Watkins, A.; Le Maitre, C.; Baird, P.; Jeziorska, M.; Knight, M.T.; Ross, E.R.; O’Brien, J.P.; Hoyland, J.A. Nerve growth factor expression and innervation of the painful intervertebral disc. J. Pathol. 2002, 197, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Lotz, J.C.; Ulrich, J.A. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: Review of animal model data. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. 2), 76–82. [Google Scholar] [CrossRef]
- Aoki, Y.; Takahashi, Y.; Ohtori, S.; Moriya, H.; Takahashi, K. Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: A review. Life Sci. 2004, 74, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Allchorne, A.; Safieh-Garabedian, B.; Poole, S. Cytokines, nerve growth factor and inflammatory hyperalgesia: The contribution of tumour necrosis factor alpha. Br. J. Pharmacol. 1997, 121, 417–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobajima, S.; Kim, J.S.; Gilbertson, L.G.; Kang, J.D. Gene therapy for degenerative disc disease. Gene Ther. 2004, 11, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Hallen, A. Hexosamine and ester sulfate content of human nucleus pulposus at different ages. Acta Chem. Scand. 1958, 12, 1869–1872. [Google Scholar] [CrossRef] [Green Version]
- Hallen, A. The collagen and ground substance of the human nucleus pulposus at different ages. Acta Chem. Scand. 1962, 16, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Vernengo, J.; Fussell, G.W.; Smith, N.G.; Lowman, A.M. Evaluation of novel injectable hydrogels for nucleus pulposus replacement. J. Biomed. Mater. Res. B. Appl. Biomater. 2008, 84, 64–69. [Google Scholar] [CrossRef]
- DiStefano, T.J.; Shmukler, J.O.; Danias, G.; Iatridis, J.C. The Functional Role of Interface Tissue Engineering in Annulus Fibrosus Repair: Bridging Mechanisms of Hydrogel Integration with Regenerative Outcomes. ACS Biomater. Sci. Eng. 2020, 6, 6556–6586. [Google Scholar] [CrossRef]
- Gullbrand, S.E.; Schaer, T.P.; Agarwal, P.; Bendigo, J.R.; Dodge, G.R.; Chen, W.; Elliott, D.M.; Mauck, R.L.; Malhotra, N.R.; Smith, L.J. Translation of an injectable triple-interpenetrating-network hydrogel for intervertebral disc regeneration in a goat model. Acta Biomater. 2017, 60, 201–209. [Google Scholar] [CrossRef]
- Vergroesen, P.P.; Bochyn Ska, A.I.; Emanuel, K.S.; Sharifi, S.; Kingma, I.; Grijpma, D.W.; Smit, T.H. A biodegradable glue for annulus closure: Evaluation of strength and endurance. Spine 2015, 40, 622–628. [Google Scholar] [CrossRef]
- Cruz, M.A.; Hom, W.W.; DiStefano, T.J.; Merrill, R.; Torre, O.M.; Lin, H.A.; Hecht, A.C.; Illien-Junger, S.; Iatridis, J.C. Cell-Seeded Adhesive Biomaterial for Repair of Annulus Fibrosus Defects in Intervertebral Discs. Tissue Eng. Part. A. 2018, 24, 187–198. [Google Scholar] [CrossRef]
- Long, R.G.; Rotman, S.G.; Hom, W.W.; Assael, D.J.; Illien-Junger, S.; Grijpma, D.W.; Iatridis, J.C. In vitro and biomechanical screening of polyethylene glycol and poly(trimethylene carbonate) block copolymers for annulus fibrosus repair. J. Tissue Eng. Regen. Med. 2018, 12, 727–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chik, T.K.; Ma, X.Y.; Choy, T.H.; Li, Y.Y.; Diao, H.J.; Teng, W.K.; Han, S.J.; Cheung, K.M.; Chan, B.P. Photochemically crosslinked collagen annulus plug: A potential solution solving the leakage problem of cell-based therapies for disc degeneration. Acta Biomater. 2013, 9, 8128–8139. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, T.J.; Shmukler, J.O.; Danias, G.; Di Pauli von Treuheim, T.; Hom, W.W.; Goldberg, D.A.; Laudier, D.M.; Nasser, P.R.; Hecht, A.C.; Nicoll, S.B.; et al. Development of a two-part biomaterial adhesive strategy for annulus fibrosus repair and ex vivo evaluation of implant herniation risk. Biomaterials 2020, 258, 120309. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Ressel, L.; Heuer, F.; Graf, N.; Rath, S. Can prevention of a reherniation be investigated? Establishment of a herniation model and experiments with an anular closure device. Spine 2013, 38, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, R.M.; Andrade, N.S.; Neuman, B.J. Lumbar Disc Herniation. Curr. Rev. Musculoskelet. Med. 2017, 10, 507–516. [Google Scholar] [CrossRef]
- Engel-Yeger, B.; Keren, A.; Berkovich, Y.; Sarfaty, E.; Merom, L. The role of physical status versus mental status in predicting the quality of life of patients with lumbar disk herniation. Disabil. Rehabil. 2018, 40, 302–308. [Google Scholar] [CrossRef]
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef]
- Seki, S.; Kawaguchi, Y.; Chiba, K.; Mikami, Y.; Kizawa, H.; Oya, T.; Mio, F.; Mori, M.; Miyamoto, Y.; Masuda, I.; et al. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat. Genet. 2005, 37, 607–612. [Google Scholar] [CrossRef]
- Long, R.G.; Zderic, I.; Gueorguiev, B.; Ferguson, S.J.; Alini, M.; Grad, S.; Iatridis, J.C. Effects of Level, Loading Rate, Injury and Repair on Biomechanical Response of Ovine Cervical Intervertebral Discs. Ann. Biomed. Eng. 2018, 46, 1911–1920. [Google Scholar] [CrossRef]
- Long, R.G.; Burki, A.; Zysset, P.; Eglin, D.; Grijpma, D.W.; Blanquer, S.B.G.; Hecht, A.C.; Iatridis, J.C. Mechanical restoration and failure analyses of a hydrogel and scaffold composite strategy for annulus fibrosus repair. Acta Biomater. 2016, 30, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Scheibler, A.G.; Gotschi, T.; Widmer, J.; Holenstein, C.; Steffen, T.; Camenzind, R.S.; Snedeker, J.G.; Farshad, M. Feasibility of the annulus fibrosus repair with in situ gelating hydrogels - A biomechanical study. PLoS ONE 2018, 13, e0208460. [Google Scholar] [CrossRef] [Green Version]
- Borde, B.; Grunert, P.; Hartl, R.; Bonassar, L.J. Injectable, high-density collagen gels for annulus fibrosus repair: An in vitro rat tail model. J. Biomed. Mater. Res. A 2015, 103, 2571–2581. [Google Scholar] [CrossRef] [Green Version]
- Sloan, S.R., Jr.; Galesso, D.; Secchieri, C.; Berlin, C.; Hartl, R.; Bonassar, L.J. Initial investigation of individual and combined annulus fibrosus and nucleus pulposus repair ex vivo. Acta Biomater. 2017, 59, 192–199. [Google Scholar] [CrossRef]
- Grunert, P.; Borde, B.H.; Towne, S.B.; Moriguchi, Y.; Hudson, K.D.; Bonassar, L.J.; Hartl, R. Riboflavin crosslinked high-density collagen gel for the repair of annular defects in intervertebral discs: An in vivo study. Acta Biomater. 2015, 26, 215–224. [Google Scholar] [CrossRef]
- Pennicooke, B.; Hussain, I.; Berlin, C.; Sloan, S.R.; Borde, B.; Moriguchi, Y.; Lang, G.; Navarro-Ramirez, R.; Cheetham, J.; Bonassar, L.J.; et al. Annulus Fibrosus Repair Using High-Density Collagen Gel: An In Vivo Ovine Model. Spine 2018, 43, 208–215. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Borde, B.; Berlin, C.; Wipplinger, C.; Sloan, S.R.; Kirnaz, S.; Pennicooke, B.; Navarro-Ramirez, R.; Khair, T.; Grunert, P.; et al. In vivo annular repair using high-density collagen gel seeded with annulus fibrosus cells. Acta Biomater. 2018, 79, 230–238. [Google Scholar] [CrossRef]
- Brockmann, W.; Geiß, P.L.; Klingen, J.; Schrder, B. Adhesion. In Adhesive Bonding; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 11–28. [Google Scholar]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef]
- Ebnesajjad, S.L. Introduction and adhesion theories. In Adhesives Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–19. [Google Scholar]
- Ebnesajjad, S. Theories of Adhesion. In Surface Treatment of Materials for Adhesive Bonding; Elsevier: Amsterdam, The Netherlands, 2014; pp. 77–91. [Google Scholar]
- Jabbari, E.P.; Peppas, N.A. Polymer-Polymer Interdiffusion and Adhesion. J. Macromol. Sci. Polym. Rev. 1994, 34, 205–241. [Google Scholar] [CrossRef]
- Epherre, R.; Goglio, G.; Mornet, S.; Duguet, E. Hybrid magnetic nanoparticles for targeted delivery. In Biocompatibility, Surface Engineering, and Delivery of Drugs, Genes and Other Molecules; Elsevier: Amsterdam, The Netherlands, 2017; Volume 4, pp. 750–771. [Google Scholar]
- Ngo, B.K.D.; Grunlan, M.A. Protein Resistant Polymeric Biomaterials. ACS Macro. Lett. 2017, 6, 992–1000. [Google Scholar] [CrossRef] [Green Version]
- Grunert, P.; Hudson, K.D.; Macielak, M.R.; Aronowitz, E.; Borde, B.H.; Alimi, M.; Njoku, I.; Ballon, D.; Tsiouris, A.J.; Bonassar, L.J.; et al. Assessment of intervertebral disc degeneration based on quantitative magnetic resonance imaging analysis: An in vivo study. Spine 2014, 39, 369–378. [Google Scholar] [CrossRef] [Green Version]


| Cell Sources | References | |
|---|---|---|
| Differentiated Cells | IVD-derived cells (nucleus pulposus (NP)-derived cells) | [25,26,27,28,29,30,31,32,33,34,35,36,37,38] |
| Chondrocyte-like cells (including chondrocytes derived articular cartilage) | [39,40,41] | |
| Stem Cells | Mesenchymal stem cells (MSCs) | |
| Bone marrow-derived MSCs | [26,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59] | |
| Adipose-derived MSCs | [34,60,61,62,63,64,65,66] | |
| Synovial-derived MSCs | [67] | |
| Nucleus pulposus-derived MSCs | [68] | |
| Induced pluripotent stem (iPS) cells | [69,70,71,72,73,74] | |
| Embryonic stem (ES) cells | [75,76,77] | |
| Bone marrow aspirate concentrate (BMAC) | [78,79,80] | |
| Cell Type | Mode | Carrier | Administration Method | Indication | n | Outcome | References | |
|---|---|---|---|---|---|---|---|---|
| Differentiated Cells | Intervertebral disc cells | Autologous | None | Percutaneous injection | Lumbar disc herniation at 12 weeks postoperatively | 112 | Improvement in pain, disc hydration improved on MRI | [28,36] |
| Activated nucleus pulposus cells | Autologous | None | Percutaneous injection | Disc degeneration adjacent to fused disc | 9 | No progression of disc degeneration | [25] | |
| Juvenile articular chondrocytes | Allogenic | Fibrin | Percutaneous injection | Degenerative disc disease with low back pain | 15 | Improvement in pain and clinical indices, and on MRI | [39] | |
| Stem Cells | Bone marrow MSCs | Autologous | Collagen sponge | Percutaneous injection | Lumbar spinal canal stenosis | 2 | Vacuum phenomenon and motion segment instability improved on radiograph, hydration improved on MRI | [51] |
| Bone marrow MSCs | Autologous | None | Percutaneous injection | Chronic low back pain | 10 | Rapid improvement in pain and disability, hydration improved on MRI | [52] | |
| Bone marrow MSCs | Autologous | None | Percutaneous injection | Degenerative disc disease with low back pain | 5 | Self-reported overall improvement, improvement in strength and mobility | [58] | |
| Bone marrow MSCs | Allogenic | None | Percutaneous injection | Degenerative disc disease with low back pain | 24 | Improvement in pain and disability, and on quantitative MRI | [59] | |
| Adipose-derived MSCs | Autologous | Hyaluronic acid | Percutaneous injection | Chronic discogenic low back pain | 10 | Improvement in pain and clinical indices | [64] | |
| Bone marrow concentrate | Autologous | None | Percutaneous injection | Discogenic low back pain | 26 | Improvement in pain and clinical indices | [79,80] | |
| Biomaterials | References | |
|---|---|---|
| Synthetic Biomaterials | Polylactic acid (PLA), Polyglycolic acid (PGA), Polylactic-co-glycolic acid (PLGA) | [38,63,123,124,125,126,127,128,129,130,131] |
| Polyethylene glycol (PEG) | [132,133,134,135,136,137,138,139,140,141,142] | |
| Polycarbonate urethane (PU) | [143,144,145,146,147,148] | |
| Poly epsilon-caprolactone (PCL) | [149,150,151,152,153,154,155] | |
| Natural Biomaterials | Alginate | [7,66,78,92,100,128,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172] |
| Agarose | [4,173,174,175,176,177,178,179] | |
| Fibrin | [39,41,49,90,101,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196] | |
| Hyaluronic acid | [57,64,132,140,142,173,185,188,197,198,199,200,201,202,203,204,205,206,207,208,209,210] | |
| Collagen | [65,132,142,197,210,211,212,213,214,215,216,217,218,219,220,221,222,223] | |
| Chitosan | [66,172,214,218,224,225,226,227,228,229,230,231] | |
| Carboxymethylcellulose | [232,233] | |
| Composition of Soft Biomaterials | Abbreviation | Clinical Trials/Preclinical | Mechanism of Regeneration | Ref. |
|---|---|---|---|---|
| Alginate | UPAL (ultra-purified alginate) | Clinical (in progress)/Preclinical (in vivo, rabbit, sheep) | Induction of endogenous NP cells and NP progenitor cells (GD2Tie2 cells), leading to endogenous IVD repair | [7,246] |
| Collagen | LASCOL (low adhesive scaffold collagen) | Preclinical (in vivo, rat) | Promotion of the formation of cell aggregative spheroids that facilitate the maintenance of the original disc NP phenotype, upregulation of the expression of chondrogenic genes | [220] |
| Fibrin | Fibrin sealant | Clinical/Preclinical (in vivo, rat) | Suppression of the acute proinflammatory cytokine (TNF-α, IL-1β, IL-6) production, increasing expression of pro-resolution cytokines (IL-4, TGF-β), inhibiting nucleotomy-induced progressive fibrosis of the NP | [192,195] |
| Hyaluronic acid | HMW HA (high molecular weight hyaluronic acid microgel) | Preclinical (in vivo, rat) | Regulation of inflammation by downregulating IFNα, reduction in cell death by suppressing expression of IGFBP3 and caspase-3 fragment p17, induction of the production of extracellular matrix | [205] |
| Composition of Soft Biomaterials | IVD Model (Ex Vivo) | Biomechanical Evaluation Method | Outcome | References | |
|---|---|---|---|---|---|
| Fibrin | Genipin cross-linked fibrin | Bovine | Cyclic axial tension–compression, torsion | Full restoration of compressive stiffness, partial restoration of tensile and torsional stiffness | [186] |
| Ovine | Cyclic axial tension–compression, torsion | Restoration of axial range of motion and torque range | [269] | ||
| Bovine | Cyclic flexion–extension, torsion, bending | Restoration of torsional stiffness, bending range of motion, low risk of herniation in bending and compression | [270] | ||
| Bovine | Ramp-to-failure test | Low risk of herniation | [271] | ||
| Collagen hydrogel | Riboflavin cross-linked collagen | Ovine | Cyclic axial tension–compression, torsion | Restoration of torsional stiffness and torque range (combined with nucleus pulposus augmentation using hyaluronic acid) | [223] |
| Rat | Axial compression (uniaxial stress-relaxation) | Improvement in effective equilibrium and instantaneous moduli (combined with nucleus pulposus augmentation using hyaluronic acid) | [273] | ||
| Rat | Axial compression (uniaxial stress-relaxation) | Improvement in effective equilibrium and instantaneous moduli | [272] | ||
| Rose Bengal cross-linked collagen | Rabbit | Cyclic axial compression, torsion push-out test | No extrusion after loading (40,320 cycles with 0.4 to 0.8 MPa compressive loading, 0–25 degree torsion) | [262] | |
| Alginate | Ultra-purified alginate (UPAL) | Ovine | Static axial compression, rotation, flexion–extension, bending, cyclic axial compression | No extrusion after loading (compression loading test up to 1000 N, or 1000 cycles with −300 N to 300 N of axial loading). Partially restored compression stiffness | [7] |
| Chitosan | Triple-interpenetrating-network hydrogel comprised of dextran, chitosan, and teleostean | Human | Cyclic axial compression | No extrusion after loading (10,000 cycles with 0.12 and 0.96 MPa compressive loading) | [230] |
| Cellulose | Carboxymethylcellulose | Bovine | Ramp-to-failure test, fatigue endurance test | Reduction in herniation risk compared to injury group, restoration of failure strength, maximum stiffness, and subsidence to failure. Restoration of fatigue endurance compared to injury group. | [233] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, K.; Iwasaki, N.; Sudo, H. Biomaterials and Cell-Based Regenerative Therapies for Intervertebral Disc Degeneration with a Focus on Biological and Biomechanical Functional Repair: Targeting Treatments for Disc Herniation. Cells 2022, 11, 602. https://doi.org/10.3390/cells11040602
Yamada K, Iwasaki N, Sudo H. Biomaterials and Cell-Based Regenerative Therapies for Intervertebral Disc Degeneration with a Focus on Biological and Biomechanical Functional Repair: Targeting Treatments for Disc Herniation. Cells. 2022; 11(4):602. https://doi.org/10.3390/cells11040602
Chicago/Turabian StyleYamada, Katsuhisa, Norimasa Iwasaki, and Hideki Sudo. 2022. "Biomaterials and Cell-Based Regenerative Therapies for Intervertebral Disc Degeneration with a Focus on Biological and Biomechanical Functional Repair: Targeting Treatments for Disc Herniation" Cells 11, no. 4: 602. https://doi.org/10.3390/cells11040602
APA StyleYamada, K., Iwasaki, N., & Sudo, H. (2022). Biomaterials and Cell-Based Regenerative Therapies for Intervertebral Disc Degeneration with a Focus on Biological and Biomechanical Functional Repair: Targeting Treatments for Disc Herniation. Cells, 11(4), 602. https://doi.org/10.3390/cells11040602






